During the 2009 influenza A (H1N1) pandemic, infants of H1N1-vaccinated mothers had 38% lower odds of being born preterm, and were 45.0 g heavier, on average, than infants of unvaccinated mothers.
Keywords: maternal immunization, influenza vaccine, prematurity, pandemic influenza, infants
Abstract
Background. Influenza infection during pregnancy is associated with adverse fetal outcomes such as preterm birth and small for gestational age (SGA). Maternal influenza immunization may prevent these adverse infant outcomes during periods of influenza circulation.
Methods. We conducted a retrospective cohort study of live births within Kaiser Permanente (KP) Georgia and Mid-Atlantic States (n = 3327) during the period of 2009 influenza A (H1N1) virus circulation. Primary outcomes were third-trimester preterm birth (27–36 weeks), birth weight, low birth weight (LBW, <2500 g), and SGA.
Results. There were 327 (9.8%) preterm, 236 (7.4%) LBW, and 267 (8.4%) SGA births. Among H1N1-vaccinated mothers (n = 1125), there were 86 (7.6%) preterm, 68 (6.4%) LBW, and 99 (9.3%) SGA births, and the mean birth weight was 3308.5 g (95% confidence interval [CI], 3276.6–3340.4). Among unvaccinated mothers (n = 1581), there were 191 (12.1%) preterm, 132 (8.8%) LBW, and 123 (8.2%) SGA births, and the mean birth weight was 3245.3 g (95% CI, 3216.5–3274.2). Infants of H1N1-vaccinated mothers had 37% lower odds of being born preterm than infants of unvaccinated mothers (adjusted odds ratio, 0.63 [95% CI, .47–.84]). The mean birth weight difference between infants of H1N1-vaccinated mothers and infants of unvaccinated mothers was 45.1 g (95% CI, 1.8–88.3). There was no significant association between maternal H1N1 influenza immunization and LBW or SGA.
Conclusions. Pregnant women who received H1N1 influenza vaccine were less likely to give birth preterm, and gave birth to heavier infants. The findings support US vaccine policy choices to prioritize pregnant women during the 2009 influenza A (H1N1) pandemic.
Influenza infection results in higher morbidity in pregnant women compared to nonpregnant women of similar age [1–3]. Influenza infection during pregnancy is associated with adverse fetal outcomes such as preterm birth and small for gestational age (SGA) birth [4–9]. During the 2009 influenza A (H1N1) pandemic, pregnant women in the United States experienced higher influenza-associated morbidity and mortality relative to the general population [10–13].
A recent population-based study in Ontario, Canada, reported that antenatal H1N1 influenza vaccination during the 2009 pandemic prevented SGA birth, preterm birth at <32 weeks, and fetal death among infants [14]. Furthermore, maternal influenza immunization may prevent adverse infant outcomes among births during periods of seasonal influenza circulation [15–17]. In a randomized controlled trial in Bangladesh, maternal influenza immunization was associated with higher mean birth weight and reduced risk of being born SGA [16, 17]. In a population-based study in the state of Georgia, women vaccinated against seasonal influenza gave birth to heavier infants and were less likely to have a preterm birth. Additionally, during periods of widespread influenza activity, vaccinated women were less likely to have infants born SGA [15].
This is the first study of the association of maternal influenza immunization and adverse infant outcomes during the 2009 influenza A (H1N1) pandemic in the United States. Our study assessed associations between maternal H1N1 influenza immunization and third-trimester preterm birth, low birth weight (LBW), and SGA within a managed care organization population.
METHODS
We conducted a retrospective cohort study based on electronic medical records (EMRs) from Kaiser Permanente (KP) managed care organization sites in Georgia and Mid-Atlantic States (including Maryland, Virginia, and the District of Columbia). The study cohort has been described elsewhere [18, 19]. We identified all live births during the study period in KP Georgia and Mid-Atlantic States, to mothers who were continuously enrolled in KP for their entire pregnancies (with permitted enrollment gaps of up to 45 days). Then, we restricted to live births to mothers who started their third trimester of pregnancy on or after the start of the study period, including only mothers who had the opportunity for third-trimester exposure to 2009 influenza A (H1N1) virus and who were not diagnosed with 2009 influenza A (H1N1) influenza prior to receiving H1N1 vaccine. We restricted our study population in this way to evaluate the impact of influenza exposure proximate to birth on infant outcomes. Earlier preterm births (eg, second-trimester preterm births) may have different causative factors than those occurring during the third trimester (eg, fetal chromosomal abnormalities, fetal and maternal anatomic factors) [20, 21]. For vaccine effectiveness (VE) calculations, we included 40 mothers who were excluded from the primary analyses because they were diagnosed with 2009 influenza A (H1N1) prior to receiving H1N1 vaccine.
The study period was defined by the timing of 2009 influenza A (H1N1) virus circulation: from the collection date in each region of the first positive laboratory test for 2009 influenza A (H1N1), up to the first week when the percentage of influenza specimens that tested positive for 2009 influenza A (H1N1) was <5%, using data previously obtained from the Centers for Disease Control and Prevention (CDC) [18]. The eligible study period was 26 April 2009 to 17 April 2010 (including births before and after the start of availability of 2009 H1N1 influenza vaccine).
The primary exposure was receipt of 2009 H1N1 influenza vaccine during any trimester of pregnancy. We used KP EMRs to identify influenza vaccines administered to mothers in our cohort during pregnancy. We identified the type of influenza vaccine administered (ie, 2009 H1N1 influenza vaccine, seasonal trivalent inactivated vaccine [TIV]) based on HL7 code and administration date. For the main analyses, we defined pregnancy as between last menstrual period (LMP) and infant's birth date. We performed sensitivity analyses to evaluate the impact of defining vaccination during pregnancy as between LMP and 7 or 14 days prior to infant's birth date (Supplementary Data 1). In the main analyses, we compared 2 vaccine exposure groups: (1) 2009 H1N1 influenza vaccine with or without 2009–2010 seasonal TIV (H1N1 vaccine) and (2) no influenza vaccine (no vaccine). The no vaccine group was used as the reference group. We performed sensitivity analyses comparing subjects who received H1N1 vaccine to subjects who did not receive H1N1 vaccine (but who may have received 2009–2010 seasonal TIV during the study period; Supplementary Data 2).
Primary outcomes were preterm birth during the third trimester of pregnancy (27–36 weeks gestation), LBW (<2500 g), and SGA. Using national birth weight percentiles defined by gestational age and gender by Oken et al, we defined SGA as birth weight <10th percentile for gestational age and sex [22].
There are several methods for adjusting for confounding in nonrandomized observational studies of influenza VE, primarily to account for differences between vaccinated and unvaccinated individuals (eg, healthy vaccinee bias) [23, 24]. We evaluated the use of a difference-in-differences approach and the approach used by Nelson et al, but concluded that we could use neither approach because there was no valid preinfluenza comparison period, given the timing of virus circulation and vaccine availability [25, 26]. In our primary analyses, we adjusted for a priori covariates of interest: maternal age, asthma, gestational diabetes, cardiovascular disease, hypertension during pregnancy, multiple birth, any pregnancy/birth complication, and KP site. In sensitivity analyses, we used a propensity score approach to efficiently control for the same set of covariates (which may have influenced influenza vaccination status) in order to obtain an unbiased estimate of the effect of exposure [27]. We used propensity score regression and matching methods to assess the impact on results of using these approaches for addressing confounding instead of adjusting for individual covariates (Supplementary Data 3).
We identified covariate data using International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code data from KP EMRs. Data on maternal underlying conditions (eg, asthma, cardiovascular disease), pregnancy and birth complications, and demographics were identified by ICD-9 diagnosis codes from 90 days prior to 90 days after LMP. Gestational diabetes was identified by ICD-9 diagnosis codes between LMP and birth. We were unable to control for confounding by mother's race, maternal smoking, and alcohol use during pregnancy due to missing or poor quality data; however, we performed sensitivity analyses to evaluate the potential effect of these confounders using available data (Supplementary Data 4 and 5). Use of antiviral medications (eg, amantadine, oseltamivir, zanamivir) was identified from outpatient dispensing records using KP electronic pharmacy files, as previously described [18]. Diagnosed influenza A (H1N1) infection was defined as having a reverse transcription polymerase chain reaction test positive for influenza, or having a medical visit during pregnancy with influenza-related ICD-9 diagnosis code during the period of 2009 influenza A (H1N1) virus circulation [18].
We used logistic regression to evaluate the association of maternal influenza vaccine and each of the primary outcomes. We performed a subanalysis using polytomous logistic regression to model early (27–33 weeks) and late preterm birth (34–36 weeks). We used linear regression to evaluate the differences in mean birth weight among births by exposure group. We did not stratify our analyses by trimester of vaccination due to limited study power.
We used the following equation to calculate the adjusted number needed to be vaccinated (aNNV) to prevent 1 preterm birth:
where UER = event rate in the unexposed [28]. We used the following expression to calculate the population prevented fraction of preterm birth:
where pc = proportion of cases vaccinated [29]. For these calculations, we used the adjusted odds ratio (AOR) for the association of maternal H1N1 vaccination with preterm birth. We calculated VE against 2009 influenza A (H1N1) infection with the equation [VE = 1 – AOR], where the OR is computed as the association between H1N1 immunization and H1N1 influenza infection adjusted for a priori covariates.
Based on an expected sample of 3000 and vaccine coverage of 30%–50%, our sample size had ≥80% or higher power to detect ORs <0.66 for associations of antenatal influenza immunization and birth outcomes. We used SAS software, version 9.2 (Cary, North Carolina) for all statistical analyses. Results were considered significant at α = .05 using 2-tailed tests. The study was reviewed and approved by the KP institutional review boards in both Georgia and Mid-Atlantic States.
RESULTS
There were a total of 4555 eligible births to 4446 mothers in KP Georgia and Mid-Atlantic States during the period of 2009 influenza A (H1N1) circulation. Of these, we excluded 27 second-trimester births, 1160 births to mothers who were already in their third trimester of pregnancy at the start of 2009 influenza A (H1N1) circulation, 1 birth to a mother who was exposed to ≤14 days of 2009 influenza A (H1N1) influenza circulation at the start of the study period, and 40 births to mothers who were diagnosed with 2009 influenza A (H1N1) before receiving 2009 H1N1 influenza vaccine. Therefore, our study population included a total of 3327 third-trimester live births to 3236 mothers between 25 May 2009 and 17 April 2010 (Figure 1).
Figure 1.
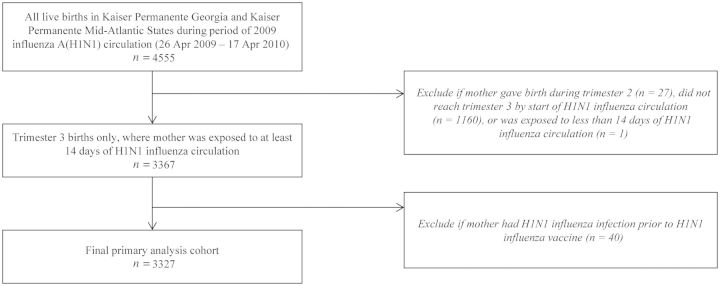
Definition of primary analysis cohort.
The H1N1 vaccine group included 1125 (33.8%) infants and the no vaccine group included 1581 (47.5%) infants (Figure 2). Additionally, 621 (18.7%) of infants were born to mothers who received only 2009–2010 seasonal TIV. Overall H1N1 influenza vaccine coverage in our cohort was 33.8% (1125/3327), and 2009–2010 seasonal TIV coverage was 41.5% (1380/3327). Among births on or after 1 October 2009 (the approximate start date of 2009 H1N1 influenza vaccine availability), H1N1 vaccine coverage was 50.1% (1125/2247), and 2009–2010 seasonal TIV coverage was 58.0% (1304/2247). The odds of having received 2009 H1N1 influenza vaccine were lower for mothers who had pregnancy/birth complications (P < .01) and mothers who gave birth within KP Georgia (P < .01) (Table 1). VE of 2009 H1N1 influenza vaccine against diagnosed 2009 influenza A (H1N1) infection during the study period among all mothers in our study cohort was 61.5% (95% confidence interval [CI], 15.5%–82.5%).
Figure 2.
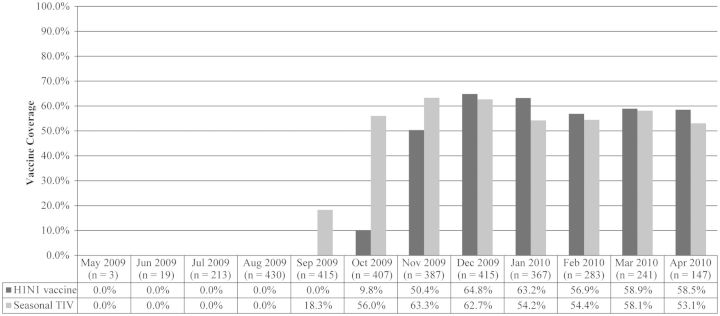
Influenza vaccine coverage among mothers of infants born during period of 2009 influenza A (H1N1) circulation, by infant's birth month. Abbreviation: TIV, trivalent inactivated vaccine.
Table 1.
Maternal Characteristics by Receipt of H1N1 Influenza Vaccine During Pregnancy
| Characteristic | All (N = 3327) | H1N1 Vaccine (n = 1125) | No Vaccine (n = 1581) | P Value |
|---|---|---|---|---|
| Maternal age, y, mean (SD) | 31.2 (5.6) | 31.5 (5.3) | 31.0 (5.7) | .01* |
| Asthma | 120 (4.0) | 44 (4.2) | 51 (3.5) | .38 |
| Maternal diabetes | 476 (15.7) | 169 (16.2) | 226 (15.6) | .72 |
| Multiple birth | 181 (5.4) | 60 (5.3) | 91 (5.8) | .64 |
| Cardiovascular disease | 183 (6.0) | 55 (5.3) | 82 (5.7) | .66 |
| Hypertension during pregnancy | 507 (15.2) | 162 (14.4) | 236 (14.9) | .70 |
| Any pregnancy complicationa | 1921 (57.7) | 602 (53.5) | 957 (60.5) | <.01* |
| Born within KP Georgia (vs KP Mid-Atlantic) | 1004 (30.2) | 286 (28.5) | 597 (59.5) | <.01* |
| Black race (subcohort) | 327 (43.1) | 62 (36.5) | 230 (46.4) | .03 |
*Indicates significant P value.
Data are No. (%) unless otherwise specified. Asthma, diabetes, and cardiovascular disease reported for births with available comorbidity data (n = 3039), including 1046 births exposed to H1N1 vaccine and 1447 births exposed to no vaccine. Black race is reported for births with available maternal race data (n = 759), including 170 births exposed to H1N1 vaccine and 496 births exposed to no vaccine.
Abbreviations: KP, Kaiser Permanente; SD, standard deviation.
a Includes fetal abnormality affecting maternal management; fetal or placental problems affecting maternal management; polyhydramnios; oligohydramnios, PROM, amnionitis; antepartum hemorrhage, abruptio placentae, and placenta previa; and antepartum complications.
There were a total of 327 (9.8%) preterm births, including 85 (2.5%) at 27–33 weeks and 242 (7.3%) at 34–36 weeks. After adjustment for a priori covariates, infants whose mothers received H1N1 vaccine had 37% lower odds of being born preterm than infants in the no vaccine group (AOR, 0.63 [95% CI, .47–.84]; Table 2). Infants whose mothers received H1N1 vaccine had 47% lower odds of 27–33 weeks preterm birth compared to infants in the no vaccine group (AOR, 0.53 [95% CI, .30–.95]), and 34% lower odds of 34–36 weeks preterm birth (AOR, 0.66 [95% CI .48–.92]). Unadjusted and propensity score adjusted analyses gave similar results (Supplementary Data 3). The adjusted number needed to vaccinate with H1N1 vaccine was 24 (95% CI, 17–58) to prevent 1 preterm birth. The population-prevented fraction, or fraction of preterm birth cases that could be prevented if the population were vaccinated against H1N1, of preterm birth by H1N1 vaccine was 15.4%.
Table 2.
Odds Ratios for Association of Maternal Influenza Immunization With Infant Outcomes, Among Infants Born During Period of 2009 Influenza A (H1N1) Virus Circulation
| H1N1 Vaccine (n = 1125) | No Vaccine (n = 1581) | Adjusteda | Unadjusted | |
|---|---|---|---|---|
| Outcome | Has Outcome, No. (%) | Has Outcome, No. | OR or Difference (95% CI) | OR or Difference (95% CI) |
| Preterm birth (27–36 wk) | 86 (7.6%) | 191 (12.1%) | .63 (.47–.84)b | .60 (.46–.79) |
| Birth at 27–33 wk | 19 (1.7%) | 52 (3.3%) | .53 (.30–.95) | .49 (.29–.83) |
| Birth at 34–36 wk | 67 (6.0%) | 139 (8.8%) | .66 (.48–.92) | .65 (.48–.87) |
| Low birth weight, <2500 gc | 68 (6.4%) | 132 (8.8%) | .79 (.56–1.10) | .71 (.52–.96) |
| Small for gestational agec | 99 (9.3%) | 123 (8.2%) | 1.26 (.94–1.69) | 1.15 (.87–1.52) |
| Birth weight, gc, mean (95% CI) | 3308.5 (3276.6–3340.4) | 3245.3 (3216.5–3274.2) | 45.1 (1.8–88.3) | 63.2 (20.0–106.3) |
Reference group for all models is group that was not exposed to any 2009–2010 inactivated influenza vaccine.
Abbreviations: CI, confidence interval; OR, odds ratio.
a Adjusted models control for a priori confounders: maternal age (years), maternal asthma, gestational diabetes, maternal cardiovascular disease, hypertension during pregnancy, any pregnancy/birth complication, multiple birth, any antiviral use during pregnancy, and Kaiser Permanente site.
b Interpretation: During the period of 2009–2010 H1N1 influenza circulation, the adjusted odds of third-trimester preterm birth were 37% lower among infants of mothers who received 2009 influenza A (H1N1) vaccine, as compared to infants of unvaccinated mothers.
c Birth weight and small for gestational age outcomes reported for 1064 births exposed to H1N1 vaccine and 1505 births exposed to no vaccine.
In the primary adjusted model, infants whose mothers received H1N1 vaccine were 45.1 g (95% CI, 1.8–88.3 g) heavier than infants in the no vaccine group (Table 2). The mean birth weight among infants whose mothers received H1N1 vaccine was 3308.5 g (95% CI, 3276.6–3340.4 g) vs 3245.3 g (95% CI, 3216.5–3274.2 g) among infants in the no vaccine group. There was no significant association between maternal receipt of 2009 H1N1 influenza vaccine and being born LBW during the period of 2009 H1N1 influenza circulation (AOR, 0.79 [95% CI, .56–1.10]; Table 2). Propensity score adjusted analyses gave similar results (Supplementary Data 3); however, the unadjusted OR was within 10% of the primary AOR but was statistically significant. There was also no significant association between maternal receipt of 2009 H1N1 influenza vaccine and being born SGA during the period of 2009 H1N1 influenza circulation (AOR, 1.26 [95% CI, .94–1.69]; Table 2). Unadjusted and propensity score adjusted analyses gave similar results (Supplementary Data 3).
We performed a series of sensitivity analyses to assess our primary results, and found similar results in each analysis. We restricted our definition of vaccine exposure to between estimated LMP and 7 or 14 days prior to infant's birth date (Supplementary Data 1). We evaluated the impact of defining the reference group as no receipt of 2009 H1N1 influenza vaccine (including mothers who only received 2009–2010 seasonal TIV; Supplement 2). We assessed the potential impact of confounding by maternal race within a subcohort of births for which race data were available, and by maternal smoking and alcohol use during pregnancy, and found no significant change in point estimates (Supplementary Data 4 and 5).
DISCUSSION
During the 2009 influenza A (H1N1) pandemic, maternal H1N1 influenza immunization was associated with reduced odds of third-trimester preterm birth. Also, infants of mothers who received 2009 H1N1 influenza vaccine weighed more at birth, on average, than infants born to unvaccinated mothers. Our VE finding of 61.5% is similar to reports from other studies showing high effectiveness of 2009 H1N1 influenza vaccine against 2009 influenza A (H1N1) infection during the pandemic (ie, 62%–84%) [30–33].
Our findings should be put into the context of recent findings describing the association between 2009 influenza A (H1N1) infection and risk of adverse infant outcomes. Several studies have shown higher risk among pregnant women infected with 2009 influenza A (H1N1) of having infants born preterm, at lower birth weight, or SGA [6, 8, 9]. However, recent findings in our study population showed no significant effect of 2009 influenza A (H1N1) infection on these birth outcomes [18], which may have been due to high use of antiviral medications within this population. Among H1N1-infected pregnant women who experienced adverse outcomes, the risk of infection and severe outcomes (eg, hospitalization, admission to intensive care, death) was higher during the second and third trimesters [7]. Our analysis of third-trimester preterm birth suggests that influenza viral infection is part of a biological pathway leading to preterm birth, putatively through infection-associated inflammation at the maternal-fetal interface [34]. Our analysis of a known vaccine effect in prevention of influenza infection is a tool for understanding the effect of influenza virus infection in pregnancy, but further studies are needed to determine the mechanism of influenza infection in birth outcomes.
Our findings that mothers vaccinated against 2009 influenza A (H1N1) had lower odds of preterm birth and gave birth to heavier infants are in line with recent findings in a Canadian population describing the preventive effect of maternal H1N1 influenza immunization during pregnancy against adverse infant outcomes. In Ontario, Canada, infants born to vaccinated mothers from November 2009 to April 2010 had lower risk of SGA birth, very preterm birth (<32 weeks), and fetal death. There was no significant effect of maternal H1N1 influenza immunization on preterm birth (<37 weeks). In our study, we found a significant reduction in the odds of preterm birth (<37 weeks) among mothers who received 2009 H1N1 influenza vaccine, consistent with a similar recent finding during seasonal influenza epidemics in the state of Georgia [15]. Also, we found no association between maternal H1N1 influenza immunization and infants being SGA, in contrast with findings in Ontario of reduced risk of SGA birth among infants of H1N1-vaccinated mothers. It has also been reported that maternal seasonal influenza immunization was protective against SGA birth during periods of widespread influenza activity [15]. Potential explanations for these differences in observations between our study and the Ontario study may include: different H1N1 vaccine products used in the United States and Canada; absolute risk of adverse outcomes (eg, 12.1% preterm birth prevalence among unvaccinated mothers in our study vs 6.4% in the Ontario study); study period (ie, our study period extended for the duration of 2009 influenza A [H1N1] virus circulation, whereas the Ontario study was conducted only during the period of vaccine availability, post-October 2009); and restriction to third-trimester live births.
We additionally found that infants of mothers vaccinated against 2009 influenza A (H1N1) weighed more than infants of unvaccinated mothers. This finding is supported by results from earlier studies in Bangladesh, the United States, and Canada showing that maternal influenza immunization is associated with increased birth weight, and maternal influenza infection is associated with decreased birth weight [15–17]. Although we found no significant association between maternal influenza immunization and being born LBW, the statistically significant difference in mean birth weight between vaccinated and unvaccinated mothers is important biologically and for informing immunization policy prioritizing pregnant women.
Our study has several strengths and limitations. We used vaccine records directly from mothers’ KP EMRs, and therefore did not rely on maternal self-report of vaccination status. Mothers may have been misclassified if vaccinated at alternative locations (eg, pharmacies, workplaces), but we believe it likely that any exposure misclassification was nondifferential. Recent CDC data on 2009–2010 influenza season vaccine uptake among pregnant women showed that they were significantly more likely to be vaccinated at the recommendation of their medical provider. Further, vaccine coverage in our study population was higher than 2009–2010 state vaccine coverage estimates for Georgia and Maryland [35]. Moreover, underestimation of coverage was likely to result in conservative OR estimates due to nondifferential misclassification of vaccine exposure.
We conducted sensitivity analyses to evaluate the effect of defining vaccination during pregnancy as between LMP and infant's date of birth. Our reported findings are more conservative than we would have obtained if this assumption had not been made. Additionally, we were unable to control for important potential confounders related to risk of preterm birth, such as previous preterm birth, medically indicated vs spontaneous preterm birth, and mother's race. In sensitivity analyses, we assessed the potential impact of confounding by maternal race, smoking, and alcohol use during pregnancy. We found qualitatively similar results, demonstrating robustness of our primary results without controlling for these potential confounders.
Our findings demonstrate the importance of vaccinating pregnant women during influenza pandemics. In this study, mothers who received 2009 H1N1 influenza vaccine were less likely to give birth preterm, and gave birth to heavier infants. Notably, our data suggest that every 24 maternal H1N1 influenza immunizations prevented 1 preterm birth—a figure that has important implications for cost-effectiveness estimates of maternal influenza immunization during pandemics, given the substantial medical and societal costs of preterm birth. This study not only reinforces previous findings about maternal influenza immunization, but supports vaccine policy choices made during the 2009 influenza A (H1N1) pandemic when pregnant women were a priority group for receiving pandemic vaccine.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. J. L. R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Financial support. This work was supported by the Kaiser Permanente Center for Effectiveness and Safety Research, the Emory Global Health Institute, and the Emory University Rollins School of Public Health Practical Experience Program.
Potential conflicts of interest. S. B. O. was awarded the Maurice R. Hilleman Early-Stage Career Investigator Award by the National Foundation for Infectious Diseases. The award was funded by an unrestricted educational grant to the National Foundation for Infectious Diseases from Merck and Co, Inc; however, S. B. O. had no direct interaction with Merck. M. C. S. has served as a consultant for Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Klein S, Passaretti C, Anker M, Olukoya P, Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol Sex Differ. 2010;1:5 doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev. 2006;28:47–53. doi: 10.1093/epirev/mxj002. [DOI] [PubMed] [Google Scholar]
- 3.Hardy JM, Azarowicz EN, Mannini A, Medearis DN, Cooke RE. The effect of Asian influenza on the outcome of pregnancy, Baltimore, 1957–1958. Am J Public Health Nations Health. 1961;51:1182–8. doi: 10.2105/ajph.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil SA, Dodds LA, Fell DB, et al. Effect of respiratory hospitalization during pregnancy on infant outcomes. Am J Obstet Gynecol. 2011;204(6 suppl):S54–7. doi: 10.1016/j.ajog.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Ács N, Bánhidy F, Puhó E, Czeizel AE. Maternal influenza during pregnancy and risk of congenital abnormalities in offspring. Birth Defects Res A Clin Mol Teratol. 2005;73:989–96. doi: 10.1002/bdra.20195. [DOI] [PubMed] [Google Scholar]
- 6.Newsome K, Williams J, Way S, Honein M, Hill H. Maternal and infant outcomes among severely ill pregnant and postpartum women with 2009 pandemic influenza. MMWR Morb Mortal Wkly Rep. 2011;60:1193–5. [PubMed] [Google Scholar]
- 7.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ. 2011;342:d3214 doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendez-Figueroa H, Raker C, Anderson BL. Neonatal characteristics and outcomes of pregnancies complicated by influenza infection during the 2009 pandemic. Am J Obstet Gynecol. 2011;204(6 suppl):S58–63. doi: 10.1016/j.ajog.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchard-Rohner G, Siegrist C-A. Vaccination during pregnancy to protect infants against influenza: why and why not? Vaccine. 2011;29:7542–50. doi: 10.1016/j.vaccine.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 12.US Centers for Disease Control and Prevention. Pandemic flu. Available at http://www.pandemicflu.gov/ Accessed 6 April 2012. [Google Scholar]
- 13.World Health Organization. Pandemic influenza in pregnant women. Available at: http://www.who.int/csr/disease/swineflu/notes/h1n1_pregnancy_20090731/en/index.html . Accessed 6 April 2012. [Google Scholar]
- 14.Fell DB, Sprague AE, Liu N, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102:e33–40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omer SB, Goodman D, Steinhoff MC, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhoff MC, Omer SB, Roy E, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ. 2012;184:645–53. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 18.Hansen C, Desai S, Bredfeldt C, et al. A large population-based study of pandemic influenza A H1N1 diagnosis during pregnancy and maternal and neonatal outcomes. J Infect Dis. 2012;206:1260–8. doi: 10.1093/infdis/jis488. [DOI] [PubMed] [Google Scholar]
- 19.Hansen C, Desai S, Cheetham C, Li D, et al. PS1-17: H1N1 flu and pregnancy: the Kaiser Permanente experience. Clin Med Res. 2011;9:155. [Google Scholar]
- 20.Gabbe S, Niebyl JR, Galan HL, et al. Obstetrics: normal and problem pregnancies. Churchill Livingstone Elsevier: Philadelphia, PA; 2007. [Google Scholar]
- 21.Michels T, Tiu AY. Second trimester pregnancy loss. Am Fam Physician. 2007;76:1341–6. [PubMed] [Google Scholar]
- 22.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 24.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35:345–52. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 25.Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;170:650–6. doi: 10.1093/aje/kwp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson JC, Jackson ML, Weiss NS, Jackson LA. New strategies are needed to improve the accuracy of influenza vaccine effectiveness estimates among seniors. J Clin Epidemiol. 2009;62:687–94. doi: 10.1016/j.jclinepi.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 28.Bender R, Blettner M. Calculating the number needed to be exposed with adjustment for confounding variables in epidemiological studies. J Clin Epidemiol. 2002;55:525–30. doi: 10.1016/s0895-4356(01)00510-8. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: principles and quantitative methods. New York: John Wiley & Sons; 1982. [Google Scholar]
- 30.Hardelid P, Fleming DM, Andrews N, et al. Effectiveness of trivalent and pandemic influenza vaccines in England and Wales 2008–2010: results from a cohort study in general practice. Vaccine. 2012;30:1371–8. doi: 10.1016/j.vaccine.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 31.Mahmud S, Hammond G, Elliott L, et al. Effectiveness of the pandemic H1N1 influenza vaccines against laboratory-confirmed H1N1 infections: population-based case–control study. Vaccine. 2011;29:7975–81. doi: 10.1016/j.vaccine.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 32.Savulescu C, Jimenez-Jorge S, de Mateo S, et al. Using surveillance data to estimate pandemic vaccine effectiveness against laboratory confirmed influenza A(H1N1)2009 infection: two case-control studies, Spain, season 2009–2010. BMC Public Health. 2011;11:899. doi: 10.1186/1471-2458-11-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uphoff H, an der Heiden M, Schweiger B, et al. Effectiveness of the AS03-adjuvanted vaccine against pandemic influenza virus A/(H1N1) 2009—a comparison of two methods; germany, 2009/10. PLoS One. 2011;6:e19932. doi: 10.1371/journal.pone.0019932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahluwalia I, Harrison L, Ding H, Austin T, et al. Influenza vaccination coverage among pregnant women—29 states and New York City, 2009–10 season. MMWR Morb Mortal Wkly Rep. 2012;61:113–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


