Abstract
Urban ecologists have demonstrated that cities are functioning ecosystems. It follows then that species living in these contexts should participate in and experience the same suite of biological processes, including evolution, that have occupied scientists for centuries in more “natural” contexts. In fact, urban ecosystems with myriad novel contexts, pressures, and species rosters provide unprecedentedly potent evolutionary stimuli. Here, we present the case for studying adaptive evolution in urban settings. We then review and synthesize techniques into a coherent approach for studying adaptive evolution in urban settings that combines observations of phenotypic divergence, measurements of fitness benefits of novel genetically based phenotypes, and experimental manipulations of potential drivers of adaptation. We believe that studying evolution in urban contexts can provide insights into fundamental evolutionary biology questions on rate, direction, and repeatability of evolution, and may inform species and ecosystem service conservation efforts.
Keywords: Anthropocene, Cities, Ecosystem services, Evolutionary ecology, Experimental evolution, Urbanization
Introduction
Humans are responsible for the fastest rates of evolution globally (Hendry and Kinnison 1999). Nowhere are humans more prevalent than in urban areas—we are in the “Century of the City” where urbanization is rapidly accelerating (Seto et al. 2010). It follows, then, that urban areas should provide unique macrocosms for testing evolutionary hypotheses in a rapidly changing world.
Furthermore, there is increasing concern about global change and the concomitant loss of biodiversity resulting in degradation of ecosystem function and services (MEA 2005). This is especially true in urban areas where many ecosystem services may be greatly diminished but are especially needed (Felson et al. 2013). However, scientists are increasingly realizing that species are capable of adapting to human effects on the landscape (e.g., industrial melanism in moths—Kettlewell 1955, battery plant metals and worms—Klerks and Levinton 1989; Levinton et al. 2003, sidewalk seed dispersal—Cheptou et al. 2008, chemical pollutants—Rasanen et al. 2003; Whitehead et al. 2010; Brady 2012; Cothran et al. 2013). This suggests that some species and communities may be more resilient to human land use than previously appreciated.
Science has progressed from studying ecology in cities, to studying the ecology of urban ecosystems, enabling us to now evaluate evolution because of urbanization. In this paper, we briefly reflect upon the insights gained over the last two decades in urban ecology as well as the scant but exciting evidence of evolutionary responses to urbanization. We then explore methods for future research programs in urban evolutionary ecology and how such studies can contribute to the study of urban ecosystems and more broadly to our understanding of evolution.
For the purposes of this piece, we define adaptive evolution as a change in the frequency of genetically based traits due to selective pressures that results in higher reproductive success (Stearns and Hoekstra 2001; Merila and Hendry 2014). Adaptive evolution takes into account adaptations both to the abiotic environment as well as to the context of its ecological community (Stearns and Hoekstra 2001). Measuring adaptive evolution in “natural” settings, biology’s historical modus operandi (Reznick et al. 1990; Grant and Grant 2002; Losos 2009), limits our capacity to understand species and community resilience in novel and rapidly changing environments. Scientists are being called upon to make predictions about future ecological communities and ecosystem functions (Schmitz 2010). In order to make these predictions, we need to better understand (1) the traits that enable species to adapt to changing, human-dominated landscapes, (2) factors influencing evolutionary rates, and (3) the repeatability of selection. In urban settings, where there is a diversity of potent drivers of selection (Fig. 1), we argue that biologists can gain the needed predictive insight into the evolutionary potential of the world’s biota.
Fig. 1.
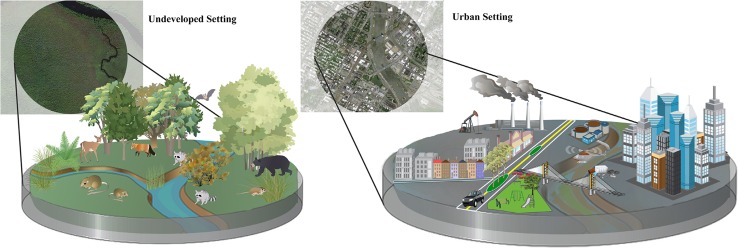
Urbanization results in diverse ecological deviations from adjacent natural settings. Hydrologic features exhibit altered flow regimes, nutrient cycling, and contaminant loading. Roads bring vehicle mortality, fossil fuel emissions, novel noise sources, and contaminants from road runoff. Trophic dynamics involve novel species interactions and exhibit reductions in taxonomic diversity and trophic levels. Vertical structure is composed of tall buildings made of glass, metal, and concrete rather than natural vegetation or geological features. Horizontal structure is a more fragmented mosaic of small patches of landscaped vegetation, roads, residential neighborhoods, and economic centers. Photoperiods are no longer strictly solar-regulated but are influenced by electrical lighting
Here, we refrain from explicitly describing the mechanics of adaptive evolution in favor of more detailed treatments elsewhere (see for examples Lande and Arnold 1983; Stearns and Hoekstra 2001; Stockwell et al. 2003; Cheptou et al. 2008). Instead, we argue for urban settings as a new macrocosm for the application of these insights. Interest in species adaptation to human influences, including climate change, is growing (e.g., Merila and Hendry 2014). With only a handful of relevant studies, we believe that the question of whether species can evolve in the face of urbanization has been largely unexplored. Studying evolution in an urban context has the potential both to inform basic evolutionary theory as well as deepen our understanding of species adaptability to a rapidly changing world. Such an undertaking should lead to a new landscape design paradigm informed by human needs, ecological function, and adaptive potential of species (sensu Felson et al. 2013). Scientists need to understand the dynamics of adaptive evolution in human-dominated contexts to make predictions about the fate of ecological systems. That research should be done in cities.
Ecology in cities
Much of urban ecology has focused on patterns of biodiversity against an urban backdrop. These studies suggest that, along an urban to rural gradient, vertebrate and invertebrate diversities peak at low levels of urbanization, while plant diversity does so at intermediate levels of urbanization (McKinney 2008). Urban diversity research has also found that environmental factors (e.g., Walsh et al. 2005; Clarke et al. 2008) and socioeconomic factors (Kinzig et al. 2005; Loss et al. 2009) influence species richness within a city. Additional lines of research have focused on the relative contributions of native and exotic species to urban biodiversity (e.g., Burghardt et al. 2008).
Complementing urban diversity research, numerous studies have expanded our knowledge of the ecology of species living in cities. For example, urban ecologists have studied novel behaviors (Slabbekoorn and Peet 2003; Rees et al. 2009), physiologies (Partecke et al. 2006; Angilletta et al. 2007), habitat-use differences between native and invasive species (Lambert et al. 2013), and changed trophic regimes in cities (Schochat 2004; Faeth et al. 2005).
In sum, this work demonstrates that cities are ecosystems, in which many organisms interact and thrive. While this brief list represents a limited cross section of research arising from the study of urban ecology, these examples illustrate the range of ecological insights gained from research in cities. Species have unique, but fully realized ecologies in urban settings, which have led to a shift in research to the ecology of cities.
Ecology of cities
As in any ecosystem, urban systems are characterized by their species assemblages as well as their chemical and physical environments, the components of which interact to govern dynamic processes such as nutrient cycling and energy flows (Schmitz 2010). However, cities are characterized by unique climates, atmospheric chemistry, hydrologic processes, soil chemistry, nutrient dynamics, and biotic communities, all of which are mutually reinforcing (Alberti et al. 2003; Walsh et al. 2005; Grimm et al. 2008). Because of humans, the dynamics of these interconnections are at times altered relative to parallel processes in less disturbed settings (Schochat et al. 2006). This has led to the realization that urban ecosystems function because of human participation, not in spite of people (Alberti et al. 2003; Grimm et al. 2008). Humans govern urban ecosystem structure and processes by introducing myriad deviations from natural systems (Fig. 1). For instance, “Urban Stream Syndrome” occurs when widespread impervious surfaces and sewer infrastructure fundamentally change the flow regimes and chemical loading of local watersheds; these urban fluvial typologies yield altered nutrient cycling and species communities (Walsh et al. 2005). Similarly, aspects of city infrastructure such as building color, street orientation, and tree planting can dramatically impact the urban climate (Watkins et al. 2007). Humans design the urban environment and govern many of its ecological processes, thereby influencing the manner in which organisms interact with the urban ecosystem. Whether species are able to adapt to these never-before-seen contexts, thrusting them into novel evolutionary trajectories, remains to be tested.
Evolution because of urbanization
To our knowledge, there are few studies over the last quarter-century, which demonstrate strong evidence of adaptive evolution in urban systems. Here, we briefly review six papers which begin to address adaptation in the urban environment. We use these papers to illustrate the methods available for investigating adaptation in urban settings and discuss the inference possible given the methods used.
Evolution has been investigated in urban birds. For example, in San Diego California, Yeh demonstrated that the extent of white tail coloration, a sexually selected trait of juncos (Junco hyemalis), is rapidly evolving relative to nearby mountain populations (Yeh 2004). While this is an example of sexual selection rather than adaptive natural selection, Yeh’s calculations show that urban populations are evolving rapidly relative to non-urban populations. If tests on whether that trait provides an adaptive advantage in urban settings have been conducted, then they have not yet been published. In Munich, European blackbird (Turdus merula) males exhibit reduced migratory behavior, a genetically based trait difference (Partecke and Gwinner 2007). There is also evidence for repeated selection on the SERT gene, which regulates anxiety behavior (e.g., harm avoidance), in European blackbirds across a suite of cities (including Madrid, Berlin, and Prague; Mueller et al. 2013). New York City mice also show genetic divergences at loci important for traits like immune response, metabolism, and spermatogenesis (Harris et al. 2013). While the latter three studies have shown a genetic basis for a trait change in urban settings, none have determined whether these traits are indeed adaptive (i.e., if there is a reproductive or fitness benefit).
In the weed Crepis sancta, urban sidewalk populations have been shown to have a higher proportion of non-dispersing seeds than nearby non-fragmented, rural population (Cheptou et al. 2008). A common garden experiment indicated that seed type (i.e., dispersing or non-dispersing) was genetically based, and another experiment indicated that urban paved surfaces are likely the drivers of non-dispersing seeds (Cheptou et al. 2008).
A final study that has demonstrated adaptive trait changes in urban populations focused on killifish (Fundulus heterclitus). In a common garden study on the effects of polychlorinated biphenyl (PCB) pollution, researchers found that killifish from a minimally developed site had reduced mortality, lower rates of developmental abnormalities, and significant differences in their functional transcriptomes (notably with genes related to cardiac toxicity) when compared to urban killifish living with three orders of magnitude more PCB pollution (Whitehead et al. 2010).
Urbanization appears to have mixed effects on gene flow, population genetic differentiation, and genetic diversity across vertebrate taxa (Hitchings and Beebee 1998; Noel et al. 2007; Bjorklund et al. 2010; Munshi-South and Kharchenko 2010; Munshi-South 2012; Munshi-South and Pehek 2013).The manner in which urbanization alters population genetic processes undoubtedly influences the capacity of populations to adapt to urban ecosystems. Urban population genetics itself is a burgeoning field and will likely yield interesting results for understanding the adaptive capacity of organisms to urbanization.
Only two of the studies highlighted in this section demonstrated the heritability or fitness relevance and driver of an observed trait difference across urban and non-urban settings (Cheptou et al. 2008; Whitehead et al. 2010). We argue that all three of these components must be explicitly addressed to demonstrate adaptive evolution in urban settings. In other words, testing for urban evolution requires a research paradigm that measures phenotypic changes in urban species, establishes the genetic basis as well as fitness benefits of an urban phenotype, and experimentally identifies which drivers induce these adaptations.
Detecting adaptive evolution in urban settings
Experimental techniques for detecting evolution in natural and laboratory settings are well established (Garland and Rose 2009) and are highly applicable to evolutionary hypotheses in human-modified settings. Accidental experiments due to humans can, in cases, enable otherwise impossible or unethical experiments on landscape scales with the potential for broad insight into evolutionary dynamics (Endler 1986). Already, important insights into evolutionary dynamics have been gained from agriculture (Cothran et al. 2013), introduced species (Stearns 1983), pollution (Rasanen et al. 2003), and climate change (Merila and Hendry 2014). Urban settings provide “accidental” experimental macrocosms with potential for studying the ability of organisms to adapt to intense human land use. While adaptive evolution is the interest here, it will be important for researchers to distinguish between differences that may be due to other process such as phenotypic plasticity (West-Eberhard 1989) or genetic drift (Stearns and Hoekstra 2001).
We recommend a three-tiered program for testing evolutionary hypotheses in urban settings. Initially, traits that vary with ecological context (e.g., urban or rural) should be identified with survey techniques. Next, to differentiate between phenotypic plasticity and selection, the genetic basis of those traits can then be tested using common garden experiments or reciprocal translocations yielding insight into the adaptive potential of the traits. Finally, experimental manipulation can be used to directly identify drivers of those trait differences. As of yet, this full research progression has not been employed in urban settings but has provided considerable insight in several non-human contexts (e.g., Guppies in Trinidad, Reznick et al. 1990; Anolis lizards in the Caribbean, Losos 2009). Here, we will elaborate on the application of this progression in urban ecosystems.
Phenotypic trait changes
Urban systems are characterized by gradients of potential adaptive drivers (e.g., thermal, food, noise, vertical structure, chemical; Fig. 1). Therefore, a first approach to detecting evolution within an urban setting is measuring trait changes across gradients both within and across urban settings. Continuous gradients can provide useful insight into potential evolutionary trajectories, for example, when tracking gene flow across urban settings (Munshi-South 2012). Categorical comparisons (urban, suburban, rural), on the other hand, can provide similarly useful insight into the context-dependence of traits (e.g., Angilletta et al. 2007; Rees et al. 2009). Understanding such phenotypic trait changes has been an important component of urban ecology, and building off of those conceptual and empirical foundations will similarly be necessary for urban evolutionary studies. We re-emphasize, though, that just because a phenotype varies as a function of urbanization does not necessarily indicate that trait variation is due to adaptation.
Trait differences with a genetic basis
One method of testing whether trait changes are genetically based (a necessary criterion for demonstrating adaptation; Merila and Hendry 2014) is using common garden experiments (e.g., Partecke and Gwinner 2007). These experiments take individuals from different source populations and rear them from birth or hatching in the same environment (i.e., a common garden). If a trait of interest varies between individuals from different demes reared in the same environment, the trait is assumed to be genetically based. It is important to note that F1 individuals used in common garden experiments may still be subject to environmental or maternal effects from their previous context. Using F2 individuals can ameliorate this caveat and provide more rigorous inference on adaptation. More complex common garden experimental designs can also simultaneously test multiple environmental factors, providing insight into trait plasticity without having to necessarily perform reciprocal transplant experiments (discussed below). While common garden experiments can indicate whether a trait is genetically linked or plastic, these tests do not necessarily determine if a trait incurs a fitness benefit—whether it is adaptive.
Genetically based traits with a fitness outcome
Theory predicts that urban populations should differ from natural populations genetically due to founders effects, genetic bottlenecks, and drift (Evans et al. 2009), as well as barriers to gene flow (Munshi-South 2012). Just because populations show genetic structure within cities and between cities and rural counterparts (Bjorklund et al. 2010; Munshi-South 2012) does not necessarily mean that the biota is adapting to the urban environment. Testing whether population genetic differences and corresponding trait differences confer a genetically based fitness advantage in novel urban settings, though, has great potential for advancing our understanding of evolution and context-dependent selection (Brady 2012)
A variation on the common garden experiment which tests for both genetically based trait changes as well as fitness is the reciprocal transplant. Such reciprocal translocations of demes are reliable manipulative experimental techniques for measuring local adaptation (Blanquart et al. 2013). The degree of local adaptation is equal to the difference between the fitness of populations on their home site and fitness of that population when moved to another site (Blanquart et al. 2013). Transplanting individuals between urban and non-urban settings, or along an urbanization gradient, can be an effective tool to test whether adaptation is occurring. Full reciprocal transplant experiments may very well be complicated by logistical and ethical concerns in urban settings, and so common garden experiments may suffice depending on the context and questions.
In some cases, common garden and reciprocal transplant experiments may not convincingly inform whether observed trait differences have a genetic basis—the foundation for testing adaptation (Merila and Hendry 2014). For example, maternal effects (Danchin et al. 2008) or epigenetics (e.g., Skinner 2011) can cause heritable changes in traits without actually resulting in a change in allele frequencies in polymorphic loci under selection. Extending a common garden experiment through the F2 generation can mitigate some of these issues; however, mapping the genes associated with a trait in addition to changes in allele frequency in response to environmental context enables far stronger inference (Merila and Hendry 2014). Genomic scanning identifies genes under positive selection by associating variation in fitness-relevant traits with genome-wide DNA polymorphisms (Storz 2005). This technique is particularly attractive, because it does not require a priori assessment of putative phenotypic traits under selection nor does it require known pedigrees or laborious experimental crosses (Storz 2005). Nonetheless, genomic scanning may be, for many projects, prohibitively challenging (Leinonen et al. 2013), and so other methodologies, such as QST–FST comparisons, are commonly used to investigate the genetic basis of adaptive traits. QST–FST comparisons contrast variation in quantitative, genetically based traits with variation in neutral genetic markers both within and among populations (Leinonen et al. 2013). While useful for differentiating between processes such as adaptation and drift, the requirements for making rigorous QST–FST comparisons can be daunting. Proper QST determination requires common garden conditions to understand the genetic underpinnings of the traits of interest, and careful selection of neutral molecular markers is imperative for determining FST (Leinonen et al. 2013). Alongside carefully constructed common garden experiments and reciprocal transplants focused on phenotypic responses, genomic scanning and QST–FST will provide exciting and useful insight toward advancing understanding of how various taxa adapt to urbanization.
Drivers of adaptation
Measuring traits across gradients can highlight trait variability in urban settings while experiments can test for the genetic basis and fitness consequences of trait differences. A final step, then, is determining drivers of the observed evolution: which aspects of the urban environment are species actually adapting to? This necessitates further manipulative experiments and may ultimately be the most challenging experimental type in the urban context.
In studies measuring evolution in natural populations, this step often involves testing field-observation-based predictions in laboratory settings to examine hypothesized mechanisms (e.g., leg length in Anolis—Losos et al. 1997). This method is equally applicable to urban settings. While this enables direct testing of the impacts of an urban feature on a trait, controlled experiments often necessarily limit the milieu of interactions that may be affecting trait variation. In designing experiments, it will be useful to acknowledge that multiple drivers in concert may yield unexpected dynamics.
An ideal test, when conditions allow, would control for the presence/absence of one focal driver (e.g., light, sound, or roads) within the contextual milieu. Latitude to do so in urban settings though will vary considerably with questions and focal species. An alternative method might be incorporated in a common garden experiment involving the experimental creation of an urban evolutionary driver. For example, such a test might involve building experimental human infrastructure in landscapes that are otherwise void of that disturbance. Stocking such experimental venues with individuals of a species from populations that have and populations that have not experienced human disturbance could yield insight into whether trait variation between source populations results in performance differences on the urban infrastructure. Another method would involve a reciprocal transplant that capitalizes on structures characterizing urban settings but which are also found external to the urban environment (e.g., roads; Brady 2012). While Brady (2012) was not examining urban evolution per se, his reciprocal transplant of spotted salamanders (Ambystoma maculatum) to and from ponds within forests and adjacent to roadsides indicated local adaptation to the road environment. Identifying drivers of urban adaptation, while challenging, is valuable for predicting which aspects of urbanization species can evolutionarily cope with.
Thus, as we have illustrated, a comprehensive research program that evaluates adaptive hypotheses in urban settings includes identifying changing traits, measuring their fitness importance and genetic basis, and ideally, identifying the drivers of their changes. These methods are certainly not unique to study adaptation to urbanization, but employing this agenda will lead to fruitful understanding of species’ adaptive capacity to increasingly human-dominated landscapes. Parallel utilization of this programme across species and between cities will provide useful insight needed for predicting ecological and evolutionary dynamics in human-dominated landscapes (Fig. 2).
Fig. 2.
Urban ecosystems both in close proximity or disparate regions around the globe show many environmental similarities (Groffman et al. 2014). Because of this, cities can serve as replicate macrocosms for urban evolution studies (sensu Mueller et al. 2013). Examining adaptive evolution across different urban settings can provide new insight into the repeatability and predictability of evolution
What Can We Learn about Evolution from Cities?
Here, we discuss just three of the many questions that research in urban settings can inform. First, can species adapt to human-modified landscapes, and if so, which traits most commonly experience selective pressure? Second, what is the rate of change of those traits? Third, does evolution repeat itself within species between urban settings? While these three questions have been productively explored in many “natural” ecological contexts, the urban petri dish offers contemporaneous, hypothetically potent selective pressures that will deepen our understanding of these questions, made more pressing with the increasing priority on conserving species and ecosystems dramatically influenced by humans.
Do species adapt to urbanized landscapes and how?
Modern urban processes are unprecedented in magnitude, rate, and dynamism (Seto et al. 2010) and rigorously determining if species can adapt to urbanization is paramount. We have outlined a plethora of potential drivers (Fig. 1) that urban residents must cope with (potentially through adaptation) but the necessary work to demonstrate adaptive trait changes has yet to be done. This is a challenging undertaking as we predict that there are multiple interacting urban effects that species adapt to. As should be clear from our arguments up to this point though, we believe there is good cause to hypothesize that the answer to this question is “yes, species can adapt to urbanization.” We call for and list the means by which to test that hypothesis.
How fast can evolution occur in human-modified landscapes?
The fastest measured rates of evolution are often associated with humans (Hendry and Kinnison 1999) but have rarely been measured relative to urbanization. In one of the few examples, Yeh (2004) calculated rates of evolution in an urban bird population commensurate with rapid evolution in Trinidadian Guppies and Galapagos Finches (Hendry and Kinnison 1999). Because urban systems often contain phylogenetically similar biological communities (McKinney 2008; Knapp et al. 2012) and ecologically similar landscapes (Groffman et al. 2014), cities of different ages can provide valuable insight into the rate at which species can adapt to urbanization. Furthermore, as modern urbanization is intensifying at rates greatly outpacing historical precedent (Seto et al. 2010), we can track species adaptation to land use change in real time. In a similar vein, cities with different structures (relatively open cities like Chicago or Saint Louis contrasted with more contiguous urbanization in Boston and Denver) provide valuable arenas for studying how urban form influences a species’ adaptive capacity. There is evidence that such vegetated areas can act as important conduits for gene flow within the urban matrix (Munshi-South 2012), but the consequences of urban landscape design on the rate, direction, and magnitude of adaptation are unknown.
The realization that evolution can occur at ecologically relevant (i.e., short) time scales (Hairston et al. 2005) led to exciting predictions about feedbacks between evolution and ecology (Post and Palkovacs 2009). Whether these eco-evolutionary feedbacks are important to the dynamics of urban ecosystems is a completely unexplored question. Theory predicts, and numerous empirical studies demonstrate, that changes in traits can have large cascading effects on ecological dynamics, with resultant effects on the evolutionary trajectory of a species (Post and Palkovacs 2009; Schmitz 2010; Palkovacs et al. 2011). Yet whether human domination of these ecological dynamics heightens or dampens these feedbacks is unknown. A greater understanding of evolutionary rates in human contexts, the potential feedbacks between trait changes and ecological dynamics, and the conditions (e.g., temperature, chemicals, vertical structure, etc.; Fig. 1) that dictate the magnitude and direction of those changes, will valuably inform conservation and management decisions with a goal of facilitating species adaptation to human-modified landscapes.
Does evolution repeat itself? Ergo, is it predictable?
Our capacity to predict adaptation to human stressors is closely tied to the question of evolution’s repeatability. Grant and Grant (2002) found that while trait changes can be anticipated in the short term, contingency and environmental stochasticity prevent predictions of longer-term evolutionary trajectories. Losos et al. (1997), on the other hand, found the opposite to be true; Anolis lizard ecomorphs in the Caribbean have repeatedly evolved predictable phenotypes. Urban settings lend themselves to further explore this question as many species are common across multiple cities (McKinney 2008), and experience similar, measurable suites of adaptive stressors (Groffman et al. 2014). If urbanization does truly lead to homogenization of ecological characteristics and biota, then we should expect repeated selection on the same species. One of the few studies capitalizing on this approach is Mueller et al. (2013), which found repeated selection on a behavioral gene in European Blackbirds across 11 cities in Europe. While this study did not provide any adaptive insight from this repeated selection, it did indicate that European urbanization can repeatedly select for a particular genotype. Urban areas thus provide useful replicates (Fig. 2) for inter-city comparisons of urban-adapted morphological, physiological, and phenological traits. Urban systems therefore may be useful arenas for testing whether evolution is repeatable.
Although not discussed here at length, phenotypic plasticity can be an adaptive trait (West-Eberhard 1989) that enables species to exist in urbanized landscapes. While plasticity represents an adaptation for a species at large, a plastic trait response, even if it repeatedly arises in populations across multiple cities, does not show that species has adapted to its context. It is important for researchers to distinguish plasticity from adaptation when exploring contemporary urban evolution. Indeed, studying replicated adaptation to urbanization is challenging and will likely result in logistical challenges associated with demonstrating natural selection recurring in similar environments. QST–FST comparisons can make inferences about natural selection on a trait without necessarily having to conduct unwieldy experiments with multiple populations. It stands, though, that urban ecosystems present a unique opportunity to study whether adaptation to similar environmental features can be repeatable.
Conservation implications
Understanding evolution on ecological time scales could be of extraordinary value to conservation (Stockwell et al. 2003). Nowhere is this truer than in the most human-dominated areas. Furthermore, we are realizing that conservation in human-dominated landscapes is increasingly important both for environmental as well as social endeavors (Kareiva and Marvier 2012). Typically, urbanization is associated with the loss of biodiversity (McKinney 2008; Groffman et al. 2014), as well as the general loss of ecosystem function (e.g., Walsh et al. 2005). However, if species can adapt to the urban environment, there is a possibility for mitigation of species loss and reclamation of services impaired by diminished biodiversity and ecosystem function. Urban planners are calling on ecologists to help design urban environments to better achieve conservation goals (Felson et al. 2013). Research that elucidates which aspects of urbanization species are able or unable to adapt to, as well as the timescales needed for adaptation, could provide valuable information for urban planners to facilitate species adaptations. Similarly, understanding drivers of urban evolution as well as the likelihood of successful adaptation could allow conservationists better predictive power for understanding a species’ ability to adapt to novel human-modified environments elsewhere on the globe. Evolutionary ecology can be a valuable tool in predicting and maintaining species adaptive capacities in an increasingly urbanized world.
Conclusions
Humans are changing global landscapes intensely and rapidly, with pressing consequences for biodiversity and ecosystem function. Whether species can rapidly adapt to, and persist within, these anthropogenic contexts remain an open question. Studying adaptive capacities in natural settings provides limited insight into this question. It is in settings where selection is strong due to intense anthropogenic change that we can best test the adaptive potential of species. Nowhere is human land use more intense than in cities. In addition, urban gradients as well as exurban and periurban suburbs may also yield valuable insights into species adaptation to human land use. Since the late 1990s when the National Science Foundation (NSF) funded Baltimore, Maryland, and Phoenix, Arizona as urban long-term ecological research sites (LTER), there has been a formal recognition that cities needed to be monitored as functioning ecosystems with interconnected social, biological, and geophysical functions. We have highlighted a body of evidence that cities act as ecosystems, and that species inhabiting urbanized landscapes exhibit novel ecologies. We suggest that it follows logically that species may adapt to urbanization.
We assert that studies of evolution in urban settings are sorely needed and present a formal research outline that would provide the needed insights. These studies should first be informed by observations of trait differences between ecological contexts. The heritable basis and fitness contribution of these traits should be identified through common garden or reciprocal transplant experiments testing for local adaptation. Finally, when possible, manipulative experiments should be performed, isolating hypothesized drivers of evolution to determine causality. This idealized research programme will yield many of the insights needed for understanding if species adapt to urbanization and the extent to which we can predict future evolution in response to anthropogenic endeavors.
By studying contemporary evolution in urban ecosystems, biologists can gain insights into if and how species can adapt to extraordinarily novel environments. Humanity has and will continue to alter the face of the world, ushering in an unprecedented era (the Anthropocene) shaped not just by the urban environment but by rapid climate change, farms of wind turbines and solar panels, intensely modified hydrology, new industrial techniques, and a plethora of additional unforeseeable alterations. A deeper understanding of whether organisms can adapt to novel human contexts could allow us to predict whether species will be able to adapt to these various environmental futures. These insights will enable scientists to begin making better-informed conservation and management decisions in anthropogenic environments.
By studying adaptive evolution to the urban environment, we can better understand evolutionary processes that were difficult, or impossible, to assess previously in “natural” settings. In this way, we will understand how urbanization influences a fundamental process of biology—evolution—and what that means for urban ecosystems.
Acknowledgments
We thank the School of Forestry and Environmental Studies for financial support and colleagues for informal discussions that influences our thinking. C. Stockwell and an anonymous reviewer made valuable contributions to the final draft.
Biographies
Colin M. Donihue
is a doctoral candidate at the Yale School of Forestry and Environmental Studies. His research focuses on how human land use affects ecological community dynamics and shapes the selective landscape in which they evolve.
Max R. Lambert
is a doctoral student at Yale University in the School of Forestry and Environmental Studies. His research interests include evolution, ecology, and developmental biology of vertebrates in urbanized ecosystems.
Footnotes
Authors contributed equally to text and are listed alphabetically.
References
- Alberti M, Marzluff JM, Schulenberger E, Bradley G, Ryan C, Zumbrunnen C. Integrating humans into ecology: Opportunities and challenges for studying urban ecosystems. BioScience. 2003;53:1169–1179. doi: 10.1641/0006-3568(2003)053[1169:IHIEOA]2.0.CO;2. [DOI] [Google Scholar]
- Angilletta MJ, Jr, Wilson RS, Niehaus AC, Sears MW, Navas CA, Ribeiro PL. Urban physiology: Urban ants possess high heat tolerance. PLoS One. 2007;2(2):e258. doi: 10.1371/journal.pone.0000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund M, Ruiz I, Senar JC. Genetic differentiation in the urban habitat: The great tits (Parus major) of the parks of Barcelona city. Biological Journal of the Linnean Society. 2010;99:9–19. doi: 10.1111/j.1095-8312.2009.01335.x. [DOI] [Google Scholar]
- Blanquart F, Kaltz O, Nuismer SL, Gandon S. A practical guide to measuring local adaptation. Ecology Letters. 2013;16:1195–1205. doi: 10.1111/ele.12150. [DOI] [PubMed] [Google Scholar]
- Brady SP. Road to evolution? Local adaptation to road adjacency in an amphibian (Ambystoma maculatum) Scientific Reports. 2012;2:235. doi: 10.1038/srep00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt KT, Tallamy DW, Shriver WG. Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conservation Biology. 2008;23:219–224. doi: 10.1111/j.1523-1739.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Carrue O, Rouifed S, Cantarel A. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proceedings of the National Academy of Sciences. 2008;105:3796–3799. doi: 10.1073/pnas.0708446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke KM, Fisher BL, LeBuhn G. The influence of urban park characteristics on ant (Hymenoptera, Formicidae) communities. Urban Ecosystems. 2008;11:317–334. doi: 10.1007/s11252-008-0065-8. [DOI] [Google Scholar]
- Cothran RD, Brown JM, Relyea RA. Proximity to agriculture is correlated with pesticide tolerance: Evidence for the evolution of amphibian resistance to modern pesticides. Evolutionary Applications. 2013;6:832–841. doi: 10.1111/eva.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin E, Giraldeau LA, Cezilly F. Behavioural ecology. New York: Oxford University Press Inc.; 2008. [Google Scholar]
- Endler JA. Natural selection in the wild. Princeton: Princeton University Press; 1986. [Google Scholar]
- Evans KL, Gaston KJ, Frantz AC, Simeoni M, Sharp SP, McGowan A, Dawson DA, Walasz K, et al. Independent colonization of multiple urban centres by a formerly forest specialist bird species. Proceedings of the Royal Society B. 2009;276:2403–2410. doi: 10.1098/rspb.2008.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeth SH, Warren PS, Shochat E, Marussich WA. Trophic dynamics in urban communities. BioScience. 2005;55:399–407. doi: 10.1641/0006-3568(2005)055[0399:TDIUC]2.0.CO;2. [DOI] [Google Scholar]
- Felson AJ, Oldfield EE, Bradford MA. Involving ecologists in shaping large-scale green infrastructure projects. BioScience. 2013;63:882–890. doi: 10.1525/bio.2013.63.11.7. [DOI] [Google Scholar]
- Garland T, Jr, Rose MR. Experimental evolution. Berkeley: University of California Press; 2009. [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin’s finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. Global change and the ecology of cities. Science. 2008;319:756–760. doi: 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- Groffman PM, Cavender-Bares J, Bettez ND, Grove JM, Hall SJ, Heffernan JB, Hobbie SE, Larson K, et al. Ecological homogenization of urban USA. Frontiers in Ecology and the Environment. 2014;12:74–81. doi: 10.1890/120374. [DOI] [Google Scholar]
- Hairston NG, Jr, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecology Letters. 2005;8:1114–1127. doi: 10.1111/j.1461-0248.2005.00812.x. [DOI] [Google Scholar]
- Harris SE, Munshi-South J, Obergfell C, O’Neill R. Signatures of rapid evolution in urban and rural transcriptomes of white-footed mice (Peromyscus leucopus) in the New York metropolitan area. PLoS One. 2013;8:e74938. doi: 10.1371/journal.pone.0074938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT. The pace of modern life: Measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.2307/2640428. [DOI] [PubMed] [Google Scholar]
- Hitchings SP, Beebee TJC. Loss of genetic diversity and fitness in common toad (Bufo bufo) populations isolated by inimical habitat. Journal of Evolutionary Biology. 1998;11:269–283. [Google Scholar]
- Kareiva P, Marvier M. What is conservation science? BioScience. 2012;62:962–969. doi: 10.1525/bio.2012.62.11.5. [DOI] [Google Scholar]
- Kettlewell HBD. Selection experiments on industrial melanism in the Lepidoptera. Heredity. 1955;9:323–342. doi: 10.1038/hdy.1955.36. [DOI] [Google Scholar]
- Kinzig AP, Warren P, Martin C, Hope D, Katti M. The effects of human socioeconomic status and cultural characteristics on urban patterns of biodiversity. Ecology and Society. 2005;10:23. [Google Scholar]
- Klerks PL, Levinton JS. Rapid evolution of metal resistance in a benthic oligochaete inhabiting a metal-polluted site. Biological Bulletin. 1989;176:135–141. doi: 10.2307/1541580. [DOI] [Google Scholar]
- Knapp S, Dinsmore L, Fissore C, Hobbie SE, Jakobsdottir I, Kattge J, King JY, Klotz S, et al. Phylogenetic and functional characteristics of household yard floras and their changes along an urbanization gradient. Ecology. 2012;93:S83–S98. doi: 10.1890/11-0392.1. [DOI] [Google Scholar]
- Lambert MR, Nielsen SN, Wright AN, Thomson RC, Shaffer HB. Habitat features determine the basking distribution of introduced red-eared sliders and native western pond turtles. Chelonian Conservation and Biology. 2013;12:192–199. doi: 10.2744/CCB-1010.1. [DOI] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.2307/2408842. [DOI] [PubMed] [Google Scholar]
- Leinonen T, Scott McCairns RJ, O’Hara RB, Merila J. QST–FST comparisons: Evolutionary and ecological insights from genomic heterogeneity. Nature Reviews Genetics. 2013;14:179–190. doi: 10.1038/nrg3395. [DOI] [PubMed] [Google Scholar]
- Levinton JS, Suatoni E, Wallace W, Junkins R, Kelaher B, Allen BJ. Rapid loss of genetically based resistance to metals after the cleanup of a Superfund site. Proceedings of the National Academy of Sciences. 2003;100:9889–9891. doi: 10.1073/pnas.1731446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB. Lizards in an evolutionary tree. Ecology and adaptive radiation of Anoles. Berkeley: University of California Press; 2009. [Google Scholar]
- Losos JB, Warheit KI, Schoener TW. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature. 1997;387:70–73. doi: 10.1038/387070a0. [DOI] [Google Scholar]
- Loss SR, Ruiz MO, Brawn JD. Relationships between avian diversity, neighborhood age, income, and environmental characteristics of an urban landscape. Biology Conservation. 2009;142:2578–2585. doi: 10.1016/j.biocon.2009.06.004. [DOI] [Google Scholar]
- McKinney ML. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosystems. 2008;11:161–176. doi: 10.1007/s11252-007-0045-4. [DOI] [Google Scholar]
- Merila J, Hendry AP. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. 2014. Evolutionary Applications. 2014;7:1–14. doi: 10.1111/eva.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment (MEA) Ecosystems and human well-being: Synthesis. Washington, DC: World Resources Institute; 2005. [Google Scholar]
- Mueller JC, Partecke J, Hatchewell BJ, Gaston KJ, Evans KL. Candidate gene polymorphisms for behavioural adaptations during urbanization in blackbirds. Molecular Ecology. 2013;22:3629–3637. doi: 10.1111/mec.12288. [DOI] [PubMed] [Google Scholar]
- Munshi-South J. Urban landscape genetics: canopy cover predicts gene flow between white-footed mouse (Peromyscus leucopus) populations in New York City. Molecular Ecology. 2012;21:1360–1378. doi: 10.1111/j.1365-294X.2012.05476.x. [DOI] [PubMed] [Google Scholar]
- Munshi-South J, Kharchenko K. Rapid, pervasive genetic differentiation or urban white-footed mouse (Peromyscus leucopus) populations in New York City. Molecular Ecology. 2010;19:4242–4254. doi: 10.1111/j.1365-294X.2010.04816.x. [DOI] [PubMed] [Google Scholar]
- Munshi-South J, Zak Y, Pehek E. Conservation genetics of extremely isolated urban populations of the northern dusky salamander (Desmognathus fuscus) in New York City. Peer J. 2013;1:e64. doi: 10.7717/peerj.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel S, Ouellet M, Galois P, Lapointe FJ. Impact of urban fragmentation on the genetic structure of the eastern red-backed salamander. Conservation Genetics. 2007;8:599–606. doi: 10.1007/s10592-006-9202-1. [DOI] [Google Scholar]
- Palkovacs EP, Wasserman BA, Kinnison MT. Eco-evolutionary trophic dynamics: Loss of top predators drives trophic evolution and ecology of prey. PLoS One. 2011;6(4):e18879. doi: 10.1371/journal.pone.0018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partecke J, Gwinner E. Increased sedentariness in European blackbirds following urbanization: A consequence of local adaptation? Ecology. 2007;88:882–890. doi: 10.1890/06-1105. [DOI] [PubMed] [Google Scholar]
- Partecke J, Schwabl I, Gwinner E. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology. 2006;87:1945–1952. doi: 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Post DM, Palkovacs EP. Eco-evolutionary feedbacks in community and ecosystem ecology: Interactions between the ecological theatre and the evolutionary play. Philosophical Transactions of the Royal Society B. 2009;364:1629–1640. doi: 10.1098/rstb.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasanen KR, Laurila A, Merila J. Geographic variation in acid stress tolerance of the moor frog, Rana arvalis. I. Local adaptation. Evolution. 2003;57:352–362. doi: 10.1554/0014-3820(2003)057[0352:GVIAST]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rees M, Roe JH, Georges A. Life in the suburbs: behavior: behavior and survival of a freshwater turtle in response to drought and urbanization. Biological Conservation. 2009;142:3172–3181. doi: 10.1016/j.biocon.2009.08.019. [DOI] [Google Scholar]
- Reznick DA, Bryga H, Endler JA. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. doi: 10.1038/346357a0. [DOI] [Google Scholar]
- Schmitz OJ. Resolving ecosystem complexity. Princeton: Princeton University Press; 2010. [Google Scholar]
- Schochat E. Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos. 2004;106:622–626. doi: 10.1111/j.0030-1299.2004.13159.x. [DOI] [Google Scholar]
- Schochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends in Ecology & Evolution. 2006;21:186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Seto KC, Sanchez-Rodriguez R, Fragkias M. The new geography of contemporary urbanization and the environment. Annual Review of Environmental Resources. 2010;35:167–194. doi: 10.1146/annurev-environ-100809-125336. [DOI] [Google Scholar]
- Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. [DOI] [PubMed] [Google Scholar]
- Stearns SC. A natural experiment in life-history evolution: Field data on the introduction of mosquitofish (Gambusia affinis) to Hawaii. Nature. 1983;37:601–617. doi: 10.1111/j.1558-5646.1983.tb05577.x. [DOI] [PubMed] [Google Scholar]
- Stearns SC, Hoekstra RF. Evolution: an introduction. New York: Oxford University Press; 2001. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. doi: 10.1016/S0169-5347(02)00044-7. [DOI] [Google Scholar]
- Storz JF. Using genome scans of DNA polymorphism to infer adaptive population divergence. Molecular Ecology. 2005;14:671–688. doi: 10.1111/j.1365-294X.2005.02437.x. [DOI] [PubMed] [Google Scholar]
- Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP., II The urban stream syndrome: Current knowledge and the search for a cure. Journal of the North American Benthological Society. 2005;24:706–723. doi: 10.1899/04-028.1. [DOI] [Google Scholar]
- Watkins R, Palmer J, Kolokotroni M. Increased temperature and intensification of the urban heat island: Implications for human comfort and urban design. Built Environment. 2007;33:85–96. doi: 10.2148/benv.33.1.85. [DOI] [Google Scholar]
- West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Annual Review of Ecology and Systematics. 1989;20:249–278. doi: 10.1146/annurev.es.20.110189.001341. [DOI] [Google Scholar]
- Whitehead A, Triant DA, Champlin D, Nacci D. Comparative transcriptomics implicates mechanisms of evolved pollution tolerance in a killifish population. Molecular Ecology. 2010;19:5186–5203. doi: 10.1111/j.1365-294X.2010.04829.x. [DOI] [PubMed] [Google Scholar]
- Yeh PJ. Rapid evolution in a sexually selected trait following population establishment in a novel habitat. Evolution. 2004;58:166–174. doi: 10.1111/j.0014-3820.2004.tb01583.x. [DOI] [PubMed] [Google Scholar]