Abstract
Objectives:
Prolotherapy is an injection-based complementary treatment, which has shown promising results in the treatment of different musculoskeletal disorders. The aim of this study was to determine the therapeutic efficacy of dextrose prolotherapy on pain, range of motion, and function in patients with knee osteoarthritis (OA).
Methods:
In this single-arm prospective study, participants with symptomatic moderate knee osteoarthritis underwent prolotherapy with intra-articular injection of 20% dextrose water at baseline, and at 4 weeks and 8 weeks later. Patients were followed for 24 weeks. Pain severity at rest and activity, according to the visual analog scale (VAS), articular range of motion (ROM), and Western Ontario and McMaster Universities arthritis index (WOMAC) scores were measured at baseline, 4, 8, and 24 weeks later.
Results:
A total of 24 female patients (average age: 58.37 ± 11.8 years old) received 3-monthly injection therapies. Before the treatment, the mean articular range of motion was 105.41 ± 11.22°. Mean VAS scale at rest and activity was 8.83 ± 1.37 and 9.37 ± 1.31, respectively. At the end of week 24, knee ROM increased by 8°. Pain severity in rest and activity decreased to 4.87 ± 1.39, 45.86%, and 44.23%, respectively (p < 0.001). Total WOMAC score and its subcategories showed a continuous improvement trend in all the evaluation sessions, so that at the end of the study, the total score decreased by 30.5 ± 14.27 points (49.58%) (p < 0.001). Improvements of all parameters were considerable until week 8, and were maintained throughout the study period.
Conclusions:
Prolotherapy with three intra-articular injections of hypertonic dextrose given 4 weeks apart for selected patients with knee OA, resulted in significant improvement of validated pain, ROM, and WOMAC-based function scores, when baseline levels were compared at 24 weeks. Further studies with randomized controlled trials involving a comparison group are suggested to confirm these findings.
Keywords: dextrose prolotherapy, intra-articular injection, knee osteoarthritis, prolotherapy
Introduction
Osteoarthritis (OA) is an age-dependent disease caused by degenerative and healing processes in subchondral tissue of articular and bone cartilage, resulting in an alteration of its biomechanical properties that eventually causes pain, stiffness, and decreased articular function [Hochberg et al. 2012; Felson, 2005; Hochberg et al. 1996]. Considering the nature of the mechanical pressure applied on the knee joint, it is a common joint for OA [Gupta et al. 2005; Felson, 2003].
There are few treatment methods for moderate to severe OA; most focus on relieving the symptoms but do little to change the biochemical environment of the joint or on the disease process. Current therapies include simple analgesics, anti-inflammatory drugs, muscle strengthening exercises, physical therapy, intra-articular injection of cartilage supplements such as hyaluronic acid agents, arthroscopic surgery, and arthroplasty [Michael et al. 2010; Toopchizadeh et al. 2012; Barron and Rubin, 2007], nevertheless no nonsurgical treatment is uniformly effective.
Prolotherapy, also known as proliferative therapy, or regeneration injection therapy, is a complementary injection treatment for musculoskeletal pains. Hypertonic dextrose is the most commonly injected solution. Although the mechanism of this treatment modality is not clearly understood, it is hypothesized that the solution creates a host inflammatory response through the upgrading of chemical mediators, which results in stronger connective tissue, improved biomechanics, and joint function and soft tissue recovery [Jensen et al. 2008; Rabago et al. 2005].
Several reports have revealed the effects of dextrose prolotherapy in treating refractory musculoskeletal disorders such as low back pain, tendonitis, lateral epicondylitis, and ligament damage [Rabago et al. 2005; Yelland et al. 2003; Klein et al. 1993]. Though prolotherapy has been used for knee OA for many decades, only recently has the efficacy of the results been studied [Hauser and Hauser, 2007; Rabago et al. 2012]. On the other hand, experimental data in this area are rather controversial and there is no general consensus about its application in joint degenerative diseases [Hashemi et al. 2010; Rabago et al. 2010].
It should be noted that the number of elderly people in society is increasing and musculoskeletal disorders, mainly OA in this population, are very common. Routine treatments for pain and disability in these patients have low efficacy, and some treatments, including hyaluronic acid injection therapy, have high costs. It is possible that prolotherapy has acceptable effects on OA in these patients. Therefore, we designed this study to investigate the effectiveness of dextrose prolotherapy in decreasing pain, improving daily functional ability, and increasing the joint range of motion (ROM) in patients with knee OA.
Methods and materials
In this single-arm trial, adult patients diagnosed with knee OA based on the clinical criteria of the American Rheumatological Association [Toledo et al. 2011; Altman, 1991], who met the inclusion and exclusion criteria, were recruited from physical medicine and rehabilitation clinics at Imam Reza Hospital, Tabriz University of Medical Sciences. We treated eligible patients and followed their status from July 2012 to November 2013.
The inclusion criteria were patients aged 45 –75 years old who had: (a) moderate or moderate to severe knee OA (grade II or III according to the radiological classification of knee OA defined by Kellgren and Lawrence [Toledo et al. 2011]); (b) lack of any inflammatory or rheumatologic diseases such as rheumatoid arthritis; (c) intractable pain and joint tenderness refractory to conservative treatment; (d) no response to medications or physiotherapy in the last 3 months; (e) consent to participate in the study.
The exclusion criteria were patients who had: (a) severe OA (grade IV according to the Kellgren–Lawrence system of classification); (b) history of rheumatologic or inflammatory diseases; (c) received oral or systemic corticosteroids during the 2 weeks prior to treatment; (d) received an intra-articular injection of hyaluronic acid agents during the previous month; (e) poorly controlled diabetes mellitus with fasting blood sugar greater than 11.1 mmol/L; (f) history of anticoagulation therapy; (g) history of prior total knee replacement surgery.
The radiological criteria of knee joint OA severities used in this study were based on the Kellgren–Lawrence classification: grade 0: normal; grade I: small osteophytes without clinical importance; grade II: definite osteophytes but normal joint space; grade III: definite osteophytes with moderate narrowing of joint space; grade IV: definite osteophytes with severe narrowing of joint space.
Research ethics
The study procedure was in accordance with the ethical standards of the responsible local committee on human experimentation of Tabriz University of Medical Sciences and it was approved by this ethics committee. The study protocol was also registered as a clinical trial in the Iranian Registry of Clinical Trials (www.irct.ir, number 201210213217 N4).
Before participating in the project, the aims of the study were explained orally to all the patients and written informed consents were obtained from all study participants.
Intervention
Each patient received three intra-articular injections at 1-month intervals in weeks 0, 4, and 8. During the procedure, each patient was placed in a supine position with the knee flexed at 10–15°, and the intra-articular injection landmark was determined below the superolateral part of the patella [Lento et al. 2011]. The injection site was located by a lateral approach; in patients without sufficient space on the lateral side, a medial approach was performed. Under sterile conditions, a composition of 8 ml of 20% dextrose and 2 ml of 1% lidocaine was injected by an expert physiatrist using a 22 gauge needle.
Outcome measures
Baseline demographic findings and Western Ontario and McMaster Universities arthritis index (WOMAC) values, knee ROM, and pain severity at rest (seated) and in activity (after walking 6 m) using the visual analogue scale (VAS) were recorded. The patients were evaluated for these parameters at the time of first injection, and 4, 8, and 24 weeks later.
Knee ROM in flexion was determined in prone position using an international standard 360º goniometer. The validity and reliability of this measuring device has been demonstrated by other researchers [Brosseau et al. 2001; Kolber et al. 2012].
Pain was measured using a 10 cm VAS. Pain intensity is classified using a range from 0 to 10, in which 0 = no pain at all and 10 = the worst possible pain. Patients were asked to sign the place on the VAS scale that corresponded to their pain level.
The WOMAC questionnaire is used to evaluate a patient’s functions when diagnosed with rheumatic diseases, especially knee OA. The WOMAC is a 24-item questionnaire with three subscales measuring pain (five items), stiffness (two items), and physical function (17 items). Answers to each of the 24 questions are scored on five-point Likert scales (none = 0, slight = 1, moderate = 2, severe = 3, extreme = 4), with total scores ranging from 0 to 96. So, the maximum possible scores for WOMAC, pain, stiffness, and function are 96 (most severe), 20, 8, and 68, respectively. Higher scores indicate greater disease severity [McConnell et al. 2001; Bellamy et al. 1988]. The WOMAC scale has been validated for use with Iranian patients [Nadrian et al. 2012].
The participants were recommended to take acetaminophen 500 mg as needed and were advised not to use nonsteroidal anti-inflammatory drugs during the following 2 weeks because of their inhibitory effects on the recovery process. They were also discouraged from taking physical therapy during the 6-month follow-up period because of its confounding effect on evaluating research in our essential treatment. The patients were not discouraged or prevented from using other medications prescribed for their underlying systemic diseases.
Data analysis
Baseline data are reported as means ± standard deviation (continuous data), or percentages and numbers (categorical data), depending on the data level. The mean changes at each level were calculated by comparing the weekly value and the baseline value as follows: mean (week n score - week 0 or baseline score). The improvement percentage is calculated by dividing the amount of change from baseline at each level on the maximum expected change (baseline score - week n score/ baseline score), and multiplying it by 100.
Achievement of minimal clinical difference with regard to similar studies was calculated as 20% for total WOMAC score and 50% for overall improvement in this score. Repeated measures analysis of variance (ANOVA) was used to evaluate the serial changes of different variables during the treatment period. All data were analyzed using the Statistical Package for Social Sciences, version 16.0; p < 0.05 was considered to be statistically significant.
Results
In this study, during a 16-month period, 24 female patients with moderate and moderately severe knee OA were enrolled, treated, and followed.
At baseline, of the 33 patients in the first screening, two patients were excluded because of poorly controlled diabetes mellitus and three other patients declined to participate. A total of 28 patients met the criteria and were enrolled in the study, but four patients failed to complete and were excluded from the analysis. These included two women who dropped out because of the pain after the first injection, and two men refused to continue therapy because of personal issues after the second injection (not related to the treatment). At the end, 24 patients were included in the final evaluation (Figure 1).
Figure 1.
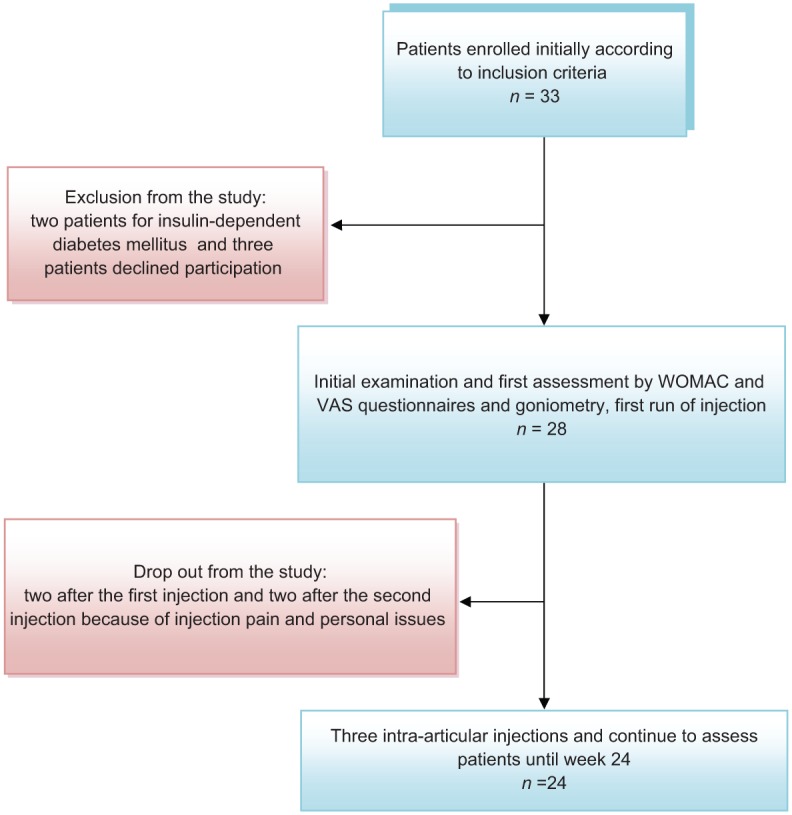
Clinical trial chart. VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities arthritis index.
The study sample consisted of female adults (58.37 ± 11.83 years old; range: 46–72 years), a majority of whom had a body mass index of 26–30 kg/m2 (Table 1).
Table 1.
Baseline participants’ characteristics (n = 28, total 48 injected knees).
| Variable | Number (%) |
|---|---|
| Age (years) | 58.37 ± 11.83 |
| Sex | 26 (92.8%) female, 2 (7.2%) male |
| Duration of knee pain (months) | 18.5 ± 8.53 |
| Body mass index (kg/m2 ), number (%) | |
| < 25 | 3 (10.7%) |
| 26–30 | 19 (67.8%) |
| > 31 | 6 (21.4%) |
| Previous physiotherapy per person | 15 (53%) |
| Previous hyaluronic acid injection per knee | 4 (8%) |
| Western Ontario and McMaster Universities arthritis index total score (0–96), | |
| point ± SD | 58.83 ± 14.52 |
| Pain (0–20) | 14.37 ± 2.88 |
| Stiffness (0–8) | 3.25 ± 2.64 |
| Function (0–68) | 41.2 ± 10.35 |
| Resting visual analog scale (0–10), point ± SD | 8.83 ± 1.37 |
| Activity visual analog scale (0–10), point ± SD | 9.37 ± 1.31 |
| Range of motion (°) | 105.41 ± 11.22 |
| X-ray Kellgren–Lawrence osteoarthritis | |
| severity, score (I–IV) | |
| Grade I–II (mild–moderate) | 6 (12%) |
| Grade II–III (moderate) | 34 (70%) |
| Grade III–IV (moderate–severe) | 8 (16%) |
SD, standard deviation.
A total of 16 patients had both knees treated, contributing 32 knees, and 8 patients had only 1 knee treated. The total sample sizes for analysis for WOMAC as well as pain VAS was 24, regardless of the number of injected knees (analysis per person); 40 knees were included for ROM evaluation (analysis per knee).
Of the 24 patients (40 injected knees) who completed the study, 30 knees were placed in the moderate (grade II–III), 6 were placed in the moderate to severe (grade III–IV), and 4 were placed in the mild to moderate (grade I–II) OA grading categories. Of the eight patients with only one treated knee, three were in the right knees, the remainder were in the left knees.
Before treatment, the mean knee ROM was 105.41° ± 11.22°. Mean VAS scale at rest and activity was 8.83 ± 1.37 and 9.37 ± 1.31, respectively. At the end of week 24, mean knee ROM increased to 113.5° ± 7.22°. Pain severity, both in rest and activity, decreased to 4.87 ± 1.39, 45.86% and 44.23%, respectively (p < 0.001). The mean WOMAC total score was 58.83 ± 14.52 points, consisting of pain subscore values 14.37 ± 2.88, stiffness subscore values 3.25 ± 2.64, and function subscore values 41.2 ± 10.35 points.
Total WOMAC score and its subcategories had a continuous improvement trend in all the evaluation sessions, so that the total score had decreased by 30.5 ± 14.27 points (49.58%) at the end of the study (p < 0.001). More than half of the participants achieved approximately 50% improvement in total WOMAC score at 24 weeks.
The baseline characteristics of the participants are presented in Table 1. Changes in knee ROM, pain VAS (rest and activity), WOMAC and its subscales during the study period and their percentage of improvement and mean changes in each time are demonstrated in Tables 2 and 3.
Table 2.
Changes in range of motion and visual analogue scale during the study periods.
| Evaluation intervals variable | At baseline |
Week 4 |
Week 8 |
Week 24 |
p value |
|---|---|---|---|---|---|
| (48 injected knees) | (44 injected knees) | (40 injected knees) | (40 injected knees) | ||
| Range of motion (°) | 105.41 ± 11.22 | 110.37 ± 9.7 | 113.08 ± 8.06 | 113.5 ± 7.22 | *< 0.001 |
| Percentage changes$ | – | (19.6%) | (25.5%) | (27.0%) | |
| Point changes‡ | – | 5.96 ± 5.7 | 7.67 ± 8.29 | 8.18 ± 9.31 | |
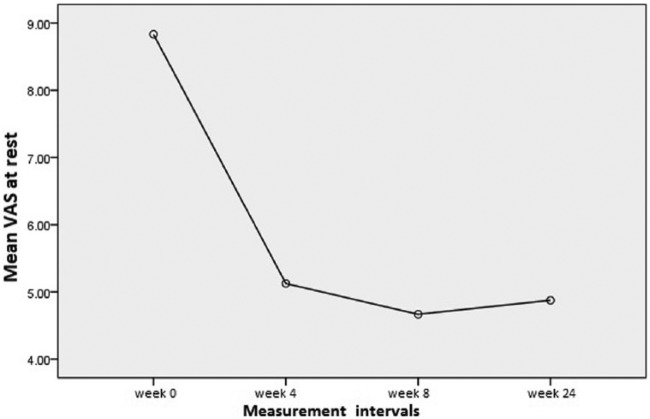
| Rest visual analogue scale | 8.83 ± 1.37 | 5.12 ± 2.02 | 4.66 ± 1.65 | 4.87 ± 1.39 | *< 0.001 |
| Percentage changes$ | – | (41.19%) | (44.78%) | (44.23%) | |
| Point changes‡ | – | −3.7 ± 2.19 | −4.16 ± 2.18 | −3.95 ± 1.57 | |
| Activity visual analogue scale | 9.37 ± 1.31 | 6.08 ± 1.95 | 5.25 ± 1.48 | 4.87 ± 1.39 | *< 0.001 |
| Percentage changes$ | – | (34.43%) | (42.32%) | (45.86%) | |
| Point changes‡ | – | −3.29 ± 1.92 | −4.12 ± 1.82 | −4.29 ± 1.39 |
p is two-sided significant (< 0.05) using repeated measures of analysis of variance statistical test.
Improvement percentage of measured values is calculated by dividing the amount of changes at each level on the maximum of expected change and multiplying it by 100.
The mean changes in each level are calculated compared with the baseline value: mean (week n score - week 0 or baseline score).
Table 3.
Changes in Western Ontario and McMaster Universities arthritis index score and its subscales during the study periods.
| Evaluation intervals variable | At baseline |
Week 4 |
Week 8 |
Week 24 |
p value |
|---|---|---|---|---|---|
| (48 injected knees) | (44 injected knees) | (40 injected knees) | (40 injected knees) | ||
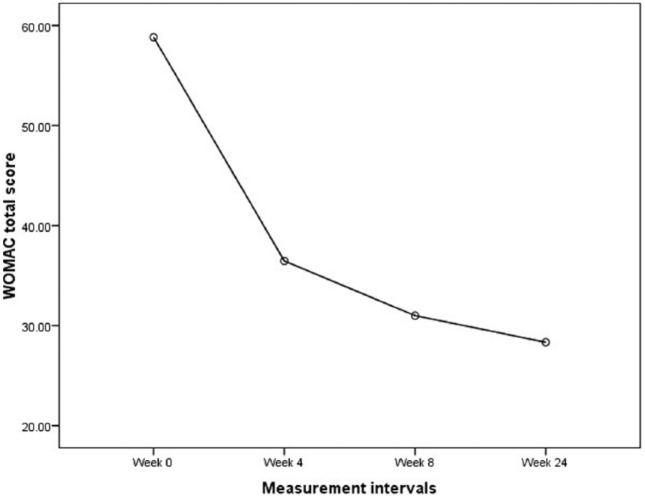
| Total Western Ontario and McMaster Universities arthritis index | 58.83 ± 14.52 | 36.45 ± 9.91 | 31 ± 7.91 | 28.33 ± 7.2 | *< 0.001 |
| Percentage changes$ | – | (34.35%) | (44.45%) | (49.58%) | |
| Point changes‡ | – | −22.37 ± 14.71 | −27.83 ± 14.71 | −30.5 ± 14.27 | |
| Pain | 14.37 ± 2.88 | 9.29 ± 2.45 | 8.16 ± 2.37 | 7.54 ± 1.99 | *< 0.001 |
| Percent changes$ | – | (34.22%) | (41.75%) | (46.19%) | |
| Point changes‡ | – | −5.07 ± 2.73 | −6.2 ± 3.18 | −6.83 ± 3.18 | |
| Stiffness | 3.25 ± 2.64 | 1.7 ± 1.47 | 0.94 ± 0.87 | 0.91 ± 0.87 | *< 0.001 |
| Percentage changes$ | – | (37.67%) | (57.42%) | (58.12%) | |
| Point changes‡ | – | −2.04 ± 2.71 | −2.87 ± 2.5 | −2.89 ± 2.1 | |
| Function | 41.2 ± 10.35 | 25.95 ± 7.88 | 22.45 ± 5.62 | 20.41 ± 5.34 | *< 0.001 |
| Percentage changes$ | – | (32.65%) | (42.25%) | (47.85%) | |
| Point changes‡ | – | −15.25 ± 10.5 | −18.75 ± 10.52 | −20.79 ± 10.3 |
p is two-sided significant (< 0.05) using repeated measures of analysis of variance statistical test.
Improvement percentage of measured values is calculated by dividing the amount of changes at each level to the maximum of expected change and multiplying it by 100.
The mean changes in each level are calculated compared with the baseline value: mean (week n score - week 0 or baseline score).
Following the prolotherapy sessions, ROM, pain VAS at rest and activity, and WOMAC scales were considerably improved until week 8 and then maintained throughout the study period. It should be noted between week 8 and the last evaluation at week 24, there was a lesser but still statistically significant improvement compared with baseline (p < 0.001). Figures 2 and 3 show these improvement trends in WOMAC total score and pain severity using VAS at various periods of the study, respectively.
Figure 2.
Changes of WOMAC total score at various periods of the study. WOMAC, Western Ontario and McMaster Universities arthritis index.
Figure 3.
Changes of pain severity using the VAS score at various periods of the study. VAS, visual analog scale.
During our study, no side effects including infection, exacerbation of inflammation, or sustained pain related to the injection or injected fluid were seen.
Discussion
Prolotherapy has been reported as a useful method in the treatment of chronic musculoskeletal and joint diseases. It is proposed that prolotherapy causes mild inflammation and cell stress in the weakened ligament or tendon area, releases cytokines and growth factors, and induces a new healing cascade in that area, which leads to activation of fibroblasts, generation of collagen precursors, and strengthening of the connective tissue [Rabago et al. 2012; Jensen et al. 2008]. It is also hypothesized that in dextrose prolotherapy, the increased extracellular glucose level and the contact of human cells with the hypertonic environment causes an increase in multiple growth factors in different cells. By these presumed mechanisms, the hypertonic dextrose solution stimulates the proliferation of chondrocytes, osteocytes, and fibroblasts. These cells then excrete extracellular matrix, which enhances the stability of the joints by tightening and strengthening the ligaments, tendons, and joint stabilizing structures [Rabago et al. 2013b; Yelland et al. 2003; Klein et al. 1993].
This single-arm clinical trial found that dextrose prolotherapy could cause significant reduction in patients’ pain at rest and during activity, and could enhance joint ROM and WOMAC scores.
There are few reports regarding the effects of prolotherapy on OA. All these studies have shown an improvement in different pain scales between 36% and 55%, as well as improved WOMAC subscales following prolotherapy [Rabago et al. 2012, 2013b; Hashemi et al. 2010; Reeves and Hassanein, 2003; Kim et al. 2002].
In the present study, knee flexion ROM increased by 8° (27%) at week 24, which is the amount of minimum detectable change that is required to be stated with 95% certainty that the change is not due to intertrial variability or measurement error [Kolber et al. 2012].
WOMAC total score in our study improved by 49.58% at the end of week 24 as well as 46.19%, 58.12%, and 47.85% reduction in pain, stiffness, and function subscale scores, respectively. This improvement exceeds the reported minimal clinical difference of 12–25% found in related studies [Tabach et al. 2005, 2009].
Similarly, Rabago and colleagues reported a 31.5% decrease in WOMAC score as well as a 34.7%, 24.4%, and 36.8% decrease in pain, stiffness, and function subscale scores, respectively at the end of 52 weeks [Rabago et al. 2012]. However, they reported less improvement in WOMAC scores compared with our findings. There is one possible explanation for this difference: we evaluated patients for 24 weeks, while Rabago and colleagues followed their patients for 52 weeks.
Although cartilage volume increases after each prolotherapy session and will remain increased for a time, it decreases over time, which has a significant correlation with the pain subscales of the WOMAC score [Rabago et al. 2013a]. Therefore, it is possible that with longer follow up, we would observe similar improvements to those reported by others.
As previously mentioned, the total WOMAC score was considerably improved until week 8 and then maintained throughout the study period. In other words, treatment effects reached a plateau after 8–12 weeks, similar to the results described by others [Rabago et al. 2012]. This observation could be due to the possibility of overuse of the knee after a temporary improvement in pain and function, and ignoring the recommendations about gradual increase of pressure on the knee.
Other studies have also reported that the improvements attenuate over time and sometimes the symptoms are exacerbated after several months, which indicate the short-term effects of the treatment, similar to the injections of hyaluronic acid agents [Samson et al. 2007]. Though post-treatment pain is not as severe as their experienced original pretreatment pain, this could suggest that these patients need several injections at intervals to keep the desired results.
Ignoring the patient’s other pain sources including joint-surrounding tendons and ligaments could be another potential cause in this regard; we did not treat enthesopathies or the ligament fibro-osseous junctions with extra-articular dextrose injections around these elements in our study. So, it appears that ligaments or other structures need to be treated to get the full benefit from prolotherapy.
Ligaments’ strength and integrity plays an important role in the function and stability of the joint, and one of the reasons for the triggering or exacerbation of OA is dysfunction of the supportive ligaments [Reeves and Hassanein, 2003; Ongley et al. 1988]. Previous studies evaluating the prolotherapy effects on OA patients with knee instability caused by injury have shown desirable results in improving pain intensity and reducing knee instability, demonstrating the positive effects of dextrose prolotherapy, both in intra-articular cartilage damage as well as extra-articular ligament injury [Rabago et al. 2012; Reeves and Hassanein, 2000, 2003]. Accordingly, mechanical instability due to injury or partial tearing of surrounding ligaments, especially the lateral collateral ligament (LCL), could be another challenging issue in patients with knee OA.
During the present study, extra-articular injection in addition to intra-articular injection was not included in the research protocol. It seems application of a dextrose injection in the insertion-to-bone site of ligaments and tendons is more investigational for knee OA and is mainly indicated in ligament laxity such as the anterior cruciate ligament (intra-articular ligament) or LCL (extra-articular ligament). In other words, the first priority in our study was a focus on the damaged cartilage and not on external ligaments or tendons. However, this would be a promising method for showing the better efficacy of prolotherapy, especially for young or middle-aged patients with ligament injury, and even for elderly patients with knee OA in whom LCL damage is not uncommon. Further studies in this area are necessary to draw a definitive conclusion.
In our study, two patients left the survey because of the pain during injections. Actually one of the difficulties that occurs in blind injections is pain during or after injection. This problem is mostly seen in obese participants or involved joints with synovial hypertrophy or major osteophytes as well as among patients with bilateral narrowing of the joint space. Therefore, it is possible that an incorrect location of the injected solution and consequent inflammation of the surrounding tissues are the main causes of pain during and after injection, respectively. Accurate localization using ultrasound-guided injection from the suprapatellar bursa seems to be an ideal choice for minimizing pain among such patients.
Strengths and limitations
We observed acceptable results on validated and patient-oriented outcomes using prolotherapy as a cost-effective treatment for patients with moderate or moderately severe OA who are refractory to conservative therapy; however, this study had some limitations. The study was a single-arm trial and could not define this method’s superiority over other methods. The sample size in our study was small and we did not include a control group to better compare the results. In addition, our assessment tools were mostly self reported and subjective, hence, more assessments such as articular cartilage thickness measurement by imaging techniques or musculoskeletal ultrasonography are recommended for objective confirmation of the clinical efficacy of prolotherapy.
Conclusion
Prolotherapy with three intra-articular injections of hypertonic dextrose given 4 weeks apart for selected patients with moderate knee OA resulted in statistically significant improvements in validated pain, ROM, as well as in WOMAC-based function scores when baseline levels were compared at 24 weeks. Further studies with randomized controlled trials involving a comparison group are suggested to confirm the obtained findings.
Acknowledgments
We greatly acknowledge Dr Morteza Ghojazadeh for his kind help in providing statistical analysis consultation. The authors are also indebted to the Physical Medicine and Rehabilitation Research Center, Tabriz University of Medical Sciences, Iran for its support.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Fariba Eslamian, Physical Medicine and Rehabilitation Research Center, Tabriz University of Medical Sciences, Imam Reza Hospital, Golgasht Ave, Tabriz, 5166615556, Iran.
Bahman Amouzandeh, Physical Medicine and Rehabilitation Department, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Altman R. (1991) Criteria for classification of clinical osteoarthritis. J Rheumatol Suppl 27: 10–12. [PubMed] [Google Scholar]
- Barron M., Rubin B. (2007) Managing osteoarthritic knee pain. J Am Osteopath Assoc 107: ES21–ES27. [PubMed] [Google Scholar]
- Bellamy N., Buchanan W., Goldsmith C., Campbell J., Stitt L. (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15: 1833–1840. [PubMed] [Google Scholar]
- Brosseau L., Balmer S., Tousignant M., O’Sullivan J., Goudreault C., Goudreault M., et al. (2001) Intra- and intertester reliability and criterion validity of the parallelogram and universal goniometers for measuring maximum active knee flexion and extension of patients with knee restrictions. Arch Phys Med Rehabil 82: 396–402. [DOI] [PubMed] [Google Scholar]
- Felson D. (2005) The sources of pain in knee osteoarthritis. Curr Opin Rheumatol 5: 624–628. [DOI] [PubMed] [Google Scholar]
- Felson D. (2003) Epidemiology of osteoarthritis. In: Brandt D., Lohmander L. (eds), Osteoarthritis. Oxford: Oxford University Press, pp: 9–16. [Google Scholar]
- Gupta S., Hawker G., Laporte A., Croxford R., Coyte P. (2005) The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology 44: 1531–1537. [DOI] [PubMed] [Google Scholar]
- Hauser R., Hauser M. (eds) (2007) Prolo Your Pain Away, Curing Chronic Pain with Prolotherapy. Updated third edition. Chicago, IL: Beulah Land Press. [Google Scholar]
- Hashemi S., Madadi F., Razavi S., Nikooseresht M., Kiyabi F., Nasiripour S. (2010) Intra-articular hyaluronic acid injections versus dextrose prolotherapy in the treatment of osteoarthritic knee pain. TU M J 70: 119–125. [Google Scholar]
- Hochberg M., Altman R., April K., Benkhalti M., Guyatt G., McGowan J., et al. (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 64: 465–474. [DOI] [PubMed] [Google Scholar]
- Hochberg M., Perlmutter D., Hudson J., Altman R. (1996) Preferences in the management of osteoarthritis of the hip and knee: results of a survey of community-based rheumatologists in the United States. Arthritis Care Res 9: 170–176. [DOI] [PubMed] [Google Scholar]
- Jensen K., Rabago D., Best T., Patterson J. (2008) Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med 36: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. (2002) The effect of prolotherapy for osteoarthritis of the knee. J Korean Acad Rehabil Med 26: 445–448. [Google Scholar]
- Klein R., Bjorn C., DeLong B., Mooney V. (1993) A randomized double-blind trial of dextrose-glycerine-phenol injections for chronic low back pain. J Spinal Disord 6: 23–33. [PubMed] [Google Scholar]
- Kolber M., Fuller C., Marshall J., Wright A., Hanney W. (2012) The reliability and concurrent validity of scapular plane shoulder elevation measurements using a digital inclinometer and goniometer. Physiother Theory Pract 28: 161–168. [DOI] [PubMed] [Google Scholar]
- Lento P., Ihm J., Kennedy D. (2011) Peripheral joint and soft tissue injection techniques. In: Braddom R. (ed.), Physical Medicine and Rehabilitation. Philadelphia, PA: Elsevier Saunders, pp: 517–540. [Google Scholar]
- McConnell S., Kolopack P., Davis A. (2001) The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Care Res 45: 453–461. [DOI] [PubMed] [Google Scholar]
- Michael J., Schlüter-Brust K., Eysel P. (2010) The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int 107: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadrian H., Moghimi N., Nadrian E., Moradzadeh R., Bahmanpour K., Iranpour A., et al. (2012) Validity and reliability of the Persian versions of WOMAC Osteoarthritis Index and Lequesne Algofunctional Index. Clin Rheumatol 31: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Ongley M., Dorman T., Eek B., Lundgren D., Klein R. (1988) Ligament instability of knees: a new approach to treatment. Manual Med 3: 152–154. [Google Scholar]
- Rabago D., Best T., Beamsley M., Patterson J. (2005) A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med 15: 376–380. [DOI] [PubMed] [Google Scholar]
- Rabago D., Kijowski R., Woods M., Patterson J., Mundt M., Zgierska A., et al. (2013a) Association between disease-specific quality of life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch Phys Med Rehabil 94: 2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabago D., Patterson J., Mundt M., Kijowski R., Grettie J., Segal N., et al. (2013b) Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med 11: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabago D., Slattengren A., Zgierska A. (2010) Prolotherapy in primary care practice. Prim Care 37: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabago D., Zgierska A., Fortney L., Kijowski R., Mundt M., Ryan M., et al. (2012) Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: results of a single-arm uncontrolled study with 1-year follow-up. J Altern Complement Med 18: 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves K., Hassanein K. (2003) Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern Ther Health Med 9: 58–62. [PubMed] [Google Scholar]
- Reeves K., Hassanein K. (2000) Randomized prospective doubleblind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med 6: 68–80. [PubMed] [Google Scholar]
- Samson D., Grant M., Ratko T., Bonnell C., Ziegler K., Aronson N. (2007) Treatment of primary and secondary osteoarthritis of the knee. Evid Rep Technol Assess 157: 1–157. [PMC free article] [PubMed] [Google Scholar]
- Tubach F., Giraudeau B., Ravaud P. (2009) The variability in minimal clinically important difference and patient acceptable symptomatic state values did not have an impact on treatment effect estimates. J Clin Epidemiol 62: 725–728. [DOI] [PubMed] [Google Scholar]
- Tubach F., Wells G., Ravaud P., Dougados M. (2005) Minimal clinically important difference, low disease activity state, and patient acceptable symptom state: methodological issues, J Rheumatol 32: 2025–2029. [PubMed] [Google Scholar]
- Toledo S., Trapani K., Feldbruegge E. (2011) Rehabilitation of patients with rheumatic disease. In: Braddom R. (ed.), Physical Medicine and Rehabilitation. Philadelphia, PA: Elsevier Saunders, pp: 769–771. [Google Scholar]
- Toopchizadeh V., Babaei-Ghazani A., Eftekhar Sadat B. (2012) Efficiency of Action Potential Simulation (APS) therapy in compare to Transcutaneous Electrical Nerve Stimulation (TENS) in knee osteoarthritis. Life Sci J 9: 3790–3794. [Google Scholar]
- Yelland M., Glasziou P., Bogduk N., Schluter P., McKernon M. (2003) Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized study. Spine 29: 9–16. [DOI] [PubMed] [Google Scholar]