Abstract
Human induced pluripotent stem cells (iPSCs) hold great promise for regenerative medicine. Generating iPSCs from immunologically immature newborn umbilical cord blood mononuclear cells (UCBMCs) is of great significance. Here we report generation of human iPSCs with great efficiency from UCBMCs using a dox-inducible lentiviral system carrying four Yamanaka factors. We generated these cells by optimizing the existing iPSC induction protocol. The UCBMC-derived iPSCs (UCB-iPSCs) have characteristics that are identical to pluripotent human embryonic stem cells (hESCs). This study highlights the use of UCBMCs to generate highly functional human iPSCs that could accelerate the development of cell-based regenerative therapy for patients suffering from various diseases.
Keywords: Human UCBMCs, Human iPSCs, Dox-inducible lentiviral system
Introduction
Human pluripotent stem cells can maintain indefinite self-renewal capacity and have the potential to differentiate into any cell types in the body. These unique properties make them useful for cell or tissue replacement therapies as well as an infinite resource for drug screening. When Yamanaka’s [1] and Thomson’s research groups [2] successfully reprogrammed human somatic cells to become induced pluripotent stem cells (iPSCs), ethical issues involved in using human embryonic stem cells (hESCs) were avoided. For cells to be successfully used for cell or tissue therapy, a perfect match of human leukocyte antigens (HLAs) [3] is required between the transplanted cells and the recipient patient’s own cells. Since there is limited availability of early-stage human embryos used for hESC derivation, it is highly difficult to obtain HLA-matched hESCs for all patients. Nonetheless, iPSCs can be derived from the patient’s own somatic cells, thus making it possible to generate HLA-matched cells for each patient. Scientists have successfully reprogrammed several somatic cell types, including fibroblasts [4,5], keratinocytes [6], extraembryonic tissues [7], umbilical cord blood mononuclear cells (UCBMCs) [8–13], peripheral blood cells [13–19] and cells obtained from urine [20,21], to iPSCs.
Several factors, including the age, origin and type of the cells used, deeply impact the reprogramming efficiency and the quality of the iPSCs generated [22]. Among all the human cell types, UCBMCs have many advantages and can be used successfully to generate iPSCs. First, unlike dermal fibroblasts, UCBMCs can be readily collected without invasive procedures. Second, accumulated nuclear and mitochondrial mutations are likely to be present in adult cells [23,24]. Such mutations cannot be corrected during the reprogramming process, and likely influence both the function and the tumor formation risk of iPSCs. In contrast, are young and are expected to carry few somatic mutations compared with adult cells. Third, UCBMCs possess low immunogenicity due to their immunological immaturity. Lastly and most importantly, primary culture of UCBMCs takes only 1–2 days, whereas primary culture of placental chorionic mesenchymal cells and dermal fibroblasts can take more than 10 days. Because the time period from primary cell collection of UCBMCs to iPSC formation is short compared with that from other cell types, and UCBMCs are subjected to less stringent criteria for HLA-donor–recipient selection compared with other cells, iPSCs derived from UCBMCs have wider potential in cell or tissue replacement therapy compared with iPSCs derived from adult cells.
In 2008, scientists successfully reprogrammed mouse B lymphocytes into iPSCs [25], paving the way for derivation of iPSCs from blood cells. Shortly after that, two groups reported that they successfully reprogrammed human cord blood cells into iPSCs [8,9], providing an easily accessible and valuable cell source for the generation of iPSCs that could be used in basic research as well as clinical applications. However, there are still several problems present in iPSCs generation that need to be solved. For example, the reported induction efficiency is extremely low. One study indicates that only five colonies were obtained from 8 × 104 CD133+ cells [8]. Haase et al. reported that the exogenous transgenes of their iPSCs were not fully silenced, implying that the iPSCs might not be completely reprogrammed [9]. Linzhao Cheng’s group used 5 or more factors, including oncogenes such as SV40LT, to reprogram CD34+ cord blood cells [13]. Aberrantly activation of these oncogenes would potentially increase the tumor formation risk posed by iPSCs.
Efficiency and safety are the two major issues involved in clinical applications of iPSCs. Scientists have undertaken major work aimed at improving iPSCs induction efficiency and increasing iPSC safety. To improve iPSC induction efficiency, many small molecules have been used in the reprogramming process, such as valproic acid (VPA) [26], butyrate [27], BIX-01294 and BayK8644 [28], vitamin C [7] and lithium [29]. Among these small molecules, several, such as histone deacetylase inhibitors [26,27] and G9a histone methyltransferase inhibitors [28], affect the activity of epigenetic regulators. The molecules applied to modulate epigenetic regulators may cause off-target effects in iPSCs. For example, when treated with histone deacetylase inhibitor TSA, embryos that have been generated by somatic cell nuclear transfer exhibit poor quality, despite high developmental efficiency [30]. Attempts to increase iPSCs safety have centered on using a minimum of factors and nonviral methods. For example, it was found that Oct4 could independently reprogram human epidermal keratinocytes into iPSCs [31]. Xiao-Bing Zhang’s group has reprogrammed CD34+ human UCBMCs into iPSCs with high efficiency using an EBNA1-based episomal vector carrying Oct4 and Sox2 expression constructs. However, the histone deacetylase inhibitor butyrate was used in the reprogramming process. Previously, our lab successfully converted primed hESCs into a naïve state using X medium [32]. Soon thereafter, we found that X medium could increase the efficiency of pig iPSC induction without the addition of small molecules that affect the activity of epigenetic regulators (Gu et al., unpublished data). We reasoned that X medium could reprogram human UCBMCs into iPSCs more efficiently than could canonical hESC medium.
Here, we describe reprogramming of human UCBMCs into iPSCs using a dox-inducible lentiviral system carrying the four Yamanaka factors with high efficiency via a modified iPSC induction procedure. These iPSCs met all pluripotency criteria commonly applied to hESCs. This high-efficiency process will yield safe iPSCs by employing modified mRNA or proteins with fewer reprogramming factors, which would further advance the development of patient-specific cell therapy using iPSCs derived from UCBMCs.
Results and discussion
Optimization of human UCBMC culture conditions to generate iPSCs
Mononuclear cells were suspended in hematopoietic stem cell (HSC) culture medium and iPSCs were adherent in the culture medium (Figure 1A). To generate human pluripotent stem cells from UCBMCs, these cells must be made to grow as an adherent population. We tried using several kinds of cellular matrix, including Matrigel, Cell Start, laminin, gelatin and MesenCult-XF Attachment Substrate, to induce attachment of UCBMCs for adherent growth. We found that MesenCult-XF Attachment Substrate had the strongest effects in promoting UCBMCs attachment to the surface (Figure 1B). Therefore, in all our subsequent experiments, we used MesenCult-XF Attachment Substrate to pre-coat the culture dishes for iPSCs induction.
Figure 1.
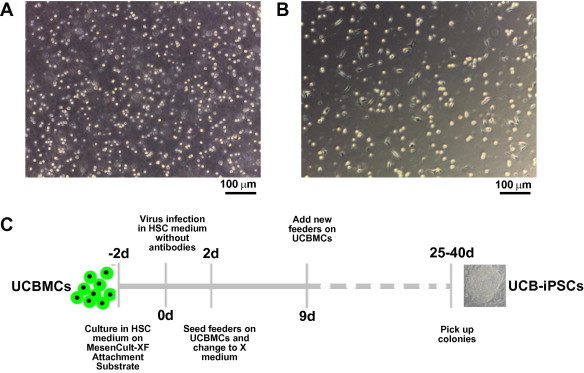
Reprogramming of human UCBMCs into UCB-iPSCs A. Morphology of primary human UCBMCs grown in suspension. Scale bar, 100 μm. B. Morphology of recovered human UCBMCs grown on MesenCult-XF Attachment Substrate. Scale bar, 100 μm. C. Time course of UCB-iPSC generation. UCBMC stands for umbilical cord blood mononuclear cell.
Generation of iPSCs from human UCBMCs with a dox-inducible lentiviral system
It has been demonstrated that adherent cells are easier to reprogram than suspension cells. As mononuclear cells are able to attach efficiently on MesenCult-XF Attachment Substrate, we supposed that iPSCs could be generated efficiently under this condition. Since cryopreserved umbilical cord blood was available in the cord blood banks worldwide, we used cryopreserved UCBMCs for iPSC induction. Human UCBMCs attached on MesenCult-XF Attachment Substrate were transduced with expression constructs for Oct4, Sox2, Klf4 and c-Myc by dox-inducible lentiviral-mediated gene transfer. Since not all blood mononuclear cells became attached to the MesenCult-XF Attachment Substrate, we speculated that passaging the infected attached blood mononuclear cells onto feeders would be difficult and that some of the cells might detach and enter into suspension, which would also influence the iPSC generation. Therefore, we attempted to improve the iPSC induction procedure (Figure 1C). Instead of passaging the infected blood mononuclear cells onto feeders two days after transduction, we added feeders onto the infected cells. Previously, our lab found that X medium can efficiently induce primed hESCs to assume a naïve state without transfection of exogenous genes [32], and, soon after, we found that X medium can also increase the efficiency of pig iPSC induction (Gu et al. unpublished data). From this observation, we reason that X medium may also improve the efficiency of human iPSC induction. Thus, we used X medium instead of the canonical hESC medium in the reprogramming procedure. Twenty-five days post infection, colonies with hESC-like morphology emerged and were picked up to generate stable cell lines.
We used the ratio of the number of ES-like colonies against the number of input blood mononuclear cells to estimate reprogramming efficiency. In our experiment, 100 colonies emerged from approximately 1 × 105 cells subjected to the infection procedure; therefore, the reprogramming efficiency was about 0.1%, which was higher than that reported by Giorgetti et al. (5 colonies from 8 × 104 CD133+ cells) [8]. Our induction efficiency was nearly the same as what Haase et al. reported [9]. However, they selectively used the cord blood endothelial cells, whereas we used cord blood mononuclear cells without selection of progenitor cells. We presume that the undivided mononuclear cells in cord blood may influence the efficiency of iPSC induction.
Without using small molecules that modulate epigenetic regulators, we were able to efficiently generate iPSCs from human UCBMCs by modifying the existing iPSC induction procedure to employ X medium. This will facilitate derivation of clinical-grade iPSCs free of exogenous genes. Our lab found that pig iPSCs can be generated more efficiently in X medium than in the canonical hESC medium. In this study, using our improved iPSC induction procedure, we failed to generate iPSC lines in hESC medium but succeeded in generating iPSCs in X medium, with greater efficiency than that reported for other reprogramming media. These results clearly indicate that X medium is better than hESC medium for the reprogramming process. Using the X medium, we could try to generate iPSCs in endangered species such as the giant panda, Tibetan antelope and tiger in order to study their developmental processes and mechanisms of drug response, which could provide information that may be employed to better protect them.
Human UCB-iPSCs express specific pluripotency markers
We obtained a total of 18 UCB-iPSC lines and selected three for further characterization, including 0627-10, 0627-12 and 0702-7.
As early as passage three, the UCB-iPSCs could be maintained in the absence of dox, which indicated that the UCB-iPSCs were not dependent on exogenous genes, and the endogenous pluripotency genes are fully activated. The UCB-iPSCs exhibited morphology consistent with that of hESCs (Figure 2A). The 0702-7 line was passaged more than 50 times without differentiation and without undergoing apoptosis. These cells expressed alkaline phosphatase (Figure 2B) and possessed a normal karyotype, with 46 (XX) chromosomes (Figure 2C).
Figure 2.
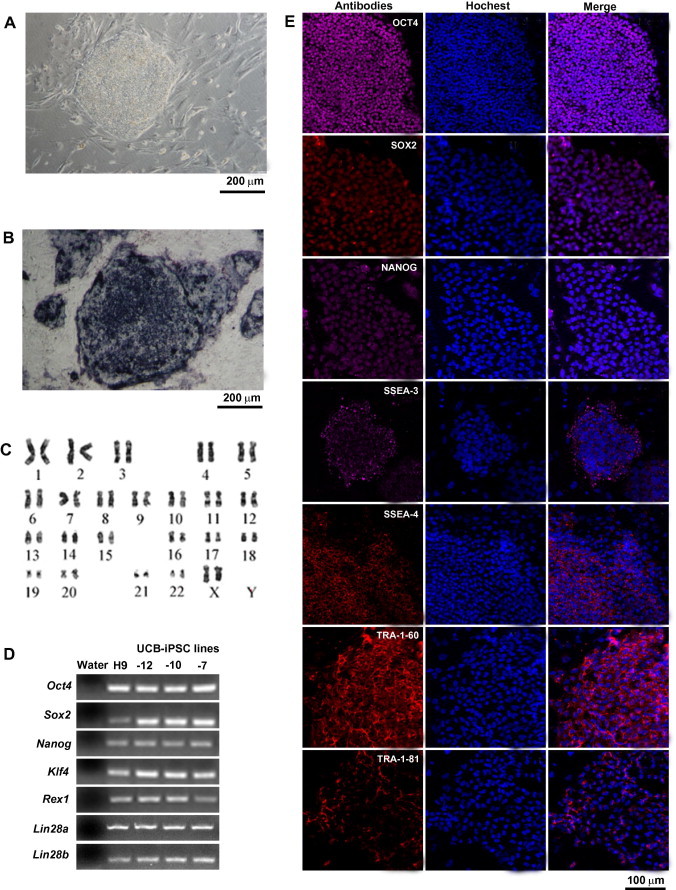
UCB-iPSCs express pluripotency-specific markers A. Morphology of UCB-iPSCs. Scale bar, 200 μm. B. UCB-iPSCs express alkaline phosphatase. Feeder cells were used as negative control. Scale bar is 200 μm. C. Karyotyping of UCB-iPSCs. D. mRNA expression in UCB-iPSC lines performed by RT-PCR. UCB-iPSCs express pluripotency markers Pou5f1 (Oct4), Sox2, Nanog, Klf4, Rex1, Lin28a and Lin28b. Water was used as a negative control, whereas H9 hESCs were used as a positive control. E. Immunofluorescence results of UCB-iPSCs. UCB-iPSCs express pluripotency markers OCT4, SOX2, NANOG, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 at the protein level. SSEA: stage-specific embryonic antigen. Scale bar, 100 μm.
RT-PCR results indicated that the UCB-iPSCs expressed pluripotency genes Oct4 (Pou5f1), Sox2, Klf4, Rex1, Lin28a and Lin28b (Figure 2D). Immunofluorescence results showed that our UCB-iPSCs expressed the pluripotent markers Oct4, Sox2, Nanog, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 at the protein level (Figure 2E).
Human UCB-iPSCs can differentiate into all three germ layers
To verify that UCB-iPSCs generated in our laboratory possess acquired pluripotency, we used the embryoid body (EB) formation assay. Three independent iPSC lines were grown in suspension without bFGF. All three populations formed round EBs the next day. Eight days after plating, we collected the EBs for further study (Figure 3A). RT-PCR analysis showed that the EBs expressed markers of all three germ layers, ectoderm (Gad1 and Pax6), mesoderm (Enolase and Osteonectin) and endoderm (Nicastrin and Alpha-fetoprotein) (Figure 3B). Teratoma formation ability is a hallmark characteristic of human pluripotent stem cells. We injected cells from three independent cell lines subcutaneously into SCID mice and found teratomas in all injected mice after 8 weeks (Figure 3C). Histological analysis revealed that the teratoma comprised tissues of all three germ layers, including epithelium (ectoderm), cartilage (mesoderm), adipose (mesoderm), muscle (mesoderm), intestine (endoderm) and gland (endoderm) (Figure 3D).
Figure 3.
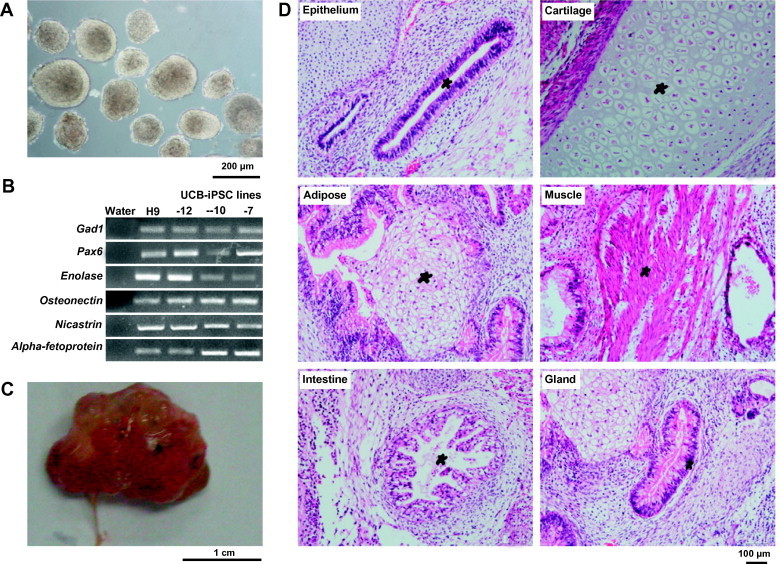
UCB-iPSCs can differentiate into all three germ layers A. Embryonic bodies (EBs) at D8 derived from 0627-10 UCB-iPSCs. Scale bar, 200 μm. B. Gene expression profile for germ-layer marker genes of the D8 EBs, ectoderm (Gad1 and Pax6), mesoderm (Enolase and Osteonectin) and endoderm (Nicastrin and Alpha-fetoprotein. C. Teratoma derived from 0627-10 UCB-iPSCs. D. Hematoxylin and eosin staining of the teratomas. Scale bar, 100 μm. The teratomas contain tissues of all three germ layers, including epithelium (ectoderm), cartilage (mesoderm), adipose (mesoderm), muscle (mesoderm), intestine (endoderm) and gland (endoderm).
In summary, we have successfully reprogrammed human UCBMCs into iPSCs. The obtained UCB-iPSCs exhibited all pluripotency characteristics and passed the testing criteria that have been defined for human pluripotent stem cells. Our findings strongly indicate that the controllable expression of exogenous genes with a dox-inducible system with high efficiency represents an advance toward clinical application of iPSCs in the near future.
There are still many problems associated with the use of iPSCs in clinical trials. For example, random insertion of the exogenous reprogramming genes into the host genome might influence the function of the resultant iPSCs. The cancer-related gene c-Myc, which is among the genes deliberately overexpressed in the reprogramming of iPSCs, might increase the neoplastic potential of the iPSCs [33]. Although Xianmei Meng et al. have successfully reprogrammed human UCBCs using a pCEP4 EBNA1/OriP-based episomal vector containing the woodchuck posttranscriptional regulatory element (WPRE) and expression constructs for Oct4 and Sox2 [34], their reprogramming efficiency was very low and they did not test whether the vector was inserted into the genome. These limitations might influence the clinical application of their iPSCs. Therefore, our future studies will focus on reprogramming human UCBMCs into iPSCs via an episomal vector or mRNA, without using oncogenes but instead using our modified high efficiency reprogramming protocol. This certainly will provide safe and easily accessible iPSCs for clinical therapeutic usage.
Materials and methods
Isolation of mononuclear cells from human umbilical cord blood
Cord blood from healthy volunteers was collected into sterile blood bags and transported to the laboratory on ice. The blood was transferred into a T75 sterile culture flask and mixed with PBS at a 1:1 ratio. Then, 1/4 volume of 0.5% methylcellulose was added to the cell suspension and the flask was incubated without shaking for 30 min at room temperature to allow red blood cells sediment. The supernatant with fewer red blood cells was collected and centrifuged at 1500 rpm at room temperature for 10 min. Then the cell pellet was collected and resuspended in PBS, mixed with Ficoll at a 1:1 ratio and centrifuged at 2000 rpm for 30 min at room temperature. The white middle layer of the solution, which contained the mononuclear cells, was collected and the cells were cultured in HSC medium (see below).
Cell culture
Human UCBMCs were cultured in HSC medium, which contains StemSpan medium (STEMCELL Technologies) supplemented with 10% fetal bovine serum (FBS, Life Technologies), 50 ng/ml TPO (R&D), 50 ng/ml FL (R&D), 50 ng/ml G-CSF (R&D), 10 ng/ml IL3 (R&D) and 10 ng/ml IL6 (R&D). Human UCB-iPSCs were initially cultured in X medium, which was prepared by mixing hESC basic medium and N2B27 medium at a 1:1 ratio and supplemented with 1000 U/ml mLIF (Millipore) and 10 ng/ml bFGF (R&D). The hESC basic medium contained knockout-DMEM (Life Technologies), 20% knockout serum replacement (KOSR, Life Technologies), 100 μM NEAA (Life Technologies), 2 mM l-glutamine (Life Technologies), 55 μM β-mercaptoethanol (Life Technologies) and 1000 U/ml penicillin/streptomycin (Life Technologies). The N2B27 medium was prepared as previously described [35]. Briefly, knockout-DMEM supplemented with N2 (Life Technologies) was combined at a 1:1 ratio with Neurobasal Medium supplemented with B27 (Life Technologies), to which was added NEAA (Life Technologies) at 100 μM, l-glutamine (Life Technologies) at 2 mM, β-mercaptoethanol (Life technologies) at 55 μM, penicillin/streptomycin (Life Technologies) at 1000 U/ml and bovine serum albumin (BSA, Sigma) at 2.5 μg/ml. At passage 5–10, the cells were transferred to hESC basic medium supplemented with 10 ng/ml bFGF. 293T cells were cultured in DMEM (high glucose) and supplemented with 10% FBS, 100 μM NEAA, 2 mM l-glutamine and 1000 U/ml penicillin/streptomycin.
Lentivirus production
Cell culture dishes were coated with 0.1 mg/ml PDL (Sigma) for 2 h at 37 °C and washed with PBS afterward. These pre-coated dishes were used to culture 293T cells. Cells were plated at 6 × 106 cells in a 100-mm pre-coated dish and incubated at 37 °C overnight. Before transfection, medium was removed and replaced with 7.0 ml opti-MEM medium (Life Technologies) per 100-mm dish. 293T cells were transfected with 8 μg PSPAX (Addgene), 4 μg PMD.2G (Addgene) and 12 μg FUW vectors carrying one of the four Yamanaka factors per 100-mm culture dish using Lipofectamine LTX and Plus reagents (Life Technologies). The transfection was done according to the manufacturer’s instruction. Twelve hours post transfection, the transfection medium was removed and 15 ml of DMEM medium (high glucose) with 1% FBS was added per 100-mm dish. Twenty-four hours later, the supernatant was collected as the first virus-containing medium and replenished with 15 ml of fresh DMEM medium supplemented with 1% FBS, which was collected after another 24 h as the second virus-containing supernatant. The virus-containing supernatant was filtered through a filter with the pore size of 0.45 μm (Millipore) and centrifuged in Amicon Ultra-15 Centrifugal Filter Units with Ultracel-100 membrane (Millipore) at 4000 g for 20 min at 4 °C to remove the cell debris and concentrate the virus. The concentrated virus was stored at −80 °C for subsequent use.
Induction of iPSCs from human UCBMCs
UCBMCs were cultured in 12-well sterile culture plates pre-coated with MesenCult-XF Attachment Substrate in HSC medium without antibodies. Each of the four concentrated lentiviral supernatants was added to the culture medium at the dosage of 10 μl per well. Eight μg/ml polybrene (Sigma) was added to the medium to enhance the infection efficiency. Twenty-four hours post transduction, the virus-containing supernatant was removed and HSC medium was added. After 24 h, the HSC medium was replaced with X medium and 1 × 105 feeder cells were added to each well. The medium was changed every day. The developed colonies were picked 25–40 days post transduction and mechanically dissociated with a needle to yield small clumps. Finally, the small clumps were transferred into 24-well plates with feeders and X medium.
Alkaline phosphatase staining
Alkaline phosphatase staining was performed using the Alkaline Phosphatase Assay Kit (Beyotime) according to the manufacturer’s instruction.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 30 min at room temperature and washed with PBS three times. Then, the cells were treated with 0.5% Triton X-100 at room temperature for 1 h for permeabilization. After being washed three times with PBS, the cells were blocked with 2% BSA for 1 h at room temperature. Primary antibodies against OCT4 (1:200 Santa), SOX2 (1:200, Millipore), NANOG (1:200, Abnova), SSEA-3 (1:200, Millipore), SSEA-4 (1:200, Millipore), TRA-1-60 (1:200, Millipore) and TRA-1-81(1:200, Millipore) were diluted in 2% BSA and applied to the cells, which were then incubated at 4 °C overnight. After incubation, cells were washed three times (for 5 min each time) with PBS, and then secondary antibodies including donkey anti-mouse IgG (H+L) conjugated to Cy3 (1:200, Jackson), donkey anti-mouse IgG conjugated to Cy5 (1:200, Jackson), donkey anti-goat IgG conjugated to Cy5 (1:200, Jackson) and donkey anti-rat IgG conjugated to Cy3 (1:200, Jackson) diluted in 2% BSA were applied to the cells, which were then incubated at room temperature for 1 h. Finally, cells were washed three times with PBS (for 5 min each time). Cells nuclei were stained with 1 mg/ml Hoechst 33342 (Invitrogen) for 5 min at room temperature and images were taken using Leica confocal microscopes.
Karyotyping and G-binding
Karytyping and G-binding was performed as previously described [36] at Chinese Academy of Medical Science & Peking Union Medical College.
RNA isolation and reverse transcription
Total RNA from UCB-iPSCs was extracted with Trizol reagent (Life Technologies) using RQ1 RNase-free DNase (Promega) to remove genomic DNA. Then the RNA was reversely transcribed to cDNA with random primers using M-MLV (Promega) according to the manufacturer’s instruction. PCR was performed in a 25 μl solution containing 12.5 μl 2X Es-Taq Master Mix (Cwbiotech), 0.5 μl forward primer (10 μM), 0.5 μl reverse primer (10 μM), 1 μl cDNA and 10 μl water. The PCR reaction was performed in a thermal cycler at 95 °C for 5 min for 1 cycle; 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s for a total number of 30 cycles; 72 °C for 10 min for 1 cycle and followed by holding at 4 °C. The PCR product was analyzed on a 1.5% agarose gel. Primer sequences used in the experiments are shown in Table S1.
EB and teratoma formation
For the EB formation assay, the confluent cells were dissociated into small clumps using 1 mg/ml dispase (Life Technologies). The dissociated small clumps were then cultured in low-attachment dishes using hESC basic medium without added growth factors. The day after plating, the cells were transferred into a new dish to remove attached cells and the medium was changed every other day. Eight days later, the EBs were collected to extract RNA and test for expression of differentiation-related genes.
For the teratoma formation assay, confluent cells in a 6-well plate were harvested by dispase, then collected into tubes, centrifuged at 1000 rpm for 2 min and resuspended in 100 μl PBS. The cells were then injected subcutaneously into SCID mice. The mice were sacrificed 8 weeks after injection according to the guidelines and regulations of the Institutional Animal Care and Use Committee (IACUC). The teratomas were fixed with PBS containing 4% paraformaldehyde, sliced and stained with hematoxylin and eosin.
Authors’ contributions
JW drafted the manuscript and participated in the establishment, culture and characterization of the obtained iPSCs. DB carried out the culture of the obtained iPSCs. QG and JH participated in the culture of UCBMCs and revised the manuscript. LL, XZ, ZL, LW and QZ proposed the idea and revised the manuscript. All authors have read and approved the final manuscript.
Competing interests
The authors have no competing interests to declare.
Acknowledgements
This study was supported in part by grants from China National Basic Research Program of China (Grant No. 2012CBA01301 to QZ, 2013CB967102 to LL and 2012CB966500 to XZ) and the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDA01030101) to LW.
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences and Genetics Society of China.
Appendix A. Supplementary material
Table S1. Primers used in this study.
References
- 1.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowry W.E., Richter L., Yachechko R., Pyle A.D., Tchieu J., Sridharan R. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 6.Aasen T., Raya A., Barrero M.J., Garreta E., Consiglio A., Gonzalez F. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 7.Esteban M.A., Wang T., Qin B., Yang J., Qin D., Cai J. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Giorgetti A., Montserrat N., Aasen T., Gonzalez F., Rodriguez-Piza I., Vassena R. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase A., Olmer R., Schwanke K., Wunderlich S., Merkert S., Hess C. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Nishishita N., Takenaka C., Fusaki N., Kawamata S. Generation of human induced pluripotent stem cells from cord blood cells. J Stem Cells. 2011;6:101–108. [PubMed] [Google Scholar]
- 11.Zaehres H., Kogler G., Arauzo-Bravo M.J., Bleidissel M., Santourlidis S., Weinhold S. Induction of pluripotency in human cord blood unrestricted somatic stem cells. Exp Hematol. 2010;38:809–818. doi: 10.1016/j.exphem.2010.05.009. 818. e1–2. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka C., Nishishita N., Takada N., Jakt L.M., Kawamata S. Effective generation of iPS cells from CD34+ cord blood cells by inhibition of p53. Exp Hematol. 2010;38:154–162. doi: 10.1016/j.exphem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Chou B.K., Mali P., Huang X., Ye Z., Dowey S.N., Resar L.M. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh Y.H., Hartung O., Li H., Guo C., Sahalie J.M., Manos P.D. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seki T., Yuasa S., Oda M., Egashira T., Yae K., Kusumoto D. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Staerk J., Dawlaty M.M., Gao Q., Maetzel D., Hanna J., Sommer C.A. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su R.J., Baylink D.J., Neises A., Kiroyan J.B., Meng X., Payne K.J. Efficient generation of integration-free iPS cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PLoS One. 2013;8:e64496. doi: 10.1371/journal.pone.0064496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sommer A.G., Rozelle S.S., Sullivan S., Mills J.A., Park S.M., Smith B.W. Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp. 2012 doi: 10.3791/4327. pii: 4327. doi: 10.3791/4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack A.A., Kroboth S., Rajesh D., Wang W.B. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS One. 2011;6:e27956. doi: 10.1371/journal.pone.0027956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou T., Benda C., Duzinger S., Huang Y., Li X., Li Y. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22:1221–1228. doi: 10.1681/ASN.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T., Benda C., Dunzinger S., Huang Y., Ho J.C., Yang J. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080–2089. doi: 10.1038/nprot.2012.115. [DOI] [PubMed] [Google Scholar]
- 22.Abyzov A., Mariani J., Palejev D., Zhang Y., Haney M.S., Tomasini L. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature. 2012;492:438–442. doi: 10.1038/nature11629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnheim N., Cortopassi G. Deleterious mitochondrial DNA mutations accumulate in aging human tissues. Mutat Res. 1992;275:157–167. doi: 10.1016/0921-8734(92)90020-p. [DOI] [PubMed] [Google Scholar]
- 24.Ono T., Uehara Y., Saito Y., Ikehata H. Mutation theory of aging, assessed in transgenic mice and knockout mice. Mech Ageing Dev. 2002;123:1543–1552. doi: 10.1016/s0047-6374(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 25.Hanna J., Markoulaki S., Schorderet P., Carey B.W., Beard C., Wernig M. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huangfu D., Maehr R., Guo W., Eijkelenboom A., Snitow M., Chen A.E. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang G., Taranova O., Xia K., Zhang Y. Butyrate promotes induced pluripotent stem cell generation. J Biol Chem. 2010;285:25516–25521. doi: 10.1074/jbc.M110.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y., Desponts C., Do J.T., Hahm H.S., Scholer H.R., Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q., Xu X., Li J., Liu J., Gu H., Zhang R. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res. 2011;21:1424–1435. doi: 10.1038/cr.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tachibana M., Amato P., Sparman M., Gutierrez N.M., Tippner-Hedges R., Ma H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu S., Li W., Zhou H., Wei W., Ambasudhan R., Lin T. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Q., Hao J., Zhao X.Y., Li W., Liu L., Wang L. Rapid conversion of human ESCs into mouse ESC-like pluripotent state by optimizing culture conditions. Protein Cell. 2012;3:71–79. doi: 10.1007/s13238-012-2007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong M., Lv Z., Liu L., Zhu H., Zheng Q.Y., Zhao X.Y. Mice generated from tetraploid complementation competent iPS cells show similar developmental features as those from ES cells but are prone to tumorigenesis. Cell Res. 2011;21:1634–1637. doi: 10.1038/cr.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giorgetti A., Montserrat N., Rodriguez-Piza I., Azqueta C., Veiga A., Izpisua Belmonte J.C. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protoc. 2010;5:811–820. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- 35.Ying Q.L., Smith A.G. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 36.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in this study.



