Abstract
Background
In breast cancer studies, many different endpoints are used. Definitions are often not provided or vary between studies. For instance, “local recurrence” may include different components in similar studies. This limits transparency and comparability of results. This project aimed to reach consensus on the definitions of local event, second primary breast cancer, regional and distant event for breast cancer studies.
Methods
The RAND-UCLA Appropriateness method (modified Delphi method) was used. A Consensus Group of international breast cancer experts was formed, including representatives of all involved clinical disciplines. Consensus was reached in two rounds of online questionnaires and one meeting.
Results
Twenty-four international breast cancer experts participated. Consensus was reached on 134 items in four categories. Local event is defined as any epithelial breast cancer or ductal carcinoma in situ (DCIS) in the ipsilateral breast, or skin and subcutaneous tissue on the ipsilateral thoracic wall. Second primary breast cancer is defined as epithelial breast cancer in the contralateral breast. Regional events are breast cancer in ipsilateral lymph nodes. A distant event is breast cancer in any other location. Therefore, this includes metastasis in contralateral lymph nodes and breast cancer involving the sternal bone. If feasible, tissue sampling of a first, solitary, lesion suspected for metastasis is highly recommended.
Conclusion
This project resulted in consensus-based event definitions for classification of recurrence in breast cancer research. Future breast cancer research projects should adopt these definitions to increase transparency. This should facilitate comparison of results and conducting reviews as well as meta-analysis.
When reporting breast cancer outcomes, many different endpoints are used. Definitions of these endpoints are not consistently provided and vary between trials (1). These inconsistencies limit transparency and comparison of study results. For instance, when interpreting different trials, it is important to know if “breast cancer–free interval” and “disease-specific survival” can be readily compared. Furthermore, even if studies use the same endpoint terminology, these endpoints may not include the same events. An endpoint such as “disease-free survival” may include local, regional, and distant events, as well as mortality and second primary cancer. Even if an endpoint consists of the same events (such as local recurrence), the specific components (eg, breast cancer in skin, metastasis in contralateral lymph node) included in these events may also vary. Therefore, the lack of consistent definition of events lies at the very root of the problem of inconsistent endpoint definitions.
These inconsistencies may compromise transparency of results. Differences in the reported outcome may reflect inconsistent endpoint definitions, rather than treatment effect. This is especially the case when the absolute number of events is low, such as in early breast cancer. When the absolute number of events is small, adding or omitting a component (eg, ipsilateral LCIS to local event) will have a proportionally larger effect on the incidence of the reported outcome.
Therefore, there is need for standardized definitions of endpoints. Several authors have addressed this problem (1–4). Efforts have been made to achieve uniform endpoint definitions in breast cancer research, specifically for the neoadjuvant and adjuvant setting (5,6). Such proposals are important steps towards overcoming this problem. Ideally, definitions are based on evidence regarding incidence, prognostic and therapeutic consequences, importance to patients, and degree to which the component is influenced by the intervention (7). However, for many events in breast cancer research, solid evidence regarding these criteria is not available. Therefore, expert consensus is a suitable alternative.
The aim of this project was to achieve consensus on the definitions of the most commonly used components in breast cancer study endpoints: local event, second primary breast cancer, regional event, and distant event, in order to improve transparency and facilitate comparison of results.
Methods
The RAND/UCLA Appropriateness Method (8) was used to assess consensus in an expert panel on the definitions of local event, second primary breast cancer, regional event, and distant event.
Consensus Methods
Several formal consensus methods are available (9,10). Among these is the Delphi method, which was introduced in the 1950s for decision making and forecasting for military purposes (11). In a Delphi study, several rounds of questionnaires are completed by an expert panel. The aim is convergence of opinions as the process advances, by allowing panel members to adapt their opinions based on input from the panel. This is done anonymously, to minimize the influence of seniority, presumptions of expertise, and dominant characters. Since the introduction, the Delphi method has been used and adapted many times. One of those adaptions is the RAND/UCLA Appropriateness Method (RAM) (8), often used for medical research. The RAM constitutes of a number of questionnaires followed by a face-to-face meeting to address unresolved disagreement.
Steps of the Consensus Process
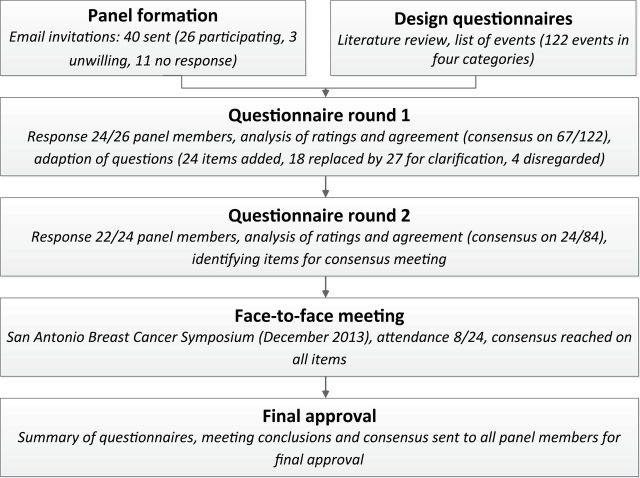
The consensus process is summarized in Figure 1. First, a limited review of the literature was performed to assess which items may be included as local events, second primary breast cancers, regional events, and distant events.
Figure 1.
Flow chart of the consensus process.
Second, breast cancer experts were contacted personally by email to assess their willingness to participate. Potential panel members were selected based on considerable experience with high impact breast cancer research (surgical treatment, radiotherapy, [neo]adjuvant systemic therapy, prognostic, and epidemiological studies), occupation of leading positions on professional boards and societies, leading positions in major breast cancer research groups, and/or leading positions in major journals.
In addition, the aim was to create a balanced panel in terms of discipline, geography, gender, and affiliation to major research groups and professional organizations.
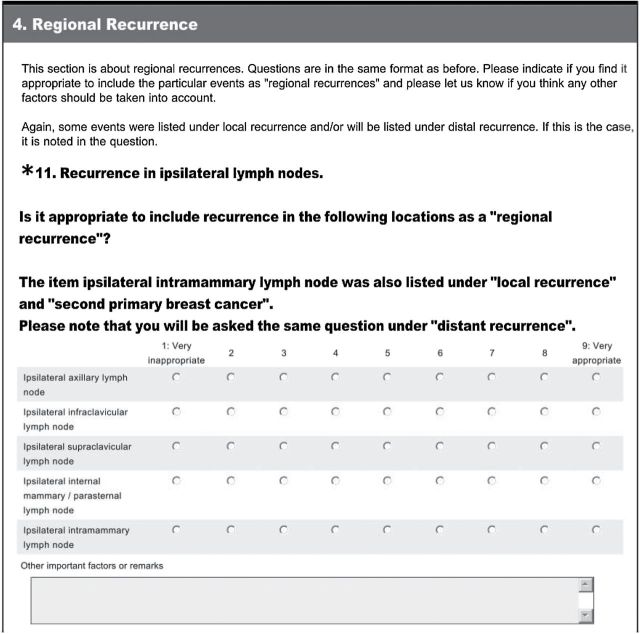
Third, the questionnaires were developed and distributed using SurveyMonkey (SurveyMonkey, Inc., Palo Alto, CA; www.surveymonkey.com). The list of items was based on the literature review, as well as suggestions from breast cancer experts. Panel members were asked to score on a nine-point scale whether they found it appropriate to include the specific item as a local event, second primary breast cancer, regional event, and distant event. No open questions were asked. Participants were encouraged to list additional items and other important factors in free text fields after each question. An example question is shown in Figure 2.
Figure 2.
Example of a question from the first questionnaire.
The second questionnaire was based on the first. Items on which consensus was reached were not repeated. Items that were unclear or ambiguous based on comments in the free text fields were adjusted and repeated. Items suggested by panel members were added. For repeated items, the median and range of the ratings, as well as any additional remarks were provided. Consequently, arguments for rating the item were available to other panel members in the second round and meeting. The results of the second questionnaire were analyzed as described above.
A face-to-face meeting was held during the San Antonio Breast Cancer Symposium in December 2013 to resolve any remaining issues. Panel members who completed the first survey were invited. After introduction of the item with presentation of the median rating, range, and any additional remarks, the item was discussed. After the discussion, panel members rated the item again on a nine-point scale. This lead resulted either in agreement that the item was appropriate or inappropriate, or in the conclusion that current evidence on the item is insufficient for the item to be incorporated into a definition. A summary of the meeting was sent to the entire panel.
Statistical Analysis
The results were exported to MS Excel 2010 (Microsoft Corporation, Redmond WA). Consensus was present if the panel rated the event appropriate or inappropriate (panel median 1–3 or 7–9) without disagreement, which was tested using the IPRAS (interpercentile range adjusted for symmetry) formula in accordance to the RAND/UCLA Appropriateness Method Manual. For more detailed information on the analysis and the definition of disagreement, see the Supplementary Methods (available online).
Results
Panel Formation
Email invitations were sent to 40 persons (10 surgical oncologists, 10 medical oncologists, eight radiation oncologists, five pathologists, three epidemiologists, and four other professionals involved in designing, publishing, or funding of breast cancer research). Of 40 persons, 26 were willing to participate and 11 did not respond. Three persons were unwilling to participate, of whom two felt that their expertise was insufficient (breast cancer currently not main field of interest); one person did not agree with the aim of the project.
Characteristics of Panel Members
The characteristics of the panel members are summarized in Table 1. All clinical breast cancer disciplines are represented.
Table 1.
Characteristics of panel members (n = 24, participants of first questionnaire)
| Characteristics | N |
|---|---|
| Discipline | |
| Epidemiology | 1 |
| Medical oncology | 8 |
| Pathology | 1 |
| Radiation oncology | 5 |
| Surgical oncology | 8 |
| Other | 1 |
| Sex | |
| Female | 8 |
| Male | 16 |
| Continent | |
| Australia | 2 |
| Europe | 12 |
| North America | 10 |
The panel members are affiliated with a variety of professional and research organizations, including American College of Surgeons Oncology Group, American Society of Breast Surgeons, American Society of Clinical Oncology, American Society for Radiation Oncology, Breast International Group, Cochrane Breast Cancer Review Group, Clinical Oncology Society Australia, European Cancer Organisation, European Organisation for Research and Treatment of Cancer, European Society for Medical Oncology, European Society for Radiotherapy and Oncology, European Registration of Cancer Care, International Breast Cancer Study Group, Medical Oncology Group of Australia, National Surgical Adjuvant Breast and Bowel Project, Royal Australian and New Zealand College of Radiologists, Society of Surgical Oncology, as well as several local and national research groups, guideline committees, and professional boards. The above listed institutions themselves were not involved in this project and do not necessarily approve of the consensus.
Participation
The first questionnaire was sent to 26 people and completed by 24. The second questionnaire was sent to all respondents of the first survey, and was completed by 22 of 24. All 24 panel members were invited to the consensus meeting, which took place at the San Antonio Breast Cancer Symposium in December 2013. Eight panel members attended.
First Questionnaire
The first questionnaire consisted of 122 items in four categories, namely local event, second primary breast cancer, regional event, and distant event. Some items were listed in multiple categories. For instance, recurrence in skin on ipsilateral thoracic wall appeared in the local, regional, and distant categories. After the first round, consensus existed on 67 of 122 items (54.9%) and disagreement or uncertainty on 33 of 122 items. Based on additional remarks, four of 122 items were disregarded, and 18 of 122 items were replaced or rephrased for clarification.
Second Questionnaire
The second questionnaire consisted of 84 items, namely items on which consensus did not exist in the first round (n = 33), items added based on additional comments (n = 24), and items which were replaced or clarified (n = 27, replacing 18 items from the first survey). After the second round, consensus existed on 24 of 84 (28.6%) items, in addition to the 67 items on which consensus was reached in the first round.
Final Meeting
In the final meeting, items on which consensus did not exist after two rounds of questionnaires were discussed. These items concerned a limited number of issues, namely classification of breast cancer in skin and subcutaneous tissue (27 items in categories local, regional, and distant event), distinction between local events and new primary ipsilateral breast cancers (13 items in local event and second primary breast cancer), contralateral lymph nodes (14 items in regional and distant event), and appropriate diagnostics of distant events (seven items).
In general, panel members preferred the word “event” over “recurrence,” as the former is more objective and less suggestive of etiology.
The first topic of debate was whether ipsilateral breast cancer should be subclassified as true recurrence or second primary. Several potential factors, such as distance from original tumor, histologic features, and molecular similarity were listed as items in the categories “local event” and “second primary breast cancer.” During the questionnaire rounds, there was disagreement regarding the appropriate classification of events occurring in another quadrant of the breast than the original tumor, events with another morphology/histologic subtype, receptor switch (particularly negative to positive), and distinction based on molecular characteristics such as loss of heterozygosity analysis. Finally, for reasons of simplicity, heterogeneity within tumors, and lack of evidence regarding prognostic significance of this distinction, the panel decided during the meeting that all ipsilateral epithelial breast cancer as well as ductal carcinoma in situ (DCIS) should be considered a local event.
The second topic of debate was isolated recurrence in contralateral lymph nodes (ie, axillary, supraclavicular, infraclavicular, parasternal, or internal mammary), in absence of synchronous malignancy in either breast or synchronous distant metastasis. Initially, a distinction was made between contralateral lymph node events after sentinel lymph node biopsy, axillary lymph node dissection, or axillary radiotherapy, as well as after a previously medially located tumor, and after inflammatory breast cancer. These distinctions were removed because of disagreement. Many panel members felt that contralateral lymph node events are associated with a worse prognosis than ipsilateral lymph node events, but a better prognosis than most distant events. Classifying metastatic contralateral nodes as a separate category was considered. During the meeting, consensus was reached that contralateral lymph node events should be considered distant events. The biology and prognostic and therapeutic consequences of contralateral lymph node events should be subject to future research.
The third topic of debate was resectability. It was suggested that irresectable recurrence should be considered distant. The panel concluded that irresectability is subjective and should not be a reason to classify an event as distant, although outcome might be worse in particular cases.
Finally, the panel discussed whether tissue sampling should be mandatory for a first, solitary lesion suspected for metastasis on imaging. The panel recommended biopsy if feasible. If tissue sampling is not possible (which the panel considered to be very rare), unconfirmed first solitary metastasis is acceptable at the discretion of the treating physician or interdisciplinary tumor board. Multiple lesions consistent with metastases on imaging are acceptable without tissue sampling, although even in these cases, histologic confirmation should be performed if feasible.
Consensus-Based Definitions
The consensus is summarized in Table 2. Consensus was reached on 134 items in four categories. All epithelial breast cancer or DCIS in the ipsilateral (former) breast, or in skin and subcutaneous tissue on the ipsilateral thoracic wall, are considered local events. Second primary breast cancer is epithelial breast cancer in the contralateral breast (with or without nodal involvement on that side). Regional events are breast cancer in ipsilateral lymph nodes (axillary, supraclavicular, infraclavicular, internal mammary, and intramammary). A distant event is breast cancer anywhere else than listed above. Thus, distant events include breast cancer involving the sternal bone, isolated contralateral lymph nodes (axillary, supraclavicular, infraclavicular, parasternal, and internal mammary) in absence of synchronous ipsilateral or contralateral breast malignancy or distant metastasis, as well as skin and subcutaneous tissue outside the ipsilateral thoracic wall. Pathology confirmation of a first, solitary lesion suspected for metastasis on imaging is highly recommended if feasible. Multiple metastases on imaging are acceptable without tissue sampling.
Table 2.
Summary of the consensus on the definition of local event, second primary breast cancer, regional event, and distant event for classification of recurrence in breast cancer research
| Term | Definition |
|---|---|
| Local event (after mastectomy or breast conserving therapy) |
Any epithelial breast cancer or DCIS in ipsilateral breast tissue Breast cancer in surgical scar Breast cancer in biopsy tract Breast cancer in skin and subcutaneous tissue on the (former) ipsilateral breast and ipsilateral thoracic wall * Should NOT include: LCIS, phyllodes tumors, any benign breast lesion, any breast cancer event involving the sternal bone |
| Second primary breast cancer | Any epithelial breast cancer in the contralateral breast (with or without lymph node metastases on that side) |
| Regional event | Breast cancer in ipsilateral axillary, infraclavicular, supraclavicular, internal mammary/ parasternal, or intramammary lymph node |
| Distant event | Breast cancer in any organ other than breast, excluding the items listed under local event, second primary breast cancer, and regional event. Therefore also including any breast cancer event involving the sternal bone Therefore also including breast cancer in contralateral lymph nodes (axillary, infraclavicular, supraclavicular, and internal mammary), in absence of synchronous ipsilateral or contralateral breast malignancy or distant metastasis Tissue sampling Pathology confirmation (histology or cytology) of a first, solitary lesion suspected for metastasis is highly recommended if feasible; if tissue sampling is impossible, unconfirmed metastasis is acceptable at discretion of the treating physician Multiple lesions consistent with metastases on imaging are acceptable without pathology confirmation |
* Ipsilateral thoracic wall: area between contralateral sternal border medially, posterior axillary line laterally, the clavicle superiorly and the (former) inframammary fold inferiorly. DCIS = ductal carcinoma in situ; LCIS = lobular carcinoma in situ.
Discussion
This project used the RAND/UCLA Appropriateness method to develop consensus-based, standardized definitions of local event, second primary breast cancer, regional event, and distant event for use in breast cancer research. Adoption of these definitions in breast cancer studies will increase transparency and facilitate comparison of results.
The definitions are designed for classification of events in research; they are not intended to guide individual patient management. For instance, a recurrence invading the chest wall after mastectomy can be treated with curative intent for one patient, considering it to be a “local” problem, whereas for the next patient it can be considered equivalent to “distant disease” as a consequence of age, comorbidity, and/or extent of the disease. Obviously, this is relevant for managing the individual patient. In contrast, registration of research data requires simplicity and consistency. Additionally, techniques for classification must be available throughout the world. A molecular technique may be promising to distinguish second primary breast cancer from true recurrence. However, if it is not universally available, incorporating it in definitions will compromise reliable comparison of results.
This consensus is based on the opinion of 24 breast cancer experts. Strengths of this approach include selection of panel members in all disciplines involved in breast cancer care and members of most major research groups and a variety of professional societies and boards. Although the number of panel members (particularly, attendance to the final meeting) is an inherent limitation of a consensus project, we consider the panel to be representative.
Results of a formal consensus project can be seen as a systematic evaluation of expert opinions. Expert opinions do not constitute the highest level of evidence, which is a second limitation of this project. If a higher level of evidence can be obtained, this is desirable. In the case of events in endpoints, this would require consistent evidence concerning prognostic and therapeutic relevance of all items. Ideally, a valid composite endpoint consists of elements that are of similar prognostic significance, importance to patients, and incidence, and are influenced by the intervention to a similar degree (7). If this is not the case, reporting the incidence of a composite endpoint may be misleading and differences in prognosis or treatment effect in study arms may not be adequately reflected. Therefore, it would have been appropriate to provide information regarding these criteria for each item. However, in the light of major changes in local treatment, systemic treatment, and diagnostics in the last decades, specific information was not available for most items. The lack of evidence concerning these criteria is both a limitation of this study and the reason why formal expert consensus is a suitable approach. Future research may illuminate prognostic and therapeutic relevance of specific items, prompting adaption of the definitions. In the meantime, however, the problem of inconsistent event definitions is so pressing that the use of standardized definitions is desirable, even if an expert consensus (with its inherent initial disagreement on some topics, as a consensus, by definition, does not reflect everybody’s initial opinion) is the highest level of evidence that can be obtained at this moment.
Using uniform definitions of events in breast cancer research is essential for transparency and reliable comparison of results. Earlier, Hudis (6) and Fumagalli (5) proposed standardized definitions of endpoints for the neoadjuvant and adjuvant setting. An additional proposal may be expected from the Definition for the Assessment of Time-to-event Endpoints in CANcer trials group (12). The current project strengthens these proposals, because uniform definition of endpoints requires uniform definition of included events. The Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials (STEEP) project by Hudis et al. (6), for instance, was specifically designed for the adjuvant setting. Although it is specific about inclusion and exclusion of noninvasive lesions in specific endpoints and distinguishes between invasive ipsilateral breast tumor recurrence and local regional recurrence, the STEEP project left room for interpretation concerning which events should be considered local, regional, and distant. The current project fills this gap. Therefore, it improves applicability in research on local and regional treatment. It also facilitates presenting incidence of specific events in addition to the primary endpoint, as was suggested by Hudis et al. Adoption of these standardized event definitions will improve transparency and will facilitate comparison of study results. This effect will be particularly pronounced when authors report the incidence of separate events (eg, number of local events, regional events) in addition to the primary endpoint. In that case, data will always be comparable, even if the primary endpoint differs.
These consensus-based definitions should be adopted in all breast cancer research using clinical outcomes. This includes research collaborative groups, national cancer institutes, and regulatory authorities. They should be integrated in coding rules for data management. They should also be used as building blocks for composite endpoints in publications. In addition, authors should report the incidence of separate events in addition to the incidence of the primary endpoint.
In conclusion, these consensus-based definitions of local event, second primary breast cancer, regional event, and distant event can serve as building blocks for endpoints in breast cancer research. They should be adopted by data managers of breast cancer studies, as well as researchers initiating, conducting, or publishing results of breast cancer research.
Funding
No funding was received for this study. Martine Moossdorff received a salary from the Cancer Research Fund Limburg (Kankeronderzoeksfonds Limburg).
Supplementary Material
Collaborators:
Members of the Maastricht Breast Cancer Endpoints Consensus Group
Prof. Stefan Aebi, medical oncologist, Luzerner Kantonsspital, Lucerne, Switzerland
Prof. David A. Cameron, medical oncologist, Edinburgh Cancer Research Centre, Western General Hospital, University of Edinburgh, Edinburgh, UK
Prof. J. Michael Dixon, surgical oncologist, Western General Hospital, Edinburgh, Scotland, UK
Prof. Armando E. Giuliano, surgical oncologist, Cedars-Sinai Medical Center, Los Angeles, CA
Prof. Bruce G. Haffty, radiation oncologist, Rutgers Cancer Institute of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ
Dr. Brigid E. Hickey, radiation oncologist, Princess Alexandra Hospital, Brisbane, Australia
Prof. Clifford A. Hudis, medical oncologist, Memorial Sloan Kettering Cancer Center, New York, NY
Prof. V. Suzanne Klimberg, surgical oncologist, Rockefeller Cancer Institute, University of Arkansas, Little Rock, AR
Prof. Bogda Koczwara, medical oncologist, Flinders Centre for Innovation in Cancer, Flinders University, Adelaide, Australia
Prof. Thorsten Kühn, head of the department of Gynecology and Obstetrics, Interdisciplinary Breast Center, Esslingen, Germany
Prof. Marc E. Lippman, medical oncologist, Sylvester Comprehensive Cancer Center/UMHC, Miami, FL
Prof. Anthony Lucci, surgical oncologist, University of Texas/M. D. Anderson Cancer Center, Houston, TX
Prof. Martine J. Piccart-Gebhart, head of medicine Department, Institut Jules Bordet, Université Libre de Bruxelles, Brussels, Belgium
Prof. Emiel J. T. Rutgers, surgical oncologist, Netherlands Cancer Institute - Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands
Dr. Benjamin D. Smith, radiation oncologist, University of Texas/M. D. Anderson Cancer Center, Houston, TX
Prof. Vivianne C. G. Tjan-Heijnen, medical oncologist, Maastricht University Medical Center, Maastricht, the Netherlands
Prof. Kimberly J. Van Zee, surgical oncologist, Memorial Sloan Kettering Cancer Center, New York, NY
Prof. Vincenzo Valentini, radiation oncologist, Università Cattolica S. Cuore, Rome, Italy
Prof. Cornelis J. H. van de Velde, surgical oncologist, Leiden University Medical Center, Leiden, the Netherlands
Prof. Jan B. Vermorken, medical oncologist, University Hospital Antwerp, Edegem, Belgium
Prof. Giuseppe Viale, pathologist, University of Milan at European Institute of Oncology, Milan, Italy
Dr. Adri C. Voogd, epidemiologist, Maastricht University Medical Center, Maastricht, the Netherlands
Prof. Irene Wapnir, surgical oncologist, Stanford University Medical Center, Stanford Women’s Cancer Center, Stanford, CA
Prof. Julia R. White, radiation oncologist, Ohio State University, James Cancer Hospital and Solove Research Institute, Columbus, OH The authors report no conflict of interest.
References
- 1. Mathoulin-Pelissier S, Gourgou-Bourgade S, Bonnetain F, Kramar A. Survival End Point Reporting in Randomized Cancer Clinical Trials: A Review of Major Journals. J Clin Oncol. 2008;26(22):3721–3726. [DOI] [PubMed] [Google Scholar]
- 2. Meropol NJ. Comparative Effectiveness Research to Inform Medical Decisions: The Need for Common Language. J Clin Oncol. 2012;30(34):4192–4193. [DOI] [PubMed] [Google Scholar]
- 3. Cuzick J. Primary endpoints for randomised trials of cancer therapy. Lancet. 2008;371(9631):2156–2158. [DOI] [PubMed] [Google Scholar]
- 4. Nout RA, Fiets WE, Struikmans H, et al. The in- or exclusion of non-breast cancer related death and contralateral breast cancer significantly affects estimated outcome probability in early breast cancer. Breast Cancer Res Treat. 2008;109(3):567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fumagalli D, Bedard PL, Nahleh Z, et al. A common language in neoadjuvant breast cancer clinical trials: proposals for standard definitions and endpoints. Lancet Oncol. 2012;13(6):e240–e248. [DOI] [PubMed] [Google Scholar]
- 6. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials: The STEEP System. J Clin Oncol. 2007;25(15):2127–2132. [DOI] [PubMed] [Google Scholar]
- 7. Montori VM, Permanyer-Miralda G, Ferreira-Gonzalez I, et al. Validity of composite end points in clinical trials. BMJ. 2005;330(7491):594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fitch K, Bernstein SJ, Aguilar MS, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND; 2001. [Google Scholar]
- 9. Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74(9):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linstone HA, Turoff M. The Delphi method: techniques and applications: Addison-Wesley Pub. Co., Advanced Book Program; 1975. [Google Scholar]
- 12. Bellera CA, Pulido M, Gourgou S, et al. Protocol of the Definition for the Assessment of Time-to-event Endpoints in CANcer trials (DATECAN) project: Formal consensus method for the development of guidelines for standardised time-to-event endpoints’ definitions in cancer clinical trials. Eur J Cancer. 2013;49(4):769–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.