Abstract
Background
The capacity to control or regulate one’s emotions, cognitions and behavior is central to competent functioning, with limitations in these abilities associated with developmental problems. Parenting appears to influence such self-regulation. Here the differential-susceptibility hypothesis is tested that the more putative ‘plasticity alleles’ adolescents carry, the more positively and negatively influenced they will be by, respectively, supportive and unsupportive parenting.
Methods
One thousand, five hundred and eighty-six (1586) adolescents (n = 754 males; n = 832 females) enrolled in the American Add Health project were scored in terms of how many of 5 putative ‘plasticity alleles’ they carried – the 10R allele of DAT1, the A1 allele of DRD2, the 7R allele of DRD4, the short allele of 5HTTLPR, and the 2R/3R alleles of MAOA. Then the effect of the resultant index (ranging from 0 to 5) of cumulative-genetic plasticity in moderating effects of parenting on adolescent self-regulation was evaluated.
Results
Consistent with differential susceptibility, the more plasticity alleles males (but not females) carried, the more and less self-regulation they manifested under, respectively, supportive and unsupportive parenting conditions.
Conclusion
Adolescent males appear to vary for genetic reasons in their susceptibility to parenting vis-à-vis self-regulation, perhaps due to epistatic and/or epigenetic processes. G×E research may benefit from compositing candidate genes. To afford comparative evaluation of differential-susceptibility vs. diathesis-stress models of environmental action, future G×E work should focus on positive as well as negative environmental conditions and developmental outcomes.
Keywords: Self-control, self-regulation, plasticity, G×E, parenting
The capacity to control or regulate one’s emotions, cognitions and behavior is central to competent functioning (Gottfredson & Hirschi, 1990; Vazsonyi & Huang, 2010). Extensive research on executive function, the brain processes that regulate cognition and action, as well as on the regulation of feelings and behavior, shows that limitations in these capacities are related to disturbances in development and functioning (Pratt & Cullen, 2000). The fact that the frontal cortex, implicated in the development of executive function, continues to develop during the adolescent years clearly suggests that experiences during the second decade of life could influence self-control (Blakemore & Choudhury, 2006). This is why the research reported herein focuses upon adolescents. Even though developmentalists often use the terms self-regulation and self-control interchangeably (Eisenberg, Champion, & Ma, 2004; Vazsonyi & Huang, 2010), for the present report we have chosen the term self-regulation.
Parenting is one well-studied source of influence on self-regulation (Cullen, Unnever, Wright, & Beaver, 2008; Lengua, Honorado, & Bush, 2007). Children and adolescents who experience warm, supportive and sensitive, even if demanding, care prove better able to attend to and concentrate on tasks, regulate their emotions under challenging circumstances and engage in goal-directed behavior than those who experience unresponsive, hostile and/or disengaged/neglectful parenting (Eisenberg et al., 2005; Maccoby, 2000; Rothbaum & Weisz, 1994). Recent research on gene×environment (G×E) interaction highlights the very real possibility, however, that some children are differentially affected by their rearing experiences (Caspi et al., 2002; Kim-Cohen et al., 2006).
The present work examining G×E interaction involving effects of maternal parenting on adolescent self-regulation diverges from most prior G×E work in two fundamental respects. The first involves the conceptual framework guiding the research. Whereas most G×E work is based on the diathesis-stress view that some individuals are more vulnerable than others to the negative effects of contextual adversity (Zuckerman, 1999; Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pluess, 2009a, b), saying nothing about responsiveness to positive environmental experiences, the research reported herein is based on the differential-susceptibility hypothesis (Belsky, 1997, 2005). This stipulates that not only are certain individuals, often for genetic reasons (Belsky & Pluess, 2009a, b; Obradović & Boyce, 2009), more prone to function poorly (e.g., become depressed) when confronted with stressful conditions (e.g., negative life events), but that the very same putatively ‘vulnerable’ individuals are also those most likely to benefit from supportive ones (Boyce & Ellis, 2005).
Just to clarify a potential source of confusion, the terminology of functioning poorly or well used in this report reflects prevailing values and norms in Western societies. Thus, low self-regulation reflects problematic functioning, whereas high self-regulation reflects competent functioning. It should be appreciated, nevertheless, especially within an evolutionary framework, that in some contexts what is regarded as limited self-regulation could be functional and adaptive and that, more generally, what may reflect adaptive behavior in one context may not do so in another (Belsky, 2007).
Recent reviews of G×E findings consistent with the differential-susceptibility hypothesis (Belsky et al., 2009; Belsky & Pluess, 2009b) underscored the need for additional research that afforded comparative evaluation of diathesis-stress vs. differential-susceptibility models of environmental action. Considered essential are assessment strategies that did not just treat the absence of contextual stress (e.g., not abused) as the positive pole of the environmental continuum being measured and the absence of a psychological disorder (e.g., not depressed) as the positive pole of a continuum of psychological/behavioral functioning. Thus, rather than just determining, for example, whether a child is harshly treated or not or whether an individual suffers from depression or not, G×E studies capable of distinguishing models of environmental action must measure indisputably supportive environmental conditions (e.g., positive parenting) and truly competent functioning (e.g., self-regulation). For this very reason, in the work presented herein a composite measure of parenting ranging from very positive and supportive to very negative/disengaged and unsupportive is used to predict a composite index of self-regulation ranging from very limited to very competent.
Virtually all G×E work to date has examined, for theoretically sensible reasons, the moderating effect of single genes on some environmental factor (Burmeister, McInnis, & Zollner, 2008). This is typically based on a well-specified theory of gene action stipulating how particular genes influence particular neurotransmitters and thus particular phenotypes (Caspi & Moffitt, 2006). In this work we adopt a somewhat different strategy given evidence that multiple genes operate as ‘plasticity genes’ in some G×E research, not just ‘vulnerability genes’ (Belsky et al., 2009), specifically the 10R allele of DAT1 (Laucht et al., 2007), the A1 allele of DRD2 (Berman & Noble, 1997), the 7R allele of DRD4 (Pluess, Belsky, & Neuman, 2009), the short allele of 5HTTLPR (Taylor et al., 2006), and the 2R/3R alleles of MAOA (Widom & Brzustowicz, 2006). In at least some G×E studies, though certainly not all of them, individuals carrying these particular alleles seem to be affected by a variety of environmental factors in a ‘for-betterand-for-worse’ manner (Belsky et al., 2007), having their functioning both disproportionately undermined and enhanced by, respectively, unsupportive and supportive environmental conditions relative to those not carrying those alleles (Belsky & Pluess, 2009b). Indeed, the latter individuals appear much less and sometimes not at all affected by the very same environmental factors under investigation.
Consider in this regard the following illustrative findings involving the genes which are the focus of the present research. In the case of the 10-repeat allele of DAT1, Laucht et al. (2007) found that German 15-year olds from a high-risk community sample carrying this allele manifest, in comparison all other genotypes, the most and least inattention when living under conditions of high and low psychosocial adversity, respectively. In the case of DRD2, Mills-Koonce and associates (2007) observed that infants with the A1 allele reared by more and less sensitive mothers had, respectively, fewer and more affective problems at three years of age than agemates with other genotypes. In the case of DRD4, Bakermans-Kranenburg and van IJzendoorn (2006) found that maternal sensitivity observed when children were 10 months predicted externalizing problems more than two years later, but only for children carrying the 7-repeat DRD4 allele. Although children with the 7-repeat DRD4 allele displayed, consistent with a diathesis-stress model, the most externalizing behavior of all children when mothers were judged insensitive, they also manifested the least externalizing behavior when mothers were highly sensitive. In the case of 5HTTPLR, Taylor and associates (2006) reported that young adults homozygous for short alleles (s/s) manifested more depressive symptoms than individuals with other allelic variants when exposed to early adversity (i.e., problematic childrearing history), as well as many recent negative life events, yet the fewest symptoms when they experienced a supportive early environment or recent positive experiences. Finally, in the case of MAOA, Kim-Cohen et al. (2006) observed that 7-year-old boys with the low-MAOA-activity variant had more mental health problems – and specifically ADHD symptoms – if they had been victims of abuse, but fewer problems if they had not, compared to boys with the high-MAOA-activity genotype. For an extensive review of studies showing such differential-susceptibility-like G×E effects which makes clear their strengths and weaknesses, see Belsky and Pluess (2009b).
Before proceeding to stipulate further the non-traditional approach to examining G×E interaction in this investigation, some comments are in order regarding the notion of ‘plasticity’ and thus ‘plasticity genes’. Plasticity is a heterogeneous concept, and one used in many of the life sciences. Neuroscientists often make reference to plasticity when referring to physical changes associated with experience, including changes in brain structure (Draganski et al., 2004), such as the pruning of neurons or the branching of dendrites, to cite but two examples. But plasticity also has long been used by developmentalists to refer to experience-induced changes in cognitive, emotional and/or behavioral development (Bornstein, 1989), something that evolutionary biologists studying animals and plants conceptualize as phenotypic plasticity (DeWitt & Scheiner, 2004). Plasticity has been used to refer, as well, to effects of environmental experiences on gene expression in epigenetic research (Cameron et al., 2005).
The use of the term plasticity in the current context derives from Belsky and associates’ (2009; Belsky & Pluess, 2009a) distinction between ‘vulnerability genes’ and ‘plasticity genes’ vis-à-vis behavioral phenotypes. The former notion is used widely in psychiatric genetic work to refer to genetic (risk) factors that affect whether or not an individual’s functioning is compromised, often in terms of developing psychopathology, by adverse environmental conditions such as child maltreatment (Rutter, 2006). As some G×E interaction work also indicates that such genes operate (in still-ill-defined ways) to make some individuals especially susceptible (or not) to both positive and negative contextual conditions, Belsky et al. (2009) proposed re-conceptualizing such genes as ‘plasticity genes’. It is thus with regard to certain genes being associated with both positive and negative changes in behavioral phenotypes in response to supportive and unsupportive environments, respectively, that the term plasticity – rather than vulnerability – is used herein.
As already noted, in most G×E work to date, one genetic polymorphism at a time has typically been studied (Burmeister et al., 2008). Rather than adopting this approach in the current inquiry and testing for a variety of singular G×E interactions which might illuminate genetic conditions under which parenting proves related to self-regulation in adolescence, we rely on a composite measure of ‘cumulative genetic plasticity’. This, then, is the second way referred to above in which this inquiry diverges from most G×E studies to date, although it converges with recent work by several investigatory teams that have created composite measures of diverse alleles to directly predict phenotypic outcomes, based on the view that individual genes have very small effects (de Quervain & Papassotiropoulos, 2006; Harlaar et al., 2005). But rather than generating a summary index of genetic risk as Beaver and associates (Beaver, Sak, Vaske, & Nilsson, 2010) did when predicting antisocial phenotypes, our cumulative-genetic-plasticity index reflects the number of putative plasticity alleles in total that an adolescent carries of the set of five delineated above. This affords testing the hypothesis that the more plasticity genes an individual carries, the stronger the parenting effect being evaluated will prove to be. Were this a priori prediction supported, it would be consistent with Sonuga-Barke et al.’s (2009) recent post-hoc demonstration that combining two genes, each of which was individually involved in a significant G×E interaction, provided even stronger evidence of genes moderating environmental effects.
Reliance on a composite measure of cumulative genetic plasticity like the one described raises the very real possibility that should findings prove consistent with the hypothesis being tested, it would likely reflect, at least in part, epistatis, or gene–gene interaction, not just gene–environment interaction. It is well appreciated that such multi-gene action is involved in many phenotypes (Carlborg & Haley, 2004; Cordell, 2002). Here we do not focus explicitly on epistatis, even if it plays a role in accounting for findings to be presented, basically because of limitations of statistical power and the desire to avoid an empirical fishing expedition. After all, with five candidate genes, not only could 32 possible 2-way interactions be tested, but so could many additional 3-, 4-, and 5-way interactions! Without strong theory regarding which of the numerous possible epistatic effects to target, exploratory analysis without a replication sample would be unwise.
Despite the selection of genes included in this inquiry being determined by evidence indicating that they operate as plasticity genes in some studies (Belsky & Pluess, 2009b), it is noteworthy that all are involved in the functioning of the dopaminergic and/or serotonergic systems. This raises the possibility that one reason they may collectively function in the way that they appear to – making some individuals more susceptible to both positive and negative environmental influences than others – is because they influence sensitivity to (a) pleasure and thus rewards or (b) displeasure and thus punishments (Bakermans-Kranenburg & van IJzendoorn, 2009; Belsky & Pluess, 2009b). This could certainly help to explain how they might contribute to some individuals being more and some less susceptible to parenting effects.
Methods
Data
Data for this study come from the National Longitudinal Study of Adolescent Health (Add Health). Detailed information about the sample and the sampling design has been published elsewhere (Harris et al., 2003; Resnick et al., 1997). Briefly, the Add Health is a four-wave, prospective, and nationally representative sample of American youth. Participants were selected through the use of a multistage stratified random sampling procedure. The initial sampling frame consisted of 26,666 public and private high schools with an eleventh grade and with an enrollment of at least 30 students. Of these schools, 132 were chosen for inclusion in the study. On a specified school day during the 1994–1995 academic year, the wave-1 in-school survey was administered to approximately 90,000 students. To gather more detailed information on a sub-sample, follow-up wave-1, in-home interviews were completed with 20,745 adolescents. Approximately one and a half years later, wave-2 surveys were administered to 14,738 adolescents who participated in the earlier in-home assessments. Nearly seven years after wave-1 data were collected, wave-3 data collection commenced when most of the participants were 18–26 years old. Finally, wave-4 surveys were administered in 2007–2008 to 15,701 respondents. Across all waves, a wide range of phenotypic measurements were collected on diverse topics (e.g., family functioning, own behavior and psychological well-being). Informed consent was secured from all participating youths at each wave of data collection.
Embedded within the Add Health data is a sample of sibling-pairs. During wave-1 interviews, respondents were asked to indicate whether they were living with a co-twin or a sibling who had the same biological parents. If they responded affirmatively, then their co-twin or sibling was randomly selected to be included in the sample. This sampling procedure netted 5,470 siblings. During wave-3 interviews, participants who were part of the sibling-pairs sample were asked to submit buccal cells to be genotyped. After providing informed consent, 2,612 participants submitted usable DNA samples. In line with previous research using the Add Health data (Haberstick et al., 2005), one twin from each monozygotic twin pair was randomly excluded from the analysis to provide conservative parameter estimates. After missing cases were removed via listwise deletion techniques, the final analytic sample was N = 1586 (n = 754 males; n = 832 females).
Measures
Parenting quality
Wave-1 measures of maternal involvement, disengagement and attachment used in other Add-Health studies are used here (Beaver, Ratchford, & Ferguson, 2009). Maternal involvement reflects the degree to which the mother was involved over the past month in 10 different aspects of the child’s life (e.g., playing a sport, shopping, going to a movie). The number of endorsed items was the maternal involvement score (Cronbach’s α = .50). Maternal disengagement was based on five questions tapping lack of maternal engagement with respect to expressions of warmth and love, frequency of talking and overall quality of the relationship (Cronbach’s α = .82). Finally, maternal attachment was measured using two questions concerning how close adolescents felt to their mothers and how much they thought their mothers cared about them. Summed responses yielded a maternal attachment score (Cronbach’s α = .64). Principal components factor analysis with varimax rotation revealed that all three scores were accounted for by a unitary factor. Following Beaver et al. (2009), after the disengagement scale was reverse-coded, scores were transformed into a weighted parenting-quality factor score.
Self-regulation
Respondents and their mothers were asked 23 questions at wave-1 interviews pertaining to the adolescents’ self-regulation of attention, feelings and behavior. For example, adolescents were asked about difficulty paying attention at school, use of a systematic method of decision making and whether difficult problems make them upset, while mothers were asked about the child’s temper and trustworthiness. Results of a principal components analysis with varimax rotation, along with inspection of the scree plot, revealed that a single factor could account for all 23 items, while confirmatory factor analysis indicated that all factor loadings were statistically significant. A composite measure of self-regulation was created by summing all items (Cronbach’s α = .76). Prior research shows that this scale has predictive validity (Beaver et al., 2009).
Cumulative genetic plasticity
Genotyping was conducted at the University of Colorado’s Institute for Behavioral Genetics, using previously established methods (Harris, Halpern, Smolen, & Haberstick, 2006). DAT1, DRD2, DRD4, 5HTTLPR, and MAOA were used in the current study because extant research has identified alleles of these polymorphisms as being associated with plasticity (Belsky et al., 2009). Specifically, the 10R allele of DAT1, the A1 allele of DRD2, the 7R allele of DRD4, the short allele of 5HTTLPR, and the 2R/3R alleles of MAOA were identified as the plasticity alleles. Each polymorphism was assigned a point if at least one putative plasticity allele was present and then these values were summed together to create a cumulative index. Because MAOA was included, the index was created separately for males and females. The 0 and 1 allele categories were collapsed together for males and females due to low frequencies, with the same being true for 4 and 5 allele categories for males. The distribution of 0 or 1, 2, 3, and 4–5 cumulative plasticity alleles for males was, respectively, 7.4% (n = 56), 33.0% (n = 249), 37.9% (n = 286), and 21.6% (n = 163). For 0 or 1, 2, 3, 4 and 5 plasticity alleles, the distribution for females was, respectively, 5.6% (n = 47), 25.6% (n = 213), 37.0% (n = 308), 25.6% (n = 213), and 6.1% (n = 51). Overall, genotyping yielded a success rate of approximately 99% or higher.
Results
The analysis for this study began by testing for gene–environment correlation between the cumulative-genetic-plasticity index and parenting quality. Correlational analysis revealed no significant bivariate association between the genetic plasticity index and parenting quality for either males (r = −.04, p = .26) or females (r = −.04, p = .26), thereby indicating that any discerned G×E interaction would not simply reflect G:E correlation and thus a possible evocative effect of genetic plasticity on parenting. Respondent’s race (0 = white, non-Hispanic; 1 = African American) was, however, significantly associated with parenting quality (r = .08, p < .05), cumulative-genetic plasticity (r = .08, p < .05), and self-regulation (r = .13, p < .01) for males, and with genetic plasticity (r = .10, p < .01) and self-regulation (r = .08, p < .05) for females. Therefore, all analyses reported were corrected for race.
Using ordinary least squares (OLS) regressions, the main effects and multiplicative interaction of cumulative genetic plasticity and parenting quality were evaluated in predicting self-regulation, separately for males and females. To account for clustering of observations, models were estimated using Huber/White standard errors. Table 1 shows that for males there were no significant main effects. Consistent with expectations, however, the two-way interaction proved significant (and positive) (β = .30, p < .01). For females, only the main effect of parenting quality proved significant (β = .51, p < .01).
Table 1.
Gene–environment interactions between cumulative genetic plasticity and parenting quality in the prediction of self-regulation by gender
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| b | Beta | SE | b | Beta | SE | ||
| Genetic plasticity × parenting quality | .95* | .30 | .41 | −.25 | −.11 | .25 | |
| Genetic plasticity | −.18 | −.01 | .30 | .00 | .00 | .26 | |
| Parenting quality | −.13 | −.01 | 1.13 | 3.78* | .51 | .84 | |
| Race | 2.24* | .12 | .74 | 1.38 | .07 | .61 | |
| N | 754 | 832 | |||||
Note: models estimated using Huber/White standard errors;
p < .01, two-tailed tests.
Follow-up analyses probed the male two-way interaction. Table 2 displays the simple slopes for each of the cumulative-genetic-plasticity subgroups. Notably and consistent with expectations, slopes for parenting quality increase monotonically in magnitude when moving from the 0 or 1 plasticity allele group all the way through the 4 or 5 allele group. Not only do males with the most plasticity alleles appear most affected by the quality of care they experience – and in a for-better-and-for-worse manner – but those with the fewest such alleles seem virtually unaffected by maternal parenting, irrespective of its quality. The right-hand side of the table presents the results of z-tests examining whether the regression slopes are significantly different from each other. These indicate that the slope for the 4-or-plasticity-allele group was significantly different from the slopes for the 3-allele group (z = 2.23, p < .05), the 2-allele group (z = 2.38, p < .05), and the 0-or-1-allele group (z = 2.64, p < .05). Slopes for all of the other subgroup comparisons were not significantly different from each other.
Table 2.
Post-hoc analysis of the gene–environment interaction between cumulative genetic plasticity and parenting quality for males
| Simple slope | Comparison group for Z-test for equality in regression slopes | |||
|---|---|---|---|---|
| Parenting qualitya | 0 or 1 plasticity alleles | 2 plasticity alleles | 3 plasticity alleles | |
| 0 or 1 plasticity alleles | −.40 | – | – | – |
| 2 plasticity alleles | 1.99* | 1.53 | – | – |
| 3 plasticity alleles | 2.46* | 1.79 | .61 | – |
| 4 or 5 plasticity alleles | 3.82* | 2.64* | 2.38* | 2.23* |
p < .05, two-tailed tests;
corrected for race.
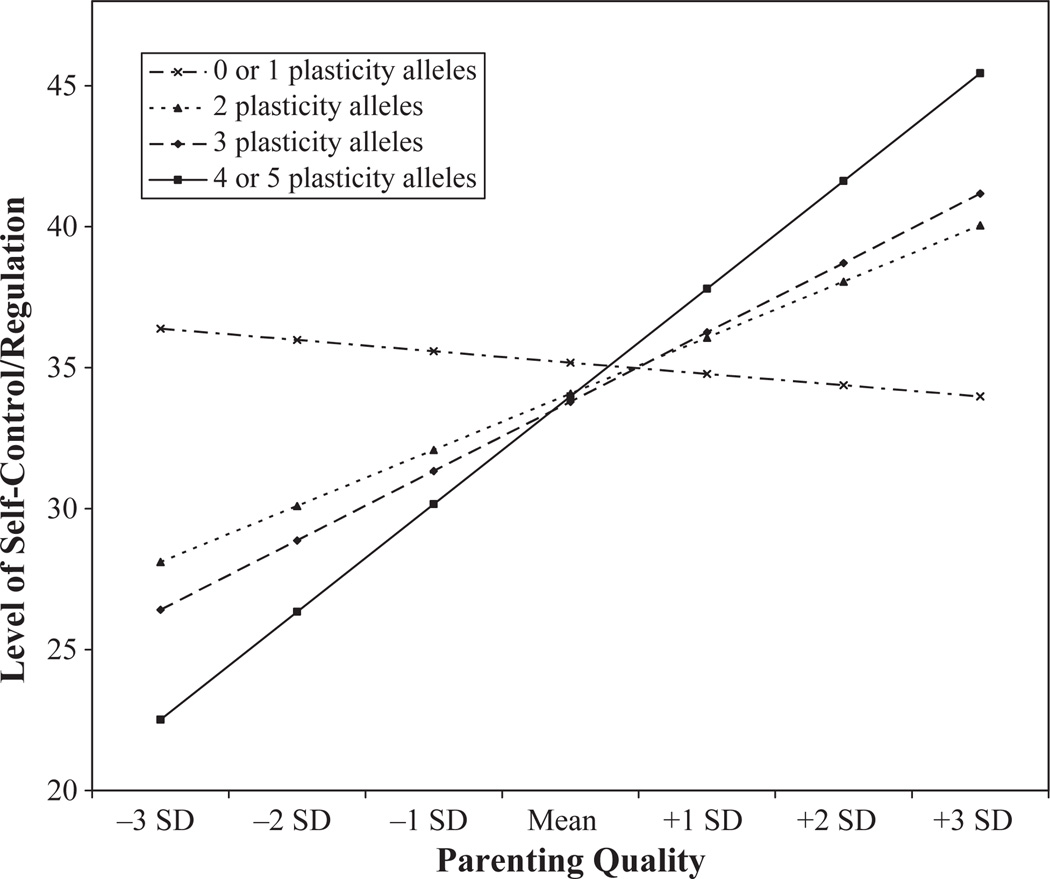
Finally, the slopes for each of the plasticity-allele subgroups were plotted across different values on the parenting quality scale. As Figure 1 shows, respondents with 4 or 5 plasticity alleles scored highest on self-control when exposed to the most supportive maternal parenting and lowest on self-control when exposed to the least supportive parenting. Although not as pronounced, respondents with 3 plasticity alleles scored the second highest on self-regulation when exposed to the most supportive maternal parenting, while respondents with 2 and 0 or 1 plasticity alleles scored, respectively, the third and fourth highest on self-regulation when exposed to the most supportive parenting. The reverse pattern was detected when examining exposure to the most unsupportive maternal parenting in that respondents with 3 plasticity alleles scored the second lowest, respondents with 2 plasticity alleles scored the third lowest, and respondents with 0 or 1 plasticity alleles scored the highest on self-regulation.
Figure 1.
Interaction between cumulative genetic plasticity and parenting quality in the prediction of self-regulation for males
Discussion
Results suggest that adolescent males (only) vary substantially – for reasons having to do with their genetic make-up – in the extent to which their self-regulation is apparently affected by the maternal parenting to which they are exposed. Moreover, rather than it just being the case that certain individuals are disproportionately adversely affected by poor parenting, as traditional diathesis-stress thinking and most G×E research to date would suggest (Zuckerman, 1999; Burmeister et al., 2008), the data prove consistent with differential-susceptibility thinking. Not only did carrying more plasticity alleles seem to amplify the adverse effects of poor maternal parenting on self-regulation, but this proved true as well of positive effects of supportive parenting. (We consider it more appropriate to refer to a ‘positive’ effect than a ‘protective’ one because it is not just the case that carrying these alleles buffers one from an adverse effect of negative parenting.) Thus, those males carrying more plasticity alleles were (apparently) affected more than those carrying fewer in a for-better-and-for-worse manner (Belsky et al., 2007). The fact that this work is not experimental in nature clearly limits any causal inferences that can be drawn.
It seems likely that the moderating effect of cumulative genetic plasticity on parenting might itself reflect unevaluated epistasis (Carlborg & Haley, 2004; Cordell, 2002), that is, gene–gene (or gene–gene–gene…) interaction (in interaction with parenting). Only when theory, statistical power and/or opportunities for replication are available would it seem appropriate for specific epistatic effects to be evaluated. For the time being, then, it seems reasonable to presume that some such gene–gene interactions underlie some of the moderating cumulative-genetic-plasticity effect discerned in this inquiry.
Besides perhaps encouraging further experimental tests of differential-susceptibility (Bakermans-Kranenburg, van IJzendoorn, Mesman, Alink, & Juffer, 2008; Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman, & Juffer, 2008; Brody, Beach, Philibert, Chen, & Murry, 2009), the research presented herein calls attention to the potential benefits of (a) insuring that measurement of both environmental factors and the behavioral phenotypes to be explained by them captures both positive and negative poles, thereby enabling a better differentiation of diathesis-stress and differential-susceptibility models of environmental action, and (b) compositing multiple genes in a well-conceptualized manner. However empirically successful – even insightful – single-gene G×E studies have proven to be, the present inquiry reveals the benefit of moving beyond a focus on a single gene at a time.
The present work also underscores the need for work on endophenotypic processes to explain how the genes in question function to make, apparently, some males more susceptible to parental influences than others. One possibility is that tested and discerned moderating effects of cumulative-genetic plasticity actually reflect mediating effects of the genes under consideration (or others) in linking parenting with self-regulation. Consistent with epigenetic thinking (Cameron et al., 2005), it could be that certain environmental exposures, including parenting experience, result in certain genes being more or less likely to be expressed, thereby influencing downstream physical, physiological, behavioral and/or psychological development. In fact, one proposition that could be tested in future work involves methylation or gene silencing. Conceivably, environmental effects on gene expression may prove stronger in the case of some individuals more than others, with those carrying more putative plasticity genes more likely to have these genes – or even others – methylated or otherwise regulated by their developmental experience.
In any event, it would be a mistake to infer that the five genes included in our cumulative-genetic-plasticity index are the only ones that qualify as ‘plasticity genes’. While this might appear to be the case on the basis of existing G×E research (Belsky & Pluess, 2009b), it remains likely that other genes will prove to moderate environmental effects in a for-better-and-for-worse manner consistent with differential susceptibility. This would seem especially so if G×E investigators move beyond operationalizing positive environments and positive functioning as just the absence of, respectively, contextual adversity (e.g., not maltreated) and psychological disturbance (e.g., not anti-social).
Certainly the most unanticipated finding of this inquiry was that the hypothesized G×E interaction involving cumulative-genetic plasticity proved significant only in the case of males. Exactly why this should be remains unclear, but it was not a statistical artifact of there being greater variance in the dependent variable in the male than female subsample, as we suspected initially on discerning the sex difference in question. Perhaps females are simply more easily socialized, so parenting affects the self-regulation of all girls to the same extent irrespective of their genetic make-up. Perhaps the difference between the male and female results stems from the fact that the challenge of self-regulation during adolescence is greater for males than females, for reasons having to do with peer pressure or the rate of development of the frontal cortex and thus executive functions. Interestingly, we are not the first to detect G×E interactions restricted to males (Frazzetto et al., 2007).
Key points.
The capacity to control or regulate one’s emotions, cognitions and behavior is central to competent functioning, with limitations in these abilities associated with developmental problems.
Parenting appears to influence such self-regulation, though gene×environment interaction research suggests this could be more the case for some than for others.
Five putative ‘plasticity genes’ were composited and this additive index of ‘cumulative genetic plasticity’ moderated the effects of parenting on adolescent self-regulation: The more such alleles, the more male (only) adolescents were apparently positively and negatively influenced by, respectively, supportive and unsupportive parenting.
Results suggest that adolescents carrying more vs. fewer plasticity alleles may benefit more from clinical and intervention efforts promoting supportive parenting.
Acknowledgements
This research uses data from Add Health, a project designed by J. Richard Udry, Peter S. Bearman and Kathleen Mullan Harris and funded by Grant PO1-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgement is due to Ronald R. Rindfuss and Barbara Entwisle for assistance in original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from Grant PO1-HD31921 for this analysis.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene–environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. The role of dopamine-related genes in G × E interaction in human development. Paper presented at the biennial meeting of the Society for Research in Child Development; Denver, CO. 2009. Apr, [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Mesman J, Alink LR, Juffer F. Effects of an attachment-based intervention on daily cortisol moderated by dopamine receptor D4: A randomized control trial on 1- to 3-year-olds screened for externalizing behavior. Development and Psychopathology. 2008;20:805–820. doi: 10.1017/S0954579408000382. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Pijlman FT, Mesman J, Juffer F. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Beaver KM, Ratchford M, Ferguson CJ. Evidence of genetic and environmental effects on the development of low self-control. Criminal Justice and Behavior. 2009;36:1158–1172. [Google Scholar]
- Beaver KM, Sak A, Vaske J, Nilsson J. Genetic risk, parent-child relations, and antisocial phenotypes in a sample of African-American males. Psychiatry Research. 2010;175:160–164. doi: 10.1016/j.psychres.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Belsky J. Variation in susceptibility to rearing influences: An evolutionary argument. Psychological Inquiry. 1997;8:182–186. [Google Scholar]
- Belsky J. Differential susceptibility to rearing influences: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guilford; 2005. pp. 139–163. [Google Scholar]
- Belsky J. Childhood experiences and reproductive strategies. In: Dunbar R, Barrett L, editors. Oxford handbook of evolutionary psychology. Oxford: Oxford University Press; 2007. pp. 237–254. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Perspectives on Psychological Science. 2009a;4:345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis-stress: Differential susceptibility to environmental influence. Psychological Bulletin. 2009b;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berman SM, Noble EP. The D2 dopamine receptor (DRD2) gene and family stress; interactive effects on cognitive functions in children. Behavior Genetics. 1997;27:33–43. doi: 10.1023/a:1025611208475. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Sensitive periods in development: Structural characteristics and causal interpretations. Psychological Bulletin. 1989;105:179–202. doi: 10.1037/0033-2909.105.2.179. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SR, Philibert RA, Chen YF, Murry VM. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene × environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: Progress amid controversy. Nature Review Genetics. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Carlborg O, Haley CS. Epistasis: Too often neglected in complex trait studies. Nature Review Genetics. 2004;5:618–625. doi: 10.1038/nrg1407. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;29:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews. Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Cordell HJ. Epistasis: What it means, what it doesn’t mean, and statistical methods to detect it in humans. Human Molecular Genetics. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- Cullen FT, Unnever JD, Wright JP, Beaver KM. Parenting and self-control. In: Goode E, editor. Out of control: Assessing the general theory of crime. Stanford, CA: Stanford University Press; 2008. pp. 61–74. [Google Scholar]
- De Quervain DJF, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. PNAS. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt TJ, Scheiner SM. Phenotypic plasticity: Functional and conceptual approaches. Oxford: Oxford University Press; 2004. [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Champion C, Ma Y. Emotion-related regulation: An emerging construct. Merrill-Palmer Quarterly. 2004;50:236–259. [Google Scholar]
- Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting and children’s effortful control and externalizing problems. Child Development. 2005;76:1055–1071. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, et al. Early trauma and increased risk for physical aggression during adulthood: The moderating role of MAOA genotype. PLoS ONE. 2007;2:e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. Stanford, CA: Stanford University Press; 1990. [Google Scholar]
- Haberstick BC, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, Hewitt JK. Monoamine oxidase A and antisocial behaviors in the presence of childhood and adolescent maltreatment. American Journal of Medical Genetics. 2005;135B:59–64. doi: 10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- Harlaar N, Butcher LM, Meaburn E, Sham P, Craig IW, Plomin R. A behavioural genomic analysis of DNA markers associated with general cognitive ability in 7-year-olds. Journal of Child Psychology and Psychiatry. 2005;46:1097–1107. doi: 10.1111/j.1469-7610.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- Harris KM, Florey F, Tabor J, Bearman PS, Jones J, Udry JR. [Retrieved June 29, 2009];The National Longitudinal Study of Adolescent Health: Research design. 2003 from http://www.cpc.unc.edu/projects/addhealth/design. [Google Scholar]
- Harris KM, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) twin data. Twin Research and Human Genetics. 2006;9:988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG, Rietschel M. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Lengua LJ, Honorado E, Bush NR. Contextual risk and parenting as predictors of effortful control and social competence in preschool children. Journal of Applied Developmental Psychology. 2007;28:40–55. doi: 10.1016/j.appdev.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccoby EE. Parenting and its effects on children. Annual Review of Psychology. 2000;51:1–27. doi: 10.1146/annurev.psych.51.1.1. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Gariepy JL, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: The family system as the unit of analyses. Development and Psychopathology. 2007;19:1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Obradović J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts. Developmental Neuroscience. 2009;31:300–308. doi: 10.1159/000216541. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Neuman RJ. Prenatal smoking and ADHD: DRD4-7R as a plasticity gene. Biological Psychiatry. 2009;66:e5–e6. doi: 10.1016/j.biopsych.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Pratt TC, Cullen FT. The empirical status of Gottfredson and Hirschi’s general theory of crime: A meta-analysis. Criminology. 2000;38:931–964. [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, Tabor J, Beuhring T, Sieving RE, Shew M, Ireland M, Bearinger LH, Udry JR. Protecting adolescents from harm: Findings from the National Longitudinal Study of Adolescent Health. Journal of the American Medical Association. 1997;278:823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Rothbaum F, Weisz JR. Parental caregiving and child externalizing behavior in nonclinical samples: A meta-analysis. Psychological Bulletin. 1994;116:55–74. doi: 10.1037/0033-2909.116.1.55. [DOI] [PubMed] [Google Scholar]
- Rutter M. Genes and behavior: Nature–nurture interplay explained. London: Blackwell; 2006. [Google Scholar]
- Sonuga-Barke EJ, Oades RD, Psychogiou L, Chen W, Franke B, Buitelaar J, et al. Dopamine and serotonin transporter genotypes moderate sensitivity to maternal expressed emotion: The case of conduct and emotional problems in attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2009;50:1052–1063. doi: 10.1111/j.1469-7610.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Vazsonyi AT, Huang L. Where self-control comes from: On the development of self-control and its relationship to deviance over time. Developmental Psychology. 2010;46:245–257. doi: 10.1037/a0016538. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the ‘cycle of violence’: Childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biological Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Vulnerability to psychopathology: A biosocial model. Washington, DC: American Psychological Association; 1999. [Google Scholar]