Abstract
Objectives
Pancreatitis is a complex inflammatory disorder, ranging from a mild attack, to severe and potentially fatal condition. Dimethyl Fumarate (DMF), a potent antioxidant and anti-inflammatory, has been used medicinally for decades. The purpose of this study was to test the hypothesis that treatment with DMF may ameliorate acute pancreatitis (AP) in a rodent model.
Methods
Rats were treated with DMF (25 mg/Kg)24 hours prior to AP induction with L-arginine (3 g/Kg). At 72 hours, the pancreas was processed for histology. Serum amylase, lactate dehydrogenase, pancreatic trypsin, and lipid peroxidation product (MDA) were evaluated. Key cytokines and chemokinesin the supernatant of LPS-stimulated splenocytes were also determined.
Results
Pancreases from DMF treated rats showed reductions in the severity of inflammatory cell infiltration, acinar damage, perilobar edema, and cell necrosis. This was associated with significantly lower amylase and MDA but not lactate dehydrogenase, or trypsin levels. The apoptotic pancreatic cells (cleaved caspase 3 positive) were significantly lower in the DMF-treated rats. LPS-stimulated splenocytes treated with DMF produced a significantly lower amount of key inflammatory mediators.
Conclusion
Administration of DMF attenuates A Pin rats.
Keywords: Dimethyl Fumarate, reactive oxygen species, pancreatitis, oxidative stress, cytokine, inflammation
Introduction
Acute Pancreatitis (AP) is characterized by the sudden onset of local pancreatic inflammation from intra-pancreatic activation of digestive enzymes. The disease carries a spectrum of severity from mild self-limiting, to a highly morbid and even fatal illness. It is believed that the initial event occurs from the disruption of acinar cells, leading to the leakage of enzymes resulting in auto digestion of the pancreas. The activated enzymes cause a local inflammatory response which if severe can result in further recruitment of inflammatory mediators and the development of a systemic response leading to multi-organ system failure and death1,2.
Fortunately, due to advances in critical care the mortality rate has decreased over the last decade however, the incidence of AP remains high worldwide 3. Recent reports have revealed an increase in the incidence of pancreatitis in developed countries including the United States4,5. Injury to the pancreatic acinar cells causes a cascade of events that includes production of reactive oxygen species (ROS) resulting in the oxidation of lipids and proteins and disruption of the pancreatic membranes6. Increased production of ROS and the resulting oxidative stress play a major role in the pathogenesis of tissue damage in AP. Under normal condition, cells respond to ROS by up-regulating expression of cytoprotective, and antioxidant enzymes and related molecules such as superoxide dismutase (SOD), catalase, Glutamate-cysteine Ligase (GCLC) and Glutathione peroxidase (GPx) which work in concert to neutralize ROS. However, when production of ROS exceeds the antioxidant capacity, it results in oxidative stress, inflammation and tissue damage7.
Currently, the treatment of AP in the United States (US) is limited to supportive care. Although there have been numerous trials exploring the efficacy of antioxidants or anti-inflammatory drugs in the treatment paradigm of AP, the results remain inconclusive. Dimethyl Fumarate (BG-12), is the methyl ester of fumaric acid and was initially recognized for its anti-cancer effects 8. Later, it became widely used in Europe for the treatment of psoriasis under the trade name Fumaderm9. Most recently, BG-12 received FDA approval in the US for the treatment of patients with multiple sclerosis. Dimethyl Fumarate is a unique and potent antioxidant and anti-inflammatory drug whose mechanism of action has yet to be elucidated. The drug has been effective for decades for the treatment of other acute and chronic inflammatory conditions; however it has never been examined specifically in the setting of pancreatitis.
The aim of the present study was to test the hypothesis that treatment with Dimethyl Fumarate may attenuate severity of AP in experimental animals by enhancing cellular antioxidant and anti-inflammatory machinery.
Materials and Methods
In vivo acute pancreatitis
Rodent studies were performed in accordance with the Institutional Animal Care and Use Committee of University of California, Irvine. (Irvine, CA). Male Sprague Dawley rats (Control-normal rats 3, L-arginine 12, L-arginine+ DMF 12, n=27) were purchased from Charles River (Wilmington, MA). Experimental agents used to produce AP were purchased from Sigma (St. Louis, MO) unless otherwise specified. Rats (250–300 g) were fed ad libitum on a standard diet with free access to water. They were maintained on a 12 h light/dark cycle. Experimental animals were given oral DMF (25 mg/kg) dissolved in methyl cellulose and fed via oral gavage 24 hours prior to initiating AP and daily thereafter until the animals were sacrificed.
L-arginine
20% L-arginine was dissolved in normal saline and filtered through a syringe filter with pH adjusted to 7.0. The solution was administered to non-fasted rats in two intraperitoneal injections at a dose of 3 g/Kg body weight, each injection separated by 1 hour. Animals, control and experimental, received Buprenorphine (Reckitt Benkiser, Richmond, VA) pain medication (0.01 mg/Kg) IM twice a day and regular food and water. Due to the experimental endpoints, anti-inflammatory medications were not administered to the animals. Experimental animals continued to receive daily DMF via gavage along with a standard diet. Control animals (L-arginine, n=12) received daily methyl cellulose. The animals were sacrificed after L-arginine induction at 24 hours for biochemical assessments (n=15, Control 3, L-arginine 6, L-arginine + DMF 6) or 72 hours for histology (n=12, L-arginine 6, L-arginine + DMF 6)10.
Histology
Rat pancreases were fixed in 10% buffered formalin, embedded in paraffin blocks, and sectioned. The pancreas tissue was processed for hematoxylin-eosin (H&E) staining using standard techniques. Using Schmidt criteria, interstitial edema, leukocyte infiltration, acinar cell destruction, and total scores were evaluated individually by two pathologists blinded to the source of the histology sections they evaluated. AP was scored using a quantitative grading system as described by Schmidt et al11. Classification (Table 1) is based on the presence of edema, leukocyte infiltration, acinar cell necrosis, and hemorrhage.
Table 1.
Quantitative grading score for pancreatitis
| Score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Interstitial edema | none | Interlobular | Lobule involved | Isolated island like acinar cells |
| Leukocyte infiltration | none | <20% | 20–50% | >50% |
| Acinar cell necrosis | none | <5% | 5–20% | >20% |
| Hemorrhage | none | 1–2 points | 3–5 points | >20% |
Immunohistochemistry for Cleaved Caspase 3
The detection system was DAKO LSAB2 system-HRP kit using the manufactures protocol (DAKO, K0675, Carpinteria, CA). Briefly, following deparaffinization and rehydration in clearing solution and graded alcohol series, samples were used for heat induce antigen retrieval procedure in citrate buffer (PH 6) for 15 minutes on a hot plate. We blocked non-specific binding sites with protein block solution (BioGenex, Fremont, CA, #HK112-9K) for 1 hour and endogenous peroxidase activity by 3% H2O2 for 10 minutes in room temperature. Sections were incubated over night at 4°C with primary antibody (cleaved caspase-3, Rabbit mAb, 1/800, cell signaling, Danvers MA). After washing in tris-buffered solution, slides were incubated with ready to use secondary Ab for 15 minutes, and then incubated with peroxidase substrate-chromogen solution (DAB) for 5–7 min. Sections were counter-stained with Meyer’s haematoxylin for 60 second, rinsed with tap water, dehydrated in graded alcohol series, cleared with clearing solution, and then covered with permount cover slips. Apoptotic cells were identified as those with a cytoplasmic brown stain. Cleaved caspase 3 positive pancreatic acinar cells (%) were quantified on 400x magnification by two pathologists. Caspase 3 index was defined as the proportion of Caspase positive cells per high powered field in a minimum of 3 high powered fields. The results corresponded to the average number of positive acinar cells per field 12.
Serum Amylase
Whole blood was obtained from anesthetized rats via cardiac puncture just prior to euthanasia. Blood was then centrifuged (3,000 RPM for 20 minutes) and the supernatant collected. Amylase level in serum was determined to indicate the severity of pancreatitis using the Phadebas Amylase test (Magle AB, Lund, Sweden). Supernatant was incubated with a 45 mg blue starch tablet which became hydrolyzed by α-amylase to form water soluble blue fragments. The absorbance (620 nm) was measured as a function of the α-amylase activity and expressed as U/L.
Pancreatic malondialdehyde content
Pancreatic malondialdehyde (MDA) was measured by thiobarbituric acid colorimetric method using MDA assay kit (Cayman Chemical Company, Ann Arbor, MI). Harvested pancreases (100 mg) were placed in lysis buffer (RIPA, Thermo Scientific, Piscataway, NJ) and homogenized (Power Gen, Fischer Scientific). Samples were then centrifuged at 1200 RPM at 4°C. The absorbance of the supernatant was measured by spectrophotometry at 535 nm for MDA content, as MDA reacted with thiobarbituric acid and turned pink after a 1 hour boil. The MDA concentration was calculated from the standard curve and expressed as μM.
Serum LDH
Lactate Dehydrogenase (LDH) was measured using a kit, In Vitro Toxicology Assay Kit, Lactate Dehydrogenase (Sigma, TOX7, St. Louis, MO). LDH assay mixture was added (1:2) to serum samples on a 96 well plate and covered with aluminum foil for 30 minutes at room temperature. The reaction was terminated with addition of 1 N HCL (1:10) to each well. The absorbance was then measured at 490 nm and then again at 690 nm and subtracted. The concentrations were compared to a standard curve and LDH was expressed as mU/ml.
Pancreatic Trypsin
Trypsin level was measured using a Tryspin Activity Assay Kit (Abcam, ab102531, Cambridge, MA) according to manufacture specifications. Pancreatic tissue (100 mg) was placed in RIPA buffer (RIPA, Thermo Scientific, Piscataway, NJ) and homogenized (Power Gen, Fischer Scientific). Samples were combined with Trypsin Assay Buffer and incubated for 10 minutes at 25 °C and measured at time zero at absorbance 405 nm, this was followed by one hour incubation at room temp and a second measurement at 405 nm (Time1). These measurements were compared to a standard curve to measure the Trypsin activity in mU/ml.
Inflammatory mediators from LPS stimulated splenocytes
Spleens were removed from normal adult rats under general anesthesia just prior to euthanasia. Spleens were cut into tiny morsels with a standard 15 blade scalpel. The spleens were then placed in an Erythrocyte lyses buffer (1.5 M NH4CL, 100 mM KHCO3, 100 mM EDTA-2Na adjusted to a ph of 7.2)13. Whole splenocytes were stimulated with endotoxin lipopolysaccharide 1μg/ml (LPS, Sigma) and incubated for 24 hours with or without DMF 20μM. The supernatant was then placed on a standard rat cytokine kit (Ray Biotech, Norcross, GA). The cytokines/chemokines: Activin A, Agrin, CINC-1, CNTF, Fas-ligand, GM-CSF, ICAM-1, IFN-gamma, IL-1α, IL-4, IL-6, IL-13, Leptin, MIP-3α, RAGE, TIMP-1, TNF-α, VEGF were analyzed. Supernatant was placed on special membranes and blocked and washed several times followed by the addition of a biotin conjugate anti-cytokine mixture incubated at room temp for 2 hours. This is followed by the addition of conjugated horseradish peroxidase labeled streptavidin (HRP-SA) and incubation at room temp for 2 hours. Membranes were washed and processed with Detection buffer and exposed with X-ray Film (Kodak, Rochester, NY). Analysis was carried out using ImageQuant TL 7.0 (GE healthcare Life Sciences, Pittsburg, PA)14.
Statistical Analysis
Student’s t test and one-way analysis of variance (ANOVA) were used in statistical analysis of the data using Excel for Windows software (Microsoft, Redmond, WA). P values equal to or less than 0.05 were considered significant. Data are expressed as mean ± SD.
Results
Dimethyl Fumarate ameliorated AP in rodents
Treatment with oral DMF in experimental pancreatitis induced by L-arginine resulted in significant reductions in infiltrating inflammatory cells, acinar architectural damage, edema, and necrosis (Figure 1A& 1B). The total severity score was statistically significant for L-arginine versus L-arginine + DMF (Table 2, p <0.001).
Figure 1.
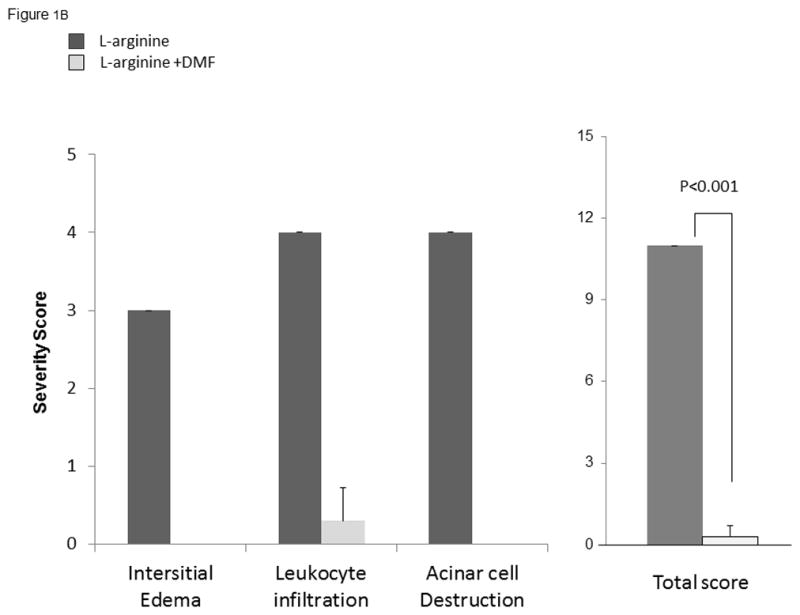
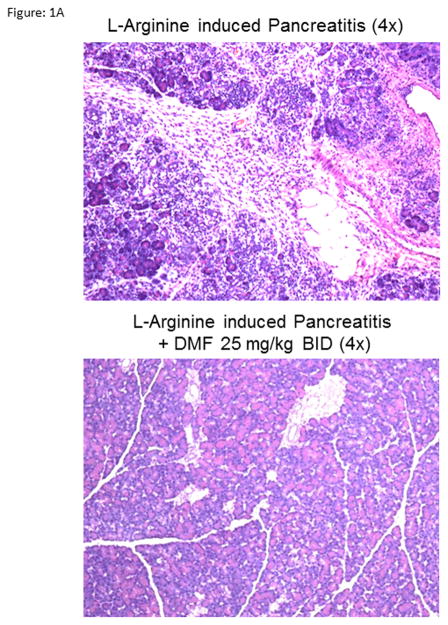
Figure 1A: Histology of L-arginine induced pancreatitis.
Representative photomicrograph of rat pancreatic H&E stained sections. DMF supplementation in rats significantly reduced pancreatic infiltration of inflammatory mediators, destruction of normal acinar architecture, perilobular edema, necrosis of cells and surrounding fat in a rodent model of pancreatitis induced by L-arginine.
Figure 1B: Quantitative pancreatitis score: interstitial edema, leukocyte infiltration, acinar cell destruction
Pancreas histology slides were evaluated by two blinded pathologists and given a score based on severity from 0–3 based on three criteria: edema, infiltration, and acinar cell destruction. Based on quantitative scores for all three criteria there was a significantly higher score for L-arginine compared to L-arginine + DMF (p < 0.001).
Table 2.
Histologic grading score for L-arginine induced pancreatitis
| Groups | Interstitial edema | Leukocyte infiltration | Acinar cell destruction | Hemorrhage | Total | P value |
|---|---|---|---|---|---|---|
| L-arginine n=6 |
3 | 4 | 4 | 0 | 11 | p <0.001 |
| L-arginine + DMF n=6 |
0 | 0.3±0.42 | 0 | 0 | 0.3±0.42 |
Treatment with oral DMF significantly reduced the proportion of Caspase-3 positive cells
Cleaved Caspase 3 antibody was used for histological identification of pancreatic acinar cell apoptosis15. Cleaved Caspase 3 was used to evaluate apoptotic cells in the pancreas of rats induced with L-arginine. Compared to the untreated group, DMF treated rats showed significantly lower caspase positive cells in pancreatic sections (Figure 2, Table 3, p <0.001).
Figure 2. Cleaved caspase-3 IHC in L-arginine induced pancreatitis.
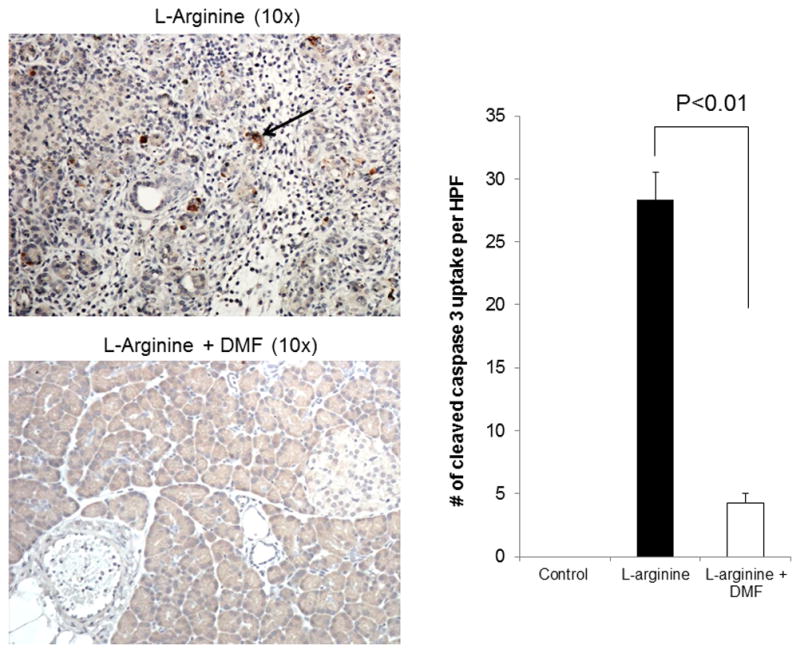
Representative photomicrograph of pancreas histology stained with cleaved caspase 3 antibody. In rats treated with DMF, the caspase-3 staining of the pancreas revealed significantly lower cleaved caspase 3 positive cells when compared with the L-arginine group (p <0.001). The arrow reveals an area of brown cytoplasmic uptake indicating cleaved caspase 3. The slides were reviewed by two blinded pathologists who scored the slides based on standardized criteria. Data are representative of three independent experiments.
Table 3.
Biochemical parameters for L-arginine induced pancreatitis
| Biochemical Parameter | Control (n=3) (mean) | L-arginine (n=6) (mean ± SD) | L-arginine + DMF (n=6) (mean ± SD) | P value (L-arginine vs L-arginine + DMF) |
|---|---|---|---|---|
| Caspase 3(# per HPF) | 0 | 28.33 ± 2.22 | 4.25 ± 0.75 | 0.001 |
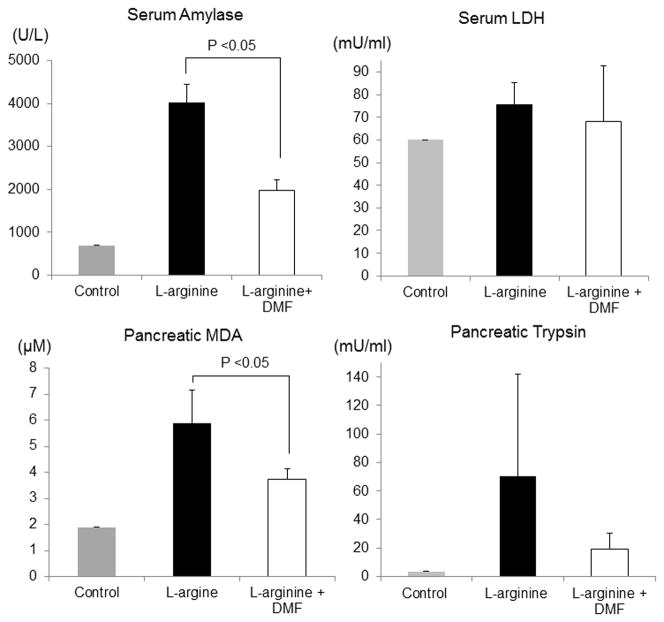
| Serum Amylase (U/L) | 700 | 4015 ± 440 | 1970 ± 260 | 0.014 |
| Pancreatic MDA (μM) | 1.9 | 5.9 ± 1.26 | 3.75 ± 0.38 | 0.04 |
| Serum LDH (mU/ml) | 60.12 | 75.65 ± 9.58 | 68.13 ± 24.64 | 0.17 |
| Pancreatic Trypsin (mU/ml) | 3.67 | 70.18 ±72 | 19.05 ± 11 | 0.18 |
Treatment with oral DMF significantly decreased serum amylase but not LDH
The serum amylase but not LDH in the DMF-treated rats was significantly lower after 24 hours of induction with L-arginine when compared to the untreated group (Figure 3, Table 3). Lower amylase level in the DMF treated rats is consistent with the observed histological improvement in rats with L-arginine induced pancreatitis.
Figure 3. Serum amylase and pancreatic MDA level.
Serum amylase and LDH and pancreatic MDA and trypsin were determined at 24 hours after induction of L-arginine. Compared to the control L-arginine rats, DMF-treated rats resulted in a significant decrease in serum amylase levels and pancreatic MDA concentration (p <0.05). Although there was a trend toward lower trypsin and LDH in DMF treated rats, the results were not statistically significant. Data are representative of at least three independent experiments.
Treatment with oral DMF significantly decreased pancreatic MDA but not Trypsin
MDA is an indicator of lipid peroxidation and cellular damage under oxidative stress. The pancreatic MDA levels in the DMF treated rats were significantly lower after 24 hours of AP induction with L-arginine when compared to the untreated group (Figure 3, Table 3 p<0.05). However, there was no significant difference in Trypsin levels however there was a propensity for lowered trypsin levels in the experimental groups.
DMF significantly lowered production of inflammatory mediators in LPS-treated rodent splenocytes
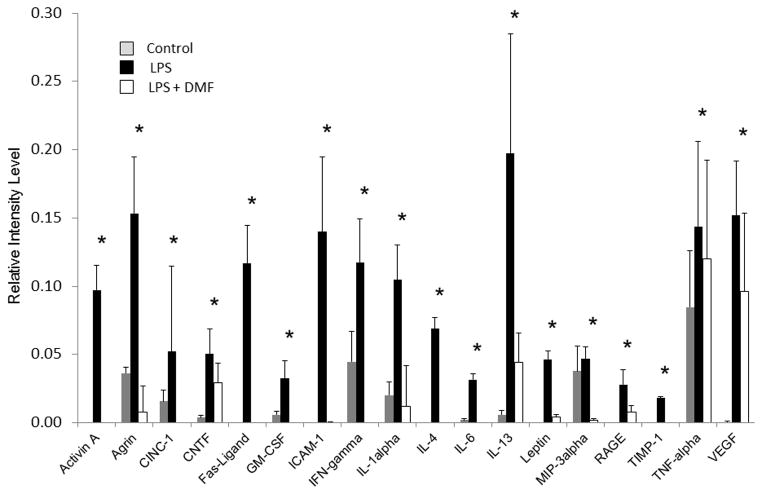
To confirm DMF’s ability to reduce inflammatory mediators, whole splenocytes from normal rats were treated with LPS alone or with DMF+ LPS 1μg/ml in vitro for 24 hours. LPS stimulated splenocytes were used to mimic an infection that can occur in the setting of clinical pancreatitis. DMF significantly decreased the production of inflammatory mediators (Activin A, Agrin, CINC-1, CNTF, Fas-ligand, GM-CSF, ICAM-1, IFN-gamma, IL-1α, IL-4, IL-6, IL-13, Leptin, MIP-3α, RAGE, TIMP-1, TNF-α, VEGF) in the supernatant of rodent splenocytes treated with LPS (Figure 4).
Figure 4. Inflammatory mediator levels from LPS stimulated rat splenocytes treated with DMF.
Rat splenocytes were isolated from normal rats and stimulated with LPS. They were cultured for 24 hours with or without DMF. DMF significantly decreased the production of key inflammatory mediators related to acute pancreatitis. Data are representative of six independent experiments.
Discussion
In this study, we evaluated the effects of DMF, on a rodent model of AP induced by, L-arginine. Our data showed that DMF attenuated the severity of tissue injury and inflammation, reduced many inflammatory mediators, and lowered specific biochemical markers of pancreatitis. Careful histological examination of the pancreas and pancreatitis scoring criteria revealed that, DMF significantly reduced acinar cell destruction. The MDA and cleaved caspase 3 levels in the pancreatic tissue were significantly lower in the DMF-treated compared to the untreated rats pointing to the efficacy of DMF in attenuating oxidative stress and cell injury in the rats with AP. Lastly, the serum amylase level which was significantly elevated in the untreated rats was reduced to the normal control rat values in the DMF treated rats.
DMF is a potent anti-inflammatory drug, as such; it can be assumed that it would exert protected effects against a number of inflammatory conditions such as pancreatitis. To our knowledge, DMF has not been previously investigated for the treatment of either clinical or experimental pancreatitis. We aimed to assess DMF’s protection against experimental AP induced by L-Arginine.
DMF has been widely used in Europe for the treatment of psoriasis vulgaris and psoriatic arthritis which are chronic inflammatory disorders, for over 20 years. Although, repurposed for human use for decades the exact mechanism of action of DMF is not clearly elucidated. The beneficial action of DMF is linked to its ability to stimulate production of anti-inflammatory cytokines in leukocytes and inhibit keratinocyte proliferation. DMF also down-regulates expression of several chemokines that are involved in the pathogenesis of psoriasis16,17. In initial investigations by Stoof et al., Monomethylfumarate (MMF), the most active metabolite of DMF, was found to selectively up-regulate expression of Th2 cytokines and suppress a Th1 response18. Another study by, Ghoreschi et al. found that in mice, the supplementation of fumarate caused type II dendritic cells to produce IL-4, resulting in Th2 expansion. Multiple sclerosis and psoriasis are two diseases in which IL-12 and IL-23 promote pathogenic T helper cell differentiation19,20. Furthermore, previous studies have found that DMF specifically inhibits transcription of NF-kB’s target gene products21. NF-kB plays a major role in transcriptional regulation of inflammatory cytokines as well as in cell differentiation and apoptosis. In a study by Meili-Butz, isolated rat endothelial cells were stimulated with TNFα which resulted in translocation of cytoplasmic NF-κB into the nucleus. TNFα-induced nuclear translocation of NF-κB was inhibited by pretreatment with DMF22. In another study DMF was shown to inhibit phosphorylation of IκB which led to a significant reduction in cardiac damage in an experimental mousemodel23.
In a study by Lehmann, DMF was found to induce immunosuppression via Glutathione depletion and induction of a potent endogenous antioxidant, heme oxygenase-1 (HO-1)24. Others have also postulated that DMF exerts its antioxidant effects as a synthetic Nrf2 activator. The Nrf2 pathway is the principal regulator of the antioxidant and anti- inflammatory responses by mediating the transcription of genes encoding hundreds of antioxidant and phase II detoxifying enzymes25,26. In summation, the effects of DMF on experimental models have been attributed to multiple factors, one being activation of Nrf2, leading to up-regulation of anti-oxidant and anti-inflammatory mediators, and down regulation of pro-inflammatory cytokines and ROS producing pathways27–29.
DMF has recently been investigated clinically in the United States for its neuroprotective effects. In a phase 3 trial for relapsing–remitting multiple sclerosis (MS), oral DMF significantly reduced the proportion of patients who had a relapse, the rate of disease progression, the annual relapse rate, and the number of gadolinium enhancing lesions and new or enlarging T2 weighted hyperintense lesions on MRI 17. In previous investigation neuroprotection was attributed predominately to the activation of the Nrf2-keap1 pathway16,28,29. Studies have revealed increased cellular levels of nicotinamide adenine dinucleotide phosphate dehydrogenase quinone 1(NQO1), HO-1, and glutamate-cysteine ligase catalytic subunit (GCLC). Glutathione (GSH) is the main cellular antioxidant against reactive oxygen species, thus upregulation of GCLC, the rate limiting enzyme in GSH biosynthesis, would increase the cells ability to survive and combat oxidative stress16,29. Given the positive outcomes of the recent clinical trial of DMF, the FDA recently approved its use for the treatment patients with multiple sclerosis.
Taken all together, studies in experimental animals and humans have demonstrated strong anti-oxidant, anti-inflammatory, and cytoprotective effects of DMF which are mediated by activation of Nrf2, inhibition of NF-κB, and suppression of T Helper 1 response. As noted above oxidative stress and inflammation play a major role in the pathogenesis and progression of acute pancreatitis and pancreatic tissue damage. Intense oxidative stress and inflammation in our animals with acute pancreatitis was accompanied by impairment of the endogenous anti-inflammatory and antioxidant responses. Given the proven efficacy of DMF in enhancing the antioxidant and anti-inflammatory defense systems in various other inflammatory disorders in humans and experimental animals, it is not surprising that it proved effective in attenuating oxidative stress, inflammation and tissue injury in our rats with acute pancreatitis.
Conclusion
In conclusion, DMF administration, a widely used anti-oxidant and anti-inflammatory medication, resulted in significant amelioration of pancreatic tissue damage, inflammation and oxidative stress in experimental AP.
Acknowledgments
Founding sources
This study was in part supported by grants from: NIH-NCRR UL1 TR000153, KL2 TR000147; the Juvenile Diabetes Research Foundation International 17-2011-609.
Footnotes
Disclosure
The authors have no conflicts of interest
References
- 1.Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343–351. doi: 10.2174/1568010023344517. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VK, Howden CW. Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta-analysis. Pancreas. 2001;22:28–31. doi: 10.1097/00006676-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Fagenholz PJ, Castillo CF, Harris NS, et al. Increasing United States hospital admissions for acute pancreatitis, 1988–2003. Ann Epidemiol. 2007;17:491–497. doi: 10.1016/j.annepidem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Murata A, Matsuda S, Mayumi T, et al. Multivariate analysis of factors influencing medical costs of acute pancreatitis hospitalizations based on a national administrative database. Dig Liver Dis. 2012;44:143–148. doi: 10.1016/j.dld.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Esrefoglu M. Experimental and clinical evidence of antioxidant therapy in acute pancreatitis. World J Gastroenterol. 2012;18:5533–5541. doi: 10.3748/wjg.v18.i39.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyakhovich VV, Vavilin VA, Zenkov NK, et al. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc) 2006;71:962–974. doi: 10.1134/s0006297906090033. [DOI] [PubMed] [Google Scholar]
- 8.Held KD, Epp ER, Clark EP, et al. Effect of dimethyl fumarate on the radiation sensitivity of mammalian cells in vitro. Radiat Res. 1988;115:495–502. [PubMed] [Google Scholar]
- 9.Mrowietz U, Altmeyer P, Bieber T, et al. Treatment of psoriasis with fumaric acid esters (Fumaderm) J Dtsch Dermatol Ges. 2007;5:716–717. doi: 10.1111/j.1610-0387.2007.06346.x. [DOI] [PubMed] [Google Scholar]
- 10.Dawra R. The Pancreapedia: Exocrine Pancreas Knowledge Base. 2012. L-Arginine-induced experimental acute pancreatitis. [Google Scholar]
- 11.Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebours V, Albuquerque M, Sauvanet A, et al. Hypoxia pathways and cellular stress activate pancreatic stellate cells: development of an organotypic culture model of thick slices of normal human pancreas. PLoS One. 2013;8:e76229. doi: 10.1371/journal.pone.0076229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown G. Protocols: ACK Lysis protocols. [Accessed November 4th, 2013];Siteman Cancer Center Web site. 2009 Mar 23; Available at: http://escore.im.wustl.edu/SubMenu_protocols/protocol8.html.
- 14.Bouzakri K, Plomgaard P, Berney T, et al. Bimodal effect on pancreatic beta-cells of secretory products from normal or insulin-resistant human skeletal muscle. Diabetes. 2011;60:1111–1121. doi: 10.2337/db10-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189–196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ghoreschi K, Bruck J, Kellerer C, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208:2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 18.Stoof TJ, Flier J, Sampat S, et al. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol. 2001;144:1114–1120. doi: 10.1046/j.1365-2133.2001.04220.x. [DOI] [PubMed] [Google Scholar]
- 19.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 20.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 21.Loewe R, Holnthoner W, Groger M, et al. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol. 2002;168:4781–4787. doi: 10.4049/jimmunol.168.9.4781. [DOI] [PubMed] [Google Scholar]
- 22.Meili-Butz S, Niermann T, Fasler-Kan E, et al. Dimethyl fumarate, a small molecule drug for psoriasis, inhibits Nuclear Factor-kappaB and reduces myocardial infarct size in rats. Eur J Pharmacol. 2008;586:251–258. doi: 10.1016/j.ejphar.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 23.Onai Y, Suzuki J, Kakuta T, et al. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;63:51–59. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann JC, Listopad JJ, Rentzsch CU, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol. 2007;127:835–845. doi: 10.1038/sj.jid.5700686. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Khor TO, Xu C, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashrafian H, Czibik G, Bellahcene M, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15(3):361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 29.Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341:274–284. doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]