Abstract
Vasculitis can affect the peripheral nervous system alone (nonsystemic vasculitic neuropathy) or can be a part of primary or secondary systemic vasculitis. In cases of pre-existing systemic vasculitis, the diagnosis can easily be made, whereas suspected vasculitic neuropathy as initial or only manifestation of vasculitis requires careful clinical, neurophysiological, laboratory and histopathological workout. The typical clinical syndrome is mononeuropathia multiplex or asymmetric neuropathy, but distal-symmetric neuropathy can frequently be seen. Standard treatments include steroids, azathioprine, methotrexate and cyclophosphamide. More recently the B-cell antibody rituximab and intravenous immunoglobulins have shown to be effective in some vasculitic neuropathy types.
Keywords: autoantibodies, polyneuropathy, rheumatic diseases, treatment, vasculitic neuropathy
Introduction
Vasculitic neuropathies are a heterogeneous group of peripheral nerve disorders associated with vasculitis, either nonsystemic (only affecting the peripheral nerve) or systemic vasculitis (Table 1). Systemic vasculitis can occur as primary vasculitis with no other reason for the vasculitis, or secondary vasculitis as a complication of other autoimmune or infectious disease [Collins, 2012]. The common feature of all vasculitic neuropathies is an inflammation of the vasa nervorum, mainly the epineural arteries of the nerve, leading to thrombosis and subsequently to ischaemic damage.
Table 1.
Classification of vasculitides associated with neuropathy (according to Collins et al. [2010]).
| Primary systemic vasculitis |
|---|
| 1. Small vessel vasculitis |
| Microscopic polyangiitis |
| Eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) |
| Granulomatosis with polyangiitis (GPA) |
| Essential mixed cryoglobulinemic (non-Hepatitis C) |
| Henoch–Schönlein purpura |
| 2. Medium vessel vasculitis |
| Polyarteritis nodosa |
| 3. Large vessel vasculitis |
| Giant cell arteritis |
| Secondary systemic vasculitis |
| 1. Connective tissue diseases |
| ➢ Rheumatoid arthritis |
| ➢ Systemic lupus erythematosus |
| ➢ Sjögren’s syndrome |
| ➢ Systemic sclerosis |
| ➢ Dermatomyositis |
| ➢ Mixed connective tissue disease |
| 2. Sarcoidosis |
| 3. Behcet’s disease |
| 4. Infections (hepatitis B and C, HIV, cytomegalovirus and others) |
| 5. Drugs |
| 6. Malignancy |
| 7. Inflammatory bowel disease |
| 8. Hypocomplementemic urticarial vasculitis syndrome |
| Nonsystemic or localized vasculitis |
| 1. Nonsystemic vasculitic neuropathy |
| 2. Diabetic radiculoplexus neuropathy (DRPLN) |
| 3. Localized cutaneous or neuropathic vasculitis |
If the neuropathy is part of an already known systemic vasculitis, diagnosis is not difficult to make. However, if the neuropathy is the first manifestation of vasculitis, diagnosis may be difficult, since only a part of the vasculitic neuropathies shows the typical clinical picture of mononeuritis multiplex. Therefore, if vasculitic neuropathy is suspected, an extensive diagnostic pathway is necessary to confirm or to exclude the diagnosis.
There are no studies, which investigated the incidence or prevalence of vasculitic neuropathy. Systemic vasculitic diseases themselves have an annual incidence of about 60–140/million, including about 30% secondary systemic vasculitis [Watts et al. 1995; Gonzalez-Gay and Garcia-Porrua, 1999]. In all patients who undergo nerve biopsy because of unclear neuropathy, about 1% overall have vasculitis [Kissel et al. 1985; Davies et al. 1996]. In some systemic vasculitis, especially large vessel vasculitic diseases, neuropathy is rare; in others neuropathy even belongs to the diagnostic criteria (Table 2) [Basu et al. 2010].
Table 2.
Frequency of neuropathy in vasculitic diseases.
Pathogenesis
Inflammation of the walls of nutrient and epineural arteries is the main pathophysiological feature in vasculitic neuropathy. However, since the underlying vasculitic diseases have different aetiologies, the common final path in the vasa nervorum is thrombosis and ischaemic damage. Although the nerve is diffusely affected by the vasculitic process, the tissue at risk is a border zone in the proximal to middle section of the nerve, where the most axonal damage occurs [Dyck et al. 1972; Morozumi et al. 2011]. In cryoglobulinemia, a direct pathogenic role of frequently detectable antisulfatide antibodies is discussed [Alpa et al. 2008]. The pain in vasculitic neuropathies may be associated with an increased expression of nerve growth factor (NGF) in the affected nerves [Yamamoto et al. 2003].
Clinical features and diagnostic procedures
About 35–65% of the vasculitic neuropathy patients show the typical clinical picture of a mononeuropathia multiplex. However, half of the patients show other clinical types, mostly painful sensorimotor axonal neuropathy or, rarely, pure sensory neuropathy, mostly with an asymmetric pattern. About 10–40% of biopsy-proven vasculitic neuropathies can occur as distal-symmetric neuropathy [Davies et al. 1996; Claussen et al. 2000; Bennett et al. 2008]. There is no association of a distinct clinical picture with the underlying vasculitic disease. Most affected nerves are the peroneal and/or tibial nerve, on the upper extremity ulnar nerve seems to be involved most frequently. Almost all vasculitic neuropathies develop acutely or subacutely, a chronic development over years can occur in rare cases. Unspecific symptoms, such as weight loss, fever or fatigue, have been reported in 80% of neuropathy with systemic vasculitis and in about 50% of patients with nonsystemic vasculitic neuropathy (NSVN).
Neurographic examination reveals multifocal axonal neuropathy with reduced compound muscle action potential (CMAP) amplitudes. In electromyography, one can see a neurogenic pattern including spontaneous muscle fibre activity, polyphasic, extended and/or high-amplitude motor unit action potentials.
If a systemic vasculitis or another underlying reason for the neuropathy has not been detected yet, a variety of laboratory tests should be performed. This includes a routine testing in all patients with neuropathy of yet unknown reason and, if inflammatory or vasculitic neuropathy is suspected, a more detailed laboratory investigation (Table 3).
Table 3.
Laboratory investigations in suspected vasculitic neuropathy.
| Basic neuropathy screening | Vasculitis suspected |
|---|---|
| Full blood count | Antinuclear antibodies (ANA) |
| Erythrocyte sedimentation rate | Antineutrophil cytoplasmic antibodies (ANCA) |
| C-reactive protein | Extractable nuclear antigens (ENA) |
| Fasting glucose (2 consecutive days) | Rheumatoid factor |
| Electrolytes | Anti-CCP antibodies |
| Renal and liver function | Cryoglobulins |
| Creatine kinase | HIV serology |
| Serum protein immunofixation | Urine analysis (microalbuminuria?) |
| Hepatitis B and C serology | Cerebrospinal fluid analysis |
| Thyroid function | Angiotensin-converting enzyme |
| Soluble interleukin-2 receptor | |
| Antineuronal antibodies | |
| Serum complement C3, C4 |
If there is no evidence of a systemic vasculitis by other parameters (clinical manifestations, autoantibodies, etc.) nerve biopsy is required. Usually, sural nerve biopsy with or without muscle biopsy has been used to detect vasculitic neuropathy. An interesting alternative is the combined biopsy of the superficial peroneal nerve together with the peroneus brevis muscle [Agadi et al. 2012]. Although controlled trials are lacking, peroneal nerve/muscle biopsy could be more effective since in the case of muscle involvement, the more distal peroneus brevis muscle may be more frequently involved than the gastrocnemius muscle.
The main pathological feature of vasculitis is a wall-damaging intramural infiltration [Collins et al. 2010] (Figure 1). The guideline on NSVN from the Peripheral Nerve Society provides diagnostic criteria for probable and definite vasculitic neuropathy. The diagnosis of definite vasculitic neuropathy includes (1) inflammatory cells in the vessel wall accompanied by pathologic evidence of acute or chronic vascular wall damage, and (2) no evidence of another primary disease that mimics vasculitis pathology (lymphoma, lymphomatoid granulomatosis or amyloidosis) [Collins et al. 2010]. Probable vasculitic neuropathy can be suspected, if (1) the criteria for definite vasculitic neuropathy are not completely fulfilled, (2) the neuropathy is predominantly axonal and (3) perivascular inflammation plus signs of vascular damage or pathological predictors of vasculitic neuropathy [Collins et al. 2010].
Figure 1.
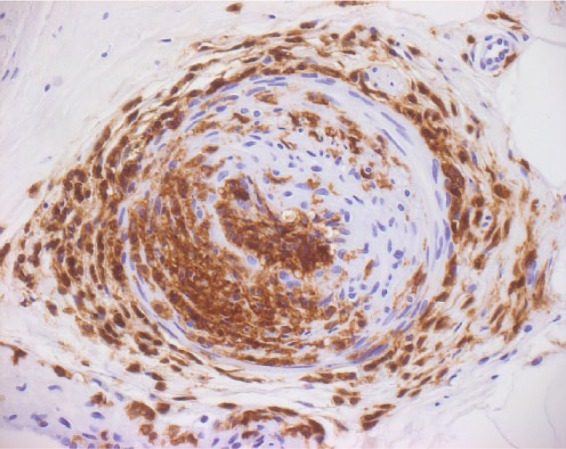
Immunohistochemistry of peripheral nerve vasculitis. Epineural vessel with extensive lymphocyte infiltration and subtotal stenosis. Staining of lymphocytes with anti-LCA (lymphocyte common antigen) antibody. (Courtesy of J. Weis, Aachen.)
Primary systemic vasculitis
Primary systemic vasculitis has been classified according to the diameter of the affected vessels, including three groups of vasculitis: small-vessel, medium-vessel and large-vessel vasculitis. The frequency of neuropathy in the different diseases varies between 5% and 80% (Table 2). However, since the most frequent diameters in nerves and muscles are between 50 and 300 µm, vasculitic neuropathy occurs mainly in the small- and medium-sized vasculitides (Table 2).
Large vessel vasculitis
The large vessel vasculitis includes two disease entities: giant cell arteritis and Takayasu arteritis. Both diseases are normally not associated with neuropathies, but with central nervous system involvement.
Medium-sized vessel vasculitis
This group includes classic polyarteritis nodosa (PAN), Kawasaki disease and thromboangiitis obliterans. Both latter diseases are not associated with neuropathy, whereas in PAN, involvement of the peripheral nervous system can frequently be seen.
Polyarteritis nodosa
The PAN is a rare systemic vasculitis (annual incidence in European countries 0.1–1.6 cases/million inhabitants) of the medium-sized arteries and not associated with glomerulonephritis [Hernandez-Rodriguez et al. 2014]. According to the definition of the Chapel Hill consensus conference, arterioles, capillaries and venules are not affected [Hiemstra et al. 2010]. PAN is an immune complex vasculitis and in about one third of the PAN patients, hepatitis B is involved in the pathogenesis. Interestingly, other chronic infections, such as parvovirus B19, hepatitis C, HIV or streptococci can also led to PAN. An observed reduction of incidence may be due to the widespread vaccination against hepatitis B. In contrast to the antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides, there are no typical autoantibodies in PAN. Histological features include infiltration of vessel walls with polymorphonuclear neutrophils, predominantly in branching points. In active lesions, fibrinoid necrosis is frequently seen. The clinical symptoms include weight loss, livedo reticularis, testicular pain, myalgia, kidney involvement and visceral artery aneurysms. PAN is mostly a monophasic disease and the prognosis depends on the involved organ systems. If the heart or the central nervous system is involved, prognosis is poor. Recently, the French vasculitis group proposed a revised five-factor score to estimate the prognosis of PAN [Guillevin et al. 2011]. Up to 75% of the PAN patients have neuropathy, which led to the inclusion of neuropathy into the American college of rheumatology diagnostic criteria [Basu et al. 2010]. Clinically, the neuropathy presents mainly as painful mononeuropathia multiplex, but typical chronic inflammatory demyelinating polyneuropathy (CIDP) has also been described in association with PAN.
Small vessel vasculitis
Churg–Strauss syndrome
Churg–Strauss syndrome (CSS) is a vasculitis of the small vessels and was first described in 1951 by Churg and Strauss. After years of prodromi (asthma, rhinitis), most patients develop an intermittent phase of eosinophilia and eosinophilic infiltrates (mainly pulmonary), then vasculitic manifestation with cutaneous, gastrointestinal, sinusitis, arthralgia and neuropathy. Involvement of PNS occurs in about 75–80% of patients, and the neuropathy is mainly mononeuropathy multiplex, less often pure sensory or axonal sensorimotor neuropathy. The histopathological picture includes granulomatous vasculitis of the small and medium-sized arteries, arterioles, capillaries and venules with eosinophilic infiltrates in the peripheral blood. Blood examination reveals eosinophilia and 40–70% of the patients have pANCA.
Granulomatosis with polyagiitis
Granulomatosis with polyagiitis (GPA) is a granulomatous disease of the upper and/or lower respiratory tract (localized), often associated with rapid-progressive glomerulonephritis and a subsequent development of systemic vasculitic disease. Rhinitis can also be observed and many patients develop fully generalized syndrome with pulmonary and renal involvement including alveolar haemorrhage. Almost all patients are positive for c-ANCA, which are mainly directed against proteinase 3. The pathophysiology of GPA is quite well understood: there is a necrotizing vasculitis of smaller vessels (arterioles, capillaries, venules), in which the ANCA induce activation and degranulation of granulocytes with consecutive endothelial damage. During this process, tumour necrosis factor (TNF)-alpha and interleukin (IL)-1 induce the translocation of proteinase 3 to the endothelial surface and adherence of neutrophils to endothelial cells, followed by degranulation of the neutrophils (→ANCA-cytokine-sequence mechanism). About 20–25% of GPA patients develop neuropathy, mostly mononeuritis multiplex or, rarely, mononeuropathy of a cranial nerve. In rare cases, the neuropathy may be the first symptom of GPA.
IgG4-related disease
IgG4-related systemic disease is a recently recognized systemic disease, which can include a variety of organ systems [Beyer et al. 2014]. This syndrome is mainly characterized by elevated IgG4 serum levels and an increased level IgG4+ plasma cells in biopsy (see the review by Perez Alamino and colleagues [Perez Alamino et al. 2013]). An IgG4-associated neuropathy has been described in single case reports, but the incidence of neuropathy in this syndrome has not been established yet [Ohyama et al. 2013a; Yokoi et al. 2014].
Microscopic polyangiitis
Microscopic polyangiitis (MPA) is a necrotizing vasculitis mainly of lung and kidney capillaries and is associated with pANCA, or, less frequently, cANCA [Schonermarck et al. 2001]. Clinically, the patients show glomerulonephritis, lung involvement, abdominal pain and skin purpura. Neuropathy has been found in 10–50% and mononeuropathia multiplex is the most common feature [Agard et al. 2003; Ribi et al. 2010; Suppiah et al. 2011].
Secondary systemic vasculitis
This is a heterogeneous group including vasculitis associated with infectious diseases, connective tissue diseases or malignancies as well as drug-induced vasculitides.
Connective tissue diseases
Systemic lupus erythematosus
Neurological involvement in systemic lupus erythematosus (SLE) includes cerebral vasculitis, transverse myelitis and peripheral neuropathy. About 20% of SLE patients have neuropathy, which can be classical distal-symmetric or mononeuropathia multiplex [Andrade et al. 2004; Collins and Periquet, 2008]. Neurophysiological studies show an axonal type in 70–80% and a more demyelinating type in 20% [Florica et al. 2011]. One third have autonomic involvement [Rafai et al. 2007].
Systemic sclerosis
Polyneuropathy in systemic sclerosis (SSc) seems to be more frequent than initially suspected. Up to 30% of the SSc patients have neuropathy, mostly sensory symptoms and small fibre dysfunction [Collins and Periquet, 2008]. Interestingly the gut motility disturbance in these patients is caused by an autonomic neuropathy of the gastrointestinal tract [Di Ciaula et al. 2008].
Sjögren’s syndrome
Sjögren’s syndrome is clinically characterized by a sicca syndrome (dry eyes/dry mouth) since the exocrine glands are affected by the autoimmune process. SS-A and/or SS-B antibodies can be found in the most patients [Beyer et al. 2014]. Sjögren’s syndrome can occur as a primary disease, but can also be a part of another connective tissue disease as secondary Sjögren’s syndrome. The incidence of neuropathy has been reported between 2% and 64%, but only a smaller part of them are vasculitic neuropathies. Clinically, symmetric and asymmetric neuropathies as well as small fibre neuropathies can be seen [Birnbaum, 2010]. In addition, the trigeminal nerve can be affected and autonomic neuropathies have been reported.
Rheumatoid arthritis
Neuropathy occurs in 15–50% of rheumatoid arthritis (RA) patients. Peripheral neuropathy in RA can have different origins, therefore, nonvasculitic neuropathy, such as entrapment mononeuropathy and drug-induced polyneuropathy must be recognised. The main clinical feature is a distal symmetric sensorimotor neuropathy [Schaublin et al. 2005].
Paraneoplastic vasculitic neuropathy
The typical paraneoplastic neuropathy is a pure sensory neuronopathy (Denny–Brown) associated with antineuronal autoantibodies (mostly anti-Hu). However, every other clinical type of neuropathy has been described as paraneoplastic. The most associated tumours are small cell lung cancer, gynaecological cancer and lymphoma. The first descriptions of paraneoplastic vasculitic neuropathy came from Torvik and Berntzen in 1968 and from Johnson and colleagues in 1979 [Torvik and Berntzen, 1968; Johnson et al. 1979]. These patients had vasculitic neuropathy associated with renal cell carcinoma, small cell lung cancer or lymphoma. A bigger series was reported by Oh, who described the clinical features of 26 patients with paraneoplastic vasculitic neuropathy, mainly associated with SCLC and lymphoma [Oh, 1997]. High cerebrospinal fluid protein content and high erythrocyte sedimentation rate were suggestive of vasculitic paraneoplastic neuropathy. Most patients with paraneoplastic vasculitic neuropathy present clinically with mononeuropathia multiplex or asymmetric polyneuropathy. In case of suspected paraneoplastic vasculitic neuropathy, the examination of antineuronal autoantibodies, especially anti-Hu, may be helpful. Anti-Hu has a high specificity for paraneoplastic aetiology and more than 90% of anti-Hu positive patients have small cell lung cancer. In contrast to most other paraneoplastic neuropathies, vasculitic neuropathies seem to respond to immunosuppressive treatment with steroids or cyclophosphamide (CYC).
Hepatitis C/cryoglobulinemia
Various chronic infections can be associated with cryoglobulinemia. Cryoglobulins are immunoglobulins that precipitate at temperatures <37°C. The cryoglobulinemia can be asymptomatic or induces a small-vessel, immune-complex-mediated vasculitis. Most cases occur in hepatitis C infections. Although most of the hepatitis C patients develop cryoglobulinemia, only 15% of them show clinical signs of vasculitic disease. Purpura of the skin, renal involvement and arthralgia are the main clinical features and 30–70% of the patients develop neuropathy [Cacoub et al. 2008]. Most of the neuropathies are mononeuropathia multiplex or symmetric sensorimotor neuropathy. In rare cases, a pure sensory neuropathy could be observed [Schaublin et al. 2005]. In some patients, autoantibodies against the GM1-ganglioside or antisulphatide antibodies could be detected [Alpa et al. 2008]. It is unclear, whether these autoantibodies are a bystander phenomenon or directly involved in the pathophysiology of the axonal damage. In infection-associated vasculitis, antigen removal by antiviral treatment is as important as immunosuppression and patients with hepatitis C virus HCV and cryoglobulinemia will receive antiviral treatment in the first line. If no improvement can be seen, combination of antiviral and immunosuppressive treatment is necessary (see treatment).
Other secondary systemic vasculitides
In sarcoidosis, mononeuropathia multiplex and typical CIDP have been described [Vital et al. 2008]. Most neuropathies respond to steroids, and (in CIDP types) to intravenous immunoglobulins.
Inflammatory bowel disease (IBD) is rarely associated with neuropathy. Figueroa and colleagues just recently reported a 20-year incidence of 0.7% in a population-based study [Figueroa et al. 2013]. Most of the IBD-associated neuropathies seem to be nonvasculitic [Kararizou et al. 2012].
Behcet’s disease is characterized by recurrent oral aphthae and several systemic clinical features, such as ocular disease, skin lesions, arthritis and genital aphthae. In most Behcet’s patients with neurological complications, central nervous system involvement is much more frequent than neuropathy [Noel et al. 2013].
Drug-induced vasculitis has been reported in a variety of drugs. Naproxene has induced a leukocytoclastic vasculitis including neuropathy [Schapira et al. 2000]. Minocycline, an antibiotic has been reported to induce a NSVN [Thaisetthawatkul et al. 2011]. Treatment with propylthiouracil, an antithyroid drug, led to an ANCA-positive vasculitis including neuropathy [Frigui et al. 2008]. Other drugs with induction of vasculitis are penicillin, cocaine, heroin, phenytoin, all in which discontinuation improved the vasculitis [Schapira et al. 2000].
Nonsystemic vasculitis of the peripheral nervous system
Vasculitic neuropathy can occur without any systemic involvement and is then called NSVN. This type of neuropathy often develops subacute, although at least one third of the patients show a progressive course. Even there is no histological examination, NSVN should be suspected if an axonal neuropathy is asymmetric, progressive and painful and is associated with disabling paresis. Neurophysiological examination reveals axonal motor or sensorimotor neuropathy. Nerve biopsy should be performed in the case of suspected NSVN. However, about 50% of patients with suspected vasculitic neuropathy are lacking histological examination. If biopsy has been performed, the main histopathological finding is intramural infiltration with vascular wall damage. The Peripheral Nerve Society recently published a guideline on the diagnosis, investigation and treatment of NSVN [Collins et al. 2010]. In this consensus report, criteria for definite, probable and possible vasculitic neuropathy have been established.
As already stated in neuropathies associated with systemic vasculitis, no randomized controlled treatment trials are available for the treatment of NSVN. There is a recommendation to treat NSVN patients with corticosteroids (start with prednisolone 1 mg/kg/day) with a slow tapering over months. Alternatively, a treatment regimen with initial high-dose prednisolone pulse (500–1000 mg prednisolone for 3–5 days), followed by tapering the dose can be used. In rapid progressive neuropathy, CYC pulse therapy can be used, followed by long-term immunosuppression with methotrexate or azathioprine. CYC should be used together with mesna to reduce the probability of haemorrhagic cystitis. For toxicity reasons, CYC should not be used for more than 6–12 months. Two cohort studies suggest that combination therapy may be more effective [Davies et al. 1996; Collins et al. 2003]. Although there is only evidence in primary vasculitis and not on NSVN, in treatment-resistant cases, rituximab, plasma exchange or intravenous immunoglobulins could be used [Levy et al. 2005; Stone et al. 2010].
Diabetic and nondiabetic lumbosacral radiculoplexus neuropathy
A mostly painful affection of lower limb nerve roots, lumbosacral plexus and peripheral nerves can occur in diabetic (diabetic lumbosacral radiculoplexus neuropathy [DLRPN]) or nondiabetic (lumbosacral radiculoplexus neuropathy [LRPN]) patients has been identified as a form of microvasculitis. Interestingly, DLRPN is not associated with increased severity of the diabetes, but affects also patients with stable diabetic situation. The disease is usually monophasic and spontaneous recovery can be seen, but is often incomplete, leaving the patients with weakness and sensory symptoms. It typically develops acute or subacute and shows an asymmetric distribution of affected nerves, plexus and/or roots, leading to the picture of a mononeuropathia multiplex of the lower limbs. Histological examination revealed nerve ischaemia, caused by microvasculitis [Dyck et al. 1999]. Upper limb involvement can occur in DLRPN/LRPN. However, a separate upper limb variant has recently been described [Massie et al. 2012].
Treatment
Primary systemic vasculitis and NSVN
In general, neuropathy associated with systemic vasculitis should be treated according to the guidelines of the disease. However, standard treatment of systemic vasculitic neuropathy (SVN) and classical NSVN are corticosteroids. A dose of 1 mg/kg daily is mostly recommended. During the following months, there should be a reduction of 5–10 mg every other week, until a maintenance therapy using 5–10 mg prednisolone daily is achieved. Osteoporosis prophylaxis should be initiated for every steroid treatment longer than 2 weeks. If patients have severe neuropathy, high-dose corticosteroids (1000 mg daily) can be initiated for 3–5 days, followed by 1 mg/kg daily. If the patient has axonal damage, a strong improvement will not be seen in the first few weeks. In patients with SVN, systemic parameters, such as erythrocyte sedimentation rate or C-reactive protein can be used as a control for effective treatment. In NSVN, no surrogate parameter has been identified yet to control treatment success. In a recent study, neuropathy has been reported to occur in about 15% of ANCA-associated vasculitis and improvement has been seen in 40% of the patients [Suppiah et al. 2011].
In many severe cases, CYC has been used additionally or subsequently to corticosteroids. Since daily orally CYC has substantial side effects, pulse therapy is recommended, mostly in dosages 0.6–0.75 g/m2 every 2–4 weeks. To avoid bladder toxicity mesna should be added to treatment. The classical long-term treatment to maintain remission includes methotrexate (20–25 mg/weekly) or azathioprine (1–2 mg/kg/day). In GPA patients, leflunomide can be used in the long-term treatment [Metzler et al. 2007].
Mycophenolate mofetil (MMF) is another immunosuppressant that has been used to treat vasculitis patients. In an open-label pilot trial MMF could induce maintained remission in 13 out of 17 patients with GPA with only mild side effects [Silva et al. 2010]. However, in another study, relapses have been more frequent and occurred more quickly using MMF treatment, when compared with azathioprine [Hiemstra et al. 2010]. Therefore, MMF use in vasculitis is still under debate and there are no data on its effect on vasculitic neuropathy. In lupus, MMF has been equally effective to azathioprine with fewer side effects [Maneiro et al. 2014].
Recently, rituximab was established as an effective treatment in patients with MPA and GPA and has been licensed for ANCA-associated vasulitis recently. Rituximab is an anti-CD20 monoclonal antibody, targeting mainly B cells. In the meantime, Rituximab is considered as first-line treatment of ANCA-associated vasculitis. In a recent study, rituximab was as effective as CYC in the treatment of ANCA-associated vasculitis (197 patients, more effective in induction of remission after relapse) [Stone et al. 2010]. Rituximab was also effective in the treatment of cryoglobulinemic vasculitis [De Vita et al. 2012]. Normal dosage is 375 mg/m2 four times every week.
Intravenous immunoglobulins (IvIg) have been described to be effective in some patients with NSVN and SVN in single case reports and smaller case series [Levy et al. 2003, 2005]. If steroids and/or CYC show no treatment effect, IvIg may be a suitable alternative.
Vasculitis associated with infections
If vasculitic neuropathy is associated with hepatitis C, antiviral treatment including pegylated interferon-alpha, ribavirin, and/or telaprevir and boceprevir [Chiche et al. 2012]. Ferri and colleagues [Ferri et al. 2011] reported 87 patients with cryoglobulinemic vasculitis, independent of hepatitis C status, which responded well to rituximab. In this study, skin purpuric lesions improved in 74%, vasculitic leg ulcers in 87%, nephropathy in 95% and neuropathy in 44% and rituximab was considered as a safe and effective treatment.
Plasma exchange is used in mixed cryoglobulinemia, since it is able to remove circulating cryoglobulins. However, no randomized controlled trials have been reported and only some of the patients seem to respond [Rockx and Clark, 2010; Ramos-Casals et al. 2012].
Concluding remarks
About 30–50% of all vasculitis patients exhibit signs of peripheral neuropathy. Neuropathies associated with systemic vasculitis should be treated according to the guidelines of the underlying disease. NSVN will be treated with steroids, or in severe/progressive cases, CYC pulse therapy. Some patients need long-term immunosuppression. Rituximab is an effective alternative to cyclophosphamide in the treatment of vasculitic neuropathies and is licensed for ANCA-associated vasculitis.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Conflict of interest statement: The author has no conflicts of interest to declare.
References
- Agadi J., Raghav G., Mahadevan A., Shankar S. (2012) Usefulness of superficial peroneal nerve/peroneus brevis muscle biopsy in the diagnosis of vasculitic neuropathy. J Clin Neurosci 19: 1392–1396. [DOI] [PubMed] [Google Scholar]
- Agard C., Mouthon L., Mahr A., Guillevin L. (2003) Microscopic polyangiitis and polyarteritis nodosa: how and when do they start? Arthritis Rheum 49: 709–715. [DOI] [PubMed] [Google Scholar]
- Agarwal V., Singh R., Wiclaf, Chauhan S., Tahlan A., Ahuja C., et al. (2008) A clinical, electrophysiological, and pathological study of neuropathy in rheumatoid arthritis. Clin Rheumatol 27: 841–844. [DOI] [PubMed] [Google Scholar]
- Allen R., Sellars R., Sandstrom P. (2003) A prospective study of 32 patients with neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 20: 118–125. [PubMed] [Google Scholar]
- Alpa M., Ferrero B., Cavallo R., Naretto C., Menegatti E., Di Simone D., et al. (2008) Anti-neuronal antibodies in patients with HCV-related mixed cryoglobulinemia. Autoimmun Rev 8: 56–58. [DOI] [PubMed] [Google Scholar]
- Andrade R., Moya Machado A., Gomez Conde S., Garcia Espinosa A., Molina Diaz M., Machado Rojas A., et al. (2004) [Neuropathies due to vasculitis in infancy]. Rev Neurol 38: 619–624. [PubMed] [Google Scholar]
- Basu N., Watts R., Bajema I., Baslund B., Bley T., Boers M., et al. (2010) EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann Rheum Dis 69: 1744–1750. [DOI] [PubMed] [Google Scholar]
- Bennett D., Groves M., Blake J., Holton J., King R., Orrell R., et al. (2008) The use of nerve and muscle biopsy in the diagnosis of vasculitis: a 5 year retrospective study. J Neurol Neurosurg Psychiatry 79: 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer G., Schwaiger T., Lerch M., Mayerle J. (2014) IgG4-related disease: a new kid on the block or an old aquaintance? United European Gastroenterol J 2: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum J. (2010) Peripheral nervous system manifestations of sjogren syndrome: clinical patterns, diagnostic paradigms, etiopathogenesis, and therapeutic strategies. Neurologist 16: 287–297. [DOI] [PubMed] [Google Scholar]
- Cacoub P., Delluc A., Saadoun D., Landau D., Sene D. (2008) Anti-CD20 monoclonal antibody (rituximab) treatment for cryoglobulinemic vasculitis: where do we stand? Ann Rheum Dis 67: 283–287. [DOI] [PubMed] [Google Scholar]
- Chen R., McLeod J. (1989) Neurological complications of sarcoidosis. Clin Exp Neurol 26: 99–112. [PubMed] [Google Scholar]
- Chia L., Fernandez A., Lacroix C., Adams D., Plante V., Said G. (1996) Contribution of nerve biopsy findings to the diagnosis of disabling neuropathy in the elderly. A retrospective review of 100 consecutive patients. Brain 119: 1091–1098. [DOI] [PubMed] [Google Scholar]
- Chiche L., Bataille S., Kaplanski G., Jourde N. (2012) The place of immunotherapy in the management of HCV-induced vasculitis: an update. Clin Dev Immunol 2012: 315167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen G., Thomas T., Goyne C., Vazquez L., Oh S. (2000) Diagnostic value of nerve and muscle biopsy in suspected vasculitis cases. J Clin Neuromuscul Dis 1: 117–123. [DOI] [PubMed] [Google Scholar]
- Collins M. (2012) The vasculitic neuropathies: an update. Curr Opin Neurol 25: 573–585. [DOI] [PubMed] [Google Scholar]
- Collins M., Dyck P., Gronseth G., Guillevin L., Hadden R., Heuss D., et al. (2010) Peripheral nerve society guideline on the classification, diagnosis, investigation, and immunosuppressive therapy of non-systemic vasculitic neuropathy: executive summary. J Peripher Nerv Syst 15: 176–184. [DOI] [PubMed] [Google Scholar]
- Collins M., Periquet M. (2008) Isolated Vasculitis of the Peripheral Nervous System. Clin Exp Rheumatol 26: S118–130. [PubMed] [Google Scholar]
- Collins M., Periquet M., Mendell J., Sahenk Z., Nagaraja H., Kissel J. (2003) Nonsystemic vasculitic neuropathy: insights from a clinical cohort. Neurology 61: 623–630. [DOI] [PubMed] [Google Scholar]
- Davies L., Spies J., Pollard J., McLeod J. (1996) Vasculitis confined to peripheral nerves. Brain 119: 1441–1448. [DOI] [PubMed] [Google Scholar]
- De Souza F., Radu Halpern A., Valente Barbas C., Shinjo S. (2010) Wegener’s granulomatosis: experience from a Brazilian tertiary center. Clin Rheumatol 29: 855–860. [DOI] [PubMed] [Google Scholar]
- De Vita S., Quartuccio L., Isola M., Mazzaro C., Scaini P., Lenzi M., et al. (2012) A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum 64: 843–853. [DOI] [PubMed] [Google Scholar]
- Delalande S., De Seze J., Fauchais A., Hachulla E., Stojkovic T., Ferriby D., et al. (2004) Neurologic manifestations in primary Sjogren syndrome: a study of 82 patients. Medicine (Baltimore) 83: 280–291. [DOI] [PubMed] [Google Scholar]
- Di Ciaula A., Covelli M., Berardino M., Wang D., Lapadula G., Palasciano G., et al. (2008) Gastrointestinal symptoms and motility disorders in patients with systemic scleroderma. BMC Gastroenterol 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck P., Conn D., Okazaki H. (1972) Necrotizing angiopathic neuropathy. Three-dimensional morphology of fiber degeneration related to sites of occluded vessels. Mayo Clin Proc 47: 461–475. [PubMed] [Google Scholar]
- Dyck P., Hunder G., Dyck P. (1997) A case-control and nerve biopsy study of crest multiple mononeuropathy. Neurology 49: 1641–1645. [DOI] [PubMed] [Google Scholar]
- Dyck P., Norell J., Dyck P. (1999) Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 53: 2113–2121. [DOI] [PubMed] [Google Scholar]
- Fain O., Hamidou M., Cacoub P., Godeau B., Wechsler B., Paries J., et al. (2007) Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum 57: 1473–1480. [DOI] [PubMed] [Google Scholar]
- Ferri C., Cacoub P., Mazzaro C., Roccatello D., Scaini P., Sebastiani M., et al. (2011) Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: result of a multicenter cohort study and review of the literature. Autoimmun Rev. 11: 48–55. [DOI] [PubMed] [Google Scholar]
- Figueroa J., Loftus E., Jr, Harmsen W., Dyck P., Klein C. (2013) Peripheral neuropathy incidence in inflammatory bowel disease: a population-based study. Neurology 80: 1693–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florica B., Aghdassi E., Su J., Gladman D., Urowitz M., Fortin P. (2011) Peripheral neuropathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum 41: 203–211. [DOI] [PubMed] [Google Scholar]
- Frigui M., Kechaou M., Haddouk S., Masmoudi A., Kaddour N., Masmoudi H., et al. (2008) [Benzylthiouracil induced ANCA-positive vasculitis: study of three cases and review of the literature]. Ann Endocrinol (Paris) 69: 517–522. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay M., Garcia-Porrua C. (1999) Systemic vasculitis in adults in Northwestern Spain, 1988–1997. Clinical and epidemiologic aspects. Medicine (Baltimore) 78: 292–308. [DOI] [PubMed] [Google Scholar]
- Guillevin L., Pagnoux C., Seror R., Mahr A., Mouthon L., Le Toumelin P. (2011) The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 90: 19–27. [DOI] [PubMed] [Google Scholar]
- Hattori N., Ichimura M., Nagamatsu M., Li M., Yamamoto K., Kumazawa K., et al. (1999) Clinicopathological features of churg-strauss syndrome-associated neuropathy. Brain 122: 427–439. [DOI] [PubMed] [Google Scholar]
- Hattori N., Mori K., Misu K., Koike H., Ichimura M., Sobue G. (2002) Mortality and morbidity in peripheral neuropathy associated Churg–Strauss syndrome and microscopic polyangiitis. J Rheumatol 29: 1408–1414. [PubMed] [Google Scholar]
- Hernandez-Rodriguez J., Alba M., Prieto-Gonzalez S., Cid M. (2014) Diagnosis and classification of polyarteritis nodosa. J Autoimmun 48–49: 84–89. [DOI] [PubMed] [Google Scholar]
- Hiemstra T., Walsh M., Mahr A., Savage C., De Groot K., Harper L., et al. (2010) Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA 304: 2381–2388. [DOI] [PubMed] [Google Scholar]
- Johnson P., Rolak L., Hamilton R., Laguna J. (1979) Paraneoplastic vasculitis of nerve: a remote effect of cancer. Ann Neurol 5: 437–444. [DOI] [PubMed] [Google Scholar]
- Kararizou E., Davaki P., Speggos K., Stamboulis E. (2012) Rare association of polyneuropathy and Crohn’s disease: a clinicopathological study of 4 cases. Pol J Pathol 63: 261–266. [DOI] [PubMed] [Google Scholar]
- Kissel J., Slivka A., Warmolts J., Mendell J. (1985) The clinical spectrum of necrotizing angiopathy of the peripheral nervous system. Ann Neurol 18: 251–257. [DOI] [PubMed] [Google Scholar]
- Koike H., Yoshida H., Ito T., Ohyama K., Hashimoto R., Kawagashira Y., et al. (2013) Demyelinating neuropathy and autoimmune hemolytic anemia in a patient with pancreatic cancer. Intern Med 52: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Levy Y., Uziel Y., Zandman G., Rotman P., Amital H., Sherer Y., et al. (2005) Response of vasculitic peripheral neuropathy to intravenous immunoglobulin. Ann N Y Acad Sci 1051: 779–786. [DOI] [PubMed] [Google Scholar]
- Levy Y., Uziel Y., Zandman G.G., Amital H., Sherer Y., Langevitz P., et al. (2003) Intravenous immunoglobulins in peripheral neuropathy associated with vasculitis. Ann Rheum Dis 62: 1221–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneiro J., Lopez-Canoa N., Salgado E., Gomez-Reino J. (2014) Maintenance therapy of lupus nephritis with mycophenolate or azathioprine: systematic review and meta-analysis. Rheumatology (Oxford) 53: 834–838. [DOI] [PubMed] [Google Scholar]
- Massie R., Mauermann M., Staff N., Amrami K., Mandrekar J., Dyck P., et al. (2012) Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 135: 3074–3088. [DOI] [PubMed] [Google Scholar]
- Metzler C., Miehle N., Manger K., Iking-Konert C., De Groot K., Hellmich B., et al. (2007) Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) 46: 1087–1091. [DOI] [PubMed] [Google Scholar]
- Morozumi S., Koike H., Tomita M., Kawagashira Y., Iijima M., Katsuno M., et al. (2011) Spatial distribution of nerve fiber pathology and vasculitis in microscopic polyangiitis-associated neuropathy. J Neuropathol Exp Neurol 70: 340–348. [DOI] [PubMed] [Google Scholar]
- Muramatsu K., Tanaka H., Taguchi T. (2008) Peripheral neuropathies of the forearm and hand in rheumatoid arthritis: diagnosis and options for treatment. Rheumatol Int 28: 951–957. [DOI] [PubMed] [Google Scholar]
- Noel N., Drier A., Wechsler B., Piette J., De Paz R., Dormont D., et al. (2013) [Neurological manifestations of Behcet’s disease.]. Rev Med Interne, in press. [DOI] [PubMed] [Google Scholar]
- Oh S. (1997) Paraneoplastic vasculitis of the peripheral nervous system. Neurol Clin 15: 849–863. [DOI] [PubMed] [Google Scholar]
- Oh S., Slaughter R., Harrell L. (1991) Paraneoplastic vasculitic neuropathy: a treatable neuropathy. Muscle Nerve 14: 152–156. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Koike H., Iijima M., Hashimoto R., Tomita M., Kawagashira Y., et al. (2013a) IgG4-related neuropathy: a case report. JAMA Neurol 70: 502–505. [DOI] [PubMed] [Google Scholar]
- Ohyama K., Yasui K., Hasegawa Y., Morozumi S., Koike H., Sobue G. (2013b) Differential recovery in cardiac and vasomotor sympathetic functional markers in a patient with acute autonomic sensory and motor neuropathy. Intern Med 52: 497–502. [DOI] [PubMed] [Google Scholar]
- Perez Alamino R., Martinez C., Espinoza L. (2013) IgG4-associated vasculitis. Curr Rheumatol Rep 15: 348. [DOI] [PubMed] [Google Scholar]
- Rafai M., Fadel H., Boulaajaj F., Gam I., El Moutawakkil B., Karkouri M., et al. (2007) [Peripheral neuropathy in systemic lupus erythematosus with epineural vasculitis and antiphospholipid antibodies]. Rev Neurol (Paris) 163: 103–106. [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M., Stone J., Cid M., Bosch X. (2012) The cryoglobulinaemias. Lancet 379: 348–360. [DOI] [PubMed] [Google Scholar]
- Ribi C., Cohen P., Pagnoux C., Mahr A., Arene J., Puechal X., et al. (2010) Treatment of polyarteritis nodosa and microscopic polyangiitis without poor-prognosis factors: a prospective randomized study of one hundred twenty-four patients. Arthritis Rheum 62: 1186–1197. [DOI] [PubMed] [Google Scholar]
- Rockx M., Clark W. (2010) Plasma exchange for treating cryoglobulinemia: a descriptive analysis. Transfus Apher Sci 42: 247–251. [DOI] [PubMed] [Google Scholar]
- Said G., Lacroix C. (2005) Primary and secondary vasculitic neuropathy. J Neurol 252: 633–641. [DOI] [PubMed] [Google Scholar]
- Schapira D., Balbir-Gurman A., Nahir A. (2000) Naproxen-induced leukocytoclastic vasculitis. Clin Rheumatol 19: 242–244. [DOI] [PubMed] [Google Scholar]
- Schaublin G., Michet C., Jr, Dyck P., Burns T. (2005) An update on the classification and treatment of vasculitic neuropathy. Lancet Neurol 4: 853–865. [DOI] [PubMed] [Google Scholar]
- Schonermarck U., Lamprecht P., Csernok E., Gross W. (2001) Prevalence and spectrum of rheumatic diseases associated with proteinase 3-antineutrophil cytoplasmic antibodies (ANCA) and myeloperoxidase-ANCA. Rheumatology (Oxford) 40: 178–184. [DOI] [PubMed] [Google Scholar]
- Servioli L., Perez C., Consani S., Suarez A., Sehabiaga G., Collazo C., et al. (2007) Prevalence and characteristics of immunomediated neuropathies in a group of patients with autoimmune diseases. J Clin Neuromuscul Dis 9: 285–290. [DOI] [PubMed] [Google Scholar]
- Silva F., Specks U., Kalra S., Hogan M., Leung N., Sethi S., et al. (2010) Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement - a prospective, open-label pilot trial. Clin J Am Soc Nephrol 5: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Merkel P., Spiera R., Seo P., Langford C., Hoffman G., et al. (2010) Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppiah R., Hadden R., Batra R., Arden N., Collins M., Guillevin L., et al. (2011) Peripheral neuropathy in ANCA-associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology (Oxford) 50: 2214–2222. [DOI] [PubMed] [Google Scholar]
- Terrier B., Lacroix C., Guillevin L., Hatron P., Dhote R., Maillot F., et al. (2007) Diagnostic and prognostic relevance of neuromuscular biopsy in primary Sjogren’s syndrome-related neuropathy. Arthritis Rheum 57: 1520–1529. [DOI] [PubMed] [Google Scholar]
- Thaisetthawatkul P., Sundell R., Robertson C., Dyck P. (2011) Vasculitic neuropathy associated with minocycline use. J Clin Neuromuscul Dis 12: 231–234. [DOI] [PubMed] [Google Scholar]
- Torvik A., Berntzen A.E. (1968) Necrotizing vasculitis without visceral involvement. postmortem examination of three cases with affection of skeletal muscles and peripheral nerves. Acta Med Scand 184: 69–77. [PubMed] [Google Scholar]
- Vital A., Lagueny A., Ferrer X., Louiset P., Canron M., Vital C. (2008) Sarcoid neuropathy: clinico-pathological study of 4 new cases and review of the literature. Clin Neuropathol 27: 96–105. [DOI] [PubMed] [Google Scholar]
- Vital C., Vital A., Canron M., Jaffre A., Viallard J., Ragnaud J., et al. (2006) Combined nerve and muscle biopsy in the diagnosis of vasculitic neuropathy. A 16-year retrospective study of 202 cases. J Peripher Nerv Syst 11: 20–29. [DOI] [PubMed] [Google Scholar]
- Voskuyl A., Hazes J., Zwinderman A., Paleolog E., Van Der Meer F., Daha M., et al. (2003) Diagnostic strategy for the assessment of rheumatoid vasculitis. Ann Rheum Dis 62: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R., Carruthers D., Scott D. (1995) Epidemiology of systemic vasculitis: changing incidence or definition? Semin Arthritis Rheum 25: 28–34. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ito Y., Mitsuma N., Hattori N., Sobue G. (2003) Pain-related differential expression of NGF, GDNF, IL-6, and their receptors in human vasculitic neuropathies. Intern Med 42: 1100–1103. [DOI] [PubMed] [Google Scholar]
- Yokoi S., Kawagashira Y., Ohyama K., Iijima M., Koike H., Watanabe H., et al. (2014) Mononeuritis multiplex with tumefactive cellular infiltration in a patient with reactive lymphoid hyperplasia with increased immunoglobulin G4-positive cells. Hum Pathol 45: 427–430. [DOI] [PubMed] [Google Scholar]
- Zwerina J. (2008) [Churg–Strauss syndrome]. Z Rheumatol 67: 137–143; quiz 144. [DOI] [PubMed] [Google Scholar]


