Abstract
Aims
Metformin’s ability to promote weight loss is believed to be at least partly attributable to decreased energy consumption. There are few data regarding the effects of metformin on energy intake in children. We therefore investigated metformin’s effects on appetite and energy intake in obese hyperinsulinemic children.
Materials and Methods
We conducted a 6-month randomized, double-blind, placebo-controlled trial to evaluate the effects of metformin 1000mg BID on body weight and energy balance in 100 obese hyperinsulinemic children aged 6–12y. Subjects ate ad libitum from standardized food arrays on two separate occasions before, and then again after, 6 months of study medication. The first test meal was consumed after an overnight fast. The second was preceded by a pre-meal load. For each test meal, energy intake was recorded, and subjects completed scales of hunger, fullness, and desire to eat.
Results
Data from the meal studies at baseline and after treatment with study medication were available for 84 children (45 metformin-treated and 39 placebo-treated). Compared with placebo, metformin treatment elicited significant reductions from baseline in adjusted mean energy intake after the pre-meal load (metformin: −104.7±83.8 kcal vs. placebo: +144.2±96.9 kcal; p=0.034) independent of changes in body composition. Metformin also significantly decreased ratings of hunger (−1.5±5.6 vs. +18.6±6.3; p=0.013) and increased ratings of fullness (+10.1±6.2 vs. −12.8±7.0; p=0.01) following the pre-meal load.
Conclusions
These data suggest that decreased perceived hunger resulting in diminished food intake are among the mechanisms by which metformin treatment reduces body weight in overweight, hyperinsulinemic children.
Keywords: Energy intake, metformin, obesity, child, insulin resistance
INTRODUCTION
Metformin is an antihyperglycemic agent approved for use in adults and children age 10 years and above for treatment of type 2 diabetes. Metformin controls hyperglycemia in patients with diabetes by decreasing hepatic glucose output, increasing intestinal glucose utilization, and by improving insulin-mediated glucose disposal in peripheral tissues [1, 2]. Because small weight losses were observed in patients with type 2 diabetes who were treated with metformin [3, 4] and in adults with prediabetes [5], metformin has also been studied for efficacy in pediatric and adolescent obesity [6–11]. For example, we found that, compared to placebo-treatment, metformin reduced body mass index (BMI) by an average of 1.09 kg/m2, and body weight by 3.38 kg, in 6–12y overweight and insulin-resistant children over a 6-month period [6].
Animal studies in both obese Zucker (fa/fa) [12] and streptozotocin-induced [13] rats with diabetes have shown that metformin decreases energy intake. Similarly, randomized controlled trials in both obese non-diabetic adults [14] and non-insulin dependent women with diabetes [15] have reported that, in addition to reducing body weight, metformin treatment leads to a greater reduction in energy intake than placebo treatment. Furthermore, Lee et al [15] showed a dose-response relationship between metformin and food intake with greater dosages of metformin leading to greater reductions in food intake among adults with type 2 diabetes. However, to our knowledge, no randomized controlled trials have evaluated the effect of metformin on energy intake in obese, nondiabetic children.
To investigate the effect of metformin on energy intake in children we conducted a secondary analysis using data from a randomized double-blind placebo-controlled trial of metformin in obese children aged 6–12 years who were hyperinsulinemic but not diabetic [6]. We hypothesized that, compared to placebo-treated children, those randomized to metformin treatment would have 1) reduced energy consumption from laboratory buffet test meals and 2) greater satiation and longer-lived satiety after a standardized meal.
MATERIALS AND METHODS
Study subjects
Obese, insulin-resistant, but otherwise healthy children, between the ages of 6 and 12y were recruited by advertisements in local newspapers and by referral from physicians. Obesity was defined as BMI at or above the 95th percentile for children of the same age and sex.[16]. Included patients were pre-pubertal or had at most early pubertal development (breast Tanner stages I, II, or III for girls; testes size less than or equal to 8 mL for boys) and were hyperinsulinemic (fasting insulin concentration ≥ 107 pmol/L [15 mIU/mL]) [6]. Patients were excluded if they had elevated baseline creatinine (≥ 1.0 mg/dl), significant cardiac or pulmonary disease, hepatic disease with elevated liver enzymes (ALT or AST > 1.5 × the upper limits of normal), evidence for impaired fasting glucose (≥100 mg/dL) or were diabetic (fasting plasma glucose 126 mg/dL or HgbA1C ≥ 6.5%), weight loss of 2% bodyweight within the past 6 months, presence of other endocrinologic disorders leading to obesity, recent use (within six months) of anorexiant medications, or medical treatment for hypertension or dyslipidemia.
Design overview
We conducted a single-center trial [6] at the National Institutes of Health (NIH) Clinical Center. All patients underwent an initial outpatient screening visit, during which eligibility for the study was determined and consent and assent were obtained. Eligible patients were admitted to the NIH Clinical Research Center for initial assessment and then entered into the 6-month randomized placebo-controlled, double-blind treatment period. After 6 months of treatment, participants were again admitted to the NIH Clinical Research Center for final assessment.
Randomization and interventions
Subjects were randomly assigned to metformin hydrochloride (U.S.P. grade, SST Corporation, Clifton, NJ) or placebo capsules, twice daily with meals in a 1:1 randomization ratio as previously described [6]. Starting at 500 mg twice daily, the study medication dose was increased over a 3-week period to a maximum of 1,000 mg twice daily based on tolerability. Medication adherence was assessed by self-reporting of medication use and by counting unused pills at monthly follow-up visits.
Dietitians met with patients and their guardian monthly to administer a weight-reduction lifestyle modification program that promoted a reduced-energy diet, increased physical activity, and decreased inactivity, as previously described [6].
Assessment of Energy intake by laboratory test meal arrays
To ensure that the foods offered at the test meals were acceptable, study subjects were asked to complete a food-preference questionnaire. They rated how much they liked 57 foods that children commonly consume [17] (including the items offered in the food array for the test meal) using 10-point Likert scales with the anchor points: 1 (“I strongly dislike the food”) and 10 (“I love the food”) [18]. At least 50% of the foods in the array had to be rated 6 or more to ensure participants found the food acceptable.
Participants were asked to consume their test meals ad libitum from a multiple-item buffet test array on two separate days during their two-day inpatient stay both at baseline and at the end of the 6-month treatment phase. During their inpatient stay subjects were restricted to sedentary activities. At baseline and at the end of the 6-month treatment phase, samples for fasting plasma glucose, serum insulin [19], and serum leptin [20] were collected.
Assessment of Satiation
After an overnight fast lasting at minimum 12 hours, at 11:00 am on day one of the inpatient stay, subjects recorded their hunger, fullness, and desire to eat on 100 mm line (visual analogue) scales [21, 22] and were brought to a testing room devoid of any sensory stimuli (e.g. pictures, TV, etc.) excluding the food array for the test meal, a chair, and instructions for the food intake study. Subjects were then presented with the food array (Food Array I) and read the food intake instructions inviting them to “let [themselves] go and eat as much as [they] would like”. The food array consisted of 28 items comprised of 9,835 kcal of energy with a wide range of macronutrient compositions including high-fat and carbohydrate foods (Table 1, 12% protein, 51% carbohydrate, 37% fat across all foods). Subjects then self-selected the quantity and types of foods they consumed from the food array. Subjects were monitored from outside the testing room throughout the food intake test. Participants were instructed to knock on the room door when they finished eating. The amount of time spent eating was measured using a stop-watch and recorded as the difference between the time the investigator left the room and the time the participant knocked on the door. This was recorded as time to satiation post-Food Array I. The amount of food consumed was assessed by weighing the food containers before and after eating. Both the nutrient information provided by food manufacturers and the USDA Nutrient Database for Standard Reference were used to determine the energy content and nutrient composition for each food consumed (Table 1). Subjects completed the rating scales on hunger, fullness, and desire to eat immediately after meal consumption.
Table 1.
Foods and beverages used in energy intake tests
| Item | Energy (kcal) |
|---|---|
| 240g American cheese | 901.1 |
| 12 slices white bread | 801.0 |
| 120g peanut butter | 711.6 |
| 90g mayonnaise | 645.1 |
| 120g tortilla chips | 601.2 |
| 120g M&M’s® candy | 590.4 |
| 150g pretzels | 571.5 |
| 12 Oreo® cookies | 566.4 |
| 200g chicken nuggets | 550.8 |
| 120g jellybeans | 440.4 |
| 850g 2% milk | 422.2 |
| 850g apple juice | 400.0 |
| 850g lemonade | 340.0 |
| 120g grape jelly | 339.6 |
| 3 medium bananas | 325.7 |
| 180g turkey | 282.6 |
| 90g light ranch dressing | 240.0 |
| 180g ham | 216.0 |
| 12 Vanilla wafer cookies | 206.3 |
| 3 medium oranges | 184.7 |
| 250g grapes | 177.5 |
| 200g baby carrots | 76.0 |
| 250g mild salsa | 70.0 |
| 90g barbeque sauce | 67.5 |
| 90g mustard | 59.4 |
| 200g tomatoes | 42.0 |
| 50g lettuce | 6.0 |
| 850g bottled water | 0.0 |
| Total | 9835 |
Assessment of satiety
Prior to their second test meal, subjects fasted overnight for a minimum of 12 hours. At 9:00 am on day two of their inpatient stay, subjects were escorted to the same testing room described above and asked to consume an entire milkshake like beverage (Scandishake, Scandipharm, Inc., Birmingham, Alabama). The prepared milkshake contained 787 kcal with 11% protein, 52% carbohydrate, and 37% protein. Subjects were encouraged to consume the entire shake within 20 minutes. Subsequently, subjects completed the visual analog scales estimating the degree of hunger, fullness, and desire to eat. Subjects self-reported onset of hunger post-shake, which was recorded as the post-shake satiety time. At least 2 hours after the pre-meal shake and after report of hunger onset, subjects were again presented with the same food items (Food Array II) used during the satiation phase of the food intake test and asked to eat liberally. The test meal procedure was exactly the same as that used during the satiation assessment. Again, the amount of food consumed was recorded and the time spent eating was recorded as time to satiation. Subjects completed the rating scales on hunger, fullness, and desire to eat as well as the onset of hunger after the food array. Satiety time post-food array was calculated as the time from cessation of food intake to onset of hunger.
The energy content and nutrient composition for each food in Food Array II was calculated as described above (Table 1).
Assessment of Body Composition and Resting Energy Expenditure
Weight was obtained with participants wearing a hospital gown using a calibrated digital scale (Life Measurement Instruments, Concord, CA) and height was obtained in triplicate using a stadiometer (Holtain Ltd., Crymych, UK) calibrated before each measurement. Whole body fat and lean mass were determined by dual-energy x-ray absorptiometry (DEXA, 4500A, Hologic Inc., Bedford MA, software version 11.2). Resting energy expenditure was measured by indirect calorimetry as previously described [23].
Outcomes
The primary study outcome was the change in energy intake during the test meal. Secondary outcome measures were changes in ratings of hunger, fullness, and desire to eat. Tertiary outcome measures were changes in duration of satiety, time to satiation, macronutrient content consumed, and obesity-related hormones (leptin and insulin, measured as previously described [20, 24]).
Statistical Analysis
Values are reported as mean ± standard error of the mean (SEM). Baseline characteristics were analyzed using t test for continuous variables and Chi-square test for categorical variables. Because lean mass is considered the major factor accounting for energy intake or expenditure, we first calculated the residuals for energy intakes at baseline using baseline lean mass and at follow-up using the 6 month lean mass. This approach allows for both the x and y intercepts of the relationship between lean mass and energy intake to be different from 0 and is thus considered superior to approaches wherein intake is simply divided by mass [25]. The changes in outcome measures from baseline to the end of the 6 month study in the metformin group were then compared to the changes for the placebo group using ANCOVA with the covariates age, sex, race/ethnicity, baseline fat mass, and change in fat mass over the 6-month interval. Thus, the analyses account for differences in lean mass and fat mass among participants. Linear regression was used to assess the relationship between change in food intake and changes in the obesity-related hormones. P<0.05 was considered significant. The reported data were analyzed using SPSS for Windows, version 18 (SPSS, Chicago, IL).
The study was approved by the National Institute of Child Health and Human Development (NICHD), NIH, and Phoenix Area Indian Health Service Institutional Review Board and was conducted in accordance with the institutional guidelines regulating subjects’ research.
RESULTS
Clinical characteristics
Clinical characteristics of metformin and placebo treated patients are shown in Table 2. One hundred children were randomly assigned to the two study groups [6]. Eighty-four of these participants (45 metformin-treated and 39 placebo-treated) had baseline and 6-month food intake data and thus were included in this analysis (Table 2). Baseline characteristics did not differ significantly between those who did and did not complete the food intake assessment (all p>0.05) or between treatment groups (all p>0.05, Table 2). Both treatment groups also had similar basal fasting serum glucose, serum insulin, and leptin concentrations (Table 2). At baseline there were no significant differences in energy intake or hunger, satiety, and desire-to-eat ratings between the metformin and placebo groups (Table 2).
Table 2.
Baseline characteristics of study participants
| Metformin | Placebo | P-value | |
|---|---|---|---|
| N | 45 | 39 | 0.55 |
| Age (years) | 10.1 ± 0.25 | 10.3 ± 0.22 | 0.66 |
| Female sex (%) | 35.9 | 44.4 | 0.43 |
| Race/ethnicity (%) | 0.76 | ||
| Non-Hispanic White | 46.7 | 51.3 | - |
| Non-Hispanic Black | 37.7 | 38.5 | - |
| Hispanic White | 6.7 | 7.7 | - |
| Other | 8.9 | 2.6 | - |
| Weight (kg) | 76.7 ± 3.6 | 80.0 ± 3.5 | 0.51 |
| BMI (kg/m2) | 34.8 ± 1.0 | 34.7 ± 1.1 | 0.94 |
| BMI SD score for age and sex | 2.9 ± 0.03 | 2.9 ± 0.04 | 0.45 |
| Total fat mass by DEXA (kg) | 38.9 ± 2.5 | 38.7 ± 2.4 | 0.85 |
| Total lean mass by DEXA (kg) | 37.4 ± 1.3 | 40.7 ± 1.4 | 0.08 |
| REE (kcal/24h) | 1696.4 ± 51.2 | 1700.8 ± 285.1 | 0.95 |
| Hemoglobin A1C (%) | 5.5 ± 0.09 | 5.4 ± 0.07 | 0.16 |
| Fasting serum insulin (pmol/L) | 144.2 ± 10.1 | 138.5 ± 11.5 | 0.51 |
| Fasting plasma glucose (mmol/L) | 5.12 ± 0.07 | 5.06 ± 0.07 | 0.54 |
| Fasting serum leptin (pmol/L) | 1820 ± 168 | 1850 ± 190 | 0.80 |
| Food array I energy intake (kcal) | 1372.3 ± 103.8 | 1350.9 ± 87.8 | 0.95 |
| Pre-Food array I ratings | |||
| Hunger | 68.5 ± 3.7 | 68.3 ± 3.5 | 0.56 |
| Fullness | 21.9 ± 4.0 | 17.9 ± 3.3 | 0.96 |
| Desire to eat | 65.3 ± 2.7 | 58.9 ± 2.4 | 0.11 |
| Post-Food array I ratings | |||
| Hunger | 5.8 ± 1.7 | 3.9 ± 0.7 | 0.25 |
| Fullness | 78.1 ± 3.9 | 80.5 ± 4.3 | 0.30 |
| Desire to eat | 11.7 ± 2.7 | 9.3 ± 3.1 | 0.30 |
| Ratings before pre-meal load | |||
| Hunger | 54.3 ± 4.3 | 57.3 ± 4.3 | 0.63 |
| Fullness | 12.5 ± 2.5 | 13.8 ± 2.7 | 0.73 |
| Desire to eat | 58.6 ± 3.2 | 55.3 ± 3.5 | 0.49 |
| Pre-meal load energy intake (kcal) | 872.0 ± 57.9 | 853.4 ± 34.1 | 0.79 |
| Ratings after pre-meal load | |||
| Hunger | 67.7 ± 4.1 | 68.7 ± 3.8 | 0.77 |
| Fullness | 11.4 ± 2.3 | 12.8 ± 2.7 | 0.61 |
| Desire to eat | 69.3 ± 3.1 | 59.9 ± 3.3 | 0.10 |
| Food array II energy intake (kcal) | 1331.5 ± 101.5 | 1262.5 ± 81.2 | 0.63 |
| Post-Food array II ratings: | |||
| Hunger | 6.6 ± 2.3 | 3.6 ± 0.6 | 0.43 |
| Fullness | 77.1 ± 4.1 | 73.2 ± 5.4 | 0.97 |
| Desire to eat | 9.3 ± 2.7 | 8.6 ± 3.2 | 0.69 |
Data are presented as means ± SEM unless otherwise indicated. At baseline, there were no significant differences between treatment groups. BMI: body mass index; REE: resting energy expenditure
Post-Treatment Results
As previously reported [6], compared to placebo, metformin treatment for 6 months was associated with significantly less gain in BMI (metformin −0.78±0.3 vs. placebo +0.32±0.3 kg/m2, P=0.006), BMI-Z score (−0.11±0.02 vs. −0.04±0.02, P=0.02), weight (+1.47±1.4 kg vs. +4.85±0.5 kg) and body fat mass (+0.48±0.7 vs. +1.88±0.9 kg, P<0.04). Children treated with metformin increased their lean mass by a significantly smaller amount (+1.19±0.36 kg) than placebo-treated children (+3.44±0.45 kg, difference −2.26 kg, CI −1.24 to −3.27, p<0.001) but showed no significant group difference in height velocity (gain in height metformin: +3.11±0.22 vs. placebo: +3.59±0.24 cm, p=0.15). Adherence to the prescribed study medication regimen did not differ significantly among the groups during the randomized phase (93.2±1.3 vs. 92.2±2.3%).
Satiation
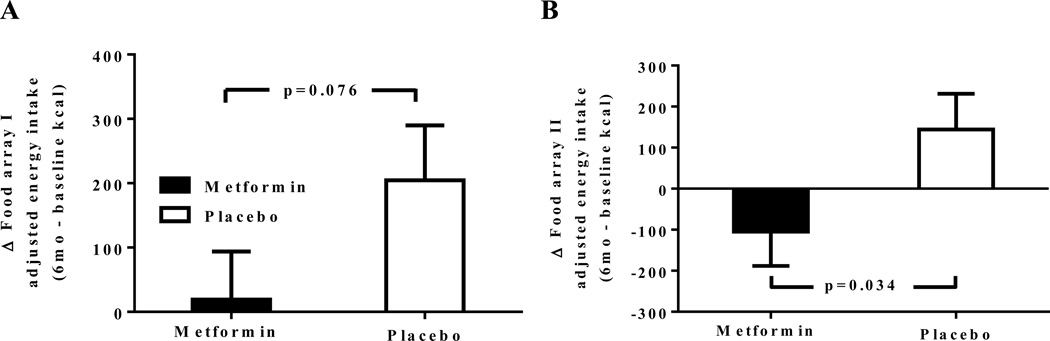
For Food Array I, following the overnight fast, there was a trend towards less of an increase in intake amongst the metformin group compared to the placebo group (Figure 1A, p=0.076). However, pre and post-food array I, changes in hunger, fullness, and desire to eat ratings were similar between both groups (Table 3, p>0.05). Also, time to satiation was similar between treatment groups (Table 3, p>0.05).
Figure 1.
Change in energy intake from baseline to 6 months of treatment adjusted for changes in body composition for Food Array I (Fig 1A, after an overnight fast) and Food Array II (Fig 1B, after a pre-load shake-like beverage). Mean ± SEM of the change in food intake is shown.
Table 3.
Post-treatment changes in hunger, fullness, and desire to eat ratings for Food Arrays
| Pre-Food array I ratings |
Post-Food array I ratings |
Pre-Shake ratings | Post-Shake ratings | Post-Food array II ratings |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | Placebo | Metformin | Placebo | Metformin | Placebo | Metformin | Placebo | Metformin | Placebo | |
| Change in hunger ratings | 5.4 ± 4.5 | −1.3 ± 5.2 | 6.1 ± 3.2 | 5.4 ± 3.6 | 13.4 ± 5.4 | 6.8 ± 6.1 | −1.5 ±5.6* | 18.6 ±6.3 | −0.4 ± 0.8* | 1.8 ±0.9 |
| Change in fullness ratings | −7.6 ± 1.4† | −3.8 ± 1.6 | −9.5 ± 5.3 | −3.4 ± 6.1 | 2.0 ± 3.9 | 2.2 ± 4.5 | 10.1 ±6.2** | −12.8 ±7.0 | −4.0 ±5.6 | −1.1 ±5.3 |
| Change in desire to eat ratings | 7.5 ± 3.5 | 10.6 ± 4.0 | 1.2 ± 3.4 | 5.7 ± 4.0 | 5.6 ± 3.6 | 4.1 ± 4.1 | −7.5 ± 4.1† | 2.5 ± 4.6 | −3.2 ±3.1 | −3.7 ±3.4 |
| Change in time to satiation | N/A | 0.7 ± 0.9 | 0.4 ± 1.0 | N/A | N/A | −2.4 ± 1.2 | −1.2 ± 1.4 | |||
Changes in hunger, fullness, and desire to eat ratings adjusted for covariates including age, race, sex, lean body weight, and fat massfor Food Array I (after an overnight fast) and Food Array II (after a pre-load shake-like beverage). Data are presented as mean ± SEM.
P≤0.01,
P≤0.05 and
P≤0.1 versus placebo-treated group. N/A – not applicable
Satiety
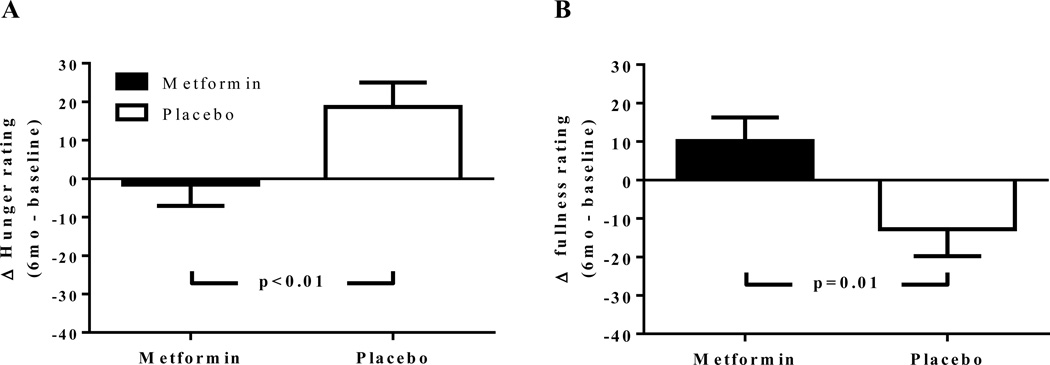
Prior to the pre-meal shake, there were no significant differences in changes for ratings of hunger, fullness, or desire to eat (Table 3, p>0.05). However, despite no significant change in shake energy consumption between groups after 6 months of treatment (metformin: +14.3±38 vs. placebo:+11.2±26 kcal, p=0.95), following the pre-meal shake the metformin group had a significant decrease in hunger ratings (−1.5±5.6 vs. +18.6±6.3; p=0.013; Figure 2A, Table 3) and increase in fullness ratings (+10.1±6.2 vs. −12.8±7.0; p=0.01; Figure 2B, Table 3) compared to the placebo group (Figure 1B). Additionally, metformin-treated children showed a trend towards lower desire to eat following the pre-meal load (p=0.09; Table 3).
Figure 2.
Change in hunger (A) and fullness (B) ratings after the pre-meal milk-shake-like beverage. Mean ± SEM of the change in hunger and fullness ratings from baseline to 6 months of treatment. *P < 0.02
After the pre-meal load, the metformin group had a significant reduction in energy consumption from Food Array II compared to the placebo group (metformin: −104.7 ± 83.8 kcal vs. placebo: +144.2±96.9 kcal; p=0.03; Figure 1). After the testing with Food Array II there were no significant differences between treatment groups in changes for ratings of hunger, fullness, desire to eat, or time to satiation, although there was a trend towards lower hunger following Food Array II for metformin-treated participants (p=0.06; Table 3).
There were no significant differences between groups in macronutrient contents consumed during either of the two food arrays. Furthermore there were no significant associations between changes in energy intake or appetite ratings and insulin or leptin concentrations amongst metformin-treated participants (p’s>0.05). Age was a significant factor predicting the change in energy intake following Food Array II (p=0.016), but the interaction of age and group assignment was not significant (p=0.88). Among participants who were randomized to metformin, 8 did not tolerate full-dose metformin at the 6 month visit and took 500–750 mg BID; 3 given placebo also took reduced doses of medication. 7 participants taking metformin reported nausea or emesis in the prior month (versus 3 taking placebo), and 7 taking metformin (versus 3 taking placebo) reported fatigue. However, the energy intakes of participants who required metformin dose reduction, reported recent nausea or emesis, or reported fatigue did not differ significantly from those of participants who had no reported adverse effects (all p’s >0.30).
DISCUSSION
Compared with placebo treatment, we found that metformin reduced energy intake among hyperinsulinemic obese children, even after adjusting for changes in body mass. Furthermore, we found that metformin improved both satiation and satiety as evidenced by the increase in fullness ratings after the pre-meal load and the reduction in hunger ratings, respectively. Taken together with previous findings that metformin treatment produces consistent reductions in body weight [6, 14, 15, 26, 27], these results suggest that decreased energy intake may be a mechanism through which metformin affects body composition.
Our results are concordant with previous animal [28] and obese adult [14, 15, 29] studies in which metformin therapy reduced both energy intake and body weight. Analogous to our findings, both Paolisso et al [14] and Lee et al [15] found that metformin treatment significantly reduced food intake amongst both healthy adults and obese adults with type 2 diabetes, respectively. Furthermore, Lee et al reported that the effect of metformin on food intake was dose dependent [15]. Similar to our findings, several prior studies have found that metformin significantly lowered hunger ratings post-meal [15] and during hypoglycemic states [30]. However our study is the first to report the inhibitory effect of metformin on food intake and hunger ratings in obese, hyperinsulinemic children.
The mechanism by which metformin inhibits energy intake remains unclear. However several studies have suggested that metformin may modulate the orexigenic neuropeptide Y (NPY) signaling pathway. Duan et al [28] found that mice that are centrally treated with metformin not only had decreased food intake, similar to our findings, but also had reduced expression of hypothalamic NPY. Similarly, Lv et al [13] found that obese diabetic rats treated orally with metformin had decreased food intake and lower expression of both NPY and another orexigenic peptide, agouti-related protein. Furthermore, they were able to demonstrate the presence of metformin in the cerebral spinal fluid of the orally treated rats; suggesting that metformin may cross the blood brain barrier. However, results are conflicting as Rouru et al reported that obese metformin-treated Zucker rats have increased hypothalamic NPY [31]. Thus, additional studies are needed before consensus can be reached regarding the mechanism through which metformin reduces energy intake.
Another hormone that is potentially affected by metformin is leptin. Kim et al found that metformin treatment of high-fat-fed obese rats with high serum leptin at baseline led to a significant reduction in leptin concentrations and that the change in leptin of metformin-treated rats was positively correlated with their reductions in food intake [32]. Paolisso et al also reported that amongst obese, non-diabetic metformin-treated patients leptin concentrations significantly decreased as food intake decreased, independent of sex and changes in body fat [14]. These studies suggested that metformin may possibly play a role in improving leptin sensitivity and lead to reduced food intake, although this result may reflect reverse causality – lower food intake leads to decreased adiposity and lower leptin secretion. We did not find a significant association between leptin and food intake amongst metformin treated participants, though we did find previously that reduction in leptin was associated with reduction in body weight among metformin-treated children [6]. Regardless, our findings suggest that altering leptin sensitivity may not be a main mechanism for metformin’s ability to decrease energy intake.
Glucagon-like peptide −1 (GLP-1) is a third energy-intake related hormone possibly altered by metformin [33]. Maida et al reported that amongst hyperglycemic obese mice, those treated with metformin had significantly higher GLP-1 plasma concentrations and mRNA expression of GLP-1 receptors, as well as a greater reduction in food intake and gastric emptying following treatment [34]. Similarly, Mannucci et al reported that non-diabetic obese men treated with metformin had a significantly greater increase in GLP-1 levels post-glucose load compared to the placebo-treated group [35]. Recently, however, Wu et al reported that while type II diabetic patients treated with metformin had greater GLP-1 plasma concentrations and greater reduction in food intake than placebo-treated patients, combination treatment with a dipeptidyl peptidase-4, a GLP-1 inhibitor, and metformin did not have a significant effect on GLP-1 levels [29]. Together these studies suggest that the effects of metformin on GLP-1 may perhaps help explain the reduction in food intake we observed amongst our hyperinsulinemic, severely obese children. Unfortunately, we did not obtain samples to measure GLP-1 pre- and post-meal. Additional studies are needed to investigate if metformin has a direct effect on GLP-1 levels and if GLP-1 concentrations correlate with food intake amongst metformin-treated subjects.
The findings from this study are limited by the relatively small sample size, which likely led to a relatively low power for some outcomes. The findings from this study are limited by the relatively small sample size, which likely led to a relatively low power for some outcomes, including perhaps energy intake for Food Array I. The finding of a significant difference in energy intake for Food Array II but only a trend for a difference for Food Array I may conceivably be related to the difference in conditions studied: Eating a lunchtime meal after a breakfast meal (Food Array II) might mimic normal consumption better than asking children to fast overnight until lunch time (Food Array I). It is also possible that our approach to control for body weight-dependent effects (by adjusting separately for lean mass at both time points before analysis as well as baseline fat mass and change in fat mass) did not fully account for the weight-related differences between groups. To our knowledge this is the largest study of the effect of metformin on energy intake and the only study done in children. Furthermore, the fact that our findings are similar to several studies in both animals [12, 13] and adults [14, 15] suggests that they are reproducible. The generalizability of these results is also subject to certain limitations. Since our study included only hyperinsulinemic severely obese children, the results of this study may not be generalizable to all obese children. The children in this study were selected for significant hyperinsulinemia, defined as the 99th percentile for fasting insulin among nonobese 6- to 12-year-old children [6] and had a mean BMI of almost 35 kg/m2. However, hyperinsulinemic non-diabetic obese children are at the greatest risk for developing obesity-related comorbid conditions; thus, they are at most need for new obesity treatments. Our study population was diverse (including 37.7% blacks, 46.7% whites, and 35.9% girls), suggesting that the results may be generalizable across these ethnic groups and both sexes. Lastly, though, to our knowledge, our 6-month study on the effect of metformin on energy intake is the longest pediatric trial reported to date, we were not able to study the longer-term effects of metformin on food intake. It is important for future studies to investigate if the effect of metformin on food intake is sustainable long-term. The observation that metformin-treated children increased their lean mass less than placebo-treated children over the six month treatment period of this study may have been caused by a lesser need for lean tissue to support their decreased adipose tissue, but it is unknown if lean tissue accumulation is adversely affected by metformin treatment in the long-term.
In conclusion, we found that the weight-loss effects of metformin treatment may be related to its ability to reduce energy intake in obese, hyperinsulinemic children. Metformin’s effects may thus be useful as an adjunct to diet, exercise, and behavioral therapy in the management of obese children.
ACKNOWLEDGEMENTS
Research support from the Intramural Research Program, National Institutes of Health (NIH), grant 1ZIAHD000641from NICHD with supplemental funding from the National Institute for Minority Health and Health Disparities (NIMHD), NIH (Dr. Yanovski) and 1ZIA-DK-069091 from NIDDK (Dr. Krakoff). MA was supported by the NIH Medical Research Scholars Program (MAA), a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from Pfizer Inc, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute. JAY, MK, and JK are Commissioned Officers in the U.S. Public Health Service, Department of Health and Human Services. The funding organizations played no role in the design or conduct of the study; the collection, management, analysis, or interpretation of data; or the preparation of the manuscript.
The authors thank the participants and their parents for their involvement in this trial. They also thank nurse practitioner Margaret Keil, NIH, post-baccalaureate research trainees Jane Elberg, NIH, Delphine Robotham, NIH, Margaret Mirch, NIH, Margaret Rutledge, NIH, and Rachael Sorg, NIH, and dietitians Nancy Sebring, NIH, Christine Salaita, NIH, Mary Hoskin NIH, and Julie Nelson, NIH, for their assistance in carrying out the study.
Footnotes
Author Contributions: The first draft of the manuscript was written by M.A.A. and J.A.Y. J.R.M, J.A.Y., J.K., and K.A.C. wrote the study protocol. J.R.M., M.K., J.K., K.A.C., S.M.B., and J.A.Y. researched data. M.A.A. and J.A.Y. performed statistical analyses. All authors reviewed/edited the manuscript. Dr. Yanovski takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Author Conflict of Interest Disclosure Summary: All of the authors (MAA, JRM, MK, JK, KAC, SMB, JAY) have nothing to declare.
REFERENCES
- 1.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15:755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2013;121:27–31. doi: 10.1055/s-0032-1327734. [DOI] [PubMed] [Google Scholar]
- 5.Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes care. 2012;35:731–737. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60:477–485. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DM, Abrams SH, Aye T, et al. Metformin extended release treatment of adolescent obesity: a 48-week randomized, double-blind, placebo-controlled trial with 48-week follow-up. Arch Pediatr Adolesc Med. 2010;164:116–123. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA : the journal of the American Medical Association. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freemark M, Bursey D. The Effects of Metformin on Body Mass Index and Glucose Tolerance in Obese Adolescents with Fasting Hyperinsulinemia and a family history of Type 2 Diabetes. Pediatrics. 2001;107:e55, 51–57. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91:2074–2080. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 11.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21:339–348. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda N, Inoue T, Nagakura T, et al. Metformin causes reduction of food intake and body weight gain and improvement of glucose intolerance in combination with dipeptidyl peptidase IV inhibitor in Zucker fa/fa rats. The Journal of pharmacology and experimental therapeutics. 2004;310:614–619. doi: 10.1124/jpet.103.064964. [DOI] [PubMed] [Google Scholar]
- 13.Lv WS, Wen JP, Li L, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain research. 2012;1444:11–19. doi: 10.1016/j.brainres.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Paolisso G, Amato L, Eccellente R, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. 1998;28:441–446. doi: 10.1046/j.1365-2362.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47–53. doi: 10.1002/j.1550-8528.1998.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Block G, Norris JC, Mandel RM, DiSogra C. Sources of energy and six nutrients in diets of low-income Hispanic-American women and their children: quantitative data from HHANES, 1982–1984. J Am Diet Assoc. 1995;95:195–208. doi: 10.1016/S0002-8223(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 18.Hetherington MRB. Methods of investigating human behavior. In: Toates FRN, editor. Feeding and Drinking. Amsterdam: Elsevier Science Publishers B.V.; 1987. pp. 77–109. [Google Scholar]
- 19.Shomaker LB, Tanofsky-Kraff M, Young-Hyman D, et al. Psychological symptoms and insulin sensitivity in adolescents. Pediatric diabetes. 2010;11:417–423. doi: 10.1111/j.1399-5448.2009.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleisch AF, Agarwal N, Roberts MD, et al. Influence of Serum Leptin on Weight and Body Fat Growth in Children at High Risk for Adult Obesity. J Clin Endocrinol Metab. 2007;92:948–954. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolls BJ, Hetherington M, Burley VJ. The specificity of satiety: the influence of foods of different macronutrient content on the development of satiety. Physiol Behav. 1988;43:145–153. doi: 10.1016/0031-9384(88)90230-2. [DOI] [PubMed] [Google Scholar]
- 22.Sepple CP, Read NW. Gastrointestinal correlates of the development of hunger in man. Appetite. 1989;13:183–191. doi: 10.1016/0195-6663(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 23.McDuffie JR, Adler-Wailes DC, Elberg J, et al. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am J Clin Nutr. 2004;80:365–373. doi: 10.1093/ajcn/80.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han JC, Rutledge MS, Kozlosky M, et al. Insulin resistance, hyperinsulinemia, and energy intake in overweight children. J Pediatr. 2008;152:612–617. 617 e611. doi: 10.1016/j.jpeds.2007.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49:968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 26.Munro JF, MacCuish AC, Marshall A, Wilson EM, Duncan LJ. Weight-reducing effect of diguanides in obese non-diabetic women. British medical journal. 1969;2:13–15. doi: 10.1136/bmj.2.5648.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontbonne A, Charles MA, Juhan-Vague I, et al. The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. BIGPRO Study Group. Diabetes Care. 1996;19:920–926. doi: 10.2337/diacare.19.9.920. [DOI] [PubMed] [Google Scholar]
- 28.Duan Y, Zhang R, Zhang M, et al. Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus. Neural Regen Res. 2013;8:2379–2388. doi: 10.3969/j.issn.1673-5374.2013.25.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu T, Ma J, Bound MJ, et al. Effects of sitagliptin on glycemia, incretin hormones, and antropyloroduodenal motility in response to intraduodenal glucose infusion in healthy lean and obese humans, and patients with type 2 diabetes treated with or without metformin. Diabetes. 2014 doi: 10.2337/db13-1627. [DOI] [PubMed] [Google Scholar]
- 30.Schultes B, Oltmanns KM, Kern W, Fehm HL, Born J, Peters A. Modulation of hunger by plasma glucose and metformin. The Journal of clinical endocrinology and metabolism. 2003;88:1133–1141. doi: 10.1210/jc.2002-021450. [DOI] [PubMed] [Google Scholar]
- 31.Rouru J, Pesonen U, Koulu M, et al. Anorectic effect of metformin in obese Zucker rats: lack of evidence for the involvement of neuropeptide Y. European journal of pharmacology. 1995;273:99–106. doi: 10.1016/0014-2999(94)00669-x. [DOI] [PubMed] [Google Scholar]
- 32.Kim YW, Kim JY, Park YH, et al. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55:716–724. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- 33.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes care. 2011;34(Suppl 2):S279–S284. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maida A, Lamont BJ, Cao X, Drucker DJ. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia. 2011;54:339–349. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 35.Mannucci E, Ognibene A, Cremasco F, et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489–494. doi: 10.2337/diacare.24.3.489. [DOI] [PubMed] [Google Scholar]