Abstract
Breast cancer survival rates decrease from 99% for patients with local disease to 25% for those with distant metastases. Matrix metalloproteinases (MMPs), including MMP2, are associated with metastatic progression. We found that loss of host MMP2 reduces the proliferation of experimental metastases in the lungs and identified fibroblasts in tumour-bearing lungs as the major source of MMP2. In vitro, spheroidal mammary tumour growth was increased by co-culture with control fibroblasts isolated from tumour-bearing lungs but not when fibroblasts with stable Mmp2 knockdown were used. This result prompted us to assess whether MMP2 was responsible for a tumour-proliferative, activated fibroblast phenotype. To test this, we evaluated (i) fibroblasts from wild-type tumour-bearing lungs with or without shRNA-mediated MMP2 knockdown and (ii) normal, quiescent fibroblasts isolated from either WT or Mmp2−/− mice. Quantitative PCR revealed that Mmp2 knockdown attenuated expression of two markers of activation (α-smooth muscle actin and vimentin), but there was minimal expression in quiescent WT or Mmp2−/− fibroblasts, as expected. Placing quiescent fibroblasts under activating conditions led to increases in activation-associated transcripts in WT but not Mmp2−/− fibroblasts. Additionally, Mmp2 knockdown fibroblasts showed significantly decreased expression of the matrix transcripts collagen I, collagen IV and fibronectin. Addition of active TGFβ was sufficient to rescue the MMP2-dependent collagen I and IV expression, while MMP2-induced collagen expression was blocked with addition of TGFβ-1 neutralizing antibody. Gene expression data in stromal cells of human breast cancers reveals that MMP2 expression is also positively correlated with activation and matrix transcripts. Thus, we present a model whereby MMP2 production in tumour fibroblasts is important for TGFβ-1 activity and subsequent activation of fibroblasts to a matrix-producing, proliferation-supportive phenotype. Overall, our results reveal a previously undefined role for MMP2 in metastatic outgrowth mediated by fibroblasts, and extend the mechanisms by which MMPs contribute to tumour progression.
Keywords: Pulmonary Metastasis, Breast cancer, Proliferation, Fibroblasts, MMP2, Collagen I, Collagen IV
INTRODUCTION
Cancer progression results from a complex interplay between tumour cells and the extracellular milieu. The influence of microenvironment is of critical importance for metastasis of cancer cells, a strong determinant of patient survival [1]. Breast cancer patients have an overall survival rate of 99% if disease is localized, but this plummets to approximately 25% if diagnosed with metastases [2]. These statistics underscore the importance of understanding and ultimately defining strategies to defeat metastasis. Multiple microenvironmental as well as tumour-derived factors can contribute to metastatic progression [3–5], and key players involved in this process include matrix metalloproteinases (MMPs) [6].
MMPs are a family of 24 zinc-dependent endopeptidases associated with extracellular matrix degradation in health and disease [7]. MMPs are also implicated in the release and processing of growth factors, as well as in angiogenesis and immune surveillance [8–10]. Matrix metalloproteinase 2 (MMP2) is a 72 kDa member of the gelatinase subfamily of MMPs. MMP2 is overexpressed in a variety of malignant tissues compared to normal tissues, such as cancers of the breast, colon, stomach, and lung [11–13]. Increased MMP2 has been associated with advanced stages of breast cancer [14], and decreased overall survival [15]. Its deficiency has been linked to a favourable prognosis in node-negative patients [16]. Although many MMP2 substrates have been identified [9], the exact roles that MMP2 plays in the progression of cancer are still being uncovered.
The majority of MMPs are produced in the tumour stroma [17–19]. Studies using in situ hybridization revealed that MMP2 mRNA is localized to the fibroblast compartment in primary breast cancer tissue [20]. Co-culture of breast cancer cells and fibroblasts enhances MMP protein production, including active MMP2, in fibroblasts [21, 22]. Reciprocally, conditioned media from fibroblasts can enhance tumour growth [22] and the inhibition of MMP2 activity in fibroblasts abolishes pro-tumorigenic effects in nude mice [23]. Further, mice in which Mmp2 was genetically ablated had significantly fewer lung tumour foci in experimental metastasis assays [24]. Collectively these data point to a role for host-derived MMP2 in the metastatic progression of breast cancer. In this study, we set out to identify the mechanism by which stromal fibroblast-derived MMP2 contributes to the outgrowth of pulmonary metastases. We chose to use an experimental metastasis model for in vivo studies, in order to focus on contributions of MMP2 to the later stages of colonization and outgrowth.
MATERIALS AND METHODS
In vivo tumour models
FVB/n and/or C57Bl/6 WT and Mmp2−/− animals were maintained in the Vanderbilt Animal Housing facility and all mouse work was conducted only after review and approval by the local institutional animal care and use committee. Six to eight week old female mice were injected with one million R221A-luc [25] or E0771 cells (CH3 Biosystems, Amherst, NY) via the tail vein. Mice were imaged using the Xenogen 200 imager at defined time points 3 minutes after retro-orbital injection of 120 mg/kg luciferin. Mice were sacrificed at 1 or 2.5 weeks post inoculation. Tumour-bearing or normal lungs were perfused with sterile PBS and used for cell isolation, fresh frozen or formalin fixed for tissue analysis.
Immunohistochemistry and immunofluorescence
After formalin fixation and paraffin embedding, lungs were cut into 5 µm sections. Immunohistochemical staining was performed as previously described [25]. Sources for antibodies used were: Ki-67 (Abcam, Cambridge, MA), phospho-histone H3 (Millipore, Billerica, MA), von Willebrand factor (Dako, Carpinteria, CA), cleaved caspase 3 (Cell Signaling, Danvers, MA), and MMP2 (Abcam). Fluorescent labeling was performed on frozen sections using the following additional antibodies: vimentin (Covance, Princeton, NJ), αSMA (Sigma, Saint Louis, MO), CD31 (BD Pharmingen, San Jose, CA), CD45 (BD Pharmingen). Further details are provided as supplemental information.
Isolation of fibroblasts
Tumour lung-derived fibroblasts were isolated from the tumour-bearing lungs of FVB/n WT mice. Briefly, lung tissue was mechanically separated by mincing and straining through a 70 µm filter, followed by enzymatic digestion (collagenase and hyaluronidase, Sigma) in serum-free DMEM:F12 medium (Life Technologies, Grand Island, NY). The tissue suspension was centrifuged and the pellet washed with sterile PBS containing 5% bovine serum. Cells were then resuspended in DMEM:F12 medium with 5% serum and plated on tissue culture plastic. After 72 hours, tumour cells were separated from fibroblast cells using differential trypsinization. Tumour-derived fibroblasts were cultured in DMEM (Life Technologies) with 10% FBS on tissue culture plastic. Quiescent fibroblasts were similarly isolated from the non-tumour-bearing lungs of WT and Mmp2−/− mice, but instead cultured in DMEM containing 1 or 2.5% FBS on collagen I-coated dishes.
Knockdown of MMP2
shRNA lentiviral particles targeting Mmp2, and control particles were obtained from Sigma, and used to infect lung tumour-derived fibroblasts from WT mice following manufacturer’s recommendations. Particles encoding different shRNA sequences were used independently. Successfully infected cells were selected by culturing in the presence of puromycin. Multiclonal populations were used for subsequent experiments.
Proliferation experiments
For media-transfer assays, R221A-luc cells were suspended in 10% Cultrex (Trevigen, Gaithersburg, MD) and added to Perfecta 3D hanging drop plates (3D Biomatrix, Ann Arbor, MI). After 48 hours, spheroids were treated with control or conditioned media from Shctl or Mmp2 KD cells and this was added every other day to respective wells. At endpoint, spheroids were transferred to flat bottom 96-well plates and fluorescence was measured using a CyQuant NF assay (Life Technologies). For 3D co-culture assays, co-cultures of mCherry-labeled R221A and/or Shctl or Mmp2 KD fibroblasts were embedded in Cultrex (Trevigen) and placed onto a MatTek dish (MatTek, Ashland, MA) pre-coated with Cultrex (Trevigen). Growth media were exchanged every other day. Spheroids were imaged with an Evos microscope (Life Technologies) at predefined intervals over 14 days. Metamorph software (Molecular Devices, Sunnyvale, CA) was used to measure area of red tumour spheroids.
Immunoblotting
Cells were lysed using RIPA buffer (0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100, 10 mM Tris pH 7.4) plus protease and phosphatase inhibitors (cOmplete Mini, EDTA-free and PhosphoSTOP; Roche, Indianapolis, IN). Following SDS-PAGE, protein was transferred to nitrocellulose, blocked and incubated with primary antibodies [MMP2 (Abcam), pSmad 2 (Cell Signaling), or Actin (Sigma)]. Secondary antibodies were directly HRP-conjugated (Cell Signaling) or biotinylated (Vector, Burlingame, CA) and detected with streptavidin-HRP. Chemiluminescent detection was achieved using Western Lightning ECL reagent (PerkinElmer, Waltham, MA).
Quantitative real time RT-PCR
RNA was isolated from fibroblasts using TRIzol reagent (Life Technologies) and an RNeasy mini-prep kit (Qiagen, Valencia, CA) or the Quick-RNA mini-prep kit (Zymo Research, Irvine, CA). Reverse transcription was performed using M-MLV (Promega, Madison, WI). Real-time PCR was performed on a BioRad iQ5 instrument using Maxima SYBR green master mix (Thermo, Pittsburg, PA) according to manufacturer’s instructions. Primer details are provided as supplemental information.
Analysis of microarray datasets
Publicly available microarray expression data for breast cancer stroma isolated by laser capture microdissection (gene set: GSE33692) was obtained from the NCBI GEO website. Excel files were uploaded and analyzed on Affymetrix Genespring GX 12.5. Following baseline normalization, expression values for a given gene were imported into Graphpad Prism for correlation analysis of gene expression in each sample, generation of linear trend-lines, and statistical analysis as previously described [26].
Statistical analysis
One way analysis of variance (One way ANOVA) was used for multiple group parametric comparisons using a Bonferroni post-hoc analysis. To compare two groups, a Student’s t-test was used for parametric analyses and Mann-Whitney for non-parametric analyses. Statistical significance was considered p<.05 and is indicated by an asterisk. All statistical analyses were conducted using Graphpad Prism software.
RESULTS
Host-derived MMP2 potentiates the proliferation of pulmonary experimental metastases
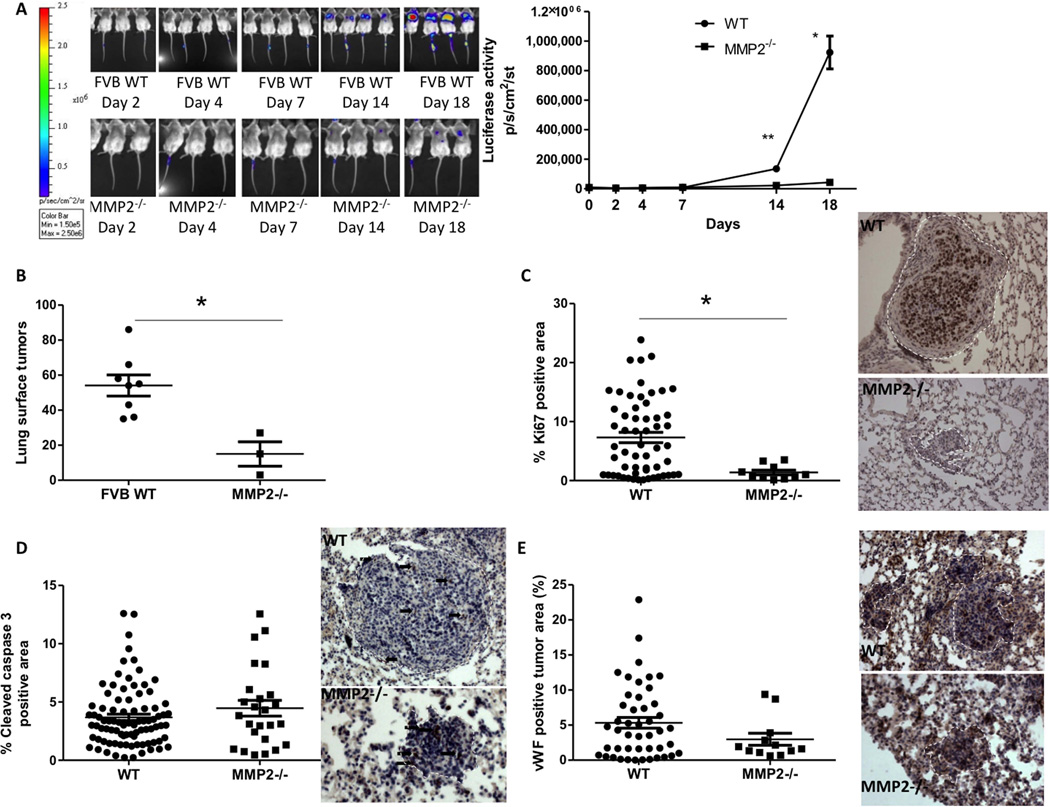
To determine the role of host MMP2 in the outgrowth of mammary-to-lung metastases, immunocompetent FVB WT or Mmp2−/− mice were intravenously injected with syngeneic R221A-luc cells [25]. In vivo outgrowth was measured over time by luciferase imaging. A significant reduction in both the luminescence signal (Figure 1A) and the number of lung surface lesions (Figure 1B) was observed in Mmp2−/− mice compared to WT mice. At the 18-day endpoint, analysis of proliferation demonstrated a significant reduction of Ki67+ staining in tumours from Mmp2−/− animals (Figure 1C), with no change in apoptosis or vascularity (Figure 1 D–E). An independent repeat of this experiment with 5 wild-type and 4 Mmp2−/− mice gave the same results (data not shown). Initial growth between WT and Mmp2−/− animals was similar until approximately day 8, suggesting that initial seeding was similar in both genotypes. To evaluate early tumour growth, a second study was conducted in which mice were sacrificed after 7 days. At this time-point, there was no discernible difference in tumour burden or growth rate as determined by luminescent signal between WT and Mmp2−/− mice (Supp Fig 1A). However, immunohistochemical analysis of the tumour foci present revealed that proliferation (phospho-histone H3 staining) was significantly lower in tumours growing in Mmp2−/− mice, confirming our previous finding (Supp Fig 1B). Additionally, apoptosis was also reduced, as measured by cleaved caspase 3 (Supp Fig 1C). The reduction in proliferation was also observed in a second, slower-growing model (E0771 cells in C57Bl/6 WT and Mmp2−/− mice) suggesting that MMP2-dependent tumour cell proliferation is a general phenomenon (Supp Fig 2). Together, these studies suggest that host MMP2 contributes to the outgrowth of mammary tumours in the lungs by stimulating tumour cell proliferation.
Figure 1. Host MMP2 contributes to the outgrowth of pulmonary metastases.
(A) Luciferase activity in WT and MMP2−/− mice was analyzed over three weeks using IVIS imaging software and the resulting average radiance is shown for each imaging time-point. *p=0.02, **p=0.01 (B) Visible macro-metastases on the lung surfaces for WT and MMP2−/− mice were manually counted and the total number obtained per mouse is shown. p=0.0055 (C) Quantitation of positive signal for Ki67 as a marker of proliferation per unit area of tumor within lung tissue sections. Examples of the staining are shown on the right with brown (diaminobenzidine) stain being a positive signal. p=0.005 (D) Quantitation of positive signal for cleaved caspase 3 as a marker of apoptosis per unit area of tumor within lung tissue sections, p=n.s. Examples of the staining are shown on the right, with brown (diaminobenzidine) stain being a positive signal. The white dashed lines indicate the tumour areas analysed. Arrows point to examples of positive cells within the tumours. (E) Quantitation of positive signal for von Willebrand factor as a marker of blood vessels per unit area of tumour (indicated by white dashed line) within lung tissue sections. Examples of the staining are shown on the right with brown (diaminobenzidine) stain being a positive signal. p=n.s.
MMP2 primarily localizes to fibroblasts
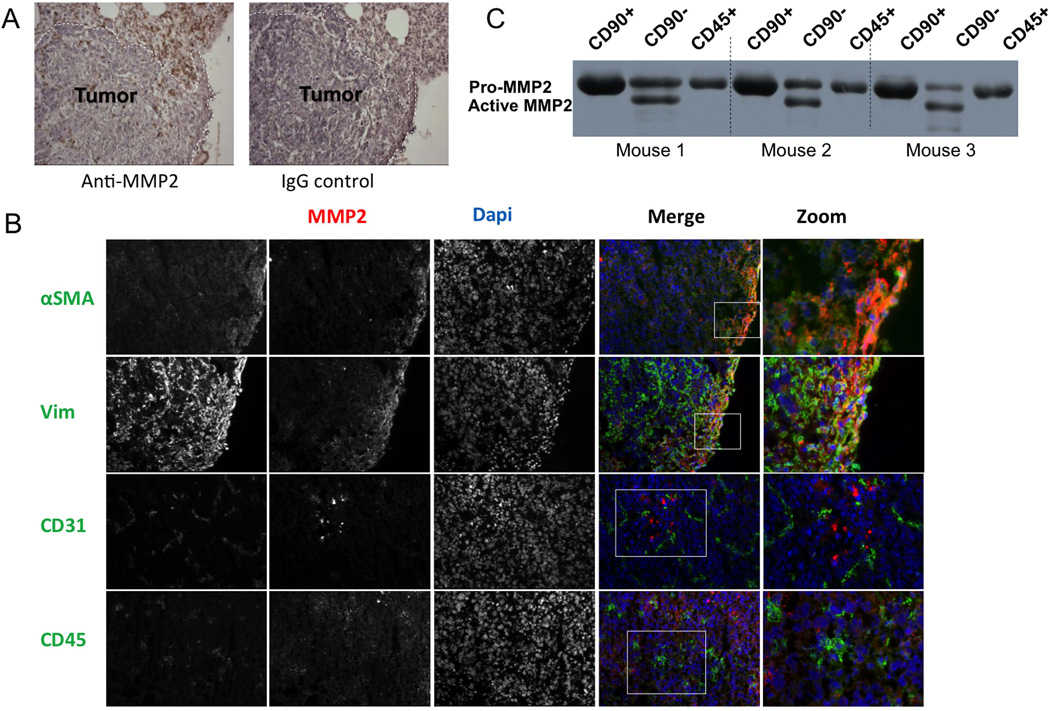
Immunohistochemistry was used to localize MMP2 in tumour-bearing lung sections. We observed MMP2 expression mostly in stromal cells around the perimeter of lung metastases (Figure 2A) and by cells between tumour cell nests. To identify the specific stromal cell types that expressed MMP2, we performed a series of dual immunofluorescence analyses in which tissue sections were co-stained for MMP2 and one of a variety of stromal cell markers. Within the tumour microenvironment, MMP2 was mainly co-expressed with vimentin and α-SMA (Figure 2B), which are commonly accepted as markers of activated fibroblasts [27, 28]. MMP2 was sporadically co-expressed with CD31 and more frequently with CD45, representing endothelial and hematopoietic cell populations, respectively (Figure 2B). Indeed, MMP2 expression has been associated with myeloid cells recruited to tumors previously [29, 30]. To further characterize the extent of MMP2 expression by hematopoietic versus other stromal cells, we prepared single cell suspensions from 3 tumour-bearing mice and isolated different stromal populations using magnetic beads conjugated to either anti-CD45 or anti-CD90 antibodies. Cells representing the CD45+, or CD45−/CD90+, or CD45−/CD90− populations were then cultured overnight in serum-free medium, or harvested for RNA. As shown in Fig 2C, the MMP2 levels were lowest in the CD45+ population, and were higher and in the active form only in the CD45−/CD90− population, which for pulmonary fibroblasts are thought to represent activated myofibroblasts [31–33]. Real-time PCR of fibroblast-specific protein (FSP) and fibroblast-activation protein (FAP) confirmed this concept (Supp Fig 3). Levels of the transcript for the polyoma viral antigen (pyvt) expressed by tumour cells were equally low in the CD45-negative samples, confirming the stromal nature of the cell populations (Supp Fig 3). Taken together, these studies suggest that tumour-associated myofibroblasts are the major source of MMP2 in our model.
Figure 2. Tumour-adjacent fibroblasts are the main cellular source of MMP2.
(A) Immunohistochemical staining of MMP2 in tumour-bearing lung sections from FVB WT mice. Positive staining (brown) was noted in the stroma surrounding tumor metastases. Image on the right shows signal obtained when an isotype control antibody is used. (B) Co-immunofluorescent staining for MMP2 and either vimentin (Vim), alpha-smooth muscle actin (aSMA), CD31 or CD45. Nuclei were counterstained with DAPI. The signal for each individual maker is shown in greyscale in the first 3 columns, with a merged image in the fourth column, where red is MMP2, blue is DAPI and green represents the specific cell-type marker. A magnified view of a portion of the merged image is shown on the right. (C) Levels of MMP2 protein (latent and active) as detected by western blotting of 24-hour conditioned media normalized for protein content, from CD45−/CD90+, CD45−/CD90−, or CD45+ stromal cells isolated from lungs of tumour-bearing mice (n=3).
Fibroblast-stimulated tumor cell proliferation requires MMP2
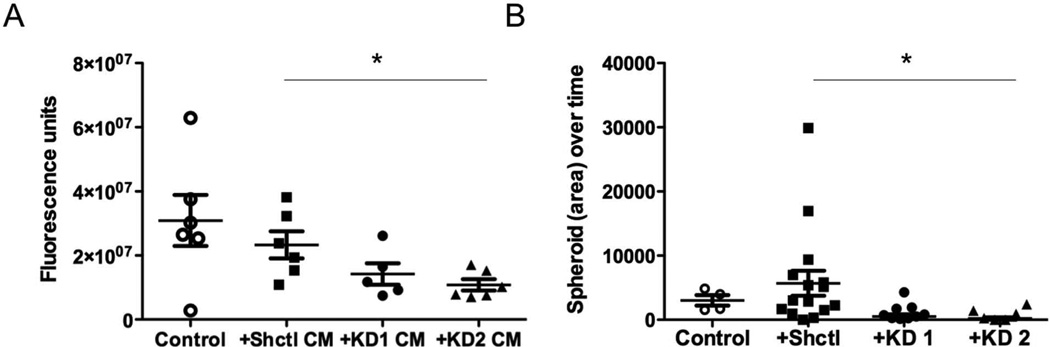
The dominant phenotype associated with loss of stromal MMP2 was reduced proliferation, and previous studies have demonstrated that tumour cell proliferation can be stimulated by tumour derived fibroblasts both in vivo and in vitro [34]. Therefore, we next investigated whether Mmp2-deficient fibroblasts could alter the growth of tumour cells in vitro compared to WT activated fibroblasts. We first isolated fibroblasts from the tumour-bearing lungs of WT mice. The mice were injected with tumour cells 14 days prior, and thus we term these fibroblasts as ‘tumour-bearing lung-derived fibroblasts’. The fibroblasts were grown on tissue culture plastic in the presence of serum, and resembled activated myofibroblasts. We then performed stable knockdown of Mmp2 mRNA and protein in the WT cells by infection with lentiviral particles carrying one of three different shRNA sequences to Mmp2 (Supp Fig 4). Because 3-dimensional (3D) conditions are more representative of the in vivo environment and are often required for proliferative effects in vitro [35], we investigated 3D tumour growth after conditioned media treatments or co-culture with Shctl or Mmp2 KD tumour-derived fibroblasts. There was a significant decrease in proliferation when tumour cells were treated with conditioned media from Mmp2 KD cells compared with parental or Shctl cells (Figure 3A). Additionally, when tumour cells were co-cultured in direct contact with fibroblasts, there was also a significant reduction in the rate of spheroid growth when the co-culture contained Mmp2 KD versus Shctl fibroblasts (Figure 3B). These results indicate that fibroblast MMP2 potentiates a tumour proliferation-enhancing function of activated fibroblasts.
Figure 3. Tumour cell proliferation is enhanced by MMP2-positive, but not MMP2-negative, lung tumour fibroblasts in vitro.
(A) Proliferation analysis of tumour spheroids embedded within basement membrane extract in the presence or absence of control or Mmp2 KD fibroblast conditioned media. Fluorescence units indicating DNA content as measured by Cyquant assay are shown. p=0.042 (B) Comparison of spheroid area over time in co-cultures of tumour cells and control or Mmp2 KD fibroblasts following embedding in basement membrane extract. p=0.0037.
Fibroblast activation status is dependent upon MMP2 expression
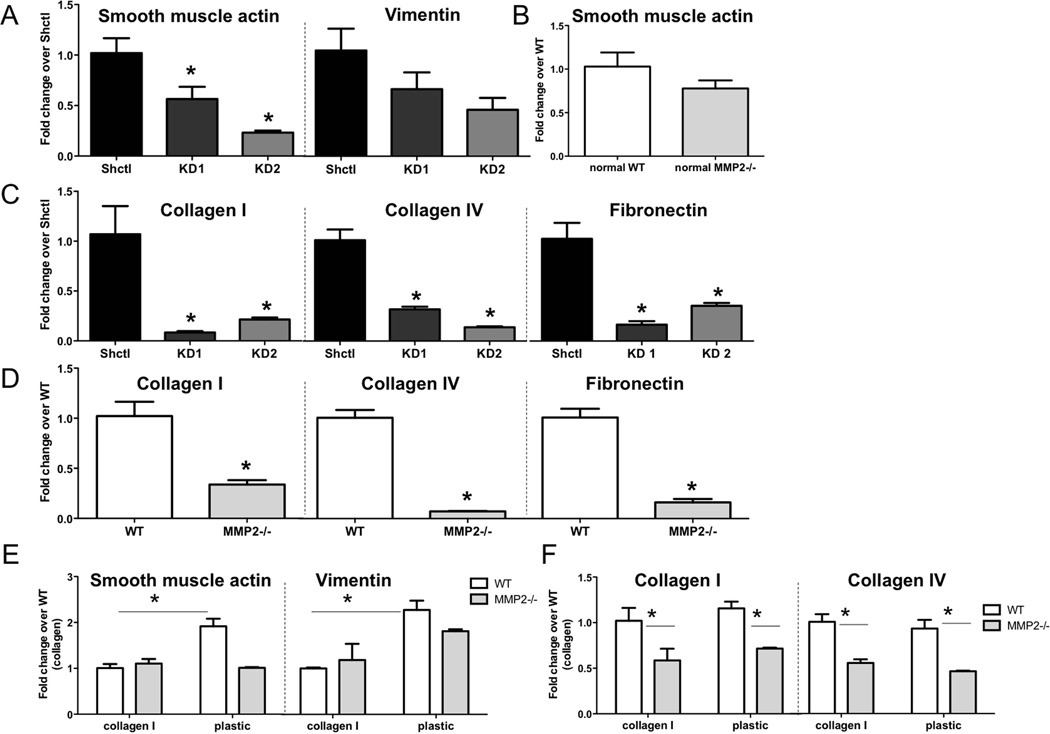
We next wanted to investigate whether MMP2 altered fibroblast phenotype. The change of fibroblasts from a quiescent to an activated state is associated with tumour-promoting effects, and we postulated that MMP2 may contribute to this change. Quantitative RT-PCR was used to measure the levels of mRNA transcripts associated with fibroblast activation state. For these analyses, we used two fibroblast models. Firstly, we isolated RNA from activated tumour-bearing lung derived fibroblasts described above that were either proficient (Shctl) or deficient in Mmp2 (Mmp2 KD). Secondly, we isolated quiescent fibroblasts from non-tumour bearing lungs of either WT or Mmp2−/− mice. In isolating these fibroblasts, we endeavoured to prevent activation as much as possible by using collagen-coated dishes to prevent contact with plastic, and minimizing serum levels [36, 37]. In activated cells, knockdown of Mmp2 led to a significant reduction in the activation status of fibroblasts compared to control cells (Figure 4A). Additionally, there was a significant reduction in the mRNA levels of matrix molecules, including collagens I and IV and fibronectin (Figure 4C). The lack of MMP2 in quiescent cells appeared to make no significant difference in baseline levels of the activation marker smooth muscle actin (Acta2) from those of WT cells (Figure 4B). However, Acta2 levels in the quiescent cells, irrespective of Mmp2 status, were significantly lower than in the Shctl fibroblasts (Supp Fig 5A), as was Mmp2 itself (Supp Fig 5B). This result was expected, since we strived to maintain the cultures in a quiescent state. Similarly to activated Mmp2 KD cells, the lack of Mmp2 in quiescent cells was also associated with significantly lower levels of transcripts for collagens I and IV as well as fibronectin (Figure 4D). We also tested whether the quiescent cells could be activated by tumour cell-derived soluble factors, as might happen within a tumour microenvironment. As shown in Supp Fig 5C, WT but not Mmp2−/− quiescent fibroblasts showed increases in several transcripts associated with the activated phenotype after exposure to tumour cell-conditioned medium.
Figure 4. MMP2 is necessary for activation signature and matrix transcript expression in fibroblasts.
(A) Expression of Acta2 and Vim transcripts in Mmp2 KD fibroblasts relative to Shctl as determined by quantitative real time PCR. Levels were normalized using Gapdh and analyzed using the comparative Ct method. *p<0.05 (B) Expression of Acta2 transcripts in quiescent Mmp2−/− fibroblasts relative to WT. p=n.s. (C–D) Expression of ColI, ColIV, and Fn1 transcripts in (C) Mmp2 KD fibroblasts and (D) quiescent Mmp2−/− fibroblasts relative to Shctl or WT cells, respectively. All values normalized to Gapdh of control cells. *p<0.01 (E–F) Expression of (E) Acta2 and Vim or (F) ColI and ColIV transcripts in fibroblasts from non-tumour-bearing lungs of WT or Mmp2−/− mice maintained under quiescent or activating culture conditions. Values are normalized to WT cells cultured under quiescent conditions. *p<0.05.
We next investigated if the lack of Mmp2 would impact the activation of quiescent fibroblasts. Quiescent WT or Mmp2−/− fibroblasts were grown on collagen-coated dishes for quiescent culture, and were switched to tissue culture plastic to allow for activation. This system models the stiffness-associated activation of fibroblasts reported in the literature [38–41]. When WT quiescent cells were switched to tissue culture plastic, there was a significant increase in Acta2 and vimentin mRNA transcripts compared to when the cells were grown on collagen, suggesting that these cells could be activated. In contrast, when Mmp2−/− quiescent cells were grown on tissue culture plastic, there was no significant difference in Acta2 or vimentin levels compared to those cultured to maintain quiescence (Figure 4E). In fact, the Acta2 transcript levels of Mmp2−/− cells grown on plastic remained at a similar level to those grown on collagen. This result indicates that these cells were not changing to an activated state. Examination of mRNA levels of collagens I and IV in quiescent fibroblasts after 24 hours on plastic revealed no change in expression from those grown on collagen (Figure 4F). However, these transcripts were lower in the Mmp2−/− cells, irrespective of culture surface. Our studies suggest that MMP2 expression regulates fibroblast activation status and expression of extracellular matrix transcripts.
MMP2-dependent fibroblast activation and collagen expression is mediated by TGFβ-1
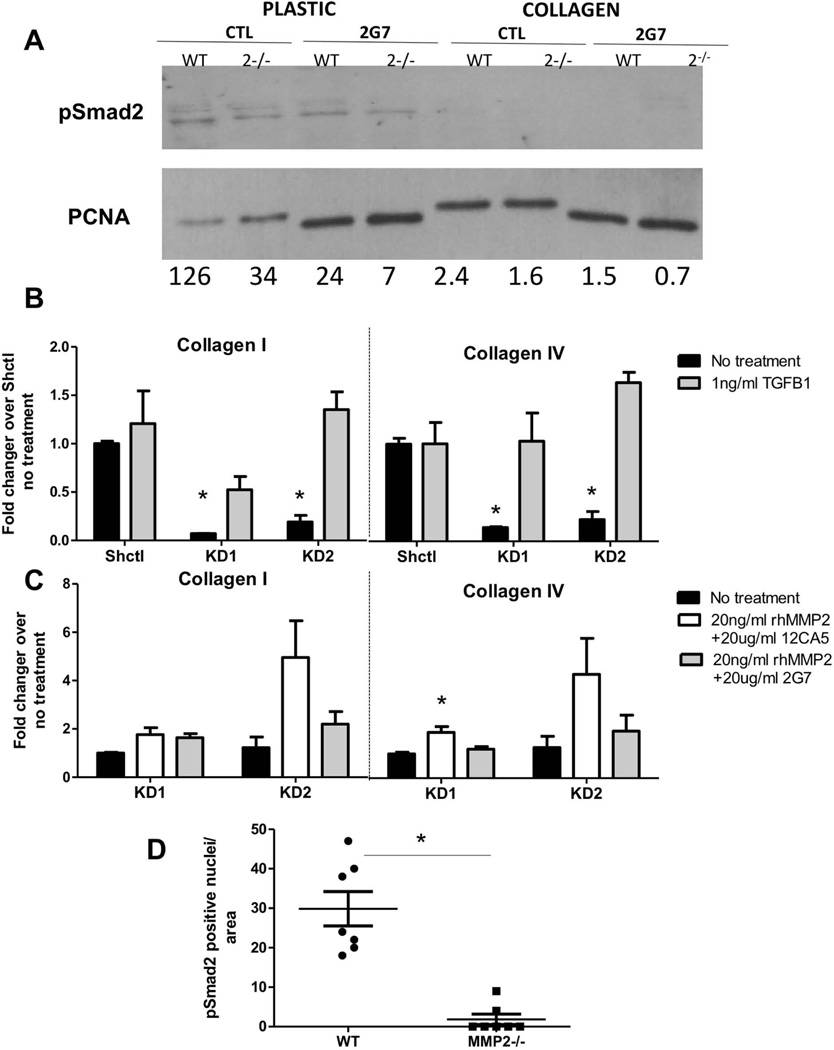
We next investigated the underlying cause of reduced fibroblast activation. TGFβ-1 is a known activator of fibroblasts in wound healing and fibrosis [42] and is a recognized promoter of the differentiation of fibroblasts to myofibroblasts in breast cancer [43]. We first tested whether the activation of quiescent fibroblasts by culture on plastic was associated with activation of the canonical TGFβ-1 signaling pathway. Lysates from quiescent WT or Mmp2−/− fibroblasts either grown on collagen or after 24 hours on plastic in high serum were analysed for presence of pSmad2, the immediate downstream effector of TGFβ–1 receptor activation [44]. As shown in Figure 5A, lysates of fibroblasts cultured on plastic, but not collagen, demonstrated measurable levels of pSmad2. To show that this was indeed directly related to TGFβ–1, we used a TGFβ-1 neutralizing antibody (2G7) or isotype control (12CA5) in these cultures and found that the increased pSmad2 associated with growth on plastic was ameliorated. We then turned to the tumour-bearing lung-derived fibroblasts to assess whether TGFβ–1 signaling was critical for their activation. Previous work from our laboratory demonstrated that MMP2 can release TGFβ-1 from its latent binding partner LTBP3, thereby allowing active TGFβ-1 to initiate signaling and exert its downstream effects [45]. We thus tested whether we could rescue the decreased matrix production phenotype in Mmp2 KD cells by the addition of active TGFβ-1. Collagen I and IV mRNA transcripts increased in Mmp2 KD fibroblasts in response to active TGFβ-1 (Figure 5B) to similar levels as shctl cells. Notably, shctl fibroblasts showed no effect, suggesting they were already maximally responsive. To test whether addition of MMP2 to Mmp2 KD cells could revert the phenotype in a manner dependent on TGFβ–1, we used exogenous recombinant active MMP2 in the presence of the 2G7 TGFβ-1 neutralizing antibody or isotype control antibody. In Mmp2 KD fibroblasts, collagen expression was stimulated by recombinant active MMP2 but not in the presence of the TGFβ-1 neutralizing antibody (Figure 5C). As expected, addition of recombinant MMP2 to shctl cells, like TGFβ-1, had no effect (data not shown). Since these data suggested a critical link between MMP2 and active TGFβ–1 signaling in fibroblasts, we returned to the tumor model to test in vivo relevance. Immunofluorescent staining of pSmad2 showed significantly higher levels associated with tumours in wild-type mice compared to Mmp2−/− mice (Figure 5D, Supp Fig 6). Taken together these results are consistent with a model whereby MMP2 regulates the phenotype of tumour-derived fibroblasts by activating TGFβ-1.
Figure 5. Active TGFB-1 is sufficient to rescue the collagen expression phenotype of lung tumour fibroblasts.
(A) Immunoblot for phosphorylated Smad2 and PCNA (loading control) from quiescent WT or Mmp2−/− fibroblasts grown on collagen or after transfer to culture on plastic, in the presence of a TGFβ-neutralizing antibody (2G7) or isotype control (12CA5). Numbers below each lane indicate level of pSmad2 corrected for loading control. (B) Expression of collagen I and collagen IV transcripts in Shctl or Mmp2 KD fibroblasts treated with 1ng/ml active mouse TGFβ-1. Values are relative to Shctl no treatment, using Gapdh levels for normalization. (C) Expression of collagen I and collagen IV transcripts in Mmp2 KD fibroblasts treated with 20 ng/ml rhMmp2 and either 20 µg/ml 12CA5 control IgG or 20 µg/ml 2G7 TGFβ–1 neutralizing antibody. Values are relative to no treatment, using Gapdh levels for normalization. *p<0.05. (D) Levels of pSmad2 in sections of tumor-bearing lungs from WT or Mmp2−/− mice. p =0.0017
MMP2 correlates with collagen signatures in stroma of breast cancer patients
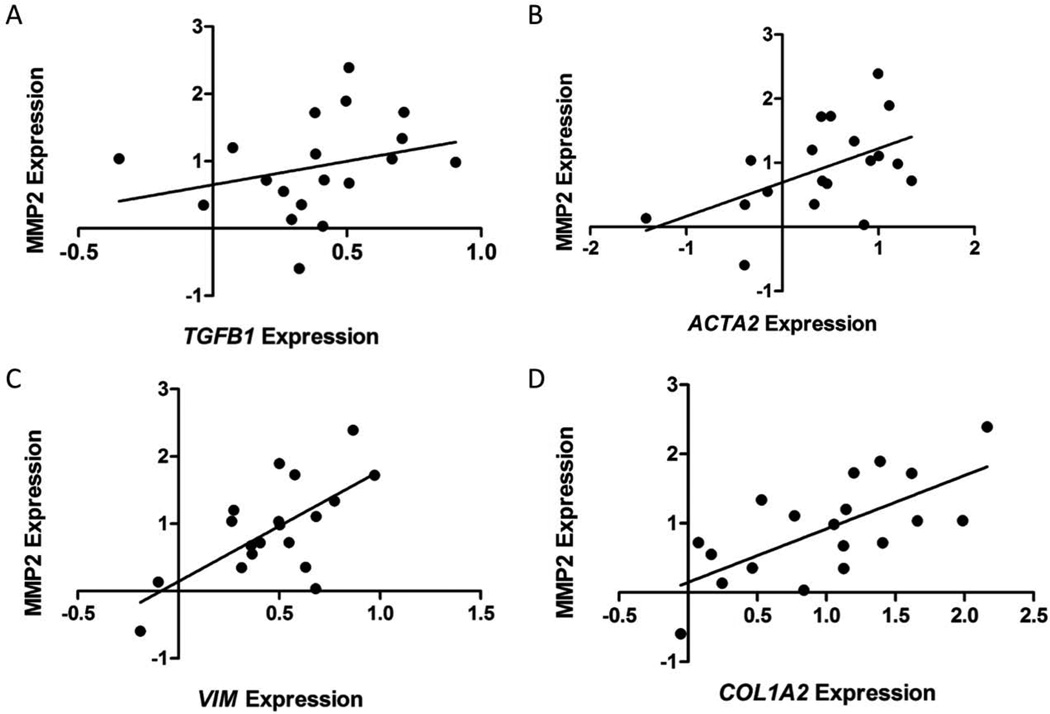
Our data are supportive of an MMP2-dependent collagen signature in mouse models. We next confirmed the relevance of these findings to human breast cancer patients. We used a publicly available dataset, GSE33692 from Knudsen and colleagues [46], which comprised stromal tissue from 45 breast cancer patients with ductal carcinoma in situ or invasive ductal carcinoma, to explore the relationships between expression of MMP2 and various stromal molecules. As expected from our proposed model of MMP2-mediated TGFβ–1 activation, there was no significant correlation between MMP2 and TGFβ-1 expression (Figure 6A). There were however, significant correlations between expression of MMP2 and the activation markers ACTA2 and vimentin (Figure 6B–C). Importantly, MMP2 expression correlated with collagen I expression in the stromal compartment of human breast cancers (Figure 6D).
Figure 6. MMP2 levels correlate with expression of several fibroblast-associated transcripts in the stromal component of tumour tissue from breast cancer patients.
Correlations between levels of MMP2 expression and (A) TGFβ1 (p=0.247), (B) ACTA2 (p=0.025), (C) VIM (p=0.0019) or (D) COL1A2 (p=0.0012) expression in breast tumour stroma from 45 patients. Data were extracted from publicly available dataset GSE33692.
DISCUSSION
Our data show that in two different models and genetic strains (PyVT-R221A cells in FVB/n mice and EO771 cells in C57BL/6 mice), lung tumours proliferate less in the absence of host-derived Mmp2. These results echo findings from other metastasis models that demonstrated reduced tumour growth when MMP2 was absent from host cells [24]. Both spontaneous and experimental metastases of B16 melanoma and Lewis Lung Carcinoma (LLC) cells were reduced in Mmp2−/− animals. In contrast to our findings, however, the effects were attributed to reduced angiogenesis in mutant animals. We observed no significant change in overall vascularity between WT and Mmp2−/− animals, but rather reduced tumor cell proliferation. Our assessment of vascularity was performed using tumour foci of similar sizes to allow direct comparison. The localization of MMP2 to fibroblasts is supported by previous studies conducted in primary breast cancers [20, 47], and suggests that these cells play an integral role in MMP2 dependent tumour cell proliferation.
Our in vitro proliferation findings show that there was a significant decrease in the growth of tumor cells treated with conditioned media from Mmp2 KD cells in 3D compared to media from control cells. Significant decreases in tumour cell proliferation were also seen when Mmp2 KD fibroblasts and tumour cells were in direct contact compared to control fibroblasts. Together these results suggest a role for soluble growth factors, and perhaps also contact-mediated effects. Identification of relevant soluble factors is ongoing; however, matrix molecule production by the fibroblasts may also play a role. Previous studies have shown that modulation of collagen density and architecture by fibroblasts leads to tumour cell proliferation and progression in mouse models of breast cancer [48, 49].
Many studies have shown that tumour-associated fibroblasts exhibit an activated phenotype that resembles differentiation into myofibroblasts. These activated cells enhance tumour growth both in vivo and in vitro [34, 50]. Because MMP2 localized to fibroblasts in vivo and its loss impacted tumour growth, we investigated if reduced proliferation could be due to altered fibroblast activation. Indeed we found that in the absence of MMP2, tumour-associated fibroblasts exhibited reduced activation, as assessed by Acta2 and matrix collagen expression. Additionally, quiescent fibroblasts exposed to activation-inducing conditions were unable to become activated in the absence of Mmp2, suggesting that Mmp2 is required for full fibroblast activation. Independent of fibroblast activation status, however, we found that reduced Mmp2 expression is associated with reduced collagen expression. In support of this, studies of cardiac fibrosis have found that Mmp2 stimulates collagen I expression in cardiac fibroblasts and that this occurs through FAK phosphorylation [51], which is necessary to mediate some TGFβ-1 dependent matrix remodeling [52].
TGFβ-1 is an important mediator of the transition from quiescent to a reactive stroma [44]. In fibrotic conditions, TGFβ-1 induces the differentiation of quiescent fibroblasts to myofibroblasts. These activated fibroblasts then release proteases and cytokines that induce the activity and production of TGFβ-1, creating a feed-forward loop and continual cycle of matrix remodeling. More importantly for our studies, activation of the TGFβ-1 pathway leads to the production of collagens. This, coupled with previous studies from our lab [45], prompted us to investigate TGFβ-1 as the molecular mediator of Mmp2-dependent collagen expression. Indeed, we found that active TGFβ-1 was sufficient to restore collagen expression in the absence of Mmp2. Additionally, Mmp2-dependent increases in collagen expression could be ablated by neutralization of TGFβ-1 and not with control antibody. Studies of fibrosis similarly found that MMP2 was essential for active TGFβ-2-induced fibrosis and matrix contraction [53].
While high mammographic density, which is associated with increased fibrillar collagen [54, 55], is a known risk factor for breast cancer development [56], there is also an association of increased collagen with aggressiveness [57] and metastatic lesions [58]. Furthermore, COL1A1 and COL1A2 were part of a 17-gene signature associated with decreased survival in multiple primary solid tumors [59]. Our correlative studies of MMP2 and collagen expression support a model in which increased MMP2 expression is associated with collagen in the stroma of breast cancer patients. These studies shed light on a novel mechanism whereby MMP2 promotes breast tumor progression by mediating increased collagen expression. Although MMP2 expression may be protective in some disease settings [60, 61], this appears not to be true in breast cancer.
Stromal MMP2 expression and its pleiotropic effects present a possible therapeutic target for breast cancer patients. Past cancer clinical trials used broad spectrum inhibitors with debilitating side effects to target multiple MMPs [62, 63]. However, we now realize the importance of using selective inhibitors, due to the detrimental effects of inhibiting protective MMPs as well as the importance of cell type-specific MMP production. Our studies reveal a new role for MMP2 in breast cancer progression by enhancing tumor cell proliferation, potentially via regulating collagen in fibroblasts. Reduction of MMP2 levels in an effort to curb tumour-promoting collagen expression might provide improved treatment modalities for breast cancer metastasis.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Julie Sterling (Vanderbilt Medical Center for Bone Biology) for generously providing TGFβ-1 neutralizing antibody and control antibody, Dr. Michael VanSaun for providing reagents and advice, and Dr. H Moses (Vanderbilt Department of Cancer Biology) for providing various reagents. This work was supported by funding from the US National Institutes of Health (R01 CA084360 to BF; U54 CA163072 to H. Moses for support of Stacy Thomas, R25 GM062459 to L Sealy for support of Andreia Bates, T32CA119925 to S Hann for support of Miranda Hallett)
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
A.L. Bates performed experiments, contributed to experimental design, analysed data, and wrote manuscript; M.W. Pickup performed human dataset analysis; M.A. Hallett assisted with experiments and manuscript revision; E.A. Dozier performed experiments; S Thomas performed experiments; and B. Fingleton conceived and oversaw experiments, and wrote manuscript. All authors were involved in editing and approving the final manuscript.
Supplemental Information
A file containing supplemental information on methods followed, antibodies and primers used and 6 supplemental figures is attached. The titles for the supplemental figures are:
Figure S1. MMP2 does not affect seeding ability or early survival of tumor cells.
Figure S2. Stromal MMP2 contributes to the outgrowth of pulmonary metastases in a secondary model.
Figure S3. Characterization of magnetic bead-isolated fractions.
Figure S4. Knockdown of MMP2 in lung-tumor derived fibroblasts.
Figure S5. Characterization of quiescent fibroblasts for Acta2 and Mmp2 mRNA expression, and responsiveness to tumour-derived soluble factors.
Figure S6. Increased pSmad2 in peritumoral regions of WT mice.
REFERENCES
- 1.Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14:2197–2205. doi: 10.1200/JCO.1996.14.8.2197. [DOI] [PubMed] [Google Scholar]
- 2.Cancer SA. Cancer Facts and Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 3.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 7.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco MM, Mourao M, Mantovani EB, et al. Expression of gelatinases A and B, stromelysin-3 and matrilysin genes in breast carcinomas: clinico-pathological correlations. Clin Exp Metastasis. 1998;16:577–585. doi: 10.1023/a:1006580415796. [DOI] [PubMed] [Google Scholar]
- 12.Murashige M, Miyahara M, Shiraishi N, et al. Enhanced expression of tissue inhibitors of metalloproteinases in human colorectal tumors. Jpn J Clin Oncol. 1996;26:303–309. doi: 10.1093/oxfordjournals.jjco.a023237. [DOI] [PubMed] [Google Scholar]
- 13.Nomura H, Fujimoto N, Seiki M, et al. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996;69:9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu SC, Yang SF, Yeh KT, et al. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clin Chim Acta. 2006;371:92–96. doi: 10.1016/j.cca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br J Cancer. 2003;89:1270–1275. doi: 10.1038/sj.bjc.6601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirvonen R, Talvensaari-Mattila A, Paakko P, et al. Matrix metalloproteinase-2 (MMP-2) in T(1–2)N0 breast carcinoma. Breast Cancer Res Treat. 2003;77:85–91. doi: 10.1023/a:1021152910976. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 18.Toi M, Ishigaki S, Tominaga T. Metalloproteinases and tissue inhibitors of metalloproteinases. Breast Cancer Res Treat. 1998;52:113–124. doi: 10.1023/a:1006167202856. [DOI] [PubMed] [Google Scholar]
- 19.Heppner KJ, Matrisian LM, Jensen RA, et al. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273–282. [PMC free article] [PubMed] [Google Scholar]
- 20.Polette M, Gilbert N, Stas I, et al. Gelatinase A expression and localization in human breast cancers. An in situ hybridization study and immunohistochemical detection using confocal microscopy. Virchows Arch. 1994;424:641–645. doi: 10.1007/BF00195779. [DOI] [PubMed] [Google Scholar]
- 21.Ito A, Nakajima S, Sasaguri Y, et al. Co-culture of human breast adenocarcinoma MCF-7 cells and human dermal fibroblasts enhances the production of matrix metalloproteinases 1, 2 and 3 in fibroblasts. Br J Cancer. 1995;71:1039–1045. doi: 10.1038/bjc.1995.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noel A, Nusgens B, Lapiere CH, et al. Interactions between tumoral MCF7 cells and fibroblasts on matrigel and purified laminin. Matrix. 1993;13:267–273. [PubMed] [Google Scholar]
- 23.Noel A, Hajitou A, L'Hoir C, et al. Inhibition of stromal matrix metalloproteases: effects on breast-tumor promotion by fibroblasts. Int J Cancer. 1998;76:267–273. doi: 10.1002/(sici)1097-0215(19980413)76:2<267::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Itoh T, Tanioka M, Yoshida H, et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 25.Martin MD, Carter KJ, Jean-Philippe SR, et al. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008;68:6251–6259. doi: 10.1158/0008-5472.CAN-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickup MW, Laklai H, Acerbi I, et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer Res. 2013;73:5336–5346. doi: 10.1158/0008-5472.CAN-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffield JS, Lupher M, Thannickal VJ, et al. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paunescu V, Bojin FM, Tatu CA, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura T, Kometani K, Hashida H, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A, Schioppa T, Porta C, et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am J Pathol. 2004;165:659–669. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorrell JM, Caplan AI. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161–214. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 34.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci U S A. 2004;101:18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang HY, Sneddon JB, Alizadeh AA, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeung T, Georges PC, Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 39.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–G118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang X, Yang N, Fiore VF, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 44.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiolloy S, Edwards JR, Fingleton B, et al. An osteoblast-derived proteinase controls tumor cell survival via TGF-beta activation in the bone microenvironment. PLoS One. 2012;7:e29862. doi: 10.1371/journal.pone.0029862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen ES, Ertel A, Davicioni E, et al. Progression of ductal carcinoma in situ to invasive breast cancer is associated with gene expression programs of EMT and myoepithelia. Breast Cancer Res Treat. 2012;133:1009–1024. doi: 10.1007/s10549-011-1894-3. [DOI] [PubMed] [Google Scholar]
- 47.Poulsom R, Hanby AM, Pignatelli M, et al. Expression of gelatinase A and TIMP-2 mRNAs in desmoplastic fibroblasts in both mammary carcinomas and basal cell carcinomas of the skin. J Clin Pathol. 1993;46:429–436. doi: 10.1136/jcp.46.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry SW, Schueckler JM, Burke K, et al. Stromal matrix metalloprotease-13 knockout alters Collagen I structure at the tumor-host interface and increases lung metastasis of C57BL/6 syngeneic E0771 mammary tumor cells. BMC Cancer. 2013;13:411. doi: 10.1186/1471-2407-13-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco OE, Jiang M, Strand DW, et al. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hori Y, Kashimoto T, Yonezawa T, et al. Matrix metalloproteinase-2 stimulates collagen-I expression through phosphorylation of focal adhesion kinase in rat cardiac fibroblasts. Am J Physiol Cell Physiol. 2012;303:C947–C953. doi: 10.1152/ajpcell.00401.2011. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Xu SW, Kennedy L, et al. FAK is required for TGFbeta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007;18:2169–2178. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eldred JA, Hodgkinson LM, Dawes LJ, et al. MMP2 activity is critical for TGFbeta2-induced matrix contraction--implications for fibrosis. Invest Ophthalmol Vis Sci. 2012;53:4085–4098. doi: 10.1167/iovs.12-9457. [DOI] [PubMed] [Google Scholar]
- 54.Alowami S, Troup S, Al-Haddad S, et al. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T, Sun L, Miller N, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 56.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 57.Jensen BV, Johansen JS, Skovsgaard T, et al. Extracellular matrix building marked by the N-terminal propeptide of procollagen type I reflect aggressiveness of recurrent breast cancer. Int J Cancer. 2002;98:582–589. doi: 10.1002/ijc.10187. [DOI] [PubMed] [Google Scholar]
- 58.Brown LF, Guidi AJ, Schnitt SJ, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 59.Ramaswamy S, Ross KN, Lander ES, et al. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 60.Onozuka I, Kakinuma S, Kamiya A, et al. Cholestatic liver fibrosis and toxin-induced fibrosis are exacerbated in matrix metalloproteinase-2 deficient mice. Biochem Biophys Res Commun. 2011;406:134–140. doi: 10.1016/j.bbrc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Takamiya Y, Fukami K, Yamagishi S, et al. Experimental diabetic nephropathy is accelerated in matrix metalloproteinase-2 knockout mice. Nephrol Dial Transplant. 2013;28:55–62. doi: 10.1093/ndt/gfs387. [DOI] [PubMed] [Google Scholar]
- 62.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 63.Fingleton B. Matrix metalloproteinases as valid clinical targets. Curr Pharm Des. 2007;13:333–346. doi: 10.2174/138161207779313551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.