Abstract
The controversy over the existence or the need for G-protein coupled receptors (GPCRs) in plant G-protein signalling has overshadowed a more fundamental quest for the role of AtGCR1, the most studied and often considered the best candidate for GPCR in plants. Our whole transcriptome microarray analysis of the GCR1-knock-out mutant (gcr1-5) in Arabidopsis thaliana revealed 350 differentially expressed genes spanning all chromosomes. Many of them were hitherto unknown in the context of GCR1 or G-protein signalling, such as in phosphate starvation, storage compound and fatty acid biosynthesis, cell fate, etc. We also found some GCR1-responsive genes/processes that are reported to be regulated by heterotrimeric G-proteins, such as biotic and abiotic stress, hormone response and secondary metabolism. Thus, GCR1 could have G-protein-mediated as well as independent roles and regardless of whether it works as a GPCR, further analysis of the organism-wide role of GCR1 has a significance of its own.
Introduction
G-protein signalling pathways are implicated in a variety of plant processes, but their upstream receptors and the role of typical G-protein coupled receptors (GPCRs) in plants are not very well understood [1,2]. The canonical GPCR gene in Arabidopsis (GCR1) was isolated independently by two groups [3,4]. The latter group initially called it a cytokinin receptor but subsequently found that cytokinin response was due to an independent mutation [5]. Others implicated GCR1 in PIPLC mediated regulation of DNA synthesis [6], abolishing seed dormancy and reducing flowering time [7]. Later, knock-out mutants of GCR1 were used to implicate it in seed germination in response to brassinosteroids and gibberellins [8], reducing drought stress, negative regulation of ABA induced gene expression, ABA and S1P-induced regulation of stomatal aperture [9], blue light response [10] etc. Additional GPCR candidates were reported based on their transmembrane domains [11–13], with the list eventually swelling upto 56 candidates, of which, GCR1 is bioinformatically considered to be the best candidate based on GPCR fold analysis [14]. Another candidate, GCR2, which was reported to be a 7TM ABA receptor [15] was later reported to be a homolog of lanthionine synthetase [16,17]; and eventually found to be a lantibiotic cyclase like protein with no role as a GPCR [18].
Conventional GPCRs act as ligand-activated guanine nucleotide exchange factors (GEF) to release GDP from the G-protein alpha subunit. Despite being the most studied candidate, the role of GCR1 as a GPCR remains biochemically unproven, due the lack of an identified ligand that binds GCR1, the lack of demonstration of GEF activity and the disagreement over its reported [9,12] physical interaction with G-protein alpha subunit, GPA1 [19,20].
Another argument against the role of GPCRs in plant G-protein signalling was based on the finding that the rate limiting step in plant G-protein signalling is the acceleration of GTP hydrolysis by another 7TM protein coded by AtRGS1, a GTPase accelerating/activating protein, and not GEF activity of GPCR [21,22]. This led to the assertions that plants neither have GPCRs nor require them for G-protein signalling, and that plant GPCRs predicted so far are mostly mis-annotated [23]. Another reasoning against plant GPCRs was that their homologs are also found in green algae that lack other components of G-protein signalling [24], but a complete G-protein complex was recently found in the green alga Chara braunii [25]. This, coupled with the lack of GAP/RGS proteins in grasses [22] and the recent report that a single pass leucine rich repeat (LRR) receptor-like protein acts like a GPCR in maize, with homologs abundant in the plant kingdom [26] point to other possibilities in receptor-G-protein coupling that cannot be completely ruled out yet. Even in the case of GCR1, the strongest GPCR candidate, there is no reason to give up hopes of finding a GCR1 ligand or GEF activity, as there is no reported evidence of failure so far.
Thus, while the controversy over the existence or requirement for G-protein coupled receptors (GPCRs) in plant G-protein signalling is far from settled, we have reframed the question, as to what is the overall role of GCR1 in Arabidopsis, rather than whether it codes for a GPCR or not. Functional genomics from a gene discovery perspective allows fundamental investigations into the genomewide role of GCR1 in Arabidopsis, including, but not limited to its role in G-protein-regulated processes. It may even contribute to resolving the current controversy to some extent. In this paper, we used whole transcriptome microarray analysis of a GCR1 knock-out mutant and its wild type in Arabidopsis for the first time to identify some important genes, pathways and responses regulated by GCR1, some of which, (but not all), are also regulated by G-proteins.
Materials and Methods
Isolation of GCR1 mutant
A T-DNA tagged mutant population from the Arabidopsis Knockout Facility at the University of Wisconsin [27] was screened by PCR for disruption of GCR1 gene: The population consisted of 72,960 BASTA (glufosinate)-resistant lines of Arabidopsis thaliana ecotype Ws2 transformed with an activation-Tag vector pSK1015 [28]. The GCR1 mutant was detected by DNA gel blot analysis of PCR-amplified products in DNA super-pool 40 of the BASTA population using combinations of GCR1-specific primers KK66 [located upstream of the ATG start codon of GCR1 ORF] (5′-AAATCGTCAATTCAATCTCTCAGATCAGT-3′) or KK68 [locates downstream of the TGA stop codon of GCR1 ORF] (5′-GCGCCGGTTTAAGTGATAGTATTTTCATA-3′) with the left T-DNA border specific primer JL202 (5′-CATTTTATAATAACGCTGCGGACATCTAC-3′). The PCR reaction contained 1X Takara Ex-Taq polymerase buffer (Takara), 0.2 mM deoxynucleotide triphosphates (dNTPs), 0.24 pmol/μl gene-specific primer, 0.24 pmol/μl JL202 primer, and 0.05 unit/μl Takara Ex-Taq polymerase. The PCR conditions were 96°C for 5 min and 36 cycles at 94°C for 15 s, 65°C for 30 s, and 72°C for 2 min each. Sequencing of KK66-JL202 and KK68-JL202 PCR products revealed multiple T-DNA integration.
Seeds corresponding to the identified super-pools and sub-pools were grown and DNA was extracted from leaves for genotyping and sequencing of the mutant lesions. The primers KK66 and KK68 were used to detect the GCR1 wild-type copy, and primers KK66 and JL202 were used to detect the presence of the T-DNA in the GCR1 gene. PCR conditions were as above. PCR products were separated on agarose gels, and individual segregating plants for the GCR1 mutant (gcr1–5) were genotyped based on the presence or absence of wild-type and T-DNA bands.
Phenotypic characterization of the mutants
Seeds of the wild type, Ws2 and GCR1 mutant (gcr1–5) were surface sterilized using 70% ethanol and washed thrice with sterile ultrapure water and stratified at 4°C for two days on half-strength B5 plates. The plates were then kept in growth chamber maintained at 22±1°C with a light intensity of 150 μM sec-1 m-2 and a photoperiod of 16:8 (light:dark). 10 day old plantlets were then transferred to 3.5 cm pots containing 1:1 mixture of soilrite and vermiculite. The pots were watered using sub-irrigation. The plants were allowed to grow for full life cycle and various phenotypic characters were measured at appropriate durations.
Plant material and RNA isolation
Arabidopsis thaliana GCR1 mutant (gcr1–5) as well as the corresponding wild type, were grown on 1X B5 medium hydroponically in a growth chamber at 22±1°C with a light intensity of 150 μM sec-1 m-2 and a photoperiod of 16:8 hours of light:dark cycle. The seeds were vernalized prior to inoculation at 4°C for 2–3 days. Total RNA was isolated from 3–4 week old whole seedlings using a modified hot phenol-LiCl method optimized on Arabidopsis [29]and reported in the context of cyanobacteria [30]. RNA samples were analyzed by Nanodrop spectrophotometer and Bioanalyzer (Agilent technologies, Santa Clara, USA) to determine the quality, quantity and suitability for microarray. RNA samples having a RIN (RNA Integrity Number) value greater than 6.0 were used for microarray experiments. The isolated RNAs were also used for validating the mutants using qPCR with gene-specific primers.
Microarray experiments and data processing
Microarray experiments were performed using Agilent 8×60k Arabidopsis array (AMADID 037661) with independent biological duplicates both the wild type, Ws2 and GCR1 mutant. Total RNA was transcribed into Cy3 labelled cRNA using Agilent Quick-Amp labelling kit as per manufacturer’s instructions. Labelled cRNA was purified using RNeasy minikit (Qiagen) and the specific activity of cRNA was determined as a quality control for all the samples. They were hybridized with the microarrays using Agilent in-situ hybridization kit as per manufacturer’s instructions. The washed slides were scanned and the images were manually verified to ensure that they are devoid of uneven hybridization, streaks, blobs and other artifacts. Hybridization across the slide was analyzed based on the number of features that were positive and significantly above background, i.e g(r) is PosAndSignif. Overall the microarray images were clean, had uniform intensity and with very low background noise. The data was then extracted from images by using Feature Extraction 10.7 software (Agilent Technologies).
Data analysis
The data was then normalized using the recommended ‘Per Chip and Per Gene Normalization’ feature of the software GeneSpring GX Version 11.5. The correlation of replicates was checked using principal component analysis and correlation coefficients were obtained. The geometric mean (geomean) fold change values are represented as log2. The average data of biological replicates was taken for final calculations. Log2fold change value of 1.0 with p-value of 0.05 was taken cut-off for differential-regulation.
Functional classification of significant genes
The differentially regulated gene lists were assigned gene ontology terms according the Arabidopsis Information Resource (TAIR 10) [31]. The differentially regulated gene lists were subjected to enriched GO categorization using AgriGO with default settings. Pathway analysis of the DEGs to obtain the list of changed pathways was done using plant MetGenMAP, which takes AraCyc as the background. Further functional classification was also carried out using Mapman tool, where the DEGs were assigned to different biological processes (bins). This tool also takes into account the log2fold change and represents it as coloured boxes on the software generated biological process map.
Data validation using qPCR
Differentially expressed genes obtained from microarray analyses were verified by RT-qPCR using Stratagene Mx3000P (Agilent technologies). Typically, total RNA was digested by RNase-free DNase (Fermantas), repurified, quantified and 5 μg of RNA was used for cDNA preparation for each biological replicate using Oligo(dT) primers and RevertAid reverse transcriptase (Fermentas). The analyses were done using biological triplicates, out of which two were the same as used for microarray. Sequences for designing the primers were obtained from TAIR. PCR amplifications were performed in 20 μl by using the BrilliantIII Ultrafast SYBR Green QPCR mastermix (Agilent Technologies) with 1.0 μl of sample cDNA and 100 nmoles of each gene-specific primer. Primer efficiency was determined by serial dilution of the template and only primers that worked at 90–110% efficiency were used for all qPCR analyses. The specificity of primer pairs was obtained by melting curve analysis of the amplicons. Actin2 (ACT2) was used as an internal control for normalization. Quantification of the relative changes in gene expression was performed by using Pffafl method [32].
Results
GCR1 mutant characterization
Sequencing of the PCR-amplified GCR1 gene confirmed the mutation in the second intron of GCR1 (Fig. 1A), similar to the mutant gcr1–3 described previously, except that it had two T-DNAs with their right borders facing each other, instead of the single T-DNA insertion reported earlier [9]. qPCR with gene-specific primers showed no expression of GCR1 transcript, confirming that this is a knock-out mutation (Fig. 1B). The mutant plants (gcr1–5) were phenotypically characterized for root length, plant height, leaf shape, etc. It was found that other than total number of siliques (S1 Fig.), gcr1–5 is barely distinguishable from the wild type as is the case with other known GCR1 mutants [8,9].
Fig 1. (a) T-DNA insertion site/orientation in the mutated gene of GCR1.
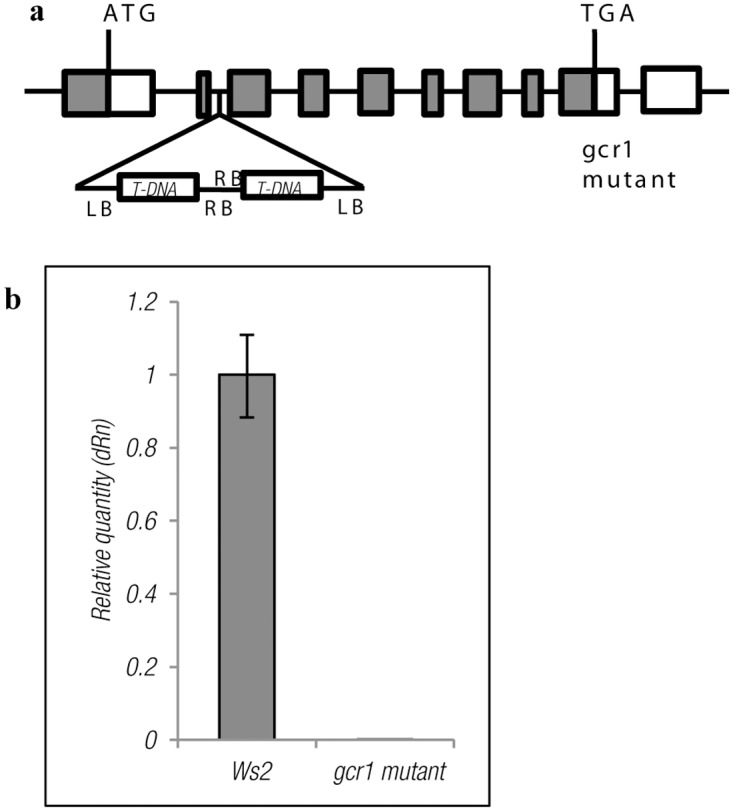
The exons are represented as boxes and the introns are represented as lines. LB and RB represent the left and right border respectively. (b) qPCR validation of the mutant. The real time RT-PCR was performed in triplicate using independent samples of total RNA and the values are represented as relative quantity ± SE.
Microarray analysis and validation
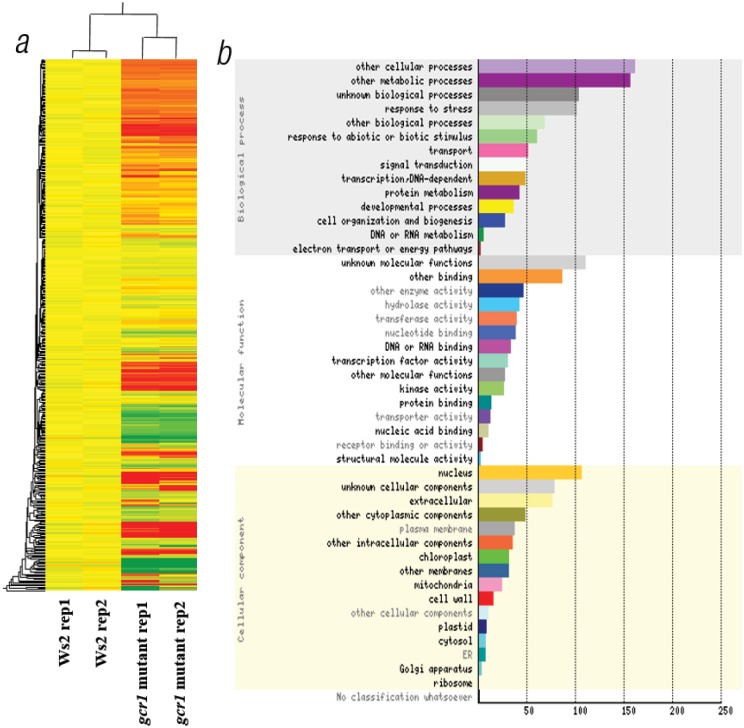
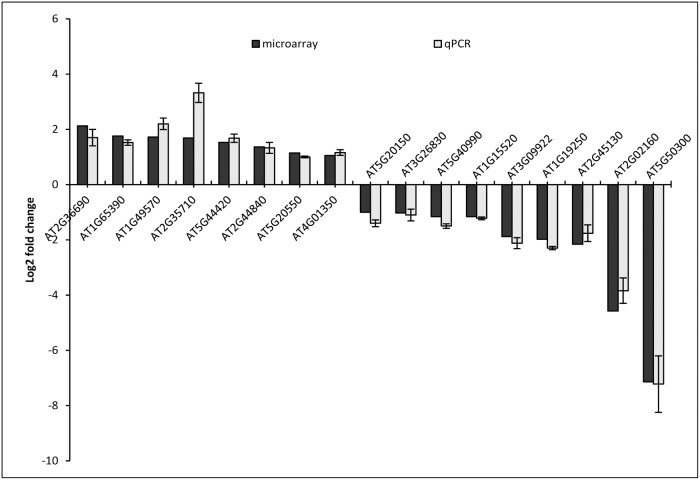
The experiment was carried out in accordance with MIAME compliance and the high correlation coefficients between biological replicates (>0.95) clearly indicate the robustness and a high level of reproducibility of the data (S1 Table). A stringent cut-off value of 1.0 (geometric mean log2) with a p-value of ≤ 0.05 was used for determining the up- or down-regulated genes in the mutant with respect to the wild type control. A total of 432 differentially regulated spots were obtained in the mutant (314 up-regulated and 118 down-regulated). These spots corresponded to 350 unique differentially expressed genes (DEGs) in the mutant (265 up-regulated and 85 down- regulated). A list of the top 20 up- and down-regulated genes are shown in Table 1. A heatmap of all the differentially regulated genes is shown in Fig. 2a. A set of 17 (8 up-regulated and 9 down regulated) genes representing all the affected biological processes were subjected to RT-qPCR using gene specific primers checked for efficiency (90–100%). The results of RT-qPCR matched with the microarray data in all the cases with a Pearson product moment correlation of 0.98 (p-value > 0.0001) (Fig. 3), validating the basic trends of regulation of gene expression on the microarray. The list of candidate genes along with the primer sequences are given in the S2 Table.
Table 1. List of the top 20 up-regulated and the top 20 down-regulated genes in the GCR1 mutant.
| Gene Description | Locus id | Accession id | Gene name | Log2FC | p-value |
|---|---|---|---|---|---|
| Up-regulated in gcr1–5 | |||||
| LOB domain-containing protein 27 | AT3G47870 | NM_114657 | LBD27 | 8.07 | 0.0116 |
| Paired amphipathic helix (PAH2) superfamily protein | AT1G24200 | NM_102266 | AT1G24200 | 5.47 | 0.0182 |
| phospholipase-like protein (PEARLI 4) family | AT5G11140 | NM_121152 | AT5G11140 | 4.57 | 0.0002 |
| Redox responsive transcription factor 1 | AT4G34410 | NM_119606 | RRTF1 | 3.81 | 0.0166 |
| Pathogenesis-related gene 1 | AT2G14610 | NM_127025 | PR1 | 3.62 | 0.0173 |
| Basic-leucine zipper (bZIP) transcription factor family protein | AT5G42910 | NM_123656 | AT5G42910 | 3.58 | 0.0348 |
| other RNA | AT2G06002 | NR_022465 | AT2G06002 | 3.48 | 0.0030 |
| C2H2 and C2HC zinc fingers superfamily protein | AT3G01030 | NM_110968 | AT3G01030 | 3.42 | 0.0118 |
| aspartyl protease family protein | AT5G48430 | NM_124218 | AT5G48430 | 3.30 | 0.0187 |
| S-locus lectin protein kinase family protein | AT1G11340 | NM_101007 | AT1G11340 | 3.25 | 0.0154 |
| unknown protein | AT3G60647 | NM_001125395 | AT3G60647 | 3.23 | 0.0055 |
| XH domain-containing protein | AT1G80970 | NM_106745 | AT1G80970 | 3.23 | 0.0363 |
| Poltergeist | AT2G46920 | NM_180132 | POL | 3.23 | 0.0211 |
| Peroxidase 4 | AT1G14540 | NM_101321 | PER4 | 3.15 | 0.0024 |
| unknown protein | AT5G66810 | NM_126079 | AT5G66810 | 3.11 | 0.0289 |
| unknown protein | AT3G55570 | NM_115414 | AT3G55570 | 3.10 | 0.0235 |
| Cytochrome p450, family 94, subfamily b, polypeptide 3 | AT3G48520 | NM_114710 | CYP94B3 | 3.01 | 0.0110 |
| Ethylene response sensor 2 | AT1G04310 | NM_100312 | ERS2 | 2.91 | 0.0420 |
| Chromomethylase 1 | AT1G80740 | NM_106722 | CMT1 | 2.87 | 0.0284 |
| Unknown protein | AT3G28870 | NM_113808 | AT3G28870 | 2.87 | 0.0060 |
| Down-regulated in gcr1–5 | |||||
| AZA-guanine resistant2 | AT5G50300 | NM_124409 | AZG2 | -7.14 | 0.0104 |
| tRNA synthetase-related | AT5G10880 | NM_121126 | AT5G10880 | -6.52 | 0.0049 |
| Protein of unknown function | AT1G04890 | NM_100367 | AT1G04890 | -6.34 | 0.0069 |
| Homeodomain-like superfamily protein | AT5G62110 | NM_125604 | AT5G62110 | -5.82 | 0.0063 |
| Leucine-rich repeat protein kinase family protein | AT5G24100 | NM_122315 | AT5G24100 | -5.26 | 0.0123 |
| unknown protein | AT2G13547 | EF183317 | AT2G13547 | -5.10 | 0.0016 |
| unknown protein | AT5G24250 | NM_122331 | AT5G24250 | -5.01 | 0.0402 |
| CCCH-type zinc finger family protein | AT2G02160 | NM_126276 | AT2G02160 | -4.57 | 0.0499 |
| F-box associated ubiquitination effector family protein | AT3G06280 | NM_111503 | AT3G06280 | -4.48 | 0.0350 |
| Methionine sulfoxide reductase b7 | AT4G21830 | CD530941 | AT4G21830 | -4.34 | 0.0277 |
| Late embryogenesis abundant (LEA) protein-related | AT5G54370 | NM_124817 | AT5G54370 | -4.08 | 0.0143 |
| Potential natural antisense gene | AT1G71828 | NR_027728 | AT1G71828 | -3.96 | 0.0391 |
| unknown protein | AT5G35870 | NM_122978 | AT5G35870 | -3.95 | 0.0019 |
| unknown protein | AT5G03440 | NM_120423 | AT5G03440 | -3.80 | 0.0082 |
| Similar to yeast POP1 | AT2G47300 | NM_001084603 | AT2G47300 | -3.74 | 0.0208 |
| Proline-rich extensin-like family protein | AT1G26250 | NM_102389 | AT1G26250 | -3.67 | 0.0041 |
| unknown protein | AT3G32896 | NM_148781 | AT3G32896 | -3.66 | 0.0199 |
| unknown protein | AT3G50674 | NM_001125338 | AT3G50674 | -3.66 | 0.0236 |
| Leucine-rich repeat protein kinase family protein | AT1G24650 | NM_102307 | AT1G24650 | -3.49 | 0.0363 |
| Similar to TSK-associating protein 1 | AT3G15950 | NM_112465 | NAI2 | -3.44 | 0.0077 |
Fig 2. (a) Heat map of differentially expressed genes.
The background-subtracted microarray data was subjected to hierarchical clustering using Genespring software ver. 11.5 to generate the heatmap. Yellow represents the control data, while red and green represent up-regulation and down regulation respectively. (b) GO categorization of DEGs. The DEGs were categorized into GO classes using classification superviewer tool of Bioarray resource (www.bar.utoronto.ca)
Fig 3. Validation and comparison of microarray results using qPCR for a few genes selected from each of the important biological processes.
The experiment was carried out using biological triplicates and the values are presented as log2FC ± SE. (AT2G36690–2-OG; AT1G65390—ATPP2-A5; AT1G49570—peroxidase family protein; AT2G35710—PGSIP7; AT5G44420—PDF1.2; AT2G44840—ERF13; AT5G20550–2-oxoglutarate; AT4G01350—Cysteine/Hisidine-rich C1 domain family protein; AT5G20150—SPX1; AT3G26830—PAD3; AT5G40990—GLIP1; AT1G15520—PDR12; AT3G09922—IPS1; AT1G19250—FMO1; AT2G45130—SPX3; AT2G02160—CCCH type zinc finger family protein; AT5G50300—AZG2.
Stress response genes among GCR1-regulated genes
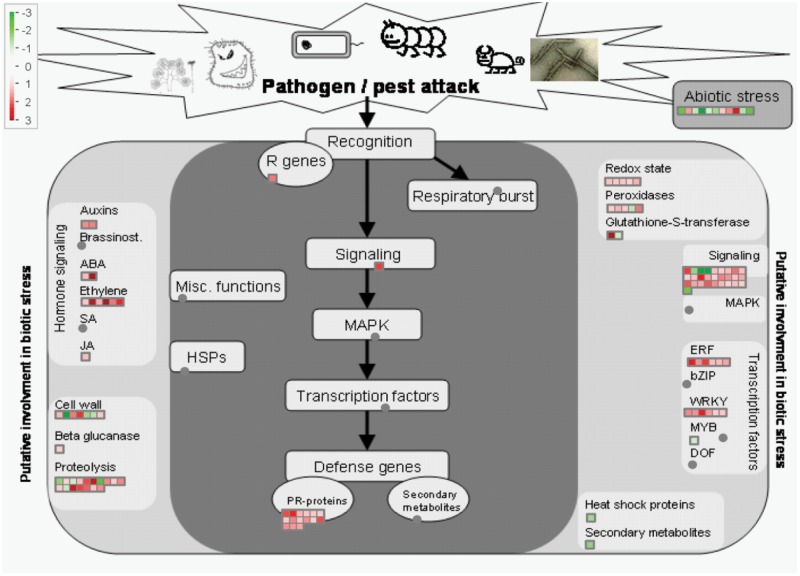
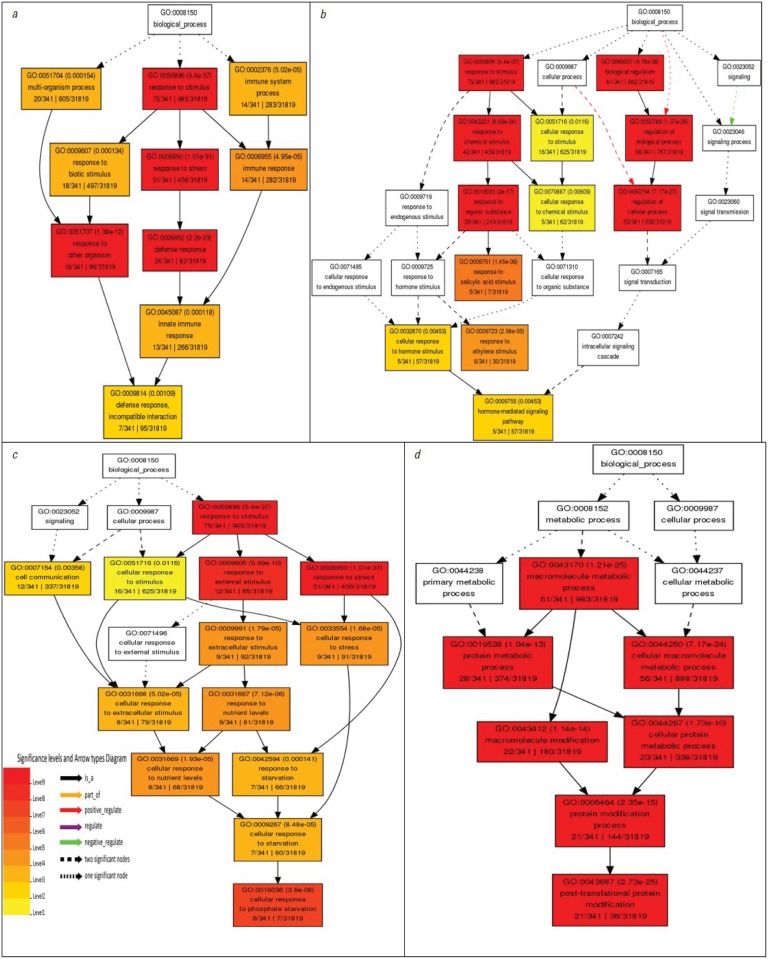
Mapman analysis mapped 119 DEGs as belonging to the biotic stress response category (Fig. 4), including some genes that are involved in both biotic and abiotic stresses and other related caterogies such as hormone signalling, transcription factors etc., with a putative role in biotic stress. This finding was substantiated by further analysis using GO slim classification. It mapped 310 genes, out of which 101 genes (32.6%) belonged to the stress responsive category, the largest among the ‘known’ processes (Fig. 2b), even though many more genes are categorized under ‘unknown’ or ‘other’ processes. Singular enrichment analysis (SEA) using AgriGO, revealed the very high significance levels of the gene clusters (S3 Table). Enriched GO classification revealed that all the classes can be broadly grouped into only four categories, i.e. response to stimulus (mainly stress), protein modification, response to hormones and response to nutrient levels or starvation (Fig. 5). The largest enriched class was that of response to stimulus with 75 genes, which included several stress-related classes like defense response, response to stress, defense response-incompatible interaction and response to stimulus as the most significant classes in their decreasing order of significance level. In terms of the number of genes per cluster, the largest clusters belong to plant defensins (PDF), mildew resistance locus O proteins (MLO), and the Toll Interleukin1 receptor-nucleotide binding site-leucine-rich repeat type R (TIR-NBS-LRR) class, which are involved in biotic stress response. In addition, there are genes involved in abiotic stress such as, Arabidopsis Zinc Finger (AZF) and Arabidopsis thaliana Phloem Protein 2 A5 (ATPP2-A5). Several peroxidases and transcription factors involved in biotic and abiotic stress response were also found to be differentially expressed in the mutant.
Fig 4. Mapman analysis of genes differentially regulated in gcr1 mutant.
Out of the total list of 350 DEGs, 119 mapped onto biotic stress response. The red dots represent the up-regulated genes, green dots represent the down-regulated genes and the grey dots represent the genes to which none of the DEGs were assigned. The level of differential regulation is according to the scale given.
Fig 5. Singular enrichment analysis (SEA) of the DEGs using AgriGO into important biological processes.
(a) Stress response (b) Hormone response (c) Response to Phosphate starvation (d) Protein modification.
Hormone biosynthesis/response genes affected in GCR1 mutant
AgriGO analysis also revealed that some of the DEGs in the GCR1 mutant are involved in hormone biosynthesis as well as in response to various hormones (S3 Table). They include cytokinin oxidase/dehyrogenase (CKX4) which is involved in cytokinin biosynthesis as well in abiotic stress response, genes involved in salicylic acid response (PDF1.2, PDR12, WRKY18, GLIP1, etc), ethylene response (ERS2, ERF13, PDR12, etc) and ABA mediated pathway (LECRKA4.2, LECRKA4.3). These results indicate the prominent role of GCR1 in hormone biosynthesis/response.
Phosphate starvation response genes among GCR1-regulated genes
Interestingly, enriched GO analysis revealed several genes related to phosphate response/starvation as downregulated in the GCR1 mutant (Table 2, Fig. 5c). They include SPX domain containing genes (SPX1, SPX3) and galactolipid/sulfolipid biosynthesis genes (MGD2, MGDC, SQD2), which are known to get differentially regulated on phosphate starvation. This finding would need further investigation.
Table 2. List of changed pathways with the genes and their log2fold change value involved in the pathway.
| Pathway name | p-value | Genes involved | Log2fold change value |
|---|---|---|---|
| fatty acid a-oxidation | 0.018437 | AT3G01420 | 1.50 |
| sulfolipid biosynthesis | 0.018437 | AT5G01220 | -1.10 |
| camalexin biosynthesis | 0.027535 | AT3G26830 | -1.03 |
| leucopelargonidin and leucocyanidin biosynthesis | 0.0322 | AT5G20550 | 1.15 |
| AT2G36690 | 1.12 | ||
| leucodelphinidin biosynthesis | 0.0322 | AT5G20550 | 1.15 |
| AT2G36690 | 1.12 | ||
| flavonoid biosynthesis | 0.038214 | AT5G20550 | 1.15 |
| AT2G36690 | 1.12 | ||
| homogalacturonan degradation | 0.038723 | AT2G41850 | 1.83 |
| AT4G02330 | 1.11 | ||
| AT1G05310 | -1.29 | ||
| glycolipid biosynthesis | 0.04549 | AT2G11810 | -1.61 |
| AT5G20410 | -1.01 | ||
| monolignolglucosides biosynthesis | 0.05435 | AT5G66690 | -1.66 |
| coniferin metabolism | 0.05435 | AT5G66690 | -1.66 |
| cytokinins degradation | 0.063132 | AT4G29740 | 1.74 |
| 13-LOX and 13-HPL pathway | 0.063132 | AT1G72520 | 1.22 |
| abscisic acid biosynthesis | 0.089016 | AT4G18350 | 1.24 |
| flavonol biosynthesis | 0.154768 | AT3G49620 | -1.19 |
| jasmonic acid biosynthesis | 0.170489 | AT1G72520 | 1.22 |
| very long chain fatty acid biosynthesis | 0.178245 | AT5G43760 | 1.74 |
| triacylglycerol degradation | 0.208588 | AT1G30370 | 1.83 |
| IAA biosynthesis I | 0.216007 | AT2G30770 | -1.78 |
| ascorbate glutathione cycle | 0.286654 | AT3G09940 | 1.17 |
| Phospholipases | 0.30004 | AT4G37070 | 1.46 |
| superpathway of flavones and derivatives biosynthesis | 0.44851 | AT3G49620 | -1.19 |
Pathways marked in bold are significantly changed with p-value ≤ 0.05.
Protein kinases/phosphatases and transcription factors regulated by GCR1
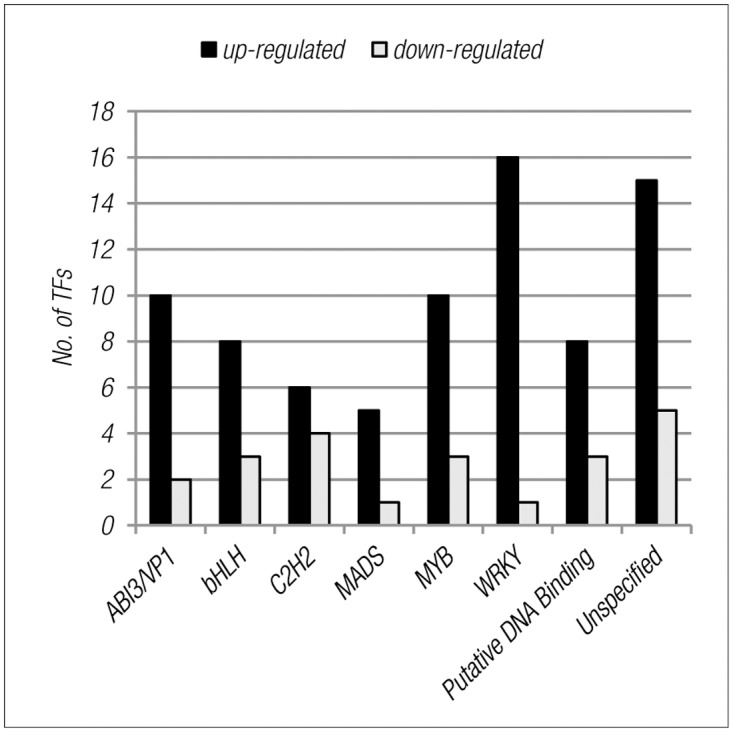
Functional classification of DEGs identified by enriched GO analysis revealed many genes involved in protein modification by phosphorylation/dephosphorylation (S3 Table, Fig. 5d). The list included several serine/threonine kinases, leucine-rich kinases and S-locus pectin kinase families, and phosphatases like PP2C and PPCK, some of which are also annotated under stress response. Transcription and gene regulation also figured as major categories in functional classification. Comparing them with the Plant transcription factor database (PlantTFDB 2.0) revealed more than 25 transcription factor families, most of which were up-regulated in the mutant. The most represented transcription factor families were WRKY, MYB, AVI3/VP1, bHLH and C2H2 (Fig. 6). A large number of putative DNA binding and unspecified TFs also figured in the list.
Fig 6. Distribution of differentially regulated transcription factors into highly represented transcription factor families.
Secondary metabolite biosynthesis among GCR1-regulated pathways
When the DEG list was mapped to AraCyc [33] using plant MetGenMAP [34], most of the pathways belonged to biosynthesis of secondary metabolites. These included pathways like sulpholipid biosynthesis, fatty acid α oxidation, camalexin biosynthesis, leucopelargonidin and leucocyanidin biosynthesis, leucodelphinidin biosynthesis, flavonoid biosynthesis, homogalacturonan degradation and glycolipid biosynthesis as significantly changed pathways (p-value ≤0.05) (Table 2). Out of the above pathways, leucopelargonindin and leucocyanidin biosynthesis, leucodelphinidin biosynthesis and flavonoid biosynthesis are part of the superpathway of flavones and derivatives biosynthesis. Both the genes encoding flavanone 3-beta hydroxylase (AT5G20550, AT2G36690) were found to be up-regulated, whereas two genes involved in glycolipid biosynthesis (AT2G11810, AT5G20410) and one each in sulpholipid biosynthesis (AT5G01220) and camalexin biosynthesis (AT3G26830) were down-regulated. The DEGs involved in all other pathways were found to be up-regulated.
Discussion
The unsettled controversy over the existence or the need for G-protein coupled receptors (GPCRs) in plant G-protein signalling has overshadowed a more fundamental quest for the overall role of GCR1 in Arabidopsis. We approached it from the gene discovery perspective, using functional genomics to investigate the genomewide role of GCR1 in Arabidopsis, including, but not limited to its more debated role in G-protein-regulated processes. For this purpose, we isolated a T-DNA knock-out mutant of GCR1 (Fig. 1, S1 Fig.) disrupted in its 2nd intron, which turned out to be similar, though not identical to gcr1–3 reported earlier [9] and also phenotypically similar to all other known GCR1 mutants [8]. It must be noted that the slightly longer, narrower leaves of this GCR1 mutant is at variance with the rounder leaves characteristic of GPA1 and AGB1 mutants that represent G-alpha and beta subunits respectively [2,35,36].
Microarray analysis of the whole plant transcriptomes of the WT and the GCR1 mutant revealed that a total of 350 genes were differentially regulated in the mutant when compared to the wild type (Fig. 2), using a stringent cut-off value of 1.0 (geometric mean log2) with a p-value of ≤ 0.05. These genes span all five chromosomes, indicating an extensive genomewide role for GCR1. A few of the upregulated and downregulated genes have been validated by RT-qPCR (Fig. 3, S2 Table) and the top 20 up/down-regulated genes are shown in Table 1. GOslim classification of differentially expressed genes (DEGs) revealed that over 50% of them are involved in metabolic processes, while 32.5% are responsive to stress, 19% to biotic and abiotic stimuli, 16.5% involved in transport and 15.4% in transcription, with several genes figuring in more than one category. A closer examination revealed that some of the GCR1-responsive genes/processes have close parallels to those attributed to G-protein signalling in Arabidopsis, whereas others have either novel roles or hither to unknown in the context of G-protein signalling, as detailed below.
Novel roles of GCR1 similar to those attributed to G-protein signalling
Stress emerged as one of the major categories and enriched GO as well as Mapman analyses revealed that most of these differentially regulated genes pertained to biotic stress, a novel finding hitherto unknown in relation to GCR1 or any other predicted GPCR. They include genes like PDF1.2 and MLO12, which are known to be involved in innate immune response and defense against pathogens [37,38]. We also found TIR-NBS-LRR class of proteins, which have been implicated in pathogen sensing and defense response in plants [39], as well as many receptor like kinases known to be involved in plant defense signalling. Our findings on the role of GCR1 in biotic stress in this study have some close parallels with reports that implicate heterotrimeric G-proteins in biotic stress response [40,41] and plant defense signalling [42–44]. One of the reports has shown that GCR1 partners with GPA1 in the regulation of root growth mediated by bacterial quorum sensing signals [45]. At least two genes identified as GCR1 responsive in our study (PDF1.2 and PAD3), have also been reported earlier in G-protein-mediated response to biotic stress and jasmonic acid [46,47]. The role of GCR1 in abiotic stress response is by no means insignificant, even though fewer genes figure in this category than in biotic stress. Our transcriptome data corroborate the role of GCR1 and/or G-proteins in several abiotic stresses reported in separate studies, such as drought [9], heat, cold, salt [48,49], oxidative stress [50].
Secondary metabolic pathways such as camalexin and flavonoid biosynthesis constitute another novel category of GCR1-responsive processes that was previously unknown in relation to plant GPCRs. In fungi, G-protein alpha subunit was recently shown to be involved in secondary metabolism/biosynthesis of secondary metabolites [51]. Therefore, it remains to be seen whether plant heterotrimeric G-proteins mediate biosynthesis of secondary metabolites and whether they partner with GCR1.
GCR1 is involved in hormonal responses, as is evident from the categorization of the DEGs. They are involved in the biosynthesis and mediation of multiple hormonal responses like cytokinin, ethylene, salicylic acid, ABA, etc. Some of them, including ABC transporter (PDR12) and transcription factors (WRKY18) mediate hormone responsive effects that are also involved in the regulation of stress response. Heterotrimeric G-proteins in general and GPA1 and GCR1 in particular have been implicated earlier in a variety of hormone responses [8,9,52,53]. Our transcriptome data not only corroborate them category-wise, but also in terms of some of the genes involved in these processes. At least 20 of them were earlier reported in G-protein-mediated ABA response [54].
G-proteins are known to be involved in the regulation of guard cell functions and root hair differentiation [9,52], as are transcription factor complexes comprised of bHLH, MYB and WD40 domains [55]. Our finding that bHLH and MYB are GCR1 responsive raises the possibility that these TF complexes mediate the regulation of guard cell and root hair differentiation via GCR1 and G-proteins, which needs experimental validation.
Further evidence regarding the upstream GCR1 ligand and downstream GEF activity in all the above processes would be needed before it can be conclusively established that GCR1 and GPA1 act together in those processes/responses in Arabidopsis.
Novel roles of GCR1 unknown or unrelated to plant G-protein signalling
MYB and WRKY class of transcription factors are known to be involved in metabolic regulation, stress and cell fate [56,57]. Our data show that the above transcription factors, as well as the above processes they regulate are GCR1 responsive, is a significant and novel observation. This is because, barring stress discussed above, the role of GPCR or G-protein has not been reported in any of them. This is also true of GCR1-regulation of AP2/EREB class of TFs found in our study, which are known to be involved in storage compound and fatty acid biosynthesis [58]. If confirmed using other G-protein mutants, it could mean that GCR1-regulation of these genes/processes works independent of GPCR/G-protein signalling.
Another novel finding of this study is that GCR1 downregulates the genes involved in response to phosphate starvation. These genes include SPX domain containing proteins (SPX1 and SPX3) and IPS1 (induced by phosphate starvation1) [59]; MGD2 and MGDC, which are involved in galactolipid metabolism during phosphate starvation [60]. Till date, no study has implicated phosphate starvation to any component of G-proteins.
Conclusions
Our transcriptome analysis shows for the first time that a) GCR1 has an extensive genomewide response, revealing many hitherto unknown genes and processes including, but not limited to those regulated by the known G-proteins in Arabidopsis; b) some of its roles, such as in biotic and abiotic stress, hormone response and secondary metabolism are among those regulated by the known heterotrimeric G-proteins; and c) GCR1 also has other important roles that are either independent of, or hitherto unattributed to G-protein signalling, such as in phosphate starvation, storage compound and fatty acid biosynthesis, cell fate etc., though the possibility of some of them being regulated by other yet-to-be identified G-proteins or their components cannot be ruled out. Overall, our results point to a serious need to revisit the role of GCR1 in G-protein signalling in Arabidopsis, including its possible role as a GPCR. Further, considering that GCR1 and Arabidopsis became the main basis for contesting the existence and the role of GPCRs in plants, our results also suggest that it may be too early to write-off plant GPCRs, or their role in plant G-protein signaling.
Supporting Information
(EPS)
(DOC)
(DOC)
(DOC)
Acknowledgments
We thank Dr. Beena Pillai, IGIB, New Delhi, for her help in the early microarray experiments. This paper is dedicated to the co-author Priyanka Sharma who is no more. We also acknowledge the help of Dr. Sunila and Jangam Annie Prasanna for critical proof-reading of the manuscript.
Data Availability
All microarray files are available from the GEO database (accession number(s) GSE 40217: GSM 988509 and GSM 988510).
Funding Statement
This work was supported by research grants to NR [60(0056)/02/EMRII and 38(1246)/10/EMRII] and research fellowships to NC [09/806(015)/2008-EMRI] and PS 60(0056)/02/EMRII] from the Council of Scientific and Industrial Research (CSIR), Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Temple BRS, Jones AM (2007) The Plant Heterotrimeric G-Protein Complex. Annual Review of Plant Biology 58: 249–266. [DOI] [PubMed] [Google Scholar]
- 2. Urano D, Chen JG, Botella JR, Jones AM (2013) Heterotrimeric G protein signalling in the plant kingdom. Open Biol 3: 120186 10.1098/rsob.120186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Josefsson L-G, Rask L (1997) Cloning of a putative G-protein-coupled receptor from Arabidopsis thaliana. European Journal of Biochemistry 249: 415–420. [DOI] [PubMed] [Google Scholar]
- 4. Plakidou-Dymock S, Dymock D, Hooley R (1998) A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Current Biology 8: 315–324. [DOI] [PubMed] [Google Scholar]
- 5. Kanyuka K, Couch D, Hooley R (2001) A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Current Biology 11: 535. [DOI] [PubMed] [Google Scholar]
- 6. Apone F, Alyeshmerni N, Wiens K, Chalmers D, Chrispeels MJ, et al. (2003) The G-Protein-Coupled Receptor GCR1 Regulates DNA Synthesis through Activation of Phosphatidylinositol-Specific Phospholipase C. Plant Physiology 133: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colucci G, Apone F, Alyeshmerni N, Chalmers D, Chrispeels MJ (2002) GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolishes seed dormancy and shortens time to flowering. Proc Natl Acad Sci U S A 99: 4736–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, et al. (2004) GCR1 Can Act Independently of Heterotrimeric G-Protein in Response to Brassinosteroids and Gibberellins in Arabidopsis Seed Germination. Plant Physiology 135: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandey S, Assmann SM (2004) The Arabidopsis Putative G Protein-Coupled Receptor GCR1 Interacts with the G Protein Subunit GPA1 and Regulates Abscisic Acid Signaling. The Plant Cell 16: 1616–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warpeha KM, Upadhyay S, Yeh J, Adamiak J, Hawkins SI, et al. (2007) The GCR1, GPA1, PRN1, NF-Y Signal Chain Mediates Both Blue Light and Abscisic Acid Responses in Arabidopsis. Plant Physiology 143: 1590–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moriyama EN, Strope PK, Opiyo SO, Chen Z, Jones AM (2006) Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biology 7: R96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gookin TE, Kim J, Assmann SM (2008) Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: computational prediction and in-vivo protein coupling. Genome Biology 9: R120 10.1186/gb-2008-9-7-r120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pandey S, Nelson DC, Assmann SM (2009) Two Novel GPCR-Type G Proteins Are Abscisic Acid Receptors in Arabidopsis. Cell 136: 136–148. 10.1016/j.cell.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 14. Taddese B, Upton GJ, Bailey GR, Jordan SR, Abdulla NY, et al. (2014) Do plants contain g protein-coupled receptors? Plant Physiology 164: 287–307. 10.1104/pp.113.228874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu X, Yue Y, Li B, Nie Y, Li W, et al. (2007) A G Protein-Coupled Receptor Is a Plasma Membrane Receptor for the Plant Hormone Abscisic Acid. Science 315: 1712–1716. [DOI] [PubMed] [Google Scholar]
- 16. Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, et al. (2007) Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. The Plant Journal 52: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 17. Johnston CA, Temple BR, Chen JG, Gao Y, Moriyama EN, et al. (2007) Comment on “A G Protein Coupled Receptor Is a Plasma Membrane Receptor for the Plant Hormone Abscisic Acid”. Science 318: 914 [DOI] [PubMed] [Google Scholar]
- 18. Illingworth CJR, Parkes KE, Snell CR, Mullineaux PM, Reynolds CA (2008) Criteria for confirming sequence periodicity identified by Fourier transform analysis: Application to GCR2, a candidate plant GPCR? Biophysical Chemistry 133: 28–35. [DOI] [PubMed] [Google Scholar]
- 19. Humphrey TV, Botella JR (2001) Re-evaluation of the cytokinin receptor role of the Arabidopsis gene GCR1. Journal of Plant Physiology 158: 645–653. [Google Scholar]
- 20. Johnston CA, Willard MD, Kimple AJ, Siderovski DP, Willard FS (2008) A sweet cycle for Arabidopsis G-proteins: Recent discoveries and controversies in plant G-protein signal transduction. Plant Signaling & Behavior 3: 1067–1076. 10.3389/fpls.2014.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, et al. (2007) GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proceedings of the National Academy of Sciences 104: 17317–17322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urano D, Jones JC, Wang H, Matthews M, Bradford W, et al. (2012) G protein activation without a GEF in the plant kingdom. PLoS Genet 8: e1002756 10.1371/journal.pgen.1002756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urano D, Jones AM (2013) "Round Up the Usual Suspects": A Comment on Nonexistent Plant G Protein-Coupled Receptors. Plant Physiology 161: 1097–1102. 10.1104/pp.112.212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradford W, Buckholz A, Morton J, Price C, Jones AM, et al. (2013) Eukaryotic G protein signaling evolved to require G protein-coupled receptors for activation. Science signaling 6: ra37 10.1126/scisignal.2003768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hackenberg D, Sakayama H, Nishiyama T, Pandey S (2013) Characterization of the heterotrimeric G-protein complex and its regulator from the green alga Chara braunii expands the evolutionary breadth of plant G-protein signaling. Plant Physiology 163: 1510–1517. 10.1104/pp.113.230425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bommert P, Je BI, Goldshmidt A, Jackson D (2013) The maize Galpha gene COMPACT PLANT2 functions in CLAVATA signalling to control shoot meristem size. Nature 502: 555–558. 10.1038/nature12583 [DOI] [PubMed] [Google Scholar]
- 27. Sussman MR, Amasino RM, Young JC, Krysan PJ, Austin-Phillips S (2000) The Arabidopsis Knockout Facility at the University of Wisconsin—Madison. Plant Physiology 124: 1465–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weigel D, Ahn JH, Bla´zquez MA, Borevitz JO, Christensen SK, et al. (2000) Activation Tagging in Arabidopsis. Plant Physiology 122: 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Etheridge N, Trusov Y, Verbelen J, Botella J (1999) Characterization of ATDRG1, a member of a new class of GTP-binding proteins in plants. Plant Molecular Biology 39: 1113–1126. [DOI] [PubMed] [Google Scholar]
- 30. Pathak RR, Lochab S (2010) A method for rapid isolation of total RNA of high purity and yield fromArthrospira platensis. Canadian Journal of Microbiology 56: 578–584. 10.1139/w10-045 [DOI] [PubMed] [Google Scholar]
- 31. Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, et al. (2011) The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Research 40: D1202–D1210. 10.1093/nar/gkr1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT—PCR. Nucleic Acids Research 29: 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mueller LA, Zhang P, Rhee SY (2003) AraCyc: a biochemical pathway database for Arabidopsis. Plant Physiology 132: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joung JG, Corbett AM, Fellman SM, Tieman DM, Klee HJ, et al. (2009) Plant MetGenMAP: an integrative analysis system for plant systems biology. Plant Physiology 151: 1758–1768. 10.1104/pp.109.145169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ullah H, Chen JG, Young JC, Im KH, Sussman MR, et al. (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069. [DOI] [PubMed] [Google Scholar]
- 36. Lease KA, Wen J, Li J, Doke JT, Liscum E, et al. (2001) A Mutant Arabidopsis Heterotrimeric G-Protein β Subunit Affects Leaf, Flower, and Fruit Development. The Plant Cell 13: 2631–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim HG, Kwon SJ, Jang YJ, Chung JH, Nam MH, et al. (2014) GDSL lipase 1 regulates ethylene signaling and ethylene-associated systemic immunity in Arabidopsis . FEBS letters 588: 1652–1658. 10.1016/j.febslet.2014.02.062 [DOI] [PubMed] [Google Scholar]
- 38. Zhou S, Jing Z, Shi J (2013) Genome-wide identification, characterization, and expression analysis of the MLO gene family in Cucumis sativus. Genetics and molecular research 12: 6565–6578. 10.4238/2013.December.11.8 [DOI] [PubMed] [Google Scholar]
- 39. Nandety RS, Caplan JL, Cavanaugh K, Perroud B, Wroblewski T, et al. (2013) The role of TIR-NBS and TIR-X proteins in plant basal defense responses. Plant physiology 162: 1459–1472. 10.1104/pp.113.219162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delgado-Cerezo M, Sanchez-Rodriguez C, Escudero V, Miedes E, Fernandez PV, et al. (2012) Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Molecular Plant 5: 98–114. 10.1093/mp/ssr082 [DOI] [PubMed] [Google Scholar]
- 41. Llorente F, Alonso-Blanco C, Sánchez-Rodriguez C, Jorda L, Molina A (2005) ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. The Plant Journal 43: 165–180. [DOI] [PubMed] [Google Scholar]
- 42. Liu J, Ding P, Sun T, Nitta Y, Dong O, et al. (2013) Heterotrimeric G Proteins Serve as a Converging Point in Plant Defense Signaling Activated by Multiple Receptor-Like Kinases. Plant Physiology 161: 2146–2158. 10.1104/pp.112.212431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trusov Y, Jordá L, Molina A, Botella J (2010) G Proteins and Plant Innate Immunity In: Yalovsky S, Baluška F, Jones A, editors. Integrated G Proteins Signaling in Plants: Springer Berlin Heidelberg; pp. 221–250. [Google Scholar]
- 44. Trusov Y, Botella J (2012) New faces in plant innate immunity: heterotrimeric G proteins. Journal of Plant Biochemistry and Biotechnology 21: 40–47. [Google Scholar]
- 45. Liu F, Bian Z, Jia Z, Zhao Q, Song S (2012) The GCR1 and GPA1 Participate in Promotion of Arabidopsis Primary Root Elongation Induced by N-Acyl-Homoserine Lactones, the Bacterial Quorum-Sensing Signals. Molecular Plant-Microbe Interactions 25: 677–683. 10.1094/MPMI-10-11-0274 [DOI] [PubMed] [Google Scholar]
- 46. Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, et al. (2006) Heterotrimeric G Proteins Facilitate Arabidopsis Resistance to Necrotrophic Pathogens and Are Involved in Jasmonate Signaling. Plant Physiology 140: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okamoto H, Gobel C, Capper RG, Saunders N, Feussner I, et al. (2009) The a-subunit of the heterotrimeric G-protein affects jasmonate responses in Arabidopsis thaliana. Journal of Experimental Botany 60: 1991–2003. 10.1093/jxb/erp060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yadav DK, Islam SS, Tuteja N (2012) Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signaling & Behavior 7: 733–740. 10.3389/fpls.2014.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yadav DK, Shukla D, Tuteja N (2013) Rice heterotrimeric G-protein alpha subunit (RGA1): In silico analysis of the gene and promoter and its upregulation under abiotic stress. Plant Physiology and Biochemistry 63: 262–271. 10.1016/j.plaphy.2012.11.031 [DOI] [PubMed] [Google Scholar]
- 50. Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. The Plant Cell 17: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Studt L, Humpf H-U, Tudzynski B (2013) Signaling governed by G proteins and cAMP is crucial for growth, secondary metabolism and sexual development in Fusarium fujikuroi. PloS one 8: e58185 10.1371/journal.pone.0058185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao Z, Stanley BA, Zhang W, Assmann SM (2010) ABA-regulated G protein signaling in Arabidopsis guard cells: a proteomic perspective. Journal of Proteome Research 9: 1637–1647. 10.1021/pr901011h [DOI] [PubMed] [Google Scholar]
- 53. Hooley R (2001) Progress towards the identification of cytokinin receptors In: Sopory SK, Oelmüller R, Maheshwari SC, editors. Signal Transduction in Plants: Current Advances: Kluwer Academic/Plenum Publishers; pp. 193–199. [Google Scholar]
- 54. Wang RS, Pandey S, Li S, Gookin TE, Zhao Z, et al. (2011) Common and unique elements of the ABA-regulated transcriptome of Arabidopsis guard cells. BMC Genomics 12: 216 10.1186/1471-2164-12-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramsay NA, Glover BJ (2005) MYB—bHLH—WD40 protein complex and the evolution of cellular diversity. Trends in Plant Science 10: 63–70. [DOI] [PubMed] [Google Scholar]
- 56. Wu K, Guo Z, Wang H, Li J (2005) The WRKY Family of Transcription Factors in Rice and Arabidopsis and Their Origins. DNA Research 12: 9–26. [DOI] [PubMed] [Google Scholar]
- 57. Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, et al. (2010) MYB transcription factors in Arabidopsis. Trends in Plant Science 15: 573–581. 10.1016/j.tplants.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 58. Cernac A, Benning C (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. The Plant Journal 40: 575–585. [DOI] [PubMed] [Google Scholar]
- 59. Lu YT, Li MY, Cheng KT, Tan CM, Su LW, et al. (2014) Transgenic Plants that Express the Phytoplasma Effector SAP11 Show Altered Phosphate Starvation and Defense Responses. Plant physiology 164: 1456–1469. 10.1104/pp.113.229740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kobayashi K, Narise T, Sonoike K, Hashimoto H, Sato N, et al. (2012) Role of galactolipid biosynthesis in coordinated development of photosynthetic complexes and thylakoid membranes during chloroplast biogenesis in Arabidopsis. The Plant Journal 73: 250–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All microarray files are available from the GEO database (accession number(s) GSE 40217: GSM 988509 and GSM 988510).