Abstract
I completed my medical studies at the Karolinska Institute in Stockholm but have always been devoted to basic research. My longstanding interest is to understand fundamental DNA repair mechanisms in the fields of cancer therapy, inherited human genetic disorders and ancient DNA. I initially measured DNA decay, including rates of base loss and cytosine deamination. I have discovered several important DNA repair proteins and determined their mechanisms of action. The discovery of uracil-DNA glycosylase defined a new category of repair enzymes with each specialized for different types of DNA damage. The base excision repair pathway was first reconstituted with human proteins in my group. Cell-free analysis for mammalian nucleotide excision repair of DNA was also developed in my laboratory. I found multiple distinct DNA ligases in mammalian cells, and led the first genetic and biochemical work on DNA ligases I, III and IV. I discovered the mammalian exonucleases DNase III (TREX1) and IV (FEN1). Interestingly, expression of TREX1 was altered in some human autoimmune diseases. I also showed that the mutagenic DNA adduct O6-methylguanine (O6mG) is repaired without removing the guanine from DNA, identifying a surprising mechanism by which the methyl group is transferred to a residue in the repair protein itself. A further novel process of DNA repair discovered by my research group is the action of AlkB as an iron-dependent enzyme carrying out oxidative demethylation.
Keywords: DNA repair, Base excision repair, DNA glycosylase, DNA exonuclease, AlkB dioxygenase
In my early research career, I observed that Epstein-Barr virus DNA is present as nonintegrated covalently-closed circles, as well as integrated viral DNA fragments, in virus-transformed cells from Burkitt lymphoma and nasopharyngeal carcinoma patients in 1975/1976 [1–23]. This work was surprising because it preceded similar studies on papilloma virus in other laboratories.
A main achievement has been to characterize and quantify spontaneous, endogenously-produced DNA damage during the 1970s and 1980s [24–44]. Surprisingly, main events, such as hydrolytic depurination, deamination of cytosine residues, oxidation of guanine and pyrimidine residues and methylation of adenine residues to 3-methyladenine, amount to 10,000 potentially mutagenic and cytotoxic changes per day in a human genome. These results strongly indicate that special DNA repair enzymes and mechanisms must exist to counteract endogenous DNA damage.
I thus became enthusiastic about understanding fundamental DNA repair mechanisms (Figure 1). A review of my work on endogenous DNA damage and its repair was published in Nature [45]. I discovered the base excision-repair pathway, the major cellular defense against endogenous DNA damage [46–57]. Later on, the two variants of base excision repair (short-patch vs long-patch repair) were reconstituted with purified proteins. I unveiled several DNA repair enzymes of previously-unknown modes of action, including (i) DNA glycosylases that catalyze the cleavage of base-sugar bonds (uracil-DNA glycosylase) [58–67], 3-methyladenine–DNA glycosylase [68–70] and DNA glycosylases that release oxidised base residues (Figure 1A-I) [29,35]; (ii) AP endonucleases that incise double-stranded DNA at base-free sugar-phosphate residues (in parallel with Prof. Walter Verly) (Figure 1A-II) [46,71–75]; (iii) the O6-methylguanine-DNA methyltransferase (MGMT, Ada protein that transfers irreversibly a promutagenic methyl group from alkylated DNA to a specific cysteine residue in the transferase itself) (Figure 1B) [27,31,32,76–83]; (iv) DNA dioxygenases (AlkB protein and its homologs) that remove certain cytotoxic methyl groups from alkylated base residues by oxidative demethylation in the presence of iron and oxoglutarate (together with Dr Barbara Sedgwick and Prof. Erling Seeberg) (Figure 1C) [84–89]. This DNA repair mechanism also resulted in the discovery of new group of enzymes FTO and ALKBH5 that demethylate a novel epigenetic marker RNA m6A (Figure 1D) [90,91].
Figure 1.
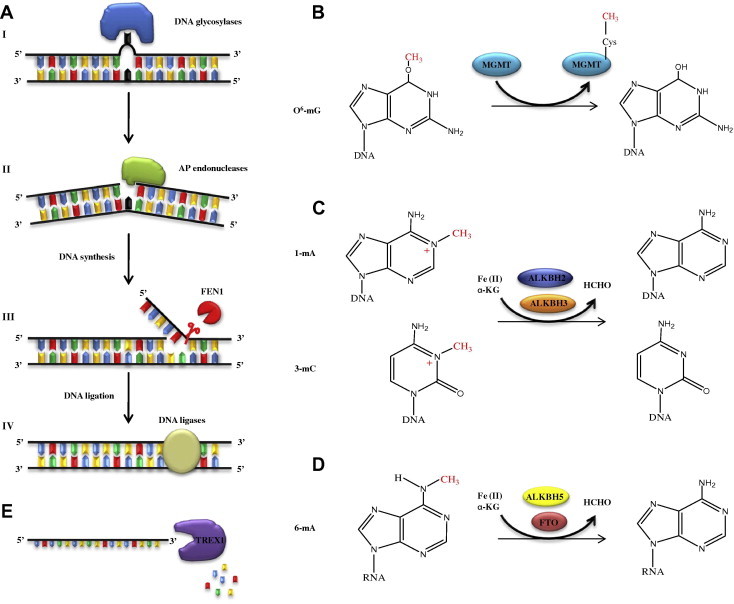
Overview of mechanistic models for enzymatic reactions A. (I) DNA glycosylases catalyze the cleavage of base-sugar bonds; (II) AP endonucleases incise double-stranded DNA at base-free sugar-phosphate residues; (III) FEN1 removes overhangs and flaps from DNA and (IV) eukaryotic DNA ligases ligate DNA ends. B. O6-methylguanine-DNA methyltransferase (MGMT) transfers irreversibly a promutagenic methyl group from alkylated DNA to a specific cysteine residue in the transferase itself. C. DNA dioxygenases remove certain cytotoxic methyl groups from alkylated base residues by oxidative demethylation in the presence of iron and oxoglutarate. D. FTO and ALKBH5 demethylate RNA m6A as a novel epigenetic marker in α-ketoglutarate (α-KG) and Fe2+-dependent manner. E. TREX1 is a 3′ to 5′ exonuclease with preference for single-stranded DNA.
It is worth mentioning that I found a complex and chemically-stable oxidative DNA lesion, cyclopurine deoxynucleoside, which is exclusively repaired by nucleotide excision repair in contrast to other oxidative DNA lesions (in collaboration with Prof. Jean Cadet) [92]. Moreover, I established a human cell-free system for ATP-dependent nucleotide excision repair (together with a senior postdoctoral fellow, Dr Rick Wood) [93]. This assay system allowed for purification of proteins such as XPA, which is missing in repair-defective xeroderma pigmentosum (XP) cells, by in vitro complementation.
It was interesting for me to identify and characterize the DNA ligases in eukaryotic cells, which require ATP rather that NAD as cofactor, in contrast to most bacterial ligases (Figure 1A-IV) [94–96]. The main DNA ligases that function in mammalian cells include DNA ligase I (DNA replication and repair) [97,98], DNA ligase III (base excision repair) [62] and DNA ligase IV (non-homologous end joining) [99–101]. The human DNA ligase I cDNA was cloned and sequenced in 1990 (in collaboration with Dr Lee Johnston) [102], which allowed for the localization of the active site for enzyme-adenylate complex formation. Early observations on alterations of DNA ligase I in human diseases prior to that year were only partially confirmed.
Furthermore, I discovered and characterized the two major DNA-specific exonucleases in mammalian cell nuclei, originally termed DNase III and IV, now called TREX1 and FEN1 (Figure 1A-III, 1E) [55], respectively. The FEN1 enzyme was shown to be a 5’ to 3’exonuclease, a replication and repair factor that removes overhangs and flaps from DNA (in parallel with Dr Michael Lieber) [55,103]. TREX1 was shown to be a 3’ to 5’ exonuclease with preference for single-stranded DNA. More recent studies established that loss of TREX1 in human cells results in a form of inherited systemic lupus erythematosus (SLE) called Aicardi-Goutières syndrome (AGS) (in collaboration with Dr Yanick Crow) [92,104–106]. In 2007, TREX1-negative cells were shown to accumulate single-stranded DNA and exhibit persistent checkpoint activation (together with coworkers, Drs Yun-Gui Yang and Deborah Barnes) [107].
Besides my discovery of several DNA repair enzymes, I also observed that self-methylation of the Ada protein [27,32,37,80–83,108], with methylation of a cysteine residue within the regulatory domain, as a consequence of DNA phosphotriester repair, converts Ada to a transcription factor. This work, published in 1986, was the first example of activation of a transcription factor by a posttranslational modification event.
Beyond my own scientific research, I also spent time to manage research laboratories, and still provide advice for their individual research concepts and directions. As the former director of the Clare Hall Laboratories at ICRF and Cancer Research UK, I was pleased to see that Clare Hall Laboratories became an internationally-renowned center of research into DNA processing. I am also very glad to see that many of my former colleagues succeed in their academic careers.
I still enjoy very much doing science. It is pleasure, it is very interesting and it is stimulating. It changes all the time. I would like to be here around hundred years to see how science develops.
Biography

Dr. Tomas Lindahl completed medical studies at the Karolinska Institute in Stockholm but has always been devoted to research. He worked on nucleic acid biochemistry with Jacques Fresco at Princeton and Gerald Edelman at Rockefeller University, and joined the faculty of the Karolinska Institute in 1969. He became Professor at the University of Gothenburg in 1978. In 1981 he was appointed Head of the Mutagenesis Laboratory at the ICRF Mill Hill Laboratories in London. From 1984 to 2006, he was Director of the Clare Hall Laboratories at ICRF and Cancer Research UK, also serving as Deputy Director of Research. Dr. Lindahl’s contributions to understanding DNA repair are fundamental and have long-lasting impact in the fields of cancer therapy, inherited human genetic disorders and ancient DNA. Beyond his own outstanding scientific achievements, his stewardship established Clare Hall Laboratories as an internationally-renowned center of research into DNA processing. The success of colleagues working together with him is a measure of his insight, support and leadership. Amongst many prestigious honours, Dr. Lindahl is a member of EMBO, a fellow of the Royal Swedish Academy of Sciences and the Royal Society. He delivered the Royal Society Croonian Lecture in 1996. Dr. Lindahl received a Royal Medal in 2007 and the prestigious Copley Medal of the Royal Society in 2010.
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
References
- 1.Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975;72:1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindahl T., Adams A., Bjursell G., Bornkamm G.W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976;102:511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- 3.Kaschka-Dierich C., Adams A., Lindahl T., Bornkamm G.W., Bjursell G., Klein G. Intracellular forms of Epstein-Barr virus DNA in human tumour cells in vivo. Nature. 1976;260:302–306. doi: 10.1038/260302a0. [DOI] [PubMed] [Google Scholar]
- 4.Adams A., Bjursell G., Gussander E., Koliais S., Falk L., Lindahl T. Size of the intracellular circular Epstein-Barr virus DNA molecules in infectious mononucleosis-derived human lymphoid cell lines. J Virol. 1979;29:815–817. doi: 10.1128/jvi.29.2.815-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams A., Bjursell G., Kaschka-Dierich C., Lindahl T. Circular Epstein-Barr virus genomes of reduced size in a human lymphoid cell line of infectious mononucleosis origin. J Virol. 1977;22:373–380. doi: 10.1128/jvi.22.2.373-380.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams A., Lindahl T. Intracellular forms of Epstein-Barr virus DNA in Raji cells. IARC Sci Publ. 1975;11(1):125–132. [PubMed] [Google Scholar]
- 7.Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973;70:2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson M., Lindahl T. Epstein-Barr virus DNA in human lymphoid cell lines: in vitro conversion. Virology. 1976;73:96–105. doi: 10.1016/0042-6822(76)90064-7. [DOI] [PubMed] [Google Scholar]
- 9.Andersson-Anvret M., Lindahl T. Integrated viral DNA sequences in Epstein-Barr virus-converted human lymphoma lines. J Virol. 1978;25:710–718. doi: 10.1128/jvi.25.3.710-718.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrand J.R., Rymo L., Walsh J.E., Bjorck E., Lindahl T., Griffin B.E. Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic Acids Res. 1981;9:2999–3014. doi: 10.1093/nar/9.13.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk L., Lindahl T., Bjursell G., Klein G. Herpesvirus papio: state and properties of intracellular viral DNA in baboon lymphoblastoid cell lines. Int J Cancer. 1979;24:75–79. doi: 10.1002/ijc.2910240113. [DOI] [PubMed] [Google Scholar]
- 12.Griffin B.E., Bjorck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Barr virus DNA in transformed cells. J Virol. 1981;40:11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jehn U., Lindahl T., Klein C. Fate of virus DNA in the abortive infection of human lymphoid cell lines by Epstein-Barr virus. J Gen Virol. 1972;16:409–412. doi: 10.1099/0022-1317-16-3-409. [DOI] [PubMed] [Google Scholar]
- 14.Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977;20:173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- 15.Klein G., Giovanella B.C., Lindahl T., Fialkow P.J., Singh S., Stehlin J.S. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974;71:4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein G., Lindahl T., Jondal M., Leibold W., Menezes J., Nilsson K. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974;71:3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koliais S., Bjursell G., Adams A., Lindahl T., Klein G. State of Epstein-Barr virus DNA in an American Burkitt’s lymphoma line. J Natl Cancer Inst. 1978;60:991–994. doi: 10.1093/jnci/60.5.991. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl T., Adams A., Andersson-Anvret M., Falk L. Integration of Epstein-Barr virus DNA. IARC Scientific publications. 1978;24(1):113–123. [PubMed] [Google Scholar]
- 19.Lindahl T., Klein G., Reedman B.M., Johansson B., Singh S. Relationship between Epstein-Barr virus (EBV) DNA and the EBV-determined nuclear antigen (EBNA) in Burkitt lymphoma biopsies and other lymphoproliferative malignancies. Int J Cancer. 1974;13:764–772. doi: 10.1002/ijc.2910130605. [DOI] [PubMed] [Google Scholar]
- 20.Luka J., Lindahl T., Klein G. Purification of the Epstein-Barr virus-determined nuclear antigen from Epstein-Barr virus-transformed human lymphoid cell lines. J Virol. 1978;27:604–611. doi: 10.1128/jvi.27.3.604-611.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno S., Luka J., Lindahl T., Klein G. Identification of a purified complement-fixing antigen as the Epstein-Barr-virus determined nuclear antigen (EBNA) by its binding to metaphase chromosomes. Proc Natl Acad Sci U S A. 1977;74:1605–1609. doi: 10.1073/pnas.74.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rymo L., Lindahl T., Adams A. Sites of sequence variability in Epstein-Barr virus DNA from different sources. Proc Natl Acad Sci U S A. 1979;76:2794–2798. doi: 10.1073/pnas.76.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rymo L., Lindahl T., Povey S., Klein G. Analysis of restriction endonuclease fragments of intracellular Epstein-Barr virus DNA and isoenzymes indicate a common origin of the Raji, NC-37, and F-265 human lymphoid cell lines. Virology. 1981;115:115–124. doi: 10.1016/0042-6822(81)90093-3. [DOI] [PubMed] [Google Scholar]
- 24.Lindahl T. Mammalian deoxyribonucleases acting on damaged DNA. Johns Hopkins Med J Suppl. 1972;1:3–13. [PubMed] [Google Scholar]
- 25.Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976;259:64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- 26.Chetsanga C.J., Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979;6:3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979;280:76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- 28.Karran P., Lindahl T. Hypoxanthine in deoxyribonucleic acid: generation by heat-induced hydrolysis of adenine residues and release in free form by a deoxyribonucleic acid glycosylase from calf thymus. Biochemistry. 1980;19:6005–6011. doi: 10.1021/bi00567a010. [DOI] [PubMed] [Google Scholar]
- 29.Breimer L., Lindahl T. A DNA glycosylase from Escherichia coli that releases free urea from a polydeoxyribonucleotide containing fragments of base residues. Nucleic Acids Res. 1980;8:6199–6211. doi: 10.1093/nar/8.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rydberg B., Lindahl T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-l-methionine is a potentially mutagenic reaction. EMBO J. 1982;1:211–216. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris A.L., Karran P., Lindahl T. O6-methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983;43:3247–3252. [PubMed] [Google Scholar]
- 32.Teo I., Sedgwick B., Demple B., Li B., Lindahl T. Induction of resistance to alkylating agents in E. coli: the ada+ gene product serves both as a regulatory protein and as an enzyme for repair of mutagenic damage. EMBO J. 1984;3:2151–2157. doi: 10.1002/j.1460-2075.1984.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breimer L.H., Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli. J Biol Chem. 1984;259:5543–5548. [PubMed] [Google Scholar]
- 34.Breimer L.H., Lindahl T. Thymine lesions produced by ionizing radiation in double-stranded DNA. Biochemistry. 1985;24:4018–4022. doi: 10.1021/bi00336a032. [DOI] [PubMed] [Google Scholar]
- 35.Breimer L.H., Lindahl T. Enzymatic excision of DNA bases damaged by exposure to ionizing radiation or oxidizing agents. Mutat Res. 1985;150:85–89. doi: 10.1016/0027-5107(85)90104-6. [DOI] [PubMed] [Google Scholar]
- 36.Karran P., Lindahl T. Cellular defense mechanisms against alkylating agents. Cancer Surv. 1985;4:583–599. [PubMed] [Google Scholar]
- 37.Teo I., Sedgwick B., Kilpatrick M.W., McCarthy T.V., Lindahl T. The intracellular signal for induction of resistance to alkylating agents in E. coli. Cell. 1986;45:315–324. doi: 10.1016/0092-8674(86)90396-x. [DOI] [PubMed] [Google Scholar]
- 38.Lindahl T. The 1987 Walter Hubert lecture: regulation and deficiencies in DNA repair. Br J Cancer. 1987;56:91–95. doi: 10.1038/bjc.1987.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehmann A.R., Willis A.E., Broughton B.C., James M.R., Steingrimsdottir H., Harcourt S.A. Relation between the human fibroblast strain 46BR and cell lines representative of Bloom’s syndrome. Cancer Res. 1988;48:6343–6347. [PubMed] [Google Scholar]
- 40.Franklin W.A., Lindahl T. DNA deoxyribophosphodiesterase. EMBO J. 1988;7:3617–3622. doi: 10.1002/j.1460-2075.1988.tb03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood R.D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988;53:97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- 42.Lindahl T., Wood R.D. DNA repair and recombination. Curr Opin Cell Biol. 1989;1:475–480. doi: 10.1016/0955-0674(89)90008-2. [DOI] [PubMed] [Google Scholar]
- 43.Karam L.R., Calsou P., Franklin W.A., Painter R.B., Olsson M., Lindahl T. Modification of deoxyribose-phosphate residues by extracts of ataxia telangiectasia cells. Mutat Res. 1990;236:19–26. doi: 10.1016/0921-8777(90)90028-4. [DOI] [PubMed] [Google Scholar]
- 44.Lindahl T. Repair of intrinsic DNA lesions. Mutat Res. 1990;238:305–311. doi: 10.1016/0165-1110(90)90022-4. [DOI] [PubMed] [Google Scholar]
- 45.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 46.Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- 47.Dianov G., Lindahl T. Preferential recognition of I.T base-pairs in the initiation of excision-repair by hypoxanthine-DNA glycosylase. Nucleic Acids Res. 1991;19:3829–3833. doi: 10.1093/nar/19.14.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satoh M.S., Jones C.J., Wood R.D., Lindahl T. DNA excision-repair defect of Xeroderma pigmentosum prevents removal of a class of oxygen free radical-induced base lesions. Proc Natl Acad Sci U S A. 1993;90:6335–6339. doi: 10.1073/pnas.90.13.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satoh M.S., Poirier G.G., Lindahl T. NAD+-dependent repair of damaged DNA by human cell extracts. J Biol Chem. 1993;268:5480–5487. [PubMed] [Google Scholar]
- 50.Dianov G., Lindahl T. Reconstitution of the DNA base excision-repair pathway. Curr Biol. 1994;4:1069–1076. doi: 10.1016/s0960-9822(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 51.Dianov G., Sedgwick B., Daly G., Olsson M., Lovett S., Lindahl T. Release of 5′-terminal deoxyribose-phosphate residues from incised abasic sites in DNA by the Escherichia coli RecJ protein. Nucleic Acids Res. 1994;22:993–998. doi: 10.1093/nar/22.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindahl T., Satoh M.S., Dianov G. Enzymes acting at strand interruptions in DNA. Philos Trans R Soc Lond B Biol Sci. 1995;347:57–62. doi: 10.1098/rstb.1995.0009. [DOI] [PubMed] [Google Scholar]
- 53.Lindahl T. Recognition and processing of damaged DNA. J Cell Sci Suppl. 1995;19:73–77. doi: 10.1242/jcs.1995.supplement_19.10. [DOI] [PubMed] [Google Scholar]
- 54.Kubota Y., Nash R.A., Klungland A., Schar P., Barnes D.E., Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 55.Klungland A., Lindahl T. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) EMBO J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindahl T. Suppression of spontaneous mutagenesis in human cells by DNA base excision-repair. Mutat Res. 2000;462:129–135. doi: 10.1016/s1383-5742(00)00024-7. [DOI] [PubMed] [Google Scholar]
- 57.Kuraoka I., Bender C., Romieu A., Cadet J., Wood R.D., Lindahl T. Removal of oxygen free-radical-induced 5′,8-purine cyclodeoxynucleosides from DNA by the nucleotide excision-repair pathway in human cells. Proc Natl Acad Sci U S A. 2000;97:3832–3837. doi: 10.1073/pnas.070471597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindahl T. Uracil-DNA glycosylase from Escherichia coli. Methods Enzymol. 1980;65:284–290. doi: 10.1016/s0076-6879(80)65038-1. [DOI] [PubMed] [Google Scholar]
- 59.Dianov G., Price A., Lindahl T. Generation of single-nucleotide repair patches following excision of uracil residues from DNA. Mol Cell Biol. 1992;12:1605–1612. doi: 10.1128/mcb.12.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nilsen H., Rosewell I., Robins P., Skjelbred C.F., Andersen S., Slupphaug G. Uracil-DNA glycosylase (UNG)-deficient mice reveal a primary role of the enzyme during DNA replication. Mol Cell. 2000;5:1059–1065. doi: 10.1016/s1097-2765(00)80271-3. [DOI] [PubMed] [Google Scholar]
- 61.Nilsen H., Haushalter K.A., Robins P., Barnes D.E., Verdine G.L., Lindahl T. Excision of deaminated cytosine from the vertebrate genome: role of the SMUG1 uracil-DNA glycosylase. EMBO J. 2001;20:4278–4286. doi: 10.1093/emboj/20.15.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nilsen H., Lindahl T., Verreault A. DNA base excision repair of uracil residues in reconstituted nucleosome core particles. EMBO J. 2002;21:5943–5952. doi: 10.1093/emboj/cdf581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rada C., Williams G.T., Nilsen H., Barnes D.E., Lindahl T., Neuberger M.S. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 64.Nilsen H., Stamp G., Andersen S., Hrivnak G., Krokan H.E., Lindahl T. Gene-targeted mice lacking the Ung uracil-DNA glycosylase develop B-cell lymphomas. Oncogene. 2003;22:5381–5386. doi: 10.1038/sj.onc.1206860. [DOI] [PubMed] [Google Scholar]
- 65.An Q., Robins P., Lindahl T., Barnes D.E. C → T mutagenesis and gamma-radiation sensitivity due to deficiency in the Smug1 and Ung DNA glycosylases. EMBO J. 2005;24:2205–2213. doi: 10.1038/sj.emboj.7600689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.An Q., Robins P., Lindahl T., Barnes D.E. 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Res. 2007;67:940–945. doi: 10.1158/0008-5472.CAN-06-2960. [DOI] [PubMed] [Google Scholar]
- 67.Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977;252:3286–3294. [PubMed] [Google Scholar]
- 68.Karran P., Lindahl T. Enzymatic excision of free hypoxanthine from polydeoxynucleotides and DNA containing deoxyinosine monophosphate residues. J Biol Chem. 1978;253:5877–5879. [PubMed] [Google Scholar]
- 69.Riazuddin S., Lindahl T. Properties of 3-methyladenine-DNA glycosylase from Escherichia coli. Biochemistry. 1978;17:2110–2118. doi: 10.1021/bi00604a014. [DOI] [PubMed] [Google Scholar]
- 70.Karran P., Lindahl T., Ofsteng I., Evensen G.B., Seeberg E. Escherichia coli mutants deficient in 3-methyladenine-DNA glycosylase. J Mol Biol. 1980;140:101–127. doi: 10.1016/0022-2836(80)90358-7. [DOI] [PubMed] [Google Scholar]
- 71.Ljungquist S., Andersson A., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem. 1974;249:1536–1540. [PubMed] [Google Scholar]
- 72.Ljungquist S., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. I. Purification and general properties. J Biol Chem. 1974;249:1530–1535. [PubMed] [Google Scholar]
- 73.Ljungquist S., Nyberg B., Lindahl T. Mammalian DNA endonuclease acting at apurinic sites: absence of associated exonuclease activity. FEBS Lett. 1975;57:169–171. doi: 10.1016/0014-5793(75)80708-3. [DOI] [PubMed] [Google Scholar]
- 74.Ljungquist S., Lindahl T., Howard-Flanders P. Methyl methane sulfonate-sensitive mutant of Escherichia coli deficient in an endonuclease specific for apurinic sites in deoxyribonucleic acid. J Bacteriol. 1976;126:646–653. doi: 10.1128/jb.126.2.646-653.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ljungquist S., Lindahl T. Relation between Escherichia coli endonucleases specific for apurinic sites in DNA and exonuclease III. Nucleic Acids Res. 1977;4:2871–2879. doi: 10.1093/nar/4.8.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980;255:10569–10571. [PubMed] [Google Scholar]
- 77.Sedgwick B., Lindahl T. A common mechanism for repair of O6-methylguanine and O6-ethylguanine in DNA. J Mol Biol. 1982;154:169–175. doi: 10.1016/0022-2836(82)90424-7. [DOI] [PubMed] [Google Scholar]
- 78.Lindahl T., Demple B., Robins P. Suicide inactivation of the E. coli O6-methylguanine-DNA methyltransferase. EMBO J. 1982;1:1359–1363. doi: 10.1002/j.1460-2075.1982.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demple B., Jacobsson A., Olsson M., Robins P., Lindahl T. Repair of alkylated DNA in Escherichia coli. Physical properties of O6-methylguanine-DNA methyltransferase. J Biol Chem. 1982;257:13776–13780. [PubMed] [Google Scholar]
- 80.Demple B., Sedgwick B., Robins P., Totty N., Waterfield M.D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985;82:2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarthy T.V., Lindahl T. Methyl phosphotriesters in alkylated DNA are repaired by the Ada regulatory protein of E. coli. Nucleic Acids Res. 1985;13:2683–2698. doi: 10.1093/nar/13.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sedgwick B., Robins P., Totty N., Lindahl T. Functional domains and methyl acceptor sites of the Escherichia coli ada protein. J Biol Chem. 1988;263:4430–4433. [PubMed] [Google Scholar]
- 83.Sedgwick B., Lindahl T. Recent progress on the Ada response for inducible repair of DNA alkylation damage. Oncogene. 2002;21:8886–8894. doi: 10.1038/sj.onc.1205998. [DOI] [PubMed] [Google Scholar]
- 84.Duncan T., Trewick S.C., Koivisto P., Bates P.A., Lindahl T., Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proc Natl Acad Sci U S A. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trewick S.C., Henshaw T.F., Hausinger R.P., Lindahl T., Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 86.Koivisto P., Duncan T., Lindahl T., Sedgwick B. Minimal methylated substrate and extended substrate range of Escherichia coli AlkB protein, a 1-methyladenine-DNA dioxygenase. J Biol Chem. 2003;278:44348–44354. doi: 10.1074/jbc.M307361200. [DOI] [PubMed] [Google Scholar]
- 87.Koivisto P., Robins P., Lindahl T., Sedgwick B. Demethylation of 3-methylthymine in DNA by bacterial and human DNA dioxygenases. J Biol Chem. 2004;279:40470–40474. doi: 10.1074/jbc.M407960200. [DOI] [PubMed] [Google Scholar]
- 88.Sedgwick B., Robins P., Lindahl T. Direct removal of alkylation damage from DNA by AlkB and related DNA dioxygenases. Methods Enzymol. 2006;408:108–120. doi: 10.1016/S0076-6879(06)08008-6. [DOI] [PubMed] [Google Scholar]
- 89.Sedgwick B., Bates P.A., Paik J., Jacobs S.C., Lindahl T. Repair of alkylated DNA: recent advances. DNA Repair. 2007;6:429–442. doi: 10.1016/j.dnarep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:1–12. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuraoka I., Robins P., Masutani C., Hanaoka F., Gasparutto D., Cadet J. Oxygen free radical damage to DNA: translesion synthesis by human DNA polymerase eta and resistance to exonuclease action at cyclopurine deoxynucleoside residues. J Biol Chem. 2001;276:49283–49288. doi: 10.1074/jbc.M107779200. [DOI] [PubMed] [Google Scholar]
- 93.Hansson J., Grossman L., Lindahl T., Wood R.D. Complementation of the Xeroderma pigmentosum DNA repair synthesis defect with Escherichia coli UvrABC proteins in a cell-free system. Nucleic Acids Res. 1990;18:35–40. doi: 10.1093/nar/18.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lasko D.D., Tomkinson A.E., Lindahl T. Eukaryotic DNA ligases. Mutat Res. 1990;236:277–287. doi: 10.1016/0921-8777(90)90011-s. [DOI] [PubMed] [Google Scholar]
- 95.Tomkinson A.E., Roberts E., Daly G., Totty N.F., Lindahl T. Three distinct DNA ligases in mammalian cells. J Biol Chem. 1991;266:21728–21735. [PubMed] [Google Scholar]
- 96.Lindahl T., Barnes D.E. Mammalian DNA ligases. Annu Rev Biochem. 1992;61:251–281. doi: 10.1146/annurev.bi.61.070192.001343. [DOI] [PubMed] [Google Scholar]
- 97.Lasko D.D., Tomkinson A.E., Lindahl T. Ligases: biosynthesis and intracellular localization of DNA ligase I. J Biol Chem. 1990;265:12618–12622. [PubMed] [Google Scholar]
- 98.Tomkinson A.E., Lasko D.D., Daly G., Lindahl T. Ligases: catalytic domain and size of DNA ligase I. J Biol Chem. 1990;265:12611–12617. [PubMed] [Google Scholar]
- 99.Robins P., Lindahl T. DNA ligase IV from HeLa cell nuclei. J Biol Chem. 1996;271:24257–24261. doi: 10.1074/jbc.271.39.24257. [DOI] [PubMed] [Google Scholar]
- 100.Barnes D.E., Stamp G., Rosewell I., Denzel A., Lindahl T. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 101.Lee Y., Barnes D.E., Lindahl T., McKinnon P.J. Defective neurogenesis resulting from DNA ligase IV deficiency requires Atm. Genes Dev. 2000;14:2576–2580. doi: 10.1101/gad.837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barnes D.E., Johnston L.H., Kodama K., Tomkinson A.E., Lasko D.D., Lindahl T. Human DNA ligase I cDNA: cloning and functional expression in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990;87:6679–6683. doi: 10.1073/pnas.87.17.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanderson R.J., Lindahl T. Down-regulation of DNA repair synthesis at DNA single-strand interruptions in poly(ADP-ribose) polymerase-1 deficient murine cell extracts. DNA Repair. 2002;1:547–558. doi: 10.1016/s1568-7864(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 104.Crow Y.J., Hayward B.E., Parmar R., Robins P., Leitch A., Ali M. Mutations in the gene encoding the 3′–5′ DNA exonuclease TREX1 cause Aicardi–Goutieres syndrome at the AGS1 locus. Nat Genet. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 105.Morita M., Stamp G., Robins P., Dulic A., Rosewell I., Hrivnak G. Gene-targeted mice lacking the Trex1 (DNase III) 3′ → 5′ DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24:6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindahl T., Barnes D.E., Yang Y.G., Robins P. Biochemical properties of mammalian TREX1 and its association with DNA replication and inherited inflammatory disease. Biochem Soc Trans. 2009;37:535–538. doi: 10.1042/BST0370535. [DOI] [PubMed] [Google Scholar]
- 107.Yang Y.G., Lindahl T., Barnes D.E. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131:873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 108.Vaughan P., Sedgwick B., Hall J., Gannon J., Lindahl T. Environmental mutagens that induce the adaptive response to alkylating agents in Escherichia coli. Carcinogenesis. 1991;12:263–268. doi: 10.1093/carcin/12.2.263. [DOI] [PubMed] [Google Scholar]



