Abstract
Anthropogenically derived nitrogen (N) has a central role in global environmental changes, including climate change, biodiversity loss, air pollution, greenhouse gas emission, water pollution, as well as food production and human health. Current understanding of the biogeochemical processes that govern the N cycle in coupled human–ecological systems around the globe is drawn largely from the long-term ecological monitoring and experimental studies. Here, we review spatial and temporal patterns and trends in reactive N emissions, and the interactions between N and other important elements that dictate their delivery from terrestrial to aquatic ecosystems, and the impacts of N on biodiversity and human society. Integrated international and long-term collaborative studies covering research gaps will reduce uncertainties and promote further understanding of the nitrogen cycle in various ecosystems.
Keywords: Atmospheric deposition, Biogeochemistry, Water quality, N2O, Nitrogen leaching
Introduction
Nitrogen (N) is an essential nutrient, but reactive N has well-known deleterious effects in high concentrations. Agriculture and industry have strongly altered the N cycle in ways that impact the environment from local to global scales by contributing to increasing greenhouse gas emissions, acidic deposition, and impairing the functioning of ecosystems through the eutrophication of soils and waters. The N cycle is thus intrinsically coupled with various environmental processes and factors including the transformation of land-use, energy and food production and consumption, climate change, exploitation of natural resources, air, soil and water pollution, human health, ecosystem services, and other natural and anthropogenic drivers (Galloway et al. 2004). Future sustainable management and stewardship of both less disturbed and highly managed ecosystems require a more integrated approach to the assessment of linkages between these systems and their interaction with the human society (Collins et al. 2011).
Long-term monitoring of N biogeochemistry is a powerful research approach to understanding the dynamic features of ecosystem behavior influenced by natural and anthropogenic drivers, locally, regionally, and globally. The Long-Term Ecological Research (LTER) program was first established in early 1980s in the USA. Since then, the LTER has been expanded to many other countries as an integrated ecological research network that enables long-term site-based research, field experiments, and database development. The US-LTER has produced important findings on the N dynamics in a broad geographical range of watersheds and landscapes, including the long-term impact of atmospheric N deposition on forest ecosystems, the impact of logging on stream N chemistry, the climate impacts on N cycles in ecosystems, effects on biodiversity, etc. (e.g., Likens et al. 1996; Clark and Tilman 2008; Fernandez et al. 2010; Driscoll et al. 2012; Groffman et al. 2012). Some European countries have also conducted long-term environmental monitoring, for example Sweden (e.g., Löfgren et al. 2011), the UK (Curtis et al. 2014), and Finland (Rask et al. 2014). In addition to the LTER sites, long-term N experiments have been also been conducted at other sites in the USA and Europe. Examples of European studies include the NITREX (Gundersen et al. 1998) and climate experiments linked with N dynamics [e.g., CLIMEX (Wright 1998), CLIMOOR (Beier et al. 2004), and VULCAN (Peñuelas et al. 2007)]. Lake manipulation experiments have been undertaken in Canada, Norway, the USA, and Finland (e.g., Carpenter et al. 2001; Harris et al. 2014). There have also been a number of snow manipulation experiments in the USA, Norway, and Germany (e.g., Kaste et al. 2008; Wipf and Rixen 2010). While contributing to our current understanding of N cycling in ecosystems, they also reveal significant gaps in knowledge which will require a continued commitment to long-term research and a broadening of international perspectives to address. The LTER program has expanded internationally since the early 1990s, and the International LTER (ILTER) was created in 1993. This currently comprises over 600 sites within 40 member networks, providing great potential for understanding altered N biogeochemistry and its impact in different environment and socio-ecological settings at an international scale. However, effective integration of this international effort has been impeded by the absence of a mechanism to bring the international research community together and the relative paucity of robust and directly comparable data that could be employed in meta-analyses at global and regional scales. Recent syntheses of nitrogen issues in the USA and Europe (e.g., Sutton et al. 2011; Suddick and Davidson 2012) bring to the fore the need to review the contributions that long-term environmental research has made to the scientific understanding of the changing N cycle and consider how it might address the current major gaps in knowledge. Here, we review current understanding of the impact of anthropogenic N on various ecosystems and environments to elucidate the consequences of globally increased N cycles for coupled social–ecological systems under a changing climate (Fig. 1). Particular attention is paid to the most recent trends in anthropogenic reactive N emissions, including nitrous oxide (N2O); the complex interaction of N with carbon (C), phosphorus (P), and other elements; impacts of N on biodiversity; seasonal and long-term trends in N biogeochemistry associated with climate variability; the N cascade process from terrestrial to aquatic ecosystem; and finally, societal challenges from ecosystem services to human health. Emerging uncertainties and further research questions are also discussed.
Fig. 1.
Conceptual framework of N biogeochemistry in coupled human and ecological systems in this review. Gray and green arrows indicate anthropogenic disturbance and ecosystem feedbacks among both systems, respectively. White arrows represent dominant reactive nitrogen (Nr) flow; Nr deposition, biological N-fixation (BNF), N leaching and emission of N2O. Blue arrow shows N cycles among plant–soil–microbe systems
Increased anthropogenic nitrogen emissions
Humans create more reactive N (Nr) than natural ecosystems do (Galloway et al. 2003), principally nitrogen oxides (NOx-N, i.e., sum of N2O, NO, and NO2) and ammonia (NH3), mostly through food and energy production and consumption, and their various byproducts. Reactive N emissions into the atmosphere contribute to increasing greenhouse gasses, acidic deposition, as well as excess inputs of N nutrients to receiving environments. One of the Nr forms, N2O, is an important greenhouse gas with an exceptionally long atmospheric half-life that is emitted through agricultural activities as well as natural processes such as denitrification within wetlands. Here, we review the long-term trends of Nr and N2O emissions and their current knowledge gaps.
Global long-term trends in anthropogenic emissions of reactive nitrogen
Reactive N emissions associated with human-induced burning of biomass and animal husbandry have been entering the atmosphere for over 10 000 years. Emissions have increased sharply since the onset of the industrial revolution due to fossil fuel combustion, while the use of synthetic N-fertilizers became especially important in the twentieth century. The global cumulative anthropogenic release of Nr to the atmosphere over the last 10 000 years has been estimated at ~17.4 Pg N, 28% of which was emitted during 1850–2000 and 42% during 1–1850 ad (Kopáček and Posch 2011). Recent global emissions of NOx from anthropogenic and natural sources have been estimated to range from 44 to 50 Tg N year−1, while the contribution from NH3 has been estimated at 54 Tg N year−1 (Kopáček and Posch 2011). About 70% of global NH3 emissions are closely related to food production and agricultural systems, predominantly livestock production and the use of synthetic N-fertilizers (Kopáček and Posch 2011). Global Nr emissions have increased sharply since the 1950s (Fig. 2). Overall, Europe is the only continent, where Nr emissions have begun to decrease in recent years (i.e., since the late 1980s) (Fig. 2). This decrease is attributed to NOx emission controls on energy production, lower NH3 emissions due to reductions in cattle production, and reduced use of synthetic N-fertilizer (Kopáček and Posch 2011).
Fig. 2.
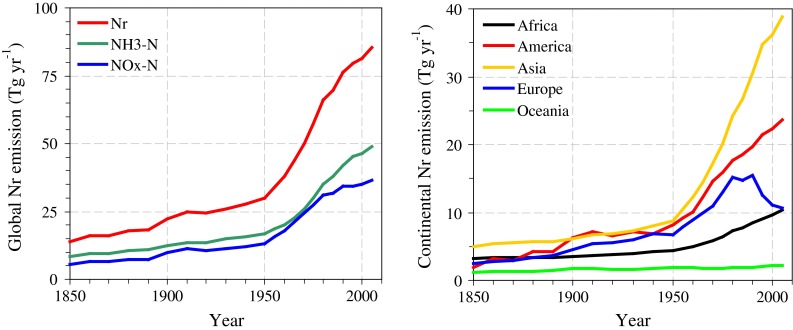
Global (left panel) and continental (right panel) annual rates of Nr (NOx–N + NH3–N) emissions (derived from Kopáček and Posch 2011)
Greenhouse gas emissions
Nitrous oxide is a potent greenhouse gas with a global warming potential that is ~300 times greater than CO2 on a per molecule basis. The production of N2O occurs during both denitrification and nitrification. N2O in the atmosphere is estimated to have increased by 18% from its pre-industrial level (IPCC 2007). The rise is attributed primarily to human activities, particularly from agriculture and land-use change.
Information on the processes influencing N2O emissions from soils is sparse, particularly that on the roles of temperature, moisture, redox potential, pH, and substrate availability (Wallenstein et al. 2006). While agricultural soils are considered a major source of N2O, the effect of N-fertilizer on soil N2O emissions remains highly uncertain (Davidson 2009; Zaehle et al. 2011). Emission factors (N2O emissions per unit N addition) have been reported to vary between 0.1 and 7% of the N applied (Skiba and Smith 2000). Since multiple processes and drivers are involved, N2O emissions are highly variable and often associated with “hotspots” (high emissions from small areas) and “hot moments” (high emissions for brief periods), making measuring, modeling, and up-scaling challenging (Groffman et al. 2009; Reay et al. 2012).
High variability in the response of N2O to N-inputs indicates nonlinearity of the response function (Hoben et al. 2011). However, there is also large global variation in levels of N-inputs to agricultural systems, ranging from 588 kg N ha−1 year−1 in a wheat-maize double-cropping system in North China to 7 kg N ha−1 year−1 in maize systems in western Kenya, resulting in very large uncertainty over global N2O emissions (Vitousek et al. 2009). Assessing a decade of measurements from an ILTER site in Michigan, USA, Robertson et al. (2000) reported that N2O fluxes were similar among different cropping systems, suggesting that N2O fluxes were driven by soil N availability, rather than by additional N-inputs. In contrast, Van Groenigen et al. (2010) found that N-inputs stimulated a dramatic increase in N2O emissions when fertilizer rates reached 301 kg N ha−1 year−1, while N2O emissions were small when fertilizer rates were ~180 to 190 kg N ha−1 year−1 or lower. Given the paucity of data for areas with low rates of fertilization such as Africa, there is currently insufficient information for establishing the response function of N2O fluxes following addition of N-inputs in these systems (Van Groenigen et al. 2010).
Clearly, there is a need for considerable clarification of the factors determining N2O emissions from N-inputs at representative sites around the globe. For example, assessments are urgently required to determine the global impact of the African Green Revolution, called for by the former United Nation secretary general Kofi Annan. Agricultural productivity across Sub-Saharan Africa is expected to increase substantially by major increases in fertilizer use (up to 100 kg ha−1 year−1, Sanchez et al. 2007). To assess the unintended N2O emissions and N leaching to the local environments, more research is urgently needed to understand the N fluxes in response to inputs.
Altered nitrogen biogeochemistry in ecosystems
Increasing anthropogenic Nr emissions are a significant source of atmospheric N deposition to land and sea (Galloway et al. 2004) and also enhance global warming through N2O emissions (IPCC 2007), disturbing N pools, cycling, and transport in and among ecosystems. In this section, we review the current understanding of the impact of increased N deposition on N biogeochemistry over a period of changing climate in various ecosystems, with special attention to ILTER’s findings.
Long-term effects of N deposition in watershed N cycles and leaching
Excess atmospheric N deposition beyond the N requirement of the biota often causes N saturation, which has been observed in many forest ecosystems of Europe and the USA (Dise and Wright 1995; Aber et al. 1998). Elevated concentrations of NO3− in surface waters derived from non-point or point sources of N pollution usually indicate that there has been sufficient anthropogenic deposition of N for catchment soils to have reached a degree of N saturation (Stoddard 1994).
In recent years, attempts in the USA and Europe to reduce atmospheric emissions of acidic precursors and other pollutants have resulted in widespread reductions in sulfur (S) deposition, with corresponding substantial reductions in sulfate concentrations in runoff. However, in Western Europe, there have been less linear changes of atmospheric deposition with respect to N. While reductions in emissions of N have led to broadly comparable reductions in ammonium (NH4+) deposition, reductions in NO3− deposition have been much lower (Fowler et al. 2007). The combination of uncertainties associated with these non-linearities between reduction of NH4+ and NO3− and the various responses of N leaching to variation in winter climate make the future prediction of NO3− leaching to surface waters and the extent to recovery from acidification very difficult.
In contrast, further increases in N emissions in the East Asia region during the next few decades have been predicted as a consequence of rapid industrialization, urbanization, and economic growth (e.g., Galloway et al. 2003, 2004; Fang et al. 2011). Fang et al. (2011) indicated that N deposition in China ranged 2.6–48 kg N ha−1 year−1, while the threshold of N deposition to N saturation in Chinese forest differs from that in the USA and Europe (i.e., the relatively high N leaching in some Chinese forests receiving low N-input). Niu et al. (2010) reported that an experimental addition of N deposition (100 kg N ha−1 year−1) for 4 years enhanced the ecosystem productivity by 27% in a temperate steppe ecosystem in China. Several comparative studies (Park et al. 2003; Fang et al. 2011; Mitchell 2011) suggest that regional climate, geology, and hydrology result in different patterns and responses to elevated N deposition in forest ecosystems, when compared to previous findings of similar studies in the USA and Europe (Ohte et al. 2001), indicating that more comparative research is needed to generalize the impact of increased N deposition on ecosystems. Long-term studies of watershed N biogeochemistry are clearly limited outside the USA and Europe, especially in East Asia, South America, and Africa, where anthropogenic N deposition will increase in future.
Complex interactions of N with other elements
The N cycle is intimately coupled to the C cycle. Soils with large pools of organic C and high C:N ratios are generally associated with N accumulation and tend to export less NO3– than soils with low C:N ratios (e.g., Aber et al. 1998; Gundersen et al. 1998). When Nr availability is elevated in an N-limited system (e.g., through atmospheric N deposition), soil inorganic N is readily utilized by plants, resulting in increased C uptake (Gruber and Galloway 2008) and reduced below-ground allocation of C (Deegan et al. 2012). Elevated Nr in soil can also change the soil microbial community by, for example, a reduction of fungal:bacterial biomass ratios (Högberg et al. 2007; Boberg et al. 2010). These studies emphasize the importance of taking N–C interactions into account when considering the possible impact of climate change on ecosystems, carbon sequestration, and in the development of earth system models (Thornton et al. 2009). Various mechanisms have been proposed to explain the changes in N–C interactions following increased N availability: biomass increase with allocation changes in plants (i.e., reduction of below-ground C allocation due to the N-increase) (Högberg et al. 2010); enhanced soil respiration reflecting an increase in soil microbial activity with an increase in N availability (Gärdenäs et al. 2011); inhibition of litter decomposition through a change in litter quality with elevated N (Knorr et al. 2005; Pregitzer et al. 2008); a change in plant uptake of organic N as a nutrient source in N-limited environments (Gärdenäs et al. 2011); altered interaction with dissolved organic carbon [e.g., enhanced mineralization of DOC due to increased abundance of electron acceptors in the form of NO3− in anoxic soil micro-sites (Kopáček et al. 2013c)]; and changes in abiotic N–C interactions in soil (e.g., abiotic reaction of nitrite with dissolved organic matter through nitration and nitrosation of aromatic ring structures) (Davidson et al. 2003).
Phosphorus (P) is also an essential nutrient for biota. N–C-P interactions in soil vary among biomes. Where P limits primary production, such as in some tropical ecosystems or acid alpine grasslands, increases in N deposition may have little impact on productivity (Matson et al. 1999), a finding that has recently been documented by field experiments at ILTER sites (Bowman et al. 2008; Cusack et al. 2011). In both N- and P-limited tundra ecosystems, C fluxes were found to respond positively to additions of both elements, although responses to P tended to be stronger than to N (Shaver et al. 1998). Bergström and Jansson (2006) have shown that increased N deposition may have changed lakes from N-limitation to P-limitation in remote and small lakes across the northern hemisphere, an observation supported by nutrient addition experiments in UK upland streams (Maberly et al. 2002). On the other hand, an assessment of long-term data from a Spanish ILTER lake site by Camarero and Catalan (2012) suggested that atmospheric P deposition may cause lakes to revert from P-limitation to N-limitation. There are clear needs for research into long-term C–N–P interactions for a much wider range of biomes.
Nitrogen deposition can serve as an acidifying as well as eutrophying agent (Oulehle et al. 2008). Bowman et al. (2008) reported that long-term acid deposition in the Western Tatra Mountains of Slovakia, central Europe has altered soil systems in alpine grasslands to an extreme level of acidification usually associated with soils exposed to acid mine drainage. They showed that increases in N deposition had resulted in a depletion of base cations, increases in aluminum (Al) and extractable iron (Fe) in soil, and a reduction in the biomass of vascular plants associated with a decrease in shoot calcium and magnesium concentrations. They suggested that acidifying soils in central Europe have reached an unprecedented level of toxicity in which Al release into soil water has been superseded by Fe release (Bowman et al. 2008).
N leaching in dormant season
Seasonal changes in nitrogen behavior of ecosystems are mostly driven by seasonal fluctuations of physical drivers (i.e., weather conditions) and biological factors (i.e., phenology in plant and microbial activity). The seasonality of plant growth in many biomes results in a seasonal N demand, while (with the exception of heavily N-saturated soils and catchments with little soil cover) most NO3− leached into surface, and ground waters are increasingly being found to have undergone soil microbial processing (Stoddard 1994; Piatek et al. 2005; Curtis et al. 2012). Consequently, the dependence of biological systems on soil microclimate can lead to strong seasonal variation in N fluxes and concentrations in soils and drainage waters.
Time series analyses from boreal to temperate forested, moorland and alpine systems emphasize the importance of winter temperatures and snow cover in determining the export of NO3− in soil, ground, and surface waters. Although winter has sometimes been considered to be the “dormant season,” due to cold temperatures, vegetation dormancy, and a persistent snow cover, microbial processes can persist and exert a critical impact on N cycling (Campbell et al. 2005; Makoto et al. 2013). Snow also allows solutes to accumulate in the soil (Kurian et al. 2012) leading to pronounced fluxes when the snow melts. Watersheds throughout the Northeast USA export more than 85% of the annual NO3− loss during winter (Mitchell et al. 1996), with most of this export occurring during spring snowmelt (Campbell et al. 2005), but mid-winter melt events and rain-on-snow events can also influence winter NO3− loads to streams (Casson et al. 2010). Individual rain-on-snow events can contribute as much as 40% of annual NO3− export from forested watersheds in southeast Canada, and the contribution of rain-on-snow events to annual and winter NO3− loads has generally increased in recent decades (Eimers et al. 2007).
The insulating properties of snow can maintain soil temperatures sufficiently high to allow root growth, microbial respiration, and other biotic activities to continue (Groffman et al. 2009). Soils devoid of a snowpack are more vulnerable to freezing and hence to physical and chemical changes, including the death of fine roots, cell lysis, and the alteration of soil microbial processes (Tierney et al. 2001; Christopher et al. 2008; Shibata et al. 2013). Experimental snow removal in alpine Europe lowered soil temperatures and increased NO3− release (Freppaz et al. 2008). In mountainous and northern regions, soil temperatures and ecosystem respiration rates tend to be higher in winters with high amounts of snowfall than in winters with less or no snow (Monson et al. 2006). On the other hand, experimental snow manipulation at a mountain site in Norway indicated that no increase of inorganic N fluxes was associated with snow removal (Kaste et al. 2008), suggesting that the effects of decreased snow on the N cycle varies among locations. Coherent patterns of variation in NO3− leaching are sometimes evident over large spatial scales and across catchments covering wide altitudinal gradients and land-use classes (e.g., Rogora et al. 2008; Evans et al. 2010). In the UK, winters when NO3− leaching to remote surface waters have been strongly associated with negative excursions in the winter North Atlantic Oscillation (NAO) Index, most likely due to low winter temperatures, lower than average rainfall and larger contributions from more polluted air masses originating from the European continent (Monteith et al. 2000; George et al. 2004). However, as soil temperature is a dominant driver, opposing inter-annual patterns in NO3− leaching may be observed in regions normally blanketed by snow in winter when they lack snow cover, since snow cover insulates the uppermost soil layer from the atmosphere (Groffman et al. 2009; Makoto et al. 2013). Consequently, relationships between NO3− leaching and the NAO index in the UK and in northern Europe may vary regionally (George et al. 2004; Blenckner et al. 2007; de Wit et al. 2008).
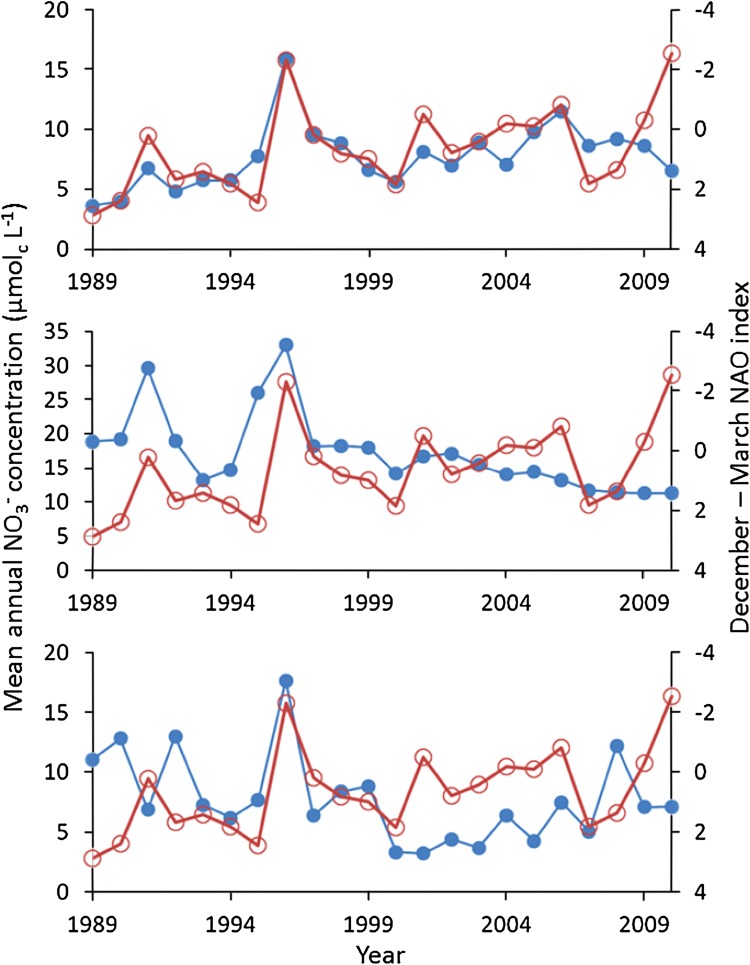
Over the last decade, trends in NO3− concentrations in waters across the UK Upland Waters Monitoring Network have lost coherence and begun to diverge (Monteith et al. 2014). Inter-annual variation in NO3− concentrations in some northern sites remains tightly linked to the winter NAO Index, and shows a long-term (>20 years) increase consistent with the long-term decline in the NAO over the same period (Fig. 3). At other sites further south, however, NO3− concentrations, while still showing evidence of influence of the NAO on short-term variability, are trending downward (Fig. 3), possibly in response to more marked reductions in N deposition in this region. The divergence provides evidence for regional differences in the relative importance of N deposition and climate variability on NO3− leaching to surface waters, with the latter exerting greater influence in areas where N deposition has been more stable.
Fig. 3.
Long-term observation of nitrate concentrations in three small upland UK lakes (The UK Upland Waters Monitoring Network). From the top, the sites are Round Loch of Glenhead (southwest Scotland), Scoat Tarn (English Lake District), and Llyn Llagi (North Wales—Snowdonia). The blue circles represent the annual means of seasonal (four samples per year) nitrate concentrations. The open red circles represent the December–March North Atlantic Oscillation Index. The NAO scale is reversed so the most negative values are uppermost
Cascading influences from terrestrial watersheds to estuaries
Nitrogen losses from agricultural land are often several times higher than those from natural systems. In typical agrarian systems, exported N represents 10–40% (~25% on average) of net anthropogenic nitrogen inputs (Howarth et al. 2011), depending on the amounts of leachable NO3– in the soil and surplus water to transport the solutes out of the watershed. Kopáček et al. (2013a, b) indicated that, in Slapy Reservoir, an ILTER site in central Europe, the reservoir of leachable NO3– in agricultural and forested watersheds originates from both external (fertilization and atmospheric deposition) and internal (mineralization of soil organic N) sources, with relative contributions dependent on topography and land-use practices such as drainage and tillage. Fluctuations in the export of N from Slapy Reservoir (Vltava river) from 1920 to 2010 were strongly related to the change in the mineralization of soil organic N enhanced by more drainage of farmlands (up to 43%) rather than to the external N sources (Kopáček et al. (2013a, b). Boyer and Howarth (2002) evaluated the anthropogenic N source in large watersheds based on intensive monitoring of N cycles, indicating that fertilizer N-inputs, N-fixation in crop land, and animal feed N imports were the dominant sources of Nr from agricultural land to the riverine N exports in sixteen large catchments of northeastern USA.
At watershed, landscape and regional scales, cascades, and interactions are key to understanding N dynamics. Riparian zones function as the interface (“buffer zone”) between terrestrial and aquatic ecosystems, and may act as either net sources or sinks of N depending on timescales, hydrological conditions, and the history of N-inputs. Anaerobic conditions in shallow groundwater in the riparian zone stimulate denitrification (microbial transformation of NO3− to reduced gaseous forms such as N2O and N2), thus reducing the potential flux of NO3− leaching to stream water (Chestnut and McDowell 2000). Hyporheic exchange, mixing saturated ground water (relatively anaerobic) with stream water (mostly aerobic), may provide “hot spots” for dynamic microbial N transformation near riparian boundaries and at the surfaces of channel beds (Shibata et al. 2004).
A substantial proportion of Nr can be buried in accumulating sediments of lakes and swamps (Noe and Hupp 2005). However, sediment anoxia may lead to the reduction of NO3− and nitrite (NO2−) to N2 (or N2O) by denitrification (Rissanen et al. 2011). Recently, “anaerobic ammonium oxidation” (anammox) has been identified as another process of N2 release under anoxic conditions (Jetten et al. 2005).
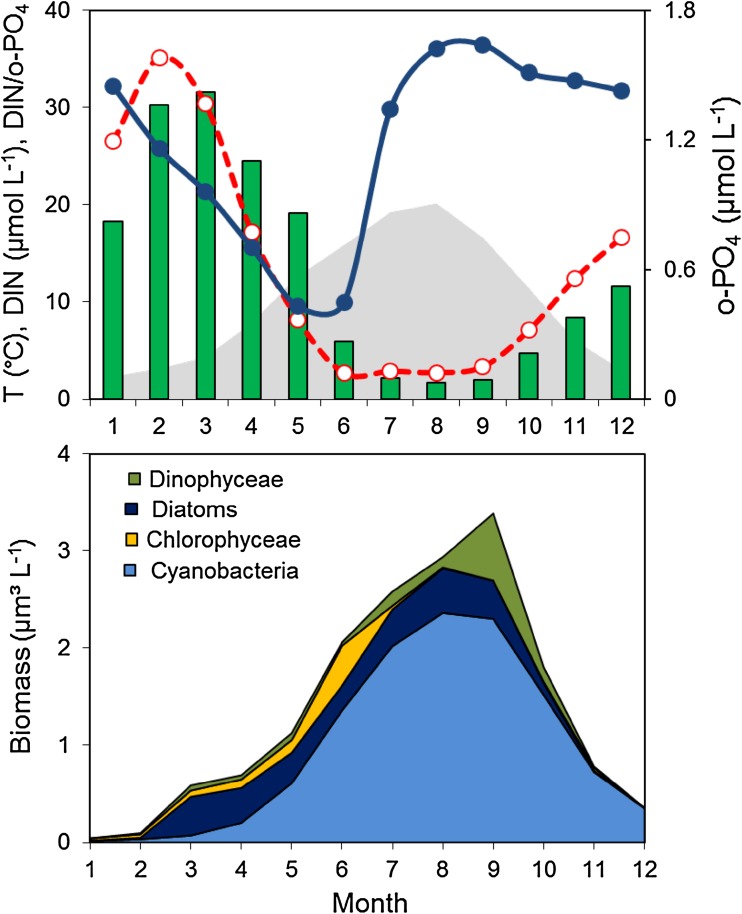
In contrast to many freshwater systems, coastal systems may be N-limited as a consequence of eutrophication associated with high P inputs (Howarth and Marino 2006). Substantial denitrification rates have even been found in N-rich downstream riverine systems, whereas no comparable P-removal process occurs (Billen et al. 2009; Vermaat et al. 2012). Consequently, this may lead to N-limitation that enhances the dominance of diazotrophic cyanobacteria in periods when the high-energy demand for N-fixation can be satisfied (Stal and Zehr 2008). This can even lead to temporal N-limitation (Schubert et al. 2010), whereby P-concentration increases during summer in response to the constant supply of P by runoff and release from the sediment (Fig. 4; Schubert and Wasmund 2005). Further investigations of denitrification rates and their driving factors including regional comparisons of diverse riverine systems and their connections to marine systems would reduce the uncertainty of N budgets of coastal systems fed by terrestrial N-inputs.
Fig. 4.
Seasonality of dissolved nutrient concentration, temperature, and phytoplankton biomass in a coastal water body. Shown are seasonal changes in dissolved inorganic nitrogen (DIN in µmol L−1; open circles), ortho-phosphate (o-PO4 in µmol L−1; closed circles), temperature (T in °C; gray background area), DIN/o-PO4 ratio (bars; upper panel), and phytoplankton biomass (µm3 L−1, lower panel, resolved for main taxonomic groups) of a coastal inlet (Kubitzer Bodden and Strelasund, Southern Baltic Sea coast, Germany). All values are averages over a 10-year period (1988–1999). For further details, see Schubert and Wasmund (2005)
Impact on biodiversity and human society
As described above, increases of anthropogenic Nr emission and deposition substantially alter N pools, cycles, and transport among ecosystems. The altered N behavior also influences structures and characteristics of organisms in natural ecosystems and anthropogenically dominated systems. Here, we review how increased Nr affects (i) biological structures in the context of biodiversity and also (ii) human society in the context of human health and ecosystem services.
Terrestrial biodiversity impacts
Numerous studies have reported a decline in species diversity (vascular plants, lichens, mosses, phytoplankton microbes, etc.) associated with both N-fertilization and N-gradients across a range of different ecosystem types in forest and semi-natural areas (Clark and Tilman 2008; Bobbink et al. 2010). Clark and Tilman (2008) demonstrated that chronic low-level nitrogen addition (10 kg N ha−1 year−1 above ambient atmospheric N deposition) reduced plant species numbers by 17% relative to controls receiving ambient N deposition based on the multi-decadal experiment of N-fertilization in the grassland ecosystem of Cedar Creek LTER. Recent studies suggested that even low-level N deposition could influence the changes in ground vegetation (Johansson et al. 2012; Hedwall et al. 2013). Nitrogen pollution stimulates competitive interactions that lead to compositional change, making conditions unfavorable for some species (Bobbink et al. 2010; Bobbink and Hettelingh 2011). Deprivation of light and nutrients resulting from the increased cover of aggressive dominant species can outweigh the potential benefits of N-fertilization for subordinate species. These changes in biodiversity can have cascading impacts on primary production, soil C storage, microbial activity, rates of decomposition, N mineralization and immobilization, tissue chemistry, trophic interactions (herbivory), and can ultimately disrupt ecosystem functions and services (Dias et al. 2011; Ochoa-Hueso et al. 2011).
The sensitivity of terrestrial biodiversity to the deposition of oxidized and reduced N provides the basis for setting critical loads for N deposition both in Europe and North America (Cape et al. 2009; Bobbink and Hettelingh 2011; Pinho et al. 2011, 2012). Independently derived critical levels for lichens and moss diversity have been found to be similar for northern and southern Europe, thus emphasizing the universal applicability of these plant groups as ecological indicators of N deposition. Pardo et al. (2011) showed that, in the USA, empirical critical loads for N tend to increase according to the following sequence: lichens and bryophytes, mycorrhizal fungi, herbaceous plants and shrubs, and trees. In several studies in the USA, lichens invariably showed the lowest NH3 critical levels (1 µg m−3) and N critical loads (1 kg N ha−1 year−1) of all biological indicators (Jovan et al. 2012).
Currently, there is no coordinated global observation system capable of providing real-time tracking of biodiversity change around the globe (Pereira et al. 2013). The scientific understanding of how biodiversity is reacting to increasing N-inputs, and how this is affecting ecosystem resilience and ecosystem services remains limited. However, biodiversity seems to be a relatively sensitive metric for measuring the effects of N at the ecosystem level, i.e., loss of particular species from an ecosystem (Cape et al. 2009). Changes in biodiversity can also be used to help identify those species most sensitive to increased N. We expect that various assessments of biodiversity will exhibit differences in scalability, temporal sensitivity, feasibility, and relevance.
Human health
Human-induced changes in response to changes in the N cycle also have both negative and positive implications for human health. The most obvious health benefit of increased use of Nr is decreased hunger and malnutrition through the use of fertilizers, while N-related air pollutants are hazardous for humans. Atmospheric N pollution can affect human health by increasing respiratory problems especially those caused by smaller particulate matter (PM2.5), since they have the ability to penetrate deeper into the respiratory tract. Approximately, 40% of PMs are NH4+ and NO3−. High levels of atmospheric NOx lead to increases in tropospheric O3 that strongly affect human respiratory function (von Mutius 2000). In addition, high concentrations of NO2 in urban air can lengthen and worsen common viral infections such as human rhinovirus, significantly elevating the risks to asthmatics and individuals with compromised immune systems (Spannhake et al. 2002).
Nitrogen also affects human health via water pollution. Concentrations of NO3− in drinking water exceeding 10 mg L−1 put children at risk of methemoglobinemia (“blue-baby” syndrome; Gupta et al. 2000). Even nitrate levels below the WHO standard may stimulate the endogenous formation of N-nitrosamines (Van Maanen et al. 1996), compounds strongly implicated in cancer risks. Long-term consumption of water with NO3− concentrations of 6.3 mg L−1 and above has been linked to a higher risk for Non-Hodgkin’s lymphoma (Ward et al. 1996). In Iowa, rising NO3− levels well below the 10 mg L−1 standard were associated with an increased risk of bladder and ovarian cancers (Weyer et al. 2001).
One way to maintain good nourishment of the human population while decreasing fertilizer consumption would be to reduce meat consumption and increase consumption of a diversity of vegetables. Typically, most NO3− exposure (86%) to humans comes from vegetables, whereas the primary contributors to nitrite (NO2−) intake are cured meats (39%), baked goods and cereals (34%), and vegetables (16%). It is possible that a diet based on a diversity of vegetables provides man with adequate levels of NO2− which contributes to the whole-body NO production and homeostasis (Landberg et al. 2011).
Global food and feed trades are one of the important drivers of global, regional, and local N circulation (Galloway et al. 2008). International trade in N has increased eightfold (from 3 to 24 Tg N) during 1961–2010 and a small number of countries (e.g., USA, Argentina, and Brazil) currently feed much of the rest of the world in terms of proteins and N (Lassaletta et al. 2014). The spatial imbalance of production and consumption of feed and food contributes to the spatial imbalance of risk for human health and environment quality (e.g., animal feed imports from Africa, where the export of N contributed to N-limitation of human food production with negative consequences for human health locally). Also, the influence of the global trade in N is more complex than merely the N flows associated with import or export of food and feed because part of the reactive N added by fertilizers and feed during the production of crop and animal products is lost to the environment, becoming a source of water and air pollution (Leach et al. 2012).
Impact on societal and economic value
The concept of ecosystem services (Millennium Ecosystem Assessment Board 2005) recognizes the vital importance of the natural environment and the biodiversity it supports in underpinning human wellbeing. The nitrogen cycle is central to several key ecosystem processes including water quality regulation (regulating services); ecosystem productivity that is often limited by N (provision of services via food, timber, and fiber); and C sequestration and control of N2O emissions (via climate regulation services). Nitrogen also indirectly impacts all ecosystem services through its influence on biodiversity (e.g., Suddick and Davidson 2012). Clearly, a range of N-related ecosystem services may be threatened by anthropogenic disturbances of biogeochemical cycles.
To date, economic valuation of N-related ecosystem services and human health has been conducted mainly in Europe and the USA (Compton et al. 2011; Sutton et al. 2011), while assessment in other region such as Asia-Pacific, Africa, and South America is still limited and entails great uncertainty. The European Nitrogen Assessment (ENA) estimated that the highest social costs of N are associated with air pollution effects of NOx on human health (10–30€ per kg of N). A similar value ($28 per kg NOx–N) relating to the USA was obtained by Compton et al. (2011). The effects of N loss to water on aquatic ecosystems were evaluated by the ENA as 5–20€ per kg of N. The ENA also estimated N-related environmental damage from agriculture in the EU to be 20–150 billion € year−1, which is comparable with a benefit of N-fertilizer for farmers of 10–100 billion € per year−1 (Sutton et al. 2011).
The need for international integration of long-term ecosystem research
Based on the above literature review, we propose that several areas require more attention to develop a better understanding and reduce uncertainties with respect to the environmental effects of N.
The long-term monitoring and analysis of N deposition, N cycles in various ecosystems, biodiversity, and N export to water are needed to provide the fundamental information necessary to address a spectrum of research questions concerning N dynamics in coupled human and ecological systems (Driscoll et al. 2012; Robertson et al. 2012). Modeling and analysis coupled with long-term monitoring and field experiments provide powerful research tools to help understand the dynamic features of the N cycle driven by multiple environmental factors, and to address new parameters to be monitored or examined (e.g., Aber et al. 2002; Driscoll et al. 2003). There are clearly regional research gaps in the context of long-term site-based research on N biogeochemistry: East Asia, South America, and Africa (e.g., Anderson et al. 2012; Urakawa et al. 2012), where increased Nr pollution has been predicted for the coming decades (Galloway et al. 2004). Also, long-term research sites in agricultural and urban ecosystems are currently limited despite their large importance in global N cycles. Increased international collaboration and integration offers the potential for further significant scientific advances, particularly with respect to the elucidation of (i) responses of N2O emission to elevated Nr inputs; (ii) biodiversity changes associated with changes in N deposition; (iii) spatial heterogeneity of temporal trends among different deposited N species; (iv) spatial patterns in N leaching from a wide spectrum of catchments including a range of altitudinal and latitudinal gradients and different land-use types; and (v) long-term trends in N concentrations in surface waters and potential linkages with inter-annual climate change.
Second, studying N dynamics at the ecosystem level should be coupled with socio-economic issues. Reactive nitrogen in the environment presents society with a global problem that must be addressed at a global scale over the long term by uniting the analyses of natural and human systems. Continued maintenance of our best long-term environmental observation systems and the development of new long-term experiments will be necessary to clarify these complex interactions and their long- and short-term impacts.
At the international scale, our environmental observation capacity remains extremely limited. Based on the analysis in this review, some research questions have emerged:
To what extent do ecosystems exhibit common or unique responses to elevated Nr across different environmental and social landscapes?
What features of socio-ecological N-interactions are likely to be most sensitive to global changes in human population and climate?
What are the political and management options to mitigate or adapt the N-related social issues (e.g., diet, human health, and ecosystem services)?
Will future climate changes have major impacts on N biogeochemistry, and what feedbacks from N cycling will be most important in influencing the climate?
Even though the answers to these questions remain unclear, some strategies need to be developed. For example, some previous studies have suggested that very sensitive organisms, such as lichens and mosses, could be effective early-warning indicators of atmospheric Nr pollution in the early stages of anthropogenic disturbance of N cycles in an ecosystem (Pinho et al. 2011). The functional diversity of lichens and/or mosses coupled with measures of their nitrogen content and isotopic composition has the potential to be explicit spatial indicators of the early effects of Nr pollution. It would, therefore, be possible to use lichens and mosses in the long-term ecological site to develop an early-warning biological monitoring system of atmospheric N pollution in regional and global scales. Other responses of ecosystem structure and functioning to altered N cycle often vary among sites, influenced not only by current driving factors but also by long-term socio-ecological legacies (Aber et al. 1998). Therefore, investigations need to include international comparisons of the impact of socio-ecological legacies on current N cycling processes by analyzing the relationship between current monitoring data and previous land history and other parameters. The historical records of site-management, land-use, natural disturbances, climate, atmospheric deposition, etc. should be used to help understand how historical factors are affecting current N cycles. Such analyses should be facilitated using the results from long-term ecological sites such as the ILTER worldwide network. Meta-analysis of comprehensive and integrated N databases and the organization of workshops on focused topics using international researchers networks (e.g., ILTER) should be encouraged. The outcomes of collaborative international research should also include analyses of the complete spectrum of socio-ecological factors related to N. This information must to be provided to both the scientific community as well as other stakeholders, including policy makers.
Acknowledgments
We thank the ILTER network for giving us this opportunity to develop the ILTER-N initiative, and for funding support to organize the workshop to develop the manuscript. We also thank all of the participants in the workshop for valuable discussions and helpful comments for this paper. This paper contributes to the synthesis of the Global Land Project (IGBP/IHDP). The synthesis activity was also partly supported by the Environmental research and technology development fund (S-9-3) of the Ministry of the Environment, Japan.
Biographies
Hideaki Shibata
is a Professor of Field Science Center for Northern Biosphere at Hokkaido University. His research interests include biogeochemistry, soil science, ecosystem ecology, and land change sciences under natural and anthropogenic disturbances.
Cristina Branquinho
is an Associate Researcher of the Centre for Environmental Biology of the Faculty of Sciences, University of Lisbon. Her research interests are developing ecological indicators as early warning of the impact in ecosystems of anthropogenic activities namely associated with atmospheric pollution including nitrogen issues; she is also interested in the ecology of semi-arid areas and on multifunctional agroecosystems.
William H. McDowell
is a Professor of Environmental Science and Presidential Chair of Department of Natural Resources and the Environment at University of New Hampshire. His research interests include Biogeochemistry and Ecosystem Ecology.
Myron J. Mitchell
is a Distinguished Professor in the Department of Environmental and Forest Biology at SUNY-College of Environmental Science and Forestry. He also directs the Council on Hydrologic Systems Science. His research areas focus on the effects of atmospheric deposition and climate change on forest ecosystems and surface waters. He has specific research interests in Eastern North America and East Asia.
Don T. Monteith
is the Research Coordinator of the UK Environmental Change Network. His research interests include freshwater acidification, the interactive effects of air pollution and climate change on upland aquatic ecosystems, and the drivers and ecological consequences of rising concentrations of dissolved organic matter.
Jianwu Tang
is an Assistant Scientist at the Ecosystems Center of the Marine Biological Laboratory in Woods Hole, USA. His research interests include ecosystem ecology, and carbon and nitrogen cycling.
Lauri Arvola
is a Professor at University of Helsinki. His research interests include aquatic ecology, catchment-lake interactions and biogeochemical processes.
Cristina Cruz
is a Professor of Faculty of Sciences at the University of Lisbon. Her research interests include ecophysiology of inorganic nitrogen assimilation by terrestrial plants, nitrogen cycle in Mediterranean ecosystems, role of symbioses in nitrogen uptake by terrestrial plants, and plant–soil interactions.
Daniela F. Cusack
is an Assistant Professor of Geography at Department of Geography, University of California - Los Angeles. She is interested in biogeochemistry, biogeography, and tropical ecology.
Lubos Halada
is a Senior Scientist and the Deputy Director at Researcher of Institute of Landscape Ecology, Slovak Academy of Sciences. His research interests include habitat classification, biodiversity assessment, vegetation ecology, ecosystem research, and landscape ecology.
Jiří Kopáček
is a Senior Scientist at Biology Centre ASCR, Institute of Hydrobiology, and Professor at University of South Bohemia. His research interests include nutrient cycling in catchment-lake systems and long-term ecological research.
Cristina Máguas
is a Professor of Center for Environmental Biology at the University of Lisbon. Her research interests include ecophysiology and isotopic ratio mass spectrometry, ecology, Mediterranean ecosystems, Lichenology, and Exotic invader plants.
Samson Sajidu
is an Associate Professor at the Department of Chemistry of the University of Malawi. His current research interests are in water/wastewater quality, particularly in development of low-cost methods of water pollution control.
Hendrik Schubert
is Professor at the University of Rostock. His research interests include ecology of brackish water ecosystems with a focus on macroalgae and phytoplankton; beside this, he works on biogeography and taxonomy of Charophytes.
Naoko Tokuchi
is a Professor of Field Science Education and Research Center at Kyoto University. Her research interests include nitrogen cycling in the forested ecosystem in a broad scale from soil microbe to ecosystem.
Jaroslav Záhora
is a Research Assistant at Mendel University in Brno. His research interests include soil microbial transformations of carbon and nitrogen.
Contributor Information
Hideaki Shibata, Phone: +81-11-706-2520, Email: shiba@fsc.hokudai.ac.jp.
Cristina Branquinho, Email: cmbranquinho@fc.ul.pt.
William H. McDowell, Email: bill.mcdowell@unh.edu
Myron J. Mitchell, Email: mitchell@syr.edu
Don T. Monteith, Email: donm@ceh.ac.uk
Jianwu Tang, Email: jtang@mbl.edu.
Lauri Arvola, Email: lauri.arvola@helsinki.fi.
Cristina Cruz, Email: ccruz@fc.ul.pt.
Daniela F. Cusack, Email: dcusack@geog.ucla.edu
Lubos Halada, Email: lubos.halada@savba.sk.
Jiří Kopáček, Email: jkopacek@hbu.cas.cz.
Cristina Máguas, Email: cmhanson@fc.ul.pt.
Samson Sajidu, Email: ssajidu@cc.ac.mw.
Hendrik Schubert, Email: hendrik.schubert@uni-rostock.de.
Naoko Tokuchi, Email: tokuchi@kais.kyoto-u.ac.jp.
Jaroslav Záhora, Email: zahora@mendelu.cz.
References
- Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, et al. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. BioScience. 1998;48:921–934. [Google Scholar]
- Aber JD, Ollinger SV, Driscoll CT, Likens GE, Holmes RT, Freuder RJ, Goodale CL. Inorganic nitrogen losses from a forested ecosystem in response to physical, chemical, biotic, and climatic perturbations. Ecosystems. 2002;5:648–658. [Google Scholar]
- Anderson CB, Celis-Diez JL, Bond BJ, Pastur GM, Little C, Armesto JJ, Ghersa C, Austin A, et al. Progress in creating a joint research agenda that allows networked long-term socio-ecological research in southern South America: Addressing crucial technological and human capacity gaps limiting its application in Chile and Argentina. Austral Ecology. 2012;37:529–536. [Google Scholar]
- Beier C, Emmett B, Gundersen P, Tietema A, Peñuelas J, Estiarte M, Gordon C, Gorissen A, et al. Novel approaches to study climate change effects on terrestrial ecosystems in the field: Drought and passive nighttime warming. Ecosystems. 2004;7:583–597. [Google Scholar]
- Bergström A, Jansson M. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Global Change Biology. 2006;12:635–643. [Google Scholar]
- Billen G, Thieu V, Garnier J, Silvestre M. Modelling the N cascade in regional watersheds: The case study of the Seine, Somme and Scheldt rivers. Agriculture, Ecosystems and Environments. 2009;133:234–246. [Google Scholar]
- Blenckner T, Adrian R, Livingstone DM, Jennings E, Weyhenmeyer GA, George DG, Jankowski T, Järvinen M, et al. Large-scale climatic signatures in lakes across Europe: A meta-analysis. Global Change Biology. 2007;13:1314–1326. [Google Scholar]
- Bobbink, R., and J.P. Hettelingh. 2011. Review and revision of empirical critical loads and dose-response relationships, Coordination Centre for Effects, National Institute for Public Health and the Environment (RIVM).
- Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecological Applications. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Boberg JB, Finlay RD, Stenlid J, Lindahl BD. Fungal C translocation restricts N-mineralization in heterogeneous environments. Functional Ecology. 2010;24:454–459. [Google Scholar]
- Bowman WD, Cleveland CC, Halada L, Hresko LJ, Baron JS. Negative impact of nitrogen deposition on soil buffering capacity. Nature Geoscience. 2008;1:767–770. [Google Scholar]
- Boyer EW, Howarth RW. The nitrogen cycle at regional to global scales. Dordrecht: Kluwer; 2002. [Google Scholar]
- Camarero L, Catalan J. Atmospheric phosphorus deposition may cause lakes to revert from phosphorus limitation back to nitrogen limitation. Nature Communications. 2012;3:1118. doi: 10.1038/ncomms2125. [DOI] [PubMed] [Google Scholar]
- Campbell JL, Mitchell MJ, Groffman PM, Christenson LM. Winter in northeastern North America: An often overlooked but critical period for ecological processes. Frontiers in Ecology and the Environment. 2005;3:314–322. [Google Scholar]
- Cape JN, Eerden LJVD, Sheppard LJ, Leith ID, Sutton MA. Evidence for changing the critical level for ammonia. Environmental Pollution. 2009;157:1033–1037. doi: 10.1016/j.envpol.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, et al. Trophic cascades, nutrients, and lake productivity: Whole-lake experiments. Ecological Monographs. 2001;71:163–186. [Google Scholar]
- Casson NJ, Eimers MC, Buttle JM. The Contribution of Rain-On-Snow Events to Nitrate Export in the Forested Landscape of South-Central Ontario, Canada. Hydrological Process. 2010;24:1985–1993. [Google Scholar]
- Chestnut TJ, McDowell WH. C and N dynamics in the riparian and hyporheic zones of a tropical stream, Luquillo mountains, Puerto Rico. Journal of the North American Benthological Society. 2000;19:199–214. [Google Scholar]
- Christopher SF, Shibata H, Ozawa M, Nakagawa Y, Mitchell MJ. The effect of soil freezing on N cycling: Comparison of two headwater subcatchments with different vegetation and snowpack conditions in the northern Hokkaido Island of Japan. Biogeochemistry. 2008;88:15–30. [Google Scholar]
- Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451:712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- Collins SL, Carpenter SR, Swinton SM, Orenstein DE, Childers DL, Gragson TL, Grimm NB, Grove JM, et al. An integrated conceptual framework for long-term social–ecological research. Frontiers in Ecology and the Environment. 2011;9:351–357. [Google Scholar]
- Compton JE, Harrison JA, Dennis RL, Greaver TL, Hill BH, Jordan SJ, Walker H, Campbell HV. Ecosystem services altered by human changes in the nitrogen cycle: A new perspective for US decision making. Ecological Letters. 2011;14:804–815. doi: 10.1111/j.1461-0248.2011.01631.x. [DOI] [PubMed] [Google Scholar]
- Curtis CJ, Heaton TEH, Simpson GL, Evans CD, Shilland J, Turner S. Dominance of biologically produced nitrate in upland waters of Great Britain indicated by stable isotopes. Biogeochemistry. 2012;111:535–554. [Google Scholar]
- Curtis, C.J., R.W. Battarbee, D.T. Monteith, and E.M. Shilland. 2014. The future of upland water ecosystems of the UK in the 21st century: A synthesis. Ecological Indicators 37(Part B): 412–430.
- Cusack DF, Silver WL, Torn MS, McDowell WH. Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry. 2011;104:203–225. [Google Scholar]
- Davidson EA. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nature Geoscience. 2009;2:659–662. [Google Scholar]
- Davidson EA, Chorover J, Dail DB. A mechanism of abiotic immobilization of nitrate in forest ecosystems: The ferrous wheel hypothesis. Global Change Biology. 2003;9:228–236. [Google Scholar]
- De Wit HA, Hindar A, Hole L. Winter climate affects long-term trends in stream water nitrate in acid-sensitive catchments in southern Norway. Hydrology and Earth System Sciences. 2008;12:393–403. [Google Scholar]
- Deegan LA, Johnson DS, Warren RS, Peterson BJ, Fleeger JW, Fagherazzi S, Wollheim WM. Coastal eutrophication as a driver of salt marsh loss. Nature. 2012;490:388–392. doi: 10.1038/nature11533. [DOI] [PubMed] [Google Scholar]
- Dias T, Malveiro S, Martins-Loução MA, Sheppard LJ, Cruz C. Linking increased N-driven biodiversity changes with soil N availability in a Mediterranean-Type Ecosystem. Plant and Soil. 2011;341:125–136. [Google Scholar]
- Dise N, Wright R. Nitrogen leaching from European forests in relation to nitrogen deposition. Forest Ecology and Management. 1995;71:153–161. [Google Scholar]
- Driscoll CT, Whitall D, Aber J, Boyer E, Castro M, Cronan C, Goodale CL, Groffman P, et al. Nitrogen Pollution in the Northeastern United States: Sources, Effects, and Management Options. BioScience. 2003;53:357–374. [Google Scholar]
- Driscoll CT, Lambert KF, Chapin FS, III, Nowak DJ, Spies TA, Swanson FJ, Kittredge DB, Hart CM. Science and society: The role of long-term studies in environmental stewardship. BioScience. 2012;62:354–366. [Google Scholar]
- Eimers MC, Buttle JM, Watmough SA. The contribution of rain-on-snow events to annual no3-n export from a forested catchment in south-central Ontario, Canada. Applied Geochemistry. 2007;22:1105–1110. [Google Scholar]
- Evans CD, Cooper DM, Monteith DT, Helliwell RC, Moldan F, Hall J, Rowe EC, Cosby BJ. Linking monitoring and modelling: Can long-term datasets be used more effectively as a basis for large-scale prediction? Biogeochemistry. 2010;101:211–227. [Google Scholar]
- Fang Y, Gundersen P, Vogt RD, Koba K, Chen F, Chen XY, Yoh M. Atmospheric deposition and leaching of nitrogen in Chinese forest ecosystems. Journal of Forest Research. 2011;16:341–350. [Google Scholar]
- Fernandez IJ, Adams MB, SanClements MD, Norton SA. Comparing decadal responses of whole-watershed manipulations at the Bear Brook and Fernow experiments. Environmental Monitoring and Assessment. 2010;171:149–161. doi: 10.1007/s10661-010-1524-2. [DOI] [PubMed] [Google Scholar]
- Fowler D, Smith R, Muller J, Cape JN, Sutton M, Erisman JW, Fagerli H. Long term trends in sulphur and nitrogen deposition in Europe and the cause of non-linearities. Water, Air, & Soil Pollution: Focus. 2007;7:41–47. [Google Scholar]
- Freppaz M, Ceili L, Marcheilli M, Zanini E. Snow removal and its influence on temperature and N dynamics in alpine soils (Vallée d’Aoste, northwest Italy) Journal of Plant Nutrition and Soil Science. 2008;171:672–680. [Google Scholar]
- Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. The nitrogen cascade. BioScience. 2003;53:341–356. [Google Scholar]
- Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, et al. Nitrogen Cycles: Past, Present and Future. Biogeochemistry. 2004;70:153–226. [Google Scholar]
- Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, et al. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- Gärdenäs AI, Ågren GI, Bird JA, Clarholm M, Hallin S, Ineson P, Kätterer T, Knicker H, Nilsson SI, et al. Knowledge gaps in soil carbon and nitrogen interactions – From molecular to global scale. Soil Biology & Biochemistry. 2011;43:702–717. [Google Scholar]
- George DG, Maberly SC, Hewitt DP. The influence of the North Atlantic Oscillation on the physical, chemical and biological characteristics of four lakes in the English Lake District. Freshwater Biology. 2004;49:760–774. [Google Scholar]
- Groffman P, Butterbach-Bahl K, Fulweiler RW, Gold AJ, Morse JL, Stander EK, Tague C, Tonitto C, et al. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry. 2009;92:49–77. [Google Scholar]
- Groffman PM, Rustad LE, Templer PH, Campbell JL, Christenson LM, Lany NK, Socci AM, Vadeboncoeur MA, et al. Long-term integrated studies show complex and surprising effects of climate change in the northern hardwood forest. BioScience. 2012;62:1056–1066. [Google Scholar]
- Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451:293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- Gundersen P, Emmett BA, Kjønaasc OJ, Koopmansd CJ, Tietemad A. Impact of nitrogen deposition on nitrogen cycling in forests: A synthesis of NITREX data. Forest Ecology and Management. 1998;101:37–55. [Google Scholar]
- Gupta SK, Gupta RC, Seth AK. Methaemoglobinaemia in areas with high nitrate concentration in drinking water. National Medical Journal of India. 2000;13:58–61. [PubMed] [Google Scholar]
- Harris TD, Wilhelm FM, Graham JL, Loftin KA. Experimental manipulation of TN:TP ratios suppress cyanobacterial biovolume and microcystin concentration in large-scale in situ microcosms. Lake Reserv Manag. 2014;30:72–83. [Google Scholar]
- Hedwall PO, Nordin A, Strengbom J, Brunet J, Olsson B. Does background nitrogen deposition affect the response of boreal vegetation to fertilization? Oecologia. 2013;173:615–624. doi: 10.1007/s00442-013-2638-3. [DOI] [PubMed] [Google Scholar]
- Hoben, J.P., R.J. Gehl, N. Millar, P.R. Grace, and G.P. Robertson. 2011. Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Global Change Biology 17: 1140–1152.
- Högberg MN, Chen Y, Högberg P. Gross nitrogen mineralisation and fungi-to-bacteria ratios are negatively correlated in boreal forests. Biology and Fertility of Soils. 2007;44:363–366. [Google Scholar]
- Högberg MN, Briones MJI, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, et al. Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytologist. 2010;187:485–493. doi: 10.1111/j.1469-8137.2010.03274.x. [DOI] [PubMed] [Google Scholar]
- Howarth RW, Marino R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnology and Oceanography. 2006;51:364–376. [Google Scholar]
- Howarth R, Swaney D, Billen G, Garnier J, Hong B, Humborg C, Johnes P, Mörth C-M, et al. Nitrogen fluxes from the landscape are controlled by net anthropogenic nitrogen inputs and by climate. Frontiers in Ecology and the Environment. 2011;10:37–43. [Google Scholar]
- IPCC. 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
- Jetten MS, Cirpus I, Kartal B, van Niftrik L, van de Pas-Schoonen KT, Sliekers O, Haaijer S, van der Star W, Schmid M, et al. 1994-2004: 10 years of research on the anaerobic oxidation of ammonium. Biochemical Society Transactions. 2005;33:119–123. doi: 10.1042/BST0330119. [DOI] [PubMed] [Google Scholar]
- Johansson O, Palmqvist K, Olofsson J. Nitrogen deposition drives lichen community changes through differential species responses. Glob Chang Biol. 2012;18:2626–2635. [Google Scholar]
- Jovan S, Riddell J, Padgett PE, Nash TH., III Eutrophic lichens respond to multiple forms of N: Implications for critical levels and critical loads research. Ecological Applications. 2012;22:1910–1922. doi: 10.1890/11-2075.1. [DOI] [PubMed] [Google Scholar]
- Kaste Ø, Austnes K, Vestgarden LS, Wright RF. Manipulation of snow in small headwater catchments at Storgama, Norway: Effects on leaching of inorganic nitrogen. AMBIO. 2008;37:29–37. doi: 10.1579/0044-7447(2008)37[29:mosish]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Knorr M, Frey SD, Curtis PS. Nitrogen additions and litter decomposition: A meta-analysis. Ecology. 2005;86:3252–3257. [Google Scholar]
- Kopáček, J., and M. Posch. 2011. Anthropogenic nitrogen emissions during the Holocene and their possible effects on remote ecosystems. Global Biogeochemical Cycles 25: GB2017. doi:10.1029/2010GB003779.
- Kopáček J, Hejzlar J, Posch M. Factors controlling the export of nitrogen from agricultural land in a large central European catchment during 1900−2010. Environmental Science and Technology. 2013;47:6400–6407. doi: 10.1021/es400181m. [DOI] [PubMed] [Google Scholar]
- Kopáček J, Hejzlar J, Posch M. Quantifying nitrogen leaching from diffuse agricultural and forest sources in a large heterogeneous catchment. Biogeochemistry. 2013;115:149–165. [Google Scholar]
- Kopáček J, Cosby BJ, Evans CD, Hruška J, Moldan F, Oulehle F, Šantrůčková H, Tahovská K, et al. Nitrogen, organic carbon and sulphur cycling in terrestrial ecosystems: Linking nitrogen saturation to carbon limitation of soil microbial processes. Biogeochemistry. 2013;115:33–51. [Google Scholar]
- Kurian LM, Lautz LK, Mitchell MJ. Winter hydrology and NO3− concentrations in a forested watershed: A detailed field study in the Adirondack Mountains of New York. Journal of the American Water Resources Association. 2012;49:264–283. [Google Scholar]
- Landberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovascular Research. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- Lassaletta L, Billen G, Grizzetti B, Garnier J, Leach AM, Galloway JN. Food and feed trade as a driver in the global nitrogen cycle: 50-year trends. Biogeochemistry. 2014;118:225–241. [Google Scholar]
- Leach AM, Galloway JN, Bleeker A, Erisman JW, Kohn R, Kitzes J. A nitrogen footprint model to help consumers understand their role in nitrogen losses to the environment. Environmental Development. 2012;1:40–66. [Google Scholar]
- Likens GE, Driscoll CT, Buso DC. Long-term effects of acid rain: Response and recovery of a forest ecosystem. Science. 1996;272:244–246. [Google Scholar]
- Löfgren S, Aastrup M, Bringmark L, Hultberg H, Lewin-Pihlblad L, Lundin L, Karlsson GP, Thunholm B. Recovery of Soil Water, Groundwater, and Streamwater from Acidification at the Swedish Integrated Monitoring Catchments. AMBIO. 2011;40:836–856. doi: 10.1007/s13280-011-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maberly SC, King L, Dent MM, Jones RI, Gibson CE. Nutrient limitation of phytoplankton and periphyton growth in upland lakes. Freshwater Biology. 2002;47:2136–2152. [Google Scholar]
- Makoto, K., T. Kajimoto, L. Koyama, G. Kudo, H. Shibata, Y. Yanai, and J.H.C. Cornelissen. 2013 Winter climate change in plant–soil systems: Summary of recent findings and future perspectives. Ecological Research (in press).
- Matson PA, McDowell WH, Townsend AR, Vitousek PM. The globalization of N deposition: Ecosystem consequences in tropical environments. Biogeochemistry. 1999;46:67–83. [Google Scholar]
- Millennium Ecosystem Assessment Board. 2005. Ecosystems and human well-being: Current state and trends, Vol. 1. Washington D.C.: Island Press.
- Mitchell MJ. Nitrate dynamics of forested watersheds: Spatial and temporal patterns in North America, Europe and Japan. Journal of Forest Research. 2011;16:333–340. [Google Scholar]
- Mitchell MJ, Driscoll CT, Kahl JS, Likens GE, Murdoch PS, Pardo LH. Climatic control of nitrate loss from forested watersheds in the northeast United States. Environmental Science and Technology. 1996;30:2609–2612. [Google Scholar]
- Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- Monteith DT, Evans CD, Reynolds B. Are temporal variations in the nitrate content of UK upland freshwaters linked to the North Atlantic Oscillation? Hydrological Processes. 2000;14:1745–1749. [Google Scholar]
- Monteith DT, Evans CD, Henrys PA, Simpson GL, Malcolm IA. Trends in the hydrochemistry of acid-sensitive surface waters in the UK 1988–2008. Ecological Indicators. 2014;37:287–303. [Google Scholar]
- Niu S, Wu M, Han Y, Xia J, Zhang Z, Yang H, Wan S. Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Global Chang Biol. 2010;16:144–155. [Google Scholar]
- Noe GB, Hupp CR. Carbon, nitrogen, and phosphorus accumulation in floodplains of Atlantic coastal plain rivers, USA. Ecological Applications. 2005;15:1178–1190. [Google Scholar]
- Ochoa-Hueso R, Allen EB, Branquinho C, Cruz C, Dias T, Fenn ME, Manrique E, Pérez-Corona ME, et al. Nitrogen deposition effects on Mediterranean-type ecosystems: An ecological assessment. Review. Environmental Pollution. 2011;159:2265–2279. doi: 10.1016/j.envpol.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Ohte N, Mitchell MJ, Shibata H, Tokuchi N, Toda H, Iwatsubo G. Comparative evaluation on nitrogen saturation of forest catchments in Japan and northeastern United States. Water, Air, and Soil pollution. 2001;131:649–654. [Google Scholar]
- Oulehle F, McDowell WH, Aitkenhead-Peterson JA, Krám P, Hruška J, Navrátil T, Buzek F, Fottová D. Long-Term Trends in Stream Nitrate Concentrations and Losses Across Watersheds Undergoing Recovery from Acidification in the Czech Republic. Ecosystems. 2008;11:410–425. [Google Scholar]
- Pardo LH, Fenn ME, Goodale CL, Geiser LH, Driscoll CT, Allen EB, Baron JS, Bobbink R, et al. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecological Applications. 2011;21:3049–3082. [Google Scholar]
- Park J, Mitchell MJ, McHale PJ, Christopher SF, Myers TP. Interactive effects of changing climate and atmospheric deposition on N and S biogeochemistry in a forested watershed of the Adirondack Mountains, New York State. Global Change Biology. 2003;9:1602–1619. [Google Scholar]
- Peñuelas J, Prieto P, Beier C, Cesaraccio C, De Angelis P, De Dato G, Emmett BA, Estiarte M, et al. Response of plant species richness and primary productivity in shrublands along a north–south gradient in Europe to seven years of experimental warming and drought: Reductions in primary productivity in the heat and drought year of 2003. Glob Chang Biol. 2007;13:2563–2581. [Google Scholar]
- Pereira HM, Ferrier S, Walters M, Geller GN, Jongman RHG, Scholes RJ, Bruford MW, Brummitt N, et al. Essential Biodiversity Variables. Science. 2013;339:277–278. doi: 10.1126/science.1229931. [DOI] [PubMed] [Google Scholar]
- Piatek KB, Mitchell MJ, Silva SR, Kendall C. Sources of nitrate in snowmelt discharge: Evidence from water chemistry and stable isotopes of nitrate. Water, Air, and Soil pollution. 2005;165:13–35. [Google Scholar]
- Pinho P, Dias T, Cruz C, Tang YS, Sutton MA, Martins-Loução M-A, Máguas C, Branquinho C. Using lichen functional-diversity to assess the effects of atmospheric ammonia in Mediterranean woodlands. Journal of Applied Ecology. 2011;48:1107–1116. [Google Scholar]
- Pinho P, Theobald MR, Dias T, Tang YS, Cruz C, Martins-Loucao MA, Maguas C, Sutton M, et al. Critical loads of nitrogen deposition and critical levels of atmospheric ammonia for semi-natural Mediterranean evergreen woodlands. Biogeosciences. 2012;9:1205–1215. [Google Scholar]
- Pregitzer KS, Burton AJ, Zak DR, Talhelm AF. Simulated chronic nitrogen deposition increases carbon storage in Northern Temperate forests. Global Change Biology. 2008;14:142–153. [Google Scholar]
- Rask M, Arvola L, Forsius M, Vuorenmaa J. Preface to the special issue “Integrated monitoring in the Valkea-Kotinen catchment during 1990–2009: Abiotic and biotic responses to changes in air pollution and climate. Boreal Environment Research. 2014;19:1–3. [Google Scholar]
- Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F, Crutzen PJ. Global agriculture and nitrous oxide emissions. Nature Climate Change. 2012;2:410–416. [Google Scholar]
- Rissanen A, Tiirola M, Ojala A. Spatial and temporal variation in denitrification and in the denitrifier community in a boreal lake. Aquatic Microbial Ecology. 2011;64:27–40. [Google Scholar]
- Robertson GP, Paul EA, Harwood RR. Greenhouse Gases in Intensive Agriculture: Contributions of Individual Gases to the Radiative Forcing of the Atmosphere. Science. 2000;289:1922–1925. doi: 10.1126/science.289.5486.1922. [DOI] [PubMed] [Google Scholar]
- Robertson GP, Collins SL, Foster DR, Brokaw N, Ducklow HW, Gragson TL, Gries C, Hamilton SK, et al. Long-Term Ecological Research in a Human-Dominated World. BioScience. 2012;62:342–353. [Google Scholar]
- Rogora M, Arese C, Balestrini R, Marchetto A. Climate control on sulphate and nitrate concentrations in alpine streams of Northern Italy along a nitrogen saturation gradient. Hydrology and Earth System Sciences. 2008;12:371–381. [Google Scholar]
- Sanchez P, Palm C, Sachs J, Denning G, Flor R, Harawa R, Jama B, Kiflemariam T, et al. The African millennium villages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16775–16780. doi: 10.1073/pnas.0700423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert H, Wasmund N. Das Phytoplankton des Strelasundes und des Kubitzer Boddens. Meer Museum. 2005;18:83–92. [Google Scholar]
- Schubert, H., N. Wasmund, and K. Sellner. 2010. Long-term investigations in brackish ecosystems. In Long-term ecological research, ed. F. Müller, C. Baessler, H. Schubert, and S. Klotz, 163–178: Dordrecht: Springer.
- Shaver GR, Johnson LC, Cades DH, Murray G, Laundre JA, Rastetter EB, Nadelhoffer KJ, Giblin AE. Biomass and CO2 flux in wet sedge tundras: Responses to nutrients, temperature, and light. Ecological Monographs. 1998;68:75–97. [Google Scholar]
- Shibata H, Sugawara O, Toyoshima H, Wondzell SM, Nakamura F, Kasahara T, Swanson FJ, Sasa K. Nitrogen dynamics in the hyporheic zone of a forested stream during a small storm, Hokkaido, Japan. Biogeochemistry. 2004;69:83–104. [Google Scholar]
- Shibata H, Hasegawa Y, Watanabe T, Fukuzawa K. Impact of snowpack decrease on net nitrogen mineralization and nitrification in forest soil of northern Japan. Biogeochemistry. 2013;116:69–82. [Google Scholar]
- Skiba A, Smith KA. The control of nitrous oxide emissions from agricultural and natural soils. Chemosphere—Global Change Science. 2000;2:379–386. [Google Scholar]
- Spannhake EW, Reddy SPM, Jacoby DB, Yu XY, Saatian B, Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environmental Health Perspectives. 2002;110:665–670. doi: 10.1289/ehp.02110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stal LJ, Zehr JP. Cyanobacterial nitrogen fixation in the ocean: Diversity, regulation and ecology. In: Herrero A, Flores E, editors. The cyanobacteria: Molecular biology, genomics and evolution. Caister: Academic Press; 2008. pp. 423–446. [Google Scholar]
- Stoddard JL. Long-term changes in watershed retention of nitrogen: Its causes and aquatic consequences. In: Bake LA, editor. Environmental chemistry of lakes and reservoirs. Washington, DC: American Chemical Society; 1994. pp. 223–284. [Google Scholar]
- Suddick, E.C., and E.A. Davidson. 2012. The Role of Nitrogen in Climate Change and the Impacts of Nitrogen-Climate Interactions on Terrestrial and Aquatic Ecosystems, Agriculture, and Human Health in the United States: A Technical Report Submitted to the US National Climate Assessment. North American Nitrogen Center of the International Nitrogen Initiative (NANC-INI), Woods Hole Research Center, 149 Woods Hole Road, Falmouth, MA.
- Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B. The European Nitrogen Assessment. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Thornton PE, Doney SC, Lindsay K, Moore JK, Mahowald N, Randerson JT, Fung I, Lamarque J-F, et al. Carbon-nitrogen interactions regulate climate-carbon cycle feedbacks: Results from an atmosphere-ocean general circulation model. Biogeosciences. 2009;6:2099–2120. [Google Scholar]
- Tierney GT, Fahey TJ, Groffman PM, Hardy JP, Fitzhugh RD, Driscoll CT. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry. 2001;56:175–190. [Google Scholar]
- Urakawa R, Toda H, Haibara K, Aiba Y. Long-term hydrochemical monitoring in an Oyasan Experimental Forest Watershed comprised of two small forested watersheds of Japanese cedar and Japanese cypress. Ecol Res (Data paper) 2012;27:245. [Google Scholar]
- Van Groenigen JW, Velthof GL, Oenema O, Van Groenigen KJ, Van Kessel C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. European Journal of Soil Science. 2010;61:903–913. [Google Scholar]
- Van Maanen JMS, Welle IJ, Hageman G, Dallinga JW, Mertens PL, Kleinjans JC. Nitrate contamination of drinking water: Relationship with HPRT variant frequency in lymphocyte DNA and urinary excretion of N- nitrosamines. Environmental Health Perspectives. 1996;104:522–528. doi: 10.1289/ehp.96104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaat, J.E., S. Broekx, B. Van Eck, G. Engelen, F. Hellmann, J.L. De Kok, H. Van der Kwast, J. Maes, W. Salomons, et al. 2012. Nitrogen source apportionment for the catchment, estuary, and adjacent coastal waters of the river Scheldt. Ecology and Society 17: 30. doi:10.5751/ES-04889-170230.
- Vitousek, P.M., R. Naylor, T. Crews, M.B. David, L.E. Drinkwater, E. Holland, P.J. Johnes, and J. Katzenberger. 2009. Nutrient imbalances in agricultural development. Science 324: 1519–1520. [DOI] [PubMed]
- Von Mutius E. Current review of allergy and immunology. Journal of Allergy and Clinical Immunology. 2000;105:9–19. doi: 10.1016/s0091-6749(00)90171-4. [DOI] [PubMed] [Google Scholar]
- Wallenstein MD, Myrold DD, Firestone M, Voytek M. Environmental controls on denitrifying communities and denitrification rates: Insights from molecular methods. Ecological Applications. 2006;16:2143–2152. doi: 10.1890/1051-0761(2006)016[2143:ecodca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ward MH, Mark SD, Cantor KP, Weisenburger DD, Correa-Villaseñor A, Zahm SH. Drinking water nitrate and the risk of non-Hodgkin’s lymphoma. Epidemiology. 1996;7:465–471. [PubMed] [Google Scholar]
- Weyer PJ, Cerhan J, Kross BC, Hallberg GR, Kantamneni J, Breuer G, Jones MP, Zheng W, et al. Municipal drinking water nitrate level and cancer risk in older women: The Iowa Women’s Health Study. Epidemiology. 2001;12:327–338. doi: 10.1097/00001648-200105000-00013. [DOI] [PubMed] [Google Scholar]
- Wipf S, Rixen C. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Research. 2010;29:95–109. [Google Scholar]
- Wrigit RF. Effect of increased carbon dioxide and temperature on runoff chemistry at a forested catchment in southern Norway (CLIMEX Project) Ecosystems. 1998;1:216–225. [Google Scholar]
- Zaehle S, Ciais P, Friend AD, Prieur V. Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nature Geoscience. 2011;4:601–605. [Google Scholar]