Abstract
BACKGROUND
Familial dysautonomia (FD) is a rare hereditary disease characterized by loss of afferent autonomic neural fiber signaling and consequent profound impairment of arterial baroreflex function and blood pressure regulation. Whether vascular endothelial dysfunction contributes to defective vasomotor control in this form of afferent autonomic failure is not known.
METHODS
We assessed blood pressure response to orthostatic stress and vascular endothelial function with brachial artery reactivity testing in 34 FD subjects with afferent autonomic failure and 34 healthy control subjects.
RESULTS
Forty-four percent of the afferent autonomic failure subjects had uncontrolled hypertension at supine rest (median systolic blood pressure = 148mm Hg, interquartile range (IQR) = 144–155mm Hg; median diastolic blood pressure = 83mm Hg, IQR = 78–105mm Hg), and 88% had abnormal response to orthostatic stress (median decrease in systolic blood pressure after upright tilt = 48mm Hg, IQR = 29–61mm Hg). Flow-mediated brachial artery reactivity did not differ in subjects with afferent autonomic failure vs. healthy control subjects (median = 6.00%, IQR = 1.86–11.77%; vs. median = 6.27%, IQR = 4.65–9.34%; P = 0.75). In afferent autonomic failure subjects, brachial artery reactivity was not associated with resting blood pressure or the magnitude of orthostatic hypotension but was decreased in association with reduced glomerular filtration rate (r = 0.62; P < 0.001).
CONCLUSIONS
Brachial artery reactivity was preserved in subjects with afferent autonomic failure despite the presence of marked blood pressure dysregulation. Comorbid renal dysfunction was associated with reduced brachial artery reactivity.
Keywords: autonomic failure, autonomic function, blood pressure, brachial reactivity, endothelial function, hypertension, labile hypertension, orthostatic hypotension
The hereditary sensory and autonomic neuropathies (HSAN) are a heterogeneous group of genetic diseases associated with abnormalities in peripheral neural fiber development and varying degrees of abnormalities in sensory perception and autonomic regulation.1 Familial dysautonomia (FD; also known as Riley-Day syndrome and HSAN type III) is a recessive trait observed most often in persons of Ashkenazi Jewish heritage and is typically associated with decreased pain and temperature perception and marked autonomic dysregulation manifest as swallowing disorders, gastroesophageal reflux, labile hypertension, and orthostatic hypotension.1–3 The specific genetic defect in almost all cases of FD is a single-point missense mutation in the IKBKAP gene that results in production of a truncated dysfunctional form of its gene product, the I-kappa-B kinase complex–associated protein (IKAP).4 Although the detailed mechanisms have not yet been elucidated, IKAP has been shown to play a critical role in the normal differentiation and survival of peripheral nerves during development.5
The IKAP-mediated defect in neural development is associated with generalized loss of the afferent fibers of the baroreceptor reflex but relative preservation of efferent sympathetic neurons.6 In contrast with patients with acquired forms of baroreflex (afferent) failure associated with selective surgical injury or radiation injury to the ninth and tenth cranial nerves,7 loss of afferent autonomic signaling in patients with FD is generalized and includes afferent input from sympathetic afferents with cell bodies in the dorsal root ganglia.8 This results in a unique phenotype characterized by resting supine hypertension, orthostatic hypotension without tachycardia, and labile hypertension associated with emotional arousal (Figure 1).6,9,10
Figure 1.
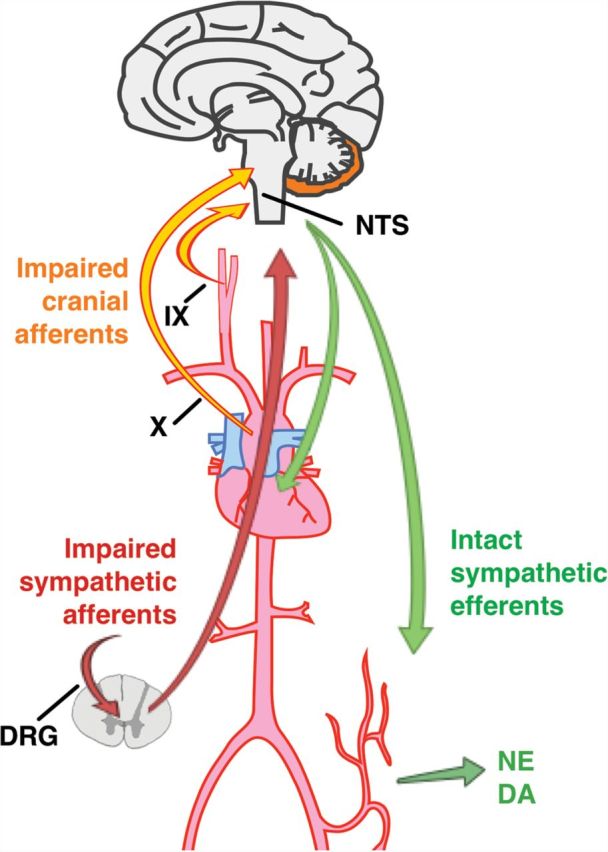
Autonomic regulatory pathways in familial dysautonomia. Involvement of afferent fibers in IX and X cranial nerves results in loss of sensory input from arterial baroreceptors to the brainstem nucleus tractus solitarius (NTS). Involvement of dorsal root ganglia (DRG) impairs sensory input from visceral organs carried in sympathetic afferent fibers, which synapse the level of the spinal cord. Efferent sympathetic fibers to the heart and vasculature remain functionally intact as evidenced by release of norepinephrine (NE) and dopamine (DA) into the circulation in response to emotional arousal. Complete failure of afferent autonomic signaling gives rise to the unique cardiovascular phenotype in familial dysautonomia.
The vascular endothelium plays an important role in the normal regulation of peripheral vasomotor tone and blood pressure.11–15 Endothelial production of nitric oxide and other flow-mediated vasoactive substances in conduit arteries is primarily regulated by the magnitude of local changes in phasic shear forces produced by pulsatile blood flow along the blood vessel wall, but modulatory influences of sympathetic activation on endothelial cell nitric oxide production and neuronal sources of nitric oxide also play important regulatory roles.13,16–18 Endothelium-dependent nitric oxide–mediated vasodilation has been reported to be enhanced in patients with acquired forms of efferent autonomic failure when compared with healthy control subjects, but vascular endothelial function in afferent autonomic failure associated with FD has not been previously described.16 Accordingly, this prospective cohort study was conducted to assess vascular endothelial function with brachial artery reactivity testing in FD subjects with congenital afferent autonomic failure and healthy control subjects.
Methods
Study sample
Afferent autonomic failure subjects (nonsmoking) were recruited from the Dysautonomia Center at the New York University Langone Medical Center. Subjects with genotype confirmed FD (HSAN type III) able to cooperate with study procedures, as described below, were enrolled as a convenience sample between October 2010 and June 2011. Healthy nonsmoking control subjects were recruited from unaffected family members of the afferent autonomic failure subjects who were not carriers of the mutation and from the general population between November 2011 and May 2014. Healthy control subjects did not have a history of chronic disease (hypertension, diabetes mellitus, inflammatory diseases) and were not taking chronic medications. The minimum risk protocol was approved by the New York University Langone Medical Center Institutional Review Board. All subjects provided written informed consent (or for minors, written assent from the child and written consent provided by at least 1 parent or legal guardian) in accordance with institutional guidelines.
Study procedures
Clinical characteristics of afferent autonomic failure subjects were extracted from review of the medical record. Estimated glomerular filtration rate was calculated with the Cockcroft–Gault equation because these estimates have been previously shown to best correlate with direct measurement of creatinine clearance in FD patients with afferent autonomic failure.19,20 Response to orthostatic stress was assessed in afferent autonomic failure subjects on a tilt table with continuous beat-to-beat measurement of blood pressure with a finger plethysmography device (Finometer/Finapres Medical Systems BV, Amsterdam, The Netherlands) calibrated to cuff blood pressure measurements made in the contralateral arm (Colin Press-Mate 7,800, San Antonio, TX), as previously described.6 Each subject rested for at least 20 minutes in a supine position and was then tilted passively to a 30-degree and then to a 60-degree angle, where they remained immobile with footplate support for at least 10 minutes. The orthostatic change in blood pressure was determined as the difference between the systolic blood pressure after 20 minutes of supine rest and the lowest blood pressure recorded during upright tilt.
Brachial arterial reactivity in response to increased blood flow was determined noninvasively with a duplex ultrasound vascular imaging system connected to an 11-MHz linear array probe (Sonosite, Bothell WA), with imaging protocol adapted from guideline recommendations, as previously described.21,22 Subjects were studied in the postabsorptive state. Medications were not discontinued because of potential risk associated with withdrawal of background therapies. Brachial artery diameter (trailing edge of anterior intima–lumen interface to leading edge of posterior lumen–intima interface) was measured in millimeters before cuff occlusion at supine rest and 1 minute after release of 5 minutes of transient distal arterial occlusion (produced by inflation of a forearm cuff to 50mm Hg above measured systolic blood pressure) at end-diastole (identified by the R wave on the electrocardiogram) with a computer-assisted edge detection image analysis system (Artery Measurement Systems, Gothenburg, Sweden) by a single investigator blinded to clinical information.23 Mean blood flow velocity (cm/sec; derived from auto-traced maximum velocity-time integral with software correction for incident angle <60o) was measured with a 1-mm pulsed Doppler sample volume placed at the center of the brachial artery before cuff occlusion at supine rest and for 15 seconds after release of cuff occlusion. The percentage change from brachial diameter before cuff occlusion to brachial artery diameter 1 minute after release of cuff occlusion was calculated as an index of endothelium-dependent, flow-mediated vasodilation in the brachial artery.24 In our laboratory, brachial artery reactivity in healthy adult subjects aged 21–45 years of age is 5.89% ± 2.88% (95% confidence interval = 4.53%–7.23%). Intraobserver coefficient of variance for brachial artery reactivity measurements of the same vessel is 0.1%–0.2%. Estimated brachial artery shear rate (1/s) before and after cuff occlusion was calculated as 4 × mean blood flow velocity/preocclusion brachial artery diameter.21 Normalized brachial artery reactivity adjusted for the shear stress stimulus was calculated as normalized brachial artery reactivity = brachial artery reactivity/estimated post–cuff occlusion shear rate.
Data analysis
All values are presented as medians with the lower boundary (25th percentile) and upper boundary (75th percentile) of the interquartile range (IQR) or as proportions. Comparisons between afferent autonomic failure subjects and healthy control subjects were made with Wilcoxon rank sum test for continuous variables or Fisher’s exact test for categorical variables (Stata 10.1 for Macintosh, College Station, TX). Linear regression was used to estimate Pearson’s correlation coefficient (r) between selected clinical characteristics and flow-mediated dilation in the brachial artery. We did not adjust for family membership in our analyses because healthy siblings were phenotypically normal and the profound phenotypic differences related to homozygosity for the IKBKAP gene mutation far outweigh other hereditability factors relevant to our reported measures. Based on prior normal control data from our laboratory, the sample size of 34 afferent autonomic failure subjects and 34 healthy control subjects provided >80% power to detect a 33% difference in brachial artery reactivity between groups with 2-tailed alpha of 0.05. For all reported analyses, statistical significance was inferred for 2-tailed P values < 0.05.
RESULTS
Clinical characteristics of study sample
The clinical characteristics of the afferent autonomic failure subjects are summarized in Table 1. Forty-four percent of the afferent autonomic failure subjects had uncontrolled hypertension at supine rest (median systolic blood pressure = 148mm Hg, IQR = 144–155mm Hg; median diastolic blood pressure = 83mm Hg, IQR = 78–105mm Hg). Healthy control subjects had similar age and sex distributions but had larger body surface area, lower blood pressure, higher glomerular filtration rate, and higher hemoglobin levels when compared with afferent failure subjects (Table 1).
Table 1.
Characteristics of familial dysautonomia subjects with afferent autonomic failure and healthy control subjects
| Characteristic | FD subjects (n = 34) | Healthy control subjects (n = 34) | P value |
|---|---|---|---|
| Age, y | 23 (15, 28) | 19 (16, 25) | 0.32 |
| Female, % | 47 | 50 | 1.00 |
| Body surface area, m2 | 1.42 (1.13, 1.51) | 1.59 (1.49, 1.88) | 0.001 |
| Supine systolic blood pressure, mm Hg | 137 (128, 147) | 117 (110,123) | <0.001 |
| Supine diastolic blood pressure, mm Hg | 77 (66, 83) | 68 (64, 71) | <0.001 |
| Supine mean blood pressure, mm Hg | 99 (83, 104) | 85 (8, 86) | <0.001 |
| Blood urinary nitrogen, mg/dl | 24 (19, 30) | 13 (12, 16) | <0.001 |
| Serum creatinine, mg/dl | 0.8 (0.7, 1.0) | 0.8 (0.7, 0.9) | 0.66 |
| Estimated glomerular filtration rate, ml/min | 79 (69, 101) | 113 (97, 127) | <0.001 |
| Proteinuria, % | 9 | NA | — |
| Blood glucose, mg/dl | 82 (73, 100) | 81 (76, 86) | 0.79 |
| Hemoglobin, g/100 ml | 12.4 (11.6, 13.2) | 14.4 (13.3, 15.2) | <0.001 |
| Medications, % | |||
| Clonidine | 35 | ||
| Beta-blocker | 6 | ||
| Amlodipine | 9 | ||
| Midodrine | 41 | ||
| Fludrocortisone | 35 | ||
| Benzodiazepines | 47 |
Data presented as median value (25th, 75th percentile) for continuous variables or percentages for dichotomous variables. P value reported for comparison of familiar dysautonomia subjects with afferent autonomic failure vs. healthy control subjects.
Abbreviations: FD, familial dysautonomia; NA, not available.
Response to orthostatic stress
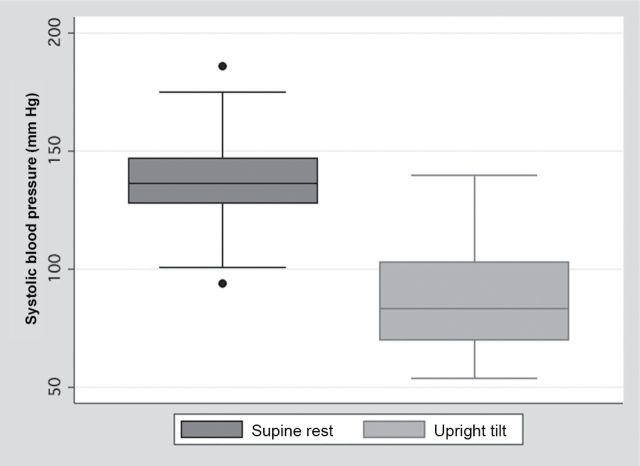
The response to orthostatic stress in the 34 afferent autonomic failure subjects is summarized in Figure 2. The response to orthostatic stress was abnormal (decrease in systolic blood pressure >20mm Hg) in 30 of the 34 subjects (88%).
Figure 2.
Box plots showing the distribution of systolic blood pressure at supine rest (dark gray) and during upright tilt (light gray) in 34 familial dysautonomia (FD) subjects with afferent autonomic failure. Box plots indicate median value (within box), interquartile range (IQR; upper and lower limits of box), values adjacent to 1.5 × IQR (box whiskers), and, if present, individual data points outside this range.
Brachial artery reactivity testing
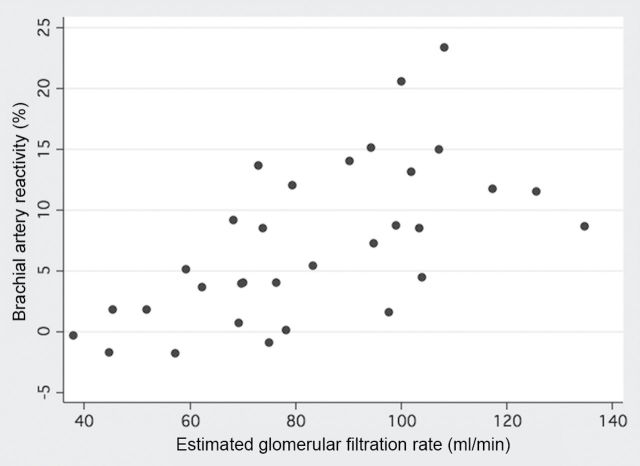
The findings of brachial artery reactivity testing for afferent autonomic failure subjects and healthy control subjects are summarized in Table 2. Brachial artery diameters before and after cuff occlusion were smaller in afferent autonomic failure subjects when compared with healthy control subjects. This difference is attributable to reduced body surface area related to growth retardation in FD because brachial artery diameter normalized to body surface area did not differ between groups. Brachial artery reactivity did not differ between afferent autonomic failure subjects and healthy control subjects. However, there were significant differences between groups in brachial artery mean blood flow velocities before and after cuff occlusion and in post–cuff occlusion estimated brachial artery shear rate. The normalized brachial artery reactivity adjusted for shear rate was significantly lower in afferent autonomic failure subjects when compared with healthy control subjects (afferent autonomic failure subjects median = 0.0069%•s, IQR = 0.0022–0.0097%•s; vs. healthy control subjects median = 0.0099%•s, IQR = 0.0077–0.0129%•s; P = 0.009). Univariate predictors of brachial artery reactivity in afferent autonomic failure subjects and healthy control subjects are listed in Table 3. In afferent autonomic failure subjects, there was no association between brachial artery reactivity and resting supine blood pressure or the decrease in blood pressure in response to orthostatic stress, but brachial artery reactivity was significantly associated with estimated glomerular filtration rate (Figure 3) and other biomarkers of renal function. Brachial artery reactivity in afferent autonomic failure subjects was not associated with use of any of the medications listed in Table 1 (data not shown). In healthy control subjects, brachial artery reactivity was significantly associated with serum creatinine but not other biomarkers of renal function.
Table 2.
Duplex ultrasound–derived variables for brachial artery reactivity testing in 34 healthy control subjects and 34 familial dysautonomia subjects with afferent autonomic failure
| Variable | Pre/post–cuff occlusion | Healthy controls (n = 34) | FD subjects (n = 34) | P value* |
|---|---|---|---|---|
| BA reactivity, % | Post–cuff occlusion | 6.27 (4.65, 9.34) | 6.00 (1.86, 11.77) | 0.75 |
| BA diameter, mm | Pre–cuff occlusion | 3.06 (2.69, 3.53) | 2.67 (2.43, 2.99) | 0.002 |
| Post–cuff occlusion | 3.30 (2.90, 3.63) | 2.83 (2.63, 3.14) | 0.004 | |
| BA diameter normalized to BSA, mm/m2 | Pre–cuff occlusion | 1.88 (1.72, 2.32) | 2.01 (1.80, 2.22) | 0.35 |
| Post–cuff occlusion | 2.03 (1.82, 2.45) | 2.18 (1.95, 2.42) | 0.22 | |
| MBFV, cm/sec | Pre–cuff occlusion | 17.6 (10.9, 25.3) | 11.9 (7.0, 15.9) | 0.01 |
| Post-cuff occlusion | 54.2 (48.3, 65.7) | 65.3 (55.5, 74.9) | 0.02 | |
| Shear rate, 1/s | Pre–cuff occlusion | 198 (106, 296) | 167 (110, 253) | 0.18 |
| Post–cuff occlusion | 705 (629, 851) | 928 (773, 1,214) | <0.001 |
Data are presented as median value (25th, 75th percentile).
Abbreviations: BA, brachial artery; BSA, body surface area; FD, familial dysautonomia; FMD, flow-mediated dilation; MBFV, mean blood flow velocity; PRH, peak reactive hyperemia.
*P values indicate comparison of familial dysautonomia subjects with healthy control subjects.
Table 3.
Regression coefficients for the association between selected clinical characteristics and brachial artery reactivity in 34 familial dysautonomia subjects with afferent autonomic failure and 34 healthy control subjects
| Characteristic | FD subjects (n = 34) | Healthy control subjects (n = 34) | ||
|---|---|---|---|---|
| r | P value | r | P value | |
| Age, y | −0.25 | 0.15 | −0.19 | 0.29 |
| Sex | −0.10 | 0.53 | −0.09 | 0.63 |
| Body surface area, m2 | −0.03 | 0.86 | −0.20 | 0.26 |
| Supine systolic blood pressure, mm Hg | 0.08 | 0.68 | −0.24 | 0.30 |
| Supine diastolic blood pressure, mm Hg | 0.03 | 0.88 | −0.34 | 0.15 |
| Supine mean blood pressure, mm Hg | 0.02 | 0.92 | −0.35 | 0.15 |
| Orthostatic change in systolic blood pressure, mm Hg | 0.07 | 0.68 | NA | NA |
| Blood urinary nitrogen, mg/dl | −0.54 | 0.002 | −0.38 | 0.12 |
| Serum creatinine, mg/dl | −0.46 | 0.008 | −0.58 | 0.01 |
| Estimated glomerular filtration rate, ml/min | 0.62 | <0.001 | 0.06 | 0.49 |
| Blood glucose, mg/dl | −0.11 | 0.60 | −0.30 | 0.23 |
| Hemoglobin, g/100 ml | −0.07 | 0.71 | −0.46 | 0.06 |
Abbreviations: FD, familiar dysautonomia; NA, not available.
Figure 3.
Scatterplot of brachial artery reactivity and estimated glomerular filtration rate in familial dysautonomia (FD) subjects with afferent autonomic failure (Pearson’s correlation coefficient = 0.62; P < 0.001).
Discussion
Our findings demonstrate that brachial artery reactivity in afferent autonomic failure subjects did not significantly differ from that of healthy control subjects and was not associated with resting blood pressure or the magnitude of orthostatic hypotension but was significantly associated with estimated glomerular filtration rate and other biomarkers of renal function.
To our knowledge, this is the first study to report brachial artery reactivity or any other measure of vascular endothelial function in FD subjects with afferent autonomic failure. The finding of preserved flow-mediated brachial artery reactivity in this population was unexpected because most afferent autonomic failure subjects have chronic supine hypertension and/or labile hypertension associated with emotional arousal. Hypertension is known to be associated with endothelial dysfunction, even in younger populations, or in patients with episodic hypertension due to pheochromocytoma.25–27 Our finding of preserved endothelium-dependent vasodilation in subjects with congenital afferent autonomic failure is in accord with a previous report in subjects with acquired forms of efferent autonomic failure.16 However, the underlying mechanisms contributing to these observations are likely divergent. In efferent autonomic failure, enhanced nitric oxide signaling may be attributable to loss of alpha-adrenergic–mediated inhibition of endothelial nitric oxide synthesis.28 In contrast, sympathetic signaling is increased at rest in FD patients with afferent autonomic failure, so preserved brachial artery reactivity must be related to other factors.6 Our findings indicate that preservation of brachial artery reactivity in subjects with afferent autonomic failure was associated with increased post-occlusion shear rate because the normalized brachial artery reactivity was significantly decreased when compared with that of healthy control subjects. Increased post–cuff occlusion shear rate in afferent autonomic failure is attributable to both reduced brachial artery diameter and increased post–cuff occlusion mean blood flow velocity. The observed reduction in brachial artery diameter was proportional to the growth retardation present in the afferent autonomic failure subjects, but an additive effect of excess adrenergic vasoconstriction cannot be excluded. The observed increase in post–cuff occlusion brachial artery mean blood flow velocity is likely due to both increased blood pressure and a greater reduction in post–ischemic vascular resistance in afferent autonomic failure subjects when compared with that of healthy control subjects. Excess metabolic vasodilation could be attributable to inability to further augment adrenergic vasoconstriction in response to ischemia-induced muscle metaboreceptor activation in patients with afferent autonomic failure.29,30
Decreased brachial artery reactivity in afferent autonomic failure was observed in association with worsening renal function, another known risk factor for endothelial dysfunction.31,32 Renal dysfunction in association with pathological evidence of glomerulosclerosis is common in FD patients with congenital afferent autonomic failure.33,34 Renal dysfunction has also been reported in older subjects with supine hypertension and efferent autonomic failure.35 The pathogenesis of renal glomerulsclerosis in afferent autonomic failure is not fully characterized but is thought to be attributable to dysregulation of intraglomerular pressure related to impaired autonomic signaling in the macula densa, episodic hypertension, and episodic intravascular volume depletion related to vomiting crises.33,34 The low prevalence of proteinuria observed in the FD population suggests that the pathogenesis of glomerular injury may differ from that in diabetes mellitus and other kidney disease populations. Worsening endothelial function in the brachial artery in the afferent autonomic failure subjects with impaired renal function might be attributable to systemic inflammation and/or increased oxidant stress associated with chronic kidney disease31,32 or could represent a common pathogenetic mechanism of vascular injury related to hypertension associated with afferent autonomic failure.20,36,37
The unique phenotype of FD subjects with congenital afferent autonomic failure provides a naturally occurring experimental setting to gain insight into the role of autonomic signaling on shear stress–mediated regulation of vasomotor tone in the intact human arterial circulation. Shear stress, induced by phasic pulsatile blood flow along the vascular endothelial cell layer, is known to be an important physiological determinant of vasomotor tone in conduit arteries.38,39 The local vasodilation produced by shear stress is mediated by release of nitric oxide, vasodilating prostanoids, and hyperpolarizing factors by the vascular endothelium.40 Shear stress–mediated vasodilation can occur in the absence of autonomic neural fibers because this response has been observed in isolated denervated blood vessels in vitro,38 but neuronal sources of nitric oxide from non-adrenergic non-cholinergic autonomic nerves and sympathetic activation have also been shown to modulate shear stress–mediated vasodilation in the intact circulation in vivo.16–18,28 Our study design cannot dissect the relative contributions of neuronal vs. endothelial sources of nitric oxide in the observed brachial artery reactivity response in afferent autonomic failure. However, upregulation of either neuronal or endothelial nitric oxide bioavailablity is unlikely because density of autonomic neural fibers has been previously shown to be decreased in the vasculature of FD subjects41 and increased vascular adrenergic signaling in afferent autonomic failure would be expected to attenuate bioavailability of endothelium-derived nitric oxide.6,10,28 Studies with alpha-adrenergic blockade are needed to further characterize the role of adrenergic signaling on vascular endothelial function in the FD population. Compensatory increase in flow-dependent prostanoid vasodilators and/or hyperpolarizing factors were not directly assessed in this study but may have contributed to preservation of flow-mediated brachial artery reactivity in the setting of decreased neuronal and endothelial sources of nitric oxide in afferent autonomic failure.42,43 Increased vascular smooth muscle responsiveness to nitric oxide signaling could also account for our findings and is consistent with a prior report of hypersensitivity to the vasodilating effects of nitroprusside in patients with acquired forms of baroreflex autonomic failure.7
Interpretation of our findings is limited by several caveats. Although medication use was not associated with brachial artery reactivity in univariate analysis, the effects of other related unmeasured clinical factors (confounding by indication) cannot be excluded. Other known correlates of endothelial dysfunction, including cholesterol levels, biomarkers of insulin sensitivity and inflammation, and levels of gonadal hormones, were not measured in our study sample, but both study groups were well within the normal range of values previously determined for our laboratory. Our sample size was powered to detect a clinically meaningful 33% difference between groups, so smaller differences with uncertain clinical impact cannot be excluded. Administration of nitroglycerin to directly assess vascular smooth muscle response to an exogenous source of nitric oxide was not incorporated into the study design because of safety concerns related to the labile blood pressure in the FD population. Accordingly, our results cannot determine whether the preserved brachial reactivity observed in FD subjects is attributable to endothelial vs. vascular smooth muscle adaptations to afferent autonomic failure. Pharmacological inhibition of nitric oxide synthesis and measurement of serum nitrate/nitrite levels were not incorporated in our study design, so the effects of nitric oxide on blood pressure regulation in afferent autonomic failure could not be directly assessed in this study.
In conclusion, vascular endothelial function, as assessed by flow-mediated brachial artery reactivity, is preserved in FD subjects with congenital afferent autonomic failure when compared with healthy controls subjects. Brachial artery reactivity was not associated with resting blood pressure or the magnitude of orthostatic hypotension but was proportionately decreased in subjects with reduced glomerular filtration rate. These novel findings suggest the hypothesis that the presence of adaptive mechanisms to preserve flow-mediated vasodilation in FD subjects may be associated with risk of hypertension-associated end-organ damage in this population. Because our cross-sectional study design does not permit causal inference based on the reported associations, additional work is needed to characterize the pathogenetic mechanisms linking vascular endothelial dysfunction, hypertension, and progression of renal disease in afferent autonomic failure.
DISCLOSURE
Dr Jelani has no conflicts or disclosures. Dr Norcliffe-Kaufmann receives research support from the National Institutes of Health (NIH) and the US Food and Drug Administration (FDA). Dr Kaufmann serves on a scientific advisory board for Chelsea Therapeutics, serves as editor-in-chief of Clinical Autonomic Research; and receives research support from the NIH, the FDA, and the Dysautonomia Foundation. Dr. Katz serves on a cardiovascular events adjudication committee for clinical trials supported by Bristol Meyers Squibb, is a consultant for Merck and Janssen Pharmaceuticals, and receives research support from the NIH and the New York State Empire Clinical Research Investigator Program.
Acknowledgments
This work was supported by the National Institutes of Health (U54NS065736 to H.K. and L.N.-K.), the US Food and Drug Administration (FD-R- 3731-01 to H.K. and L.N.-K.) and the Dysautonomia Foundation (to H.K.).
References
- 1. Axelrod FB, Gold-von Simson G. Hereditary sensory and autonomic neuropathies: types II, III, and IV. Orphanet J Rare Dis 2007; 2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dancis J, Smith AA. Familial dysautonomia. N Engl J Med 1966; 274:207–209. [DOI] [PubMed] [Google Scholar]
- 3. Riley CM. Familial autonomic dysfunction. J Am Med Assoc 1952; 149:1532–1535. [DOI] [PubMed] [Google Scholar]
- 4. Anderson SL, Coli R, Daly IW, Kichula EA, Rork MJ, Volpi SA, Ekstein J, Rubin BY. Familial dysautonomia is caused by mutations of the IKAP gene. Am J Hum Genet 2001; 68:753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunnicutt BJ, Chaverra M, George L, Lefcort F. IKAP/ELP1 is required in vivo for neurogenesis and neuronal survival, but not for neural crest migration. PLoS One 2012; 7:e32050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norcliffe-Kaufmann L, Axelrod F, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology 2010; 75:1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heusser K, Tank J, Luft FC, Jordan J. Baroreflex failure. Hypertension 2005; 45:834–839. [DOI] [PubMed] [Google Scholar]
- 8. Pearson J, Pytel BA, Grover-Johnson N, Axelrod F, Dancis J. Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci 1978; 35:77–92. [DOI] [PubMed] [Google Scholar]
- 9. Mason DT, Kopin IJ, Braunwald E. Abnormalities in reflex control of the circulation in familial dysautonomia. Effects of changes in posture on venous and arterial constriction in normal subjects and in patients with dysautonomia. Am J Med 1966; 41:898–909. [DOI] [PubMed] [Google Scholar]
- 10. Macefield VG, Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H. Cardiac-locked bursts of muscle sympathetic nerve activity are absent in familial dysautonomia. J Physiol 2013; 591:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haynes WG, Noon JP, Walker BR, Webb DJ. Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens 1993; 11:1375–1380. [DOI] [PubMed] [Google Scholar]
- 12. Vanhoutte PM, Mombouli JV. Vascular endothelium: Vasoactive mediators. Prog Cardiovasc Dis 1996; 39:229–238. [DOI] [PubMed] [Google Scholar]
- 13. Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape S, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. Hypertension 2007; 49:170–177. [DOI] [PubMed] [Google Scholar]
- 14. Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002; 105:213–217. [DOI] [PubMed] [Google Scholar]
- 15. Katz SD, Krum H, Khan T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J Am Coll Cardiol 1996; 28:585–590. [DOI] [PubMed] [Google Scholar]
- 16. Gamboa A, Shibao C, Diedrich A, Paranjape SY, Farley G, Christman B, Raj SR, Robertson D, Biaggioni I. Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. Hypertension 2008; 51:1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 2008; 117:1991–1996. [DOI] [PubMed] [Google Scholar]
- 18. Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev 2003; 55:271–324. [DOI] [PubMed] [Google Scholar]
- 19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 20. Norcliffe-Kaufmann L, Axelrod FB, Kaufmann H. Developmental abnormalities, blood pressure variability and renal disease in riley day syndrome. J Hum Hypertens 2013; 27:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 2010; 55:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng H, Cable R, Spencer B, Votto N, Katz SD. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol 2005; 25:1577–1583. [DOI] [PubMed] [Google Scholar]
- 23. Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke 1997; 28:2195–2200. [DOI] [PubMed] [Google Scholar]
- 24. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 25. Panza JA, Quyyumi AA, Brush JE, Jr., Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990; 323:22–27. [DOI] [PubMed] [Google Scholar]
- 26. Higashi Y, Sasaki S, Nakagawa K, Kimura M, Sasaki S, Noma K, Matsuura H, Hara K, Goto C, Oshima T, Chayama K. Excess norepinephrine impairs both endothelium-dependent and -independent vasodilation in patients with pheochromocytoma. Hypertension 2002; 39:513–518. [DOI] [PubMed] [Google Scholar]
- 27. Juonala M, Viikari JSA, Ronnemaa T, Helenius H, Taittonen L, Raitakari OT. Elevated blood pressure in adolescent boys predicts endothelial dysfunction—the Cardiovascular Risk in Young Finns Study. Hypertension 2006; 48:424–430. [DOI] [PubMed] [Google Scholar]
- 28. Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 2002; 39:683–688. [DOI] [PubMed] [Google Scholar]
- 29. Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 1996; 98:584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinoway LI, Wilson JS, Zelis R, Shenberger J, McLaughlin DP, Morris DL, Day FP. Sympathetic tone affects human limb vascular resistance during a maximal metabolic stimulus. Am J Physiol 1988; 255:H937–H946. [DOI] [PubMed] [Google Scholar]
- 31. Bolton CH, Downs LG, Victory JGG, Dwight JF, Tomson CRV, Mackness MI, Pinkney JH. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant 2001; 16:1189–1197. [DOI] [PubMed] [Google Scholar]
- 32. Thambyrajah J, Landray MJ, McGlynn FJ, Jones HJ, Wheeler DC, Townend JN. Abnormalities of endothelial function in patients with predialysis renal failure. Heart 2000; 83:205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pearson J, Gallo G, Gluck M, Axelrod F. Renal disease in familial dysautonomia. Kidney Int 1980; 17:102–112. [DOI] [PubMed] [Google Scholar]
- 34. Elkayam L, Matalon A, Tseng CH, Axelrod F. Prevalence and severity of renal disease in familial dysautonomia. Am J Kidney Dis 2006; 48:780–786. [DOI] [PubMed] [Google Scholar]
- 35. Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL, Biaggioni I, Robertson D. Renal impairment of pure autonomic failure. Hypertension 2009; 54:1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 1992; 90:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perticone F, Maio R, Perticone M, Sciacqua A, Shehaj E, Naccarato P, Sesti G. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation 2010; 122:379–384. [DOI] [PubMed] [Google Scholar]
- 38. Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 1986; 250:H1145–H1149. [DOI] [PubMed] [Google Scholar]
- 39. Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res 1996; 79:984–991. [DOI] [PubMed] [Google Scholar]
- 40. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J Physiol (London) 2005; 568:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grover-Johnson N, Pearson J. Deficient vascular innervation in familial dysautonomia, an explanation for vasomotor instability. Neuropathol Appl Neurobiol 1976; 2:217–224. [Google Scholar]
- 42. Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C, Joannides R. Crucial role of no and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension 2006; 48:1088–1094. [DOI] [PubMed] [Google Scholar]
- 43. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 1999; 100:1951–1957. [DOI] [PubMed] [Google Scholar]