Implementation of a rapid molecular diagnostic for the detection of multidrug-resistant tuberculosis (MDR-TB) led to decreased times to initiation of second-line drug treatment, culture conversion, and decreased the length of time MDR-TB patients were hospitalized on drug-susceptible TB wards.
Keywords: tuberculosis, drug-resistance, MTBDRplus, clinical outcomes
Abstract
Background. There are limited data on the clinical impact of rapid diagnostic tests to detect multidrug-resistant tuberculosis (MDR-TB). We sought to determine whether the use of a molecular diagnostic test to detect MDR-TB improves clinical outcomes.
Methods. A quasi-experimental study was conducted to analyze the impact of the Genotype MTBDRplus assay on clinical outcomes among patients with culture-confirmed pulmonary MDR-TB. Patients received treatment at the National Center for Tuberculosis and Lung Diseases in Tbilisi, Georgia. Time to MDR-TB treatment initiation, culture conversion, and infection control measures were compared to a time period prior to the implementation of the molecular test.
Results. Of 152 MDR-TB patients, 72 (47%) were from prior to and 80 (53%) following implementation of the MTBDRplus assay (“post-implementation group”). Patients in the post-implementation group initiated a second-line treatment regimen more rapidly than those in the pre-implementation group (18.2 vs 83.9 days, P < .01). Among patients admitted to a “drug-susceptible” tuberculosis ward, those from the post-implementation group spent significantly fewer days on the drug-susceptible ward compared to patients in the pre-implementation group (10.0 vs 58.3 days, P < .01). Among patients with 24 weeks follow-up (n = 119), those in the post-implementation group had a higher rate of culture conversion at 24 weeks (86% vs 63%, P < .01) and a more rapid rate of time to culture conversion (adjusted hazard ratio [aHR] 4.15, 95% confidence interval [CI], 2.5–6.9).
Conclusions. The implementation of a rapid molecular diagnostic test led to significant clinical improvements including reduced time to initiation of MDR-TB treatment, culture conversion, and improved infection control practices.
Multidrug-resistant tuberculosis (MDR-TB), defined as resistance to isoniazid and rifampicin, is an enormous public health problem. The World Health Organization (WHO) estimated 450 000 new cases of MDR-TB in 2012 and 170 000 MDR-TB related deaths [1]. WHO also indicated that current control efforts are “off-track” in managing MDR-TB and that addressing the epidemic should be a public health priority [1]. In addition to the advancement of new drugs, an integral issue in the successful management of MDR-TB is the implementation of accurate rapid tests for detection of drug resistance.
The highest rates of MDR-TB have been reported from Eastern Europe, primarily in former Soviet republics [1]. This includes Georgia, one of 27 high-burden MDR-TB countries as designated by WHO. In 2012, 9% of newly diagnosed and 31% of retreatment cases had MDR-TB [1]. In 2008, Georgia became one of the first lower-middle-income countries to achieve universal access to diagnosis and treatment of MDR-TB. However, the rate of favorable outcomes among the first MDR-TB cohort was low (54%), in part due to a large number of chronic tuberculosis cases [2]. In an effort to improve MDR-TB care, the Georgian National Tuberculosis Program validated and subsequently implemented the use of a molecular diagnostic test, the Genotype MTBDRplus line probe assay (LPA), in 2009–2010 [3].
Globally, fewer than 25% of patients with MDR-TB have drug resistance detected [1]. In settings where drug resistance testing is available, culture-based drug-susceptibility testing (DST) remains the most commonly utilized method. Although culture-based DST is considered the gold standard, it requires up to 8 weeks for results. The delay in detecting drug resistance may postpone the administration of second-line drug (SLD) regimens used to treat MDR-TB and increase the likelihood of poor clinical outcomes and disease transmission. Two rapid molecular diagnostic tests have recently been developed and endorsed by WHO including a LPA (MTBDRplus) and the Xpert MTB/RIF assay [4, 5]. Both assays use detection of genetic mutations to detect rifampicin resistance (Xpert) or both rifampicin and isoniazid resistance (MTBDRplus). Several studies have demonstrated that the assays perform well in detecting Mycobacterium tuberculosis and associated drug resistance; especially among acid fast bacilli (AFB) smear-positive specimens [6, 7]. However, there are limited data on the clinical impact of these tests. A recent policy paper by the Infectious Diseases Society of America emphasizes the importance and relevance of measuring the clinical impact of new diagnostic tests [8]. Data on the clinical utility of new tuberculosis drug resistance detection tests is needed to inform National Tuberculosis Programs on how best to allocate limited resources and implement new testing algorithms. In this study, we sought to determine the impact of MTBDRplus implementation on clinical outcomes including time to MDR-TB treatment initiation, culture conversion, and infection control measures.
METHODS
Population
We conducted a quasi-experimental study [9] of patients with pulmonary MDR-TB treated at the National Center for Tuberculosis Lung Diseases (NCTLD) in Tbilisi, Georgia. All patients had sputum culture positive tuberculosis disease with MDR confirmed by conventional DST. Consecutive patients with MDR-TB initially detected with the Genotype MTBDRplus LPA, from June 2010 to October 2012 (post-implementation group) were compared to consecutive patients that had MDR-TB detected by conventional culture and DST from March 2009 to May 2010 prior to implementation of the LPA (pre-implementation group). Approval for this study was received from the Georgian NCTLD and Emory University Institutional Review Board.
Cultures and Drug-Susceptibility Testing
Direct sputum smears with Ziehl–Neelsen staining were examined by light microscopy and each patient had 1 AFB smear positive sample sent to the National Reference Laboratory (NRL) in Tbilisi for processing [3]. The processed specimen was inoculated onto both Löwenstein–Jensen (LJ) based solid medium and the BACTEC MGIT 960 broth culture system. Positive cultures by either method were confirmed to be M. tuberculosis complex (MTBC) using the MTBDRplus assay along with colony morphology [3]. DST for isoniazid (INH) and rifampicin (RIF) was performed using either the absolute concentration method on LJ medium (INH 0.2 µg/mL, RIF 40 µg/mL) or in 7H9 broth with the BACTECT MGIT 960 system (INH 0.1 µg/mL, RIF 1 µg/mL) [3]. DST to SLDs was performed using the proportion method on LJ medium as previously described [10]. The NRL undergoes annual external quality assessment by the Antwerp WHO Supranational Tuberculosis Reference Laboratory and in 2012 there was 100% accuracy for all drugs tested including INH and RIF. As per standard of care, follow-up sputum cultures were performed monthly during the intensive phase of MDR-TB treatment (minimum 6 months).
Molecular Diagnostic Test
The MTBDRplus assay was performed directly on sputum samples and according to the manufacturer's instructions [11]. A portion of the same sputum specimen was used for both molecular testing and culture at the NRL. A 500 µL portion of decontaminated sample was used for DNA isolation, subsequent amplification, and hybridization [11]. Each test strip consists of 27 reaction zones (bands) including controls that were interpreted to determine test validity, MTBC identification, and resistance to INH and RIF. The LPA was performed 2–3 times per week with between 2 and 8 samples used per run.
Laboratory technicians filled out a MTBDRplus result data form and delivered them to the treating physician or department head for patients treated at the NCTLD (ambulatory department or hospital). For patients treated at other outpatient tuberculosis clinics, forms were delivered by a laboratory transport courier. After delivery, forms were included in the medical records.
Data Collection
Data were abstracted from the following sources: medical charts, patient treatment cards, the national tuberculosis database, and reference laboratory database. Information was collected pertaining to sociodemographic characteristics; tuberculosis history and current tuberculosis presentation; patient treatment follow-up; and laboratory results. As some study patients remained on treatment, final treatment outcomes were not collected for these analyses.
Definitions
The time to MDR-TB treatment initiation was defined as the time from initial sputum collection to start of SLD therapy. The time to culture conversion was defined as the time from sputum collection to the date of the first of 2 consecutive negative sputum cultures performed at least 1 month apart. An empiric treatment regimen (ETR) was defined as a SLD regimen that was initiated before receiving the results of DST while an individualized treatment regimen (ITR) was defined as a tailored drug regimen based on DST results. Initial MDR-TB treatment was defined as any drug received within 30 days of starting a SLD regimen. Treatment interruption was defined as a continuous interruption of SLDs for ≥1 week.
Treatment
The NCTLD drug resistance tuberculosis treatment committee provides initial guidance on choosing a SLD regimen for MDR-TB patients. An ETR was initiated before second-line DST results were available. After final DST results, treatment regimens were individualized based on the results and guided by WHO recommendations. When possible, regimens were designed to include at least 4 drugs to which the patient's M. tuberculosis isolate was susceptible. All treatment regimens included a fluoroquinolone, an injectable agent, and pyrazinamide. All patients received treatment through directly observed therapy (DOT). Upon initiating MDR-TB treatment, patients were recommended to receive initial care as an inpatient and were recommended to remain hospitalized until at least achieving smear and/or culture conversion. Drug-susceptible tuberculosis patients are hospitalized at their physician's discretion. Patients with unknown or pending DST results are generally admitted to a drug-susceptible ward while those with known MDR are cohorted to a separate drug-resistant ward.
Data Analysis
Data analyses were performed using SAS software, version 9.3. For descriptive statistics, differences in categorical variables were tested using either the χ2 or Fisher exact test and for continuous variables a 2-sample t-test was used. A 2-sided P-value of <.05 was considered significant. A Cox proportional hazards model was used to estimate the adjusted association between cohorts in the pre- and post-implementation phases and the rate of culture conversion. Patients without culture conversion at 24 weeks were censored at this time point. Cox model building and covariate selection was based on the purposeful selection of patient level factors as previously described [12].
RESULTS
Patient Characteristics
In total, 152 AFB sputum smear positive patients with MDR-TB were included in the study, including 72 pre- and 80 post-implementation of the LPA (Figure 1). The mean age was 38 years, and most patients were male (73%; Table 1). Most patients were unemployed (86%), and 28% had a history of imprisonment. In regards to comorbidities, 13% of patients had diabetes mellitus, 13% had hepatitis C virus (HCV) infection, and 3% were coinfected with HIV. Almost half (45%) of all patients had a prior history of tuberculosis, including 9% who had been previously treated for MDR-TB. One fifth of patients had cavitary disease, and the mean number of anti-TB drugs that were resistant by DST was 5.5. Nearly all patients received the following SLDs as part of their initial MDR-TB treatment regimen: pyrazinamide, prothionamide, levofloxacin, cycloserine, and para-aminosalicylic acid (Table 1). Additionally, the majority of patients initially received kanamycin (61%).
Figure 1.
Study diagram.
Table 1.
A Comparison of Patient Characteristics Among MDR-TB Patients From Pre- and Post-Implementation of a Rapid Molecular Diagnostic Test to Detect Drug-Resistant Tuberculosis (N = 152)
| Characteristic | Overall N = 152 (%) |
Molecular Diagnostic Test Implementation Period |
P Value | |
|---|---|---|---|---|
| Pre N = 72 (%) |
Post N = 80 (%) |
|||
| Mean age [IQR] | 37.8 [27–47] | 39.2 [26–53] | 36.5 [29–43] | .23 |
| Male | 111 (73) | 52 (72) | 59 (74) | .83 |
| Married | 77 (51) | 36 (50) | 41 (51) | .88 |
| Employed | 21 (14) | 12 (17) | 9 (11) | .33 |
| History of imprisonment | 42 (28) | 18 (25) | 24 (30) | .49 |
| Diabetes | 19 (13) | 8 (11) | 11 (14) | .62 |
| Hepatitis C | 20 (13) | 4 (6) | 16 (20) | <.01 |
| HIV | 5 (3) | 1 (1) | 4 (5) | .21 |
| BMI (kg/m2) [IQR] | 20.4 [19–22] | 20.6 [19–23] | 20.3 [18–22] | .53 |
| History of tuberculosis | 69 (45) | 27 (38) | 42 (53) | .06 |
| Prior tuberculosis treatment | .02 | |||
| None | 83 (55) | 45 (63) | 38 (48) | |
| First-line treatment | 55 (36) | 25 (35) | 30 (38) | |
| Second-line treatment | 14 (9) | 2 (3) | 12 (15) | |
| Cavitary disease | 31 (20) | 13 (18) | 18 (23) | .50 |
| Mean number of resistant drugs by DST [IQR] | 5.5 [5–6] | 5.3 [4–6] | 5.7 [5–7] | .03 |
| Any Fluoroquinolone Resistant | 22 (15) | 7 (10) | 15 (19) | .11 |
| Extensively drug resistant | 11 (7) | 4 (6) | 7 (9) | .45 |
| Initial treatment site | <.01 | |||
| Inpatient | 50 (33) | 16 (22) | 34 (43) | |
| Outpatient | 102 (67) | 56 (78) | 46 (58) | |
| Initial MDR-TB treatment | ||||
| Pyrazinamide | 150 (99) | 72 (100) | 78 (98) | .18 |
| Prothionamide | 152 (100) | 72 (100) | 80 (100) | … |
| Kanamycin | 92 (61) | 32 (44) | 60 (75) | <.01 |
| Capreomycin | 67 (44) | 47 (65) | 20 (25) | <.01 |
| Levofloxacin | 140 (92) | 67 (93) | 73 (91) | .68 |
| Cycloserine | 145 (95) | 69 (96) | 76 (95) | .81 |
| Para-aminosalicyclic acid | 151 (99) | 71 (99) | 80 (100) | .29 |
| Treatment interruption | 60 (40) | 24 (33) | 36 (45) | .14 |
Abbreviations: BMI, body mass index; DST, drug susceptibility testing; HIV, human immunodeficiency virus; IQR, interquartile range; MDR-TB, multidrug resistant-tuberculosis.
There were a few significant differences in characteristics between patients in the pre- and post-implementation groups (Table 1). Patients in the post-implementation group had higher rates of coinfection with HCV (20% vs 6%, P < .01) and were more likely to receive initial treatment as an inpatient (43% vs 22%, P < .01) vs patients in the pre-implementation group. Those in the post-implementation group had higher rates of receiving MDR-TB treatment in the past (15% vs 3%, P = .02) and were resistant to more drugs by DST (5.7 vs 5.3, P = .03) as compared to the pre-implementation group. In regards to initial MDR-TB treatment, patients in the post-implementation group were more likely to have received kanamycin (75% vs 44%, P < .01), whereas those in the pre-implementation group were more likely to have received capreomycin (65% vs 25%, P < .01).
Treatment
Overall, 88% of patients received a first-line drug regimen for some period of time with more patients in the pre- vs post-implementation group having received first-line drugs (99% vs 78%, P < .01; Table 2). Of those patients receiving first-line anti-tuberculosis drugs, the average days on treatment was 11.2 days in the post-implementation group compared to 74.5 days in the pre-implementation group (P < .01). Compared to the post-implementation group, patients in the pre-implementation group had a longer delay in starting a SLD regimen (83.9 vs 18.2 days, P < .01).
Table 2.
Clinical Outcomes Among MDR-TB Patients Before and After Implementation of a Rapid Diagnostic Test to Detect Drug-Resistant Tuberculosis (N = 152)
| Characteristic | Overall N = 152 | Molecular Diagnostic Test Implementation Period |
P Value | |
|---|---|---|---|---|
| Pre (N = 72) | Post (N = 80) | |||
| Treatment | ||||
| Patients receiving a first-line drug regimen (%)a | 133 (88) | 71 (99) | 62 (78) | <.01 |
| Mean days on first-line treatment (n = 133) [IQR] | 45 [11–77] | 74.5 [45–95] | 11.2 [7–15] | <.01 |
| Mean days on first-line treatment (n = 152) [IQR] | 39 [7–72] | 73.4 [45–95] | 8.7 [2–14] | <.01 |
| Mean days until starting a treatment regimen for MDR-TB with SLDs [IQR] | 49 [17–83] | 83.9 [56–106] | 18.2 [11–24] | <.01 |
| ITR regimen different than ETR (%) | 103 (68) | 53 (74) | 50 (63) | .14 |
| Infection Control Measures | ||||
| Placed on ward for patients with drug-susceptible tuberculosis (%) | 39 (26) | 20 (28) | 19 (24) | .57 |
| Mean days on drug-susceptible tuberculosis ward [IQR] (n = 39) | 34.7 [8–69] | 58.3 [35–74] | 10.0 [6–14] | <.01 |
| Mean total days of hospitalization [IQR] (n = 39) | 179.0 [84–215] | 207.5 [124–216] | 149.1 [56–184] | .20 |
| Total days of hospitalization [IQR] (n = 152) | 102.4 [45–135] | 123.5 [49–165] | 83.5 [43–93] | <.01 |
| Days until hospitalization among patients receiving first-line treatment and outpatient therapy (n = 102) | 54.8 [18–84] | 85.3 [48–107] | 17.7 [14–22] | <.01 |
Abbreviations: ETR, empiric treatment regimen; IQR, interquartile range; ITR, individualized treatment regimen; MDR-TB, multidrug resistant-tuberculosis; SLD, second-line drug.
a First-line drug regimen consisted of rifampin, isoniazid, ethambutol, and pyrazinamide.
Infection Control Measures
All 152 patients were eventually hospitalized for MDR-TB treatment and placed on a drug-resistant tuberculosis ward. However, 39 of 152 (26%) patients were hospitalized before their drug-resistant status was known and placed on a drug-susceptible ward, with no differences between the 2 groups (Table 2). Among these 39 patients with MDR-TB, those in the pre-implementation group spent more time on a drug-susceptible ward (58.3 vs 10.0 days, P < .01) and tended to have longer overall hospital stays (207.5 vs 149.1 days, P = .20) vs patients in the post-implementation group. A total of 102 AFB sputum smear positive MDR-TB patients initiated first-line drug treatment as an outpatient before their drug-resistant status was known. Of these 102 patients, the mean days until hospitalization and initiation of SLD treatment was 54.8 days: 17.7 days in the post-implementation group compared to 85.3 days in the pre-implementation group (P < .01).
Sputum Conversion
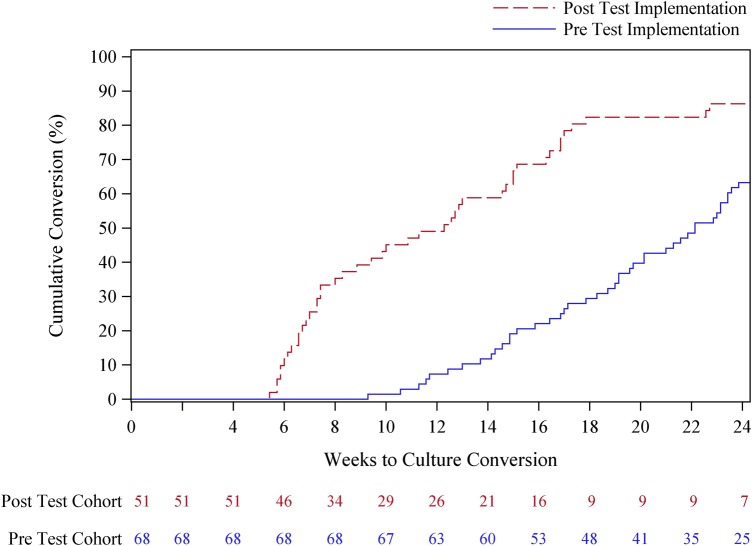
A total of 119 patients had at least 24 weeks of follow-up time and were included in the analysis of conversion rates. Of the 33 patients with <24 weeks of follow-up, the main reason for exclusion was treatment default (n = 27) followed by death (n = 6). Among the 119 patients who could be evaluated for conversion, a total of 82% and 73% achieved sputum smear and culture conversion by 6 months, respectively (Table 3). Patients in the post-implementation group were significantly more likely to have culture conversion by 24 weeks compared to those in the pre-implementation group (86% vs 63%, P = .01) and had higher but nonsignificant rates of smear conversion at 24 weeks (90% vs 77%, P = .05). The use of the LPA in clinical management was significantly associated with increased rate of culture conversion in both univariate (hazard ratio [HR] 2.99, 95% confidence interval [CI], 2.0–4.6) and multivariate analysis (adjusted HR [aHR] 4.24, 95% CI, 2.7–6.8). Variables significantly associated with decreased rate of culture conversion in multivariate analysis included increasing age and ofloxacin resistance (Table 4). A cumulative conversion graph shows the decreased time to culture conversion among patients in the post-implementation vs pre-implementation group (Figure 2).
Table 3.
Cumulative Sputum Culture Conversion by Selected Characteristics (N = 119)
| Covariate | Cumulative Culture Conversion |
P Value | |||
|---|---|---|---|---|---|
| 12 wks |
24 wks |
||||
| n/N | % | n/N | % | ||
| OVERALL | 30/119 | 25 | 87/119 | 73 | … |
| Group | |||||
| Pre-test implementation | 5/68 | 7 | 43/68 | 63 | <.01 |
| Post-test implementation | 25/51 | 49 | 44/51 | 86 | |
| Gender | |||||
| Male | 17/82 | 21 | 58/82 | 71 | .20 |
| Female | 13/37 | 35 | 29/37 | 78 | |
| BMI (kg/m2) | |||||
| ≤18.5 | 10/31 | 32 | 20/31 | 65 | .60 |
| >18.5 | 20/88 | 23 | 67/88 | 76 | |
| Tuberculosis history | |||||
| Yes | 12/48 | 25 | 31/48 | 65 | .18 |
| No | 18/71 | 25 | 56/71 | 79 | |
| Diabetes mellitus | |||||
| Yes | 3/15 | 20 | 12/15 | 80 | .74 |
| No | 27/104 | 26 | 75/104 | 72 | |
| Hepatitis C | |||||
| Yes | 2/9 | 22 | 4/9 | 44 | .16 |
| No | 28/110 | 26 | 83/110 | 76 | |
| Cavitary disease | |||||
| Yes | 3/21 | 14 | 12/21 | 57 | .11 |
| No | 27/98 | 28 | 75/98 | 77 | |
| XDR | |||||
| Yes | 0/7 | 0 | 5/7 | 71 | .63 |
| No | 30/112 | 27 | 82/112 | 73 | |
| Ofloxacin resistance | |||||
| Yes | 0/13 | 0 | 6/13 | 46 | .03 |
| No | 30/106 | 28 | 81/106 | 76 | |
| Capreomycin or kanamycin resistance | |||||
| Yes | 14/42 | 33 | 32/42 | 76 | .36 |
| No | 16/77 | 21 | 55/77 | 71 | |
| Initial treatment with kanamycin | |||||
| Yes | 20/68 | 29 | 52/68 | 77 | .11 |
| No | 10/51 | 20 | 35/51 | 69 | |
| Treatment interruption | |||||
| Yes | 13/46 | 28 | 34/46 | 74 | .72 |
| No | 17/73 | 23 | 53/73 | 73 | |
Abbreviations: BMI, body mass index; XDR, extensively drug-resistant.
Table 4.
Univariate and Multivariate of Predictors for Culture Conversion Among Patients With Multidrug-Resistant Tuberculosis (n = 119)a
| Predictor | Univariate Analysis |
Multivariate Analysisb
|
||
|---|---|---|---|---|
| HR | 95% CI | aHR | 95% CI | |
| Use of MTDBRplus test | 2.99 | (1.95–4.59) | 4.24 | (2.66–6.75) |
| Age, per year | 0.98 | (.97–.99) | 0.98 | (.96–.99) |
| Male | 0.75 | (.48–1.16) | … | … |
| BMI ≤ 18.5 kg/m2 | 0.88 | (.53–1.44) | … | … |
| Prior history of tuberculosis | 0.75 | (.48–1.16) | 0.63 | (.40–1.00) |
| Diabetes | 1.11 | (.60–2.04) | … | … |
| Hepatitis C | 0.52 | (.19–1.42) | … | … |
| Cavitary disease | 0.60 | (.33–1.11) | … | … |
| XDR | 0.80 | (.33–1.99) | … | … |
| Ofloxacin resistance | 0.41 | (.18–.93) | 0.25 | (.10–.59) |
| Capreomycin or Kanamycin resistance | 1.23 | (.79–1.90) | … | … |
| Initial treatment with kanamycin | 1.42 | (.93–2.19) | ||
| Treatment interruption | 1.08 | (.70–1.67) | … | … |
Abbreviations: aHR, adjusted hazard ratio; BMI, body mass index; CI, confidence interval; HR, hazard ratio; XDR, extensively drug-resistant.
a 33 patients excluded due to <24 weeks of follow-up time.
b Final model included use of MTBDRplus test, age, prior history of tuberculosis, and ofloxacin resistance.
Figure 2.
Proportion of patients with positive sputum cultures and time to culture conversion from date of sputum collection.
DISCUSSION
We found the implementation of a rapid molecular diagnostic test, the Genotype MTBDRplus, into routine clinical practice had a significant impact on improving the clinical care among patients with MDR-TB patients in Georgia, a country with high rates of drug-resistant tuberculosis. Following implementation of the LPA there was a >75% reduction in time to initiation of SLD regimens (83.9–18.2 days) and correspondingly an increased rate of culture conversion during the first 24 weeks after initial sputum collection. Additionally, in a setting where tuberculosis patients are frequently hospitalized during the initiation of therapy, we found the implementation of the LPA led to improved infection control practices. After LPA implementation, there were significantly less days that patients with MDR-TB were hospitalized on drug-susceptible tuberculosis wards. This rapid separation of patients with drug-susceptible and drug-resistant tuberculosis has the potential to decrease nosocomial transmission of MDR-TB; previous reports have noted nosocomial transmission of MDR-TB to those with drug susceptible tuberculosis [13]. The impact of the MTBDRplus assay on time to treatment initiation with second-line drugs for MDR-TB, culture conversion, and patient cohorting highlights how use of this rapid molecular test improved patient care and safety.
There is very limited data on the impact of rapid molecular diagnostic tests on clinical outcomes. There are only 2 prior published studies that reported measures of clinical impact using a LPA for detecting MDR-TB [14, 15]. Both studies took place in South Africa and evaluated the time to initiation of MDR-TB treatment. Jacobson et al [15] reported that time to MDR-TB treatment decreased from 80 to 55 days after MTBDRplus implementation, whereas Hanrahan et al [14] reported a similar decrease from 78 to 62 days. They both cite clinical and laboratory operational issues for delaying the delivery and use of LPA results. Our study reports a much more robust clinical impact of a LPA for detection of MDR-TB. In our study, the laboratory communicated the results of the LPA to physicians in a timely manner, and this led to a marked and clinically significant reduction in time to initiation of SLDs for the treatment of MDR-TB (83.9–18.2 days). A standard method to provide test results to physicians in a timely manner likely facilitated the quick use of LPA results for drug resistance and highlights the importance of developing an implementation policy to maximize the benefits of rapid diagnostics.
After controlling for confounders, we found a >4-fold increased rate of achieving culture conversion in a cohort of patients following implementation of a LPA. Previous studies have not evaluated time to culture conversion. While Hanrahan et al [14] reported an increased proportion of patients had converted their sputum cultures to negative by 8 months, they did not evaluate time to culture conversion. The significant decrease in time to culture conversion shown in our study was likely due in large part to the earlier initiation of SLD regimens. Additionally, the association of phenotypic ofloxacin resistance with delayed culture conversion has been shown elsewhere and demonstrates the importance of fluoroquinolones in MDR-TB treatment [16, 17]. Earlier culture conversion is an important finding as it has been shown to increase the likelihood of a favorable outcome [2] and also decreases the time when a patient is infectious and can spread drug-resistant tuberculosis to close contacts. Additional benefits of starting appropriate MDR-TB treatment earlier include reducing costs and potential adverse effects of unnecessary first-line drugs as well as minimizing further drug resistance.
Prevention of nosocomial transmission of tuberculosis has been a long neglected area in tuberculosis control efforts in low- and middle-income countries [18]. Given the delay in tuberculosis diagnosis and subsequent detection of drug resistance, hospitalized patients with MDR-TB may transmit M. tuberculosis to highly susceptible patients without tuberculosis or to patients with drug-susceptible tuberculosis [19]. This problem has been highlighted by the outbreak of XDR-TB among HIV-infected patients in Tugela Ferry, South Africa, where transmission was predominantly due to nosocomial transmission [20]. Our results show that the use of a rapid molecular diagnostic test to detect MDR-TB can greatly improve the proper cohorting of patients and significantly reduce the time patients with MDR-TB spend on a ward with patients with drug-susceptible tuberculosis. Additionally, we also found that use of a rapid test for MDR-TB detection decreased the time patients were out in the community receiving inadequate first-line drug treatment and at risk for transmitting drug-resistant tuberculosis. This is important as household contacts of MDR-TB patients have been found to be at high risk of acquiring tuberculosis infection and disease [21].
A limitation of our study was the use of a quasi-experimental design. Although there could be biases from using a historical cohort, we feel it was limited due to the following: no changes in Georgia's MDR treatment protocols, NCTLD drug resistance committee, or MDR-TB doctors throughout the study period. The only appreciable clinical practice difference revealed by our data was the increased use of kanamycin vs capreomycin during the post-implementation period, which did not affect culture conversion in our survival analyses. Additionally, patients in the post-implementation group had higher rates of coinfection with hepatitis C virus, prior tuberculosis treatment, and treatment interruption, which are risk factors for worse outcomes.
In summary, in a setting with high rates of MDR-TB, implementation of a rapid molecular diagnostic test for detection drug-resistant tuberculosis into routine clinical care significantly decreased the time to initiation of MDR-TB treatment, to culture conversion, and improved timely cohorting of patients with MDR-TB. These findings are some of the first to demonstrate improved clinical outcomes following implementation of a rapid molecular diagnostic test to detect drug-resistant tuberculosis. Other National Tuberculosis Programs in low- and middle-income countries, especially those with high rates of drug-resistant tuberculosis, should explore implementation of such a test in order to improve patient care and enhance tuberculosis infection control efforts.
Notes
Financial support. This work was supported in part by the National Institutes of Health (NIH) Fogarty International Center (D43TW007124), NIH National Institute of Allergy and Infectious Diseases (K23AI103044), the Atlanta Clinical and Translational Science Institute (NIH/National Center for Advancing Translational Sciences UL1TR000454), and the Emory University Global Health Institute.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. Global Tuberculosis Report 2013. WHO/HTM/20131.
- 2.Gegia M, Kalandadze I, Kempker RR, Magee MJ, Blumberg HM. Adjunctive surgery improves treatment outcomes among patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis. 2012;16:e391–6. doi: 10.1016/j.ijid.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One. 2012;7:e31563. doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Policy Statement: Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis. 2008.
- 5.WHO. Policy statement: Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO/HTM/TB/2011.4. [PubMed]
- 6.Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD009593.pub2. CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multi drug resistant tuberculosis: A meta-analysis. BMC Infect Dis. 2009;9:67. doi: 10.1186/1471-2334-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caliendo AM, Gilbert DN, Ginocchio CC, et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin Infect Dis. 2013;57(suppl 3)):S139–70. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris AD, Bradham DD, Baumgarten M, Zuckerman IH, Fink JC, Perencevich EN. The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis. 2004;38:1586–91. doi: 10.1086/420936. [DOI] [PubMed] [Google Scholar]
- 10.Parsons LM, Somoskovi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: Challenges and opportunities. Clin Microbiol Rev. 2011;24:314–50. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nehren, Germany: Hain Lifescience; Genotype MTBDRplus, product page. Available at: http://www.hain-lifescience.de/en/products/microbiology/mycobacteria/genotype-mtbdrplus.html. Accessed 1 December 2013. [Google Scholar]
- 12.Hosmer DW, Lemeshow S, May S. Model development. 2nd ed. Wiley-Interscience; 2008. Applied survival analysis: regression modeling of time-to-event data. [Google Scholar]
- 13.Shenoi SV, Escombe AR, Friedland G. Transmission of drug-susceptible and drug-resistant tuberculosis and the critical importance of airborne infection control in the era of HIV infection and highly active antiretroviral therapy rollouts. Clin Infect Dis. 2010;50(suppl 3):S231–7. doi: 10.1086/651496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanrahan CF, Dorman SE, Erasmus L, Koornhof H, Coetzee G, Golub JE. The impact of expanded testing for multidrug resistant tuberculosis using genotype [correction of geontype] MTBDRplus in South Africa: An observational cohort study. PLoS One. 2012;7:e49898. doi: 10.1371/journal.pone.0049898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KR, Theron D, Kendall EA, et al. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis. 2013;56:503–8. doi: 10.1093/cid/cis920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurbatova EV, Gammino VM, Bayona J, et al. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:1335–43. doi: 10.5588/ijtld.11.0811. [DOI] [PubMed] [Google Scholar]
- 17.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: A systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abubakar I, Zignol M, Falzon D, et al. Drug-resistant tuberculosis: Time for visionary political leadership. Lancet Infect Dis. 2013;13:529–39. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 19.Nardell E, Dharmadhikari A. Turning off the spigot: reducing drug-resistant tuberculosis transmission in resource-limited settings. Int J Tuberc Lung Dis. 2010;14:1233–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi NR, Weissman D, Moodley P, et al. Nosocomial transmission of extensively drug-resistant tuberculosis in a rural hospital in South Africa. J Infect Dis. 2013;207:9–17. doi: 10.1093/infdis/jis631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: Systematic review and meta-analysis. Clin Infect Dis. 2014;58:381–91. doi: 10.1093/cid/cit643. [DOI] [PMC free article] [PubMed] [Google Scholar]