Abstract
Just as mating patterns can promote speciation or hybridization, the presence of hybridization can shape mating patterns within a population. In this study, we characterized patterns of multiple mating and reproductive skew in a naturally hybridizing swordtail fish species, Xiphophorus birchmanni. We quantified multiple mating using microsatellite markers to genotype embryos from 43 females collected from 2 wild populations. We also used a suite of single-nucleotide polymorphism markers to categorize females and their inferred mates as either parental X. birchmanni or as introgressed individuals, which carried alleles from a sister species, X. malinche. We found that parental and introgressed X. birchmanni females mated multiply with both parental and introgressed males. We found no difference in mating patterns or reproductive skew between parental and introgressed X. birchmanni females. However, nonintrogressed X. birchmanni males mated more often with large, fecund females. These females also had the greatest levels of skew in fertilization success of males. Thus, our results show that X. birchmanni has a polygynandrous mating system and that introgression of X. malinche alleles has only subtle effects on mating patterns in this species.
Key words: hybrid zone, microsatellite, parentage, polygynandry, swordtail
Individual reproductive success is shaped by a number of factors, including ecological, social, and individual effects (Jones and Ratterman 2009; Alonzo 2010; Kuijper et al. 2012). These factors contribute to the evolution of complex traits, such as male ornaments and female mate choice, as well as to the ecological and evolutionary dynamics of populations. For example, sexual selection can act to drive and subsequently reinforce the evolution of pre- and postmating mechanisms of reproductive isolation during speciation (Panhuis et al. 2001). Alternatively, in some cases positive selection may act in favor of the introgression of a trait found in 1 parental species into another (Parsons et al. 1993) or favoring hybrid phenotypes not found in either parental species (Rosenfield and Kodric-Brown 2003). In these cases, the presence of hybrid phenotypes changes the phenotypic distribution upon which selection can act, sometimes resulting in trait values that lie outside of the range of variation observed in either parental species.
Just as the introduction of heterospecific alleles may change the distribution of traits and preferences related to precopulatory mate choice, hybridization may impact phenotypic diversity in traits linked to sperm competitive ability (Joly et al. 1997) and thus alter reproductive success via postcopulatory processes. These factors may affect within-brood reproductive skew, which is the bias of a female’s reproductive effort towards 1 or a few of her mates, due to unequal sperm transfer, sperm competition or sperm viability (Birkhead and Pizzari 2002; Evans 2010). Females can also contribute to reproductive skew via mechanisms of cryptic female choice, either through differential sperm use, which impacts the fertilization success of individual males (Eberhard 1996), or by differential investment in offspring size and number before or after fertilization, which affects offspring fitness (Sheldon 2000). In species with sperm storage, the female’s mating strategy, including the frequency of mating and timing of fertilization, could well influence the fertilization success of a given male. Empirical evidence in guppies suggests that fresh sperm has a competitive advantage over stored sperm (Hildemann and Wagner 1954). Therefore, in hybridizing species, skewed mating success of males of a certain genotypic or phenotypic class is potentially influenced by several pre- and postcopulatory processes.
Pre- and postcopulatory processes interact to drive variation in the mating success of males in live-bearing poeciliid fish species. Poeciliids have both polyandry and internal fertilization, allowing for the possibility of sperm storage, sperm competition, cryptic female choice, and a high degree of intersexual conflict (Soucy and Travis 2003; Evans et al. 2003; Smith 2014). Multiple paternity of broods is common in this family, though its frequency varies within and among species depending on the mating system and life history (Herdman et al. 2004; Travis et al. 1990; Trexler et al. 1997; Kelly et al. 1999; Avise and Liu 2010).
Precopulatory processes have been studied extensively in swordtails and platyfishes (Poeciliidae: Xiphophorus). Natural variation in male phenotypes and female preferences occurs within and among populations and species, and may be shaped by both natural and sexual selection (Ryan and Rosenthal 2001). The swordtails Xiphophorus birchmanni and X. malinche are sister species (Cui et al. 2013) endemic to the Río Pánuco basin of the eastern slopes of the Sierra Madre Oriental in central Mexico (Rauchenberger et al. 1990). Recently discovered natural hybrid zones between X. birchmanni and X. malinche (Rosenthal et al. 2003; Culumber et al. 2011) offer a unique opportunity to study how sexual selection affects male and female reproductive success within a hybrid zone. Previous research has demonstrated that female choice for novel male phenotypes is likely to occur in X. birchmanni populations (Rosenthal 2013), which could explain the observed introgression of X. malinche alleles into these X. birchmanni populations.
Hybridization influences premating processes in both X. birchmanni and X. malinche (Verzijden et al. 2012). Sexually dimorphic traits of males found in the hybrid zones exhibit phenotypic diversity outside of the parental range due to the introgression of heterospecific alleles (Rosenthal et al. 2003). The presence of greater male phenotypic diversity in populations with introgressed X. malinche alleles may impact male mating success by producing multivariate male phenotypes that are more attractive to females than either parental morph (Fisher et al. 2009; Rosenthal 2013). Similarly, hybrid or introgressed females may exhibit mate preferences that differ from parental females (Rosenthal 2013). Natural selection may also shape patterns of introgression and hybridization within a population, as the fitness of parental and hybrid morphs also vary across environmental gradients (Culumber et al. 2012). Experimental evidence has shown that the female preferences and reproductive allocation of the parental species are also influenced by their experience with males (Verzijden and Rosenthal 2011; Willis et al. 2011; Verzijden et al. 2012; Kindsvater et al. 2013) but the effect of introgression on male mating success is unclear. Although we have little evidence that introgressed offspring are less viable than offspring with parental species’ genomes, the persistence of parental species’ genotypes in populations with hybridization suggest that pre- or postcopulatory processes could influence hybrid fitness (Culumber et al. 2011).
Based on previous studies of female choice, we expect that X. birchmanni females with introgressed X. malinche alleles will exhibit different preferences than parental X. birchmanni females, and that X. birchmanni males with introgressed X. malinche alleles may have greater mating success than non-introgressed X. birchmanni males (Fisher et al. 2009; Rosenthal 2013). Nevertheless, we still do not know how postmating processes affect reproductive success in these species or how a history of hybridization may further complicate these processes. Postcopulatory processes may work to favor the same individuals that succeed in accruing mating success (Evans et al. 2003; Rose et al. 2013), or may favor individuals of a different phenotypic class (Evans 2010). As a result, interactions between these phases may result in a complex pattern of parentage.
In this study, we asked how mating success of parental and introgressed individuals played out in a natural X. birchmanni population with a history of hybridization between X. birchmanni and X. malinche. Our objectives were to use microsatellite markers to characterize the patterns of multiple mating and reproductive skew in females of 2 populations of X. birchmanni. We then used single nucleotide polymorphism (SNP)-based markers to characterize differences between parental (or non-introgressed) and introgressed individuals in: 1) mating and reproductive success, 2) female investment in reproductive traits, and 3) interaction effects between introgression in males, introgression in females, and female reproductive allocation. In this way, we can address whether pre- and postcopulatory processes vary among parental and introgressed X. birchmanni individuals.
Methods
Sample Collection
Adult female X. birchmanni were collected and preserved whole in ethanol from previously sampled locations near the CICHAZ field station in Calnali, Hidalgo, Mexico. Our analyses focused on samples collected at the San Pedro site (N = 31, abbreviated “SP”, Table 1), a population with a known history of introgression of X. malinche alleles at low levels (from Culumber et al. 2011, 14 of 39 collected individuals, or 36%, had 1 or 2 heterospecific alleles out of a possible 7). We pooled samples collected at San Pedro across 2 years (N = 23 in 2008, N = 8 in 2010). We saw no effect of annual variation in our data: females from 2008 and 2010 did not differ in number of offspring (Welch’s t-test, P = 0.63, 95% CI, lower bound: −7.51, upper bound: 4.76), number of mates (Welch’s t-test, P = 0.08, 95% CI, lower bound: −1.39, upper bound:0.08), or reproductive skew (Welch’s t-test, P = 0.85, 95% CI, lower bound: −0.52, upper bound: 0.06). A previous analysis showed no effect of annual variation in female size or embryo size in this population (Kindsvater et al. 2012). Although annual variation may exist in this population, our data shows no detectable difference in trait values so year was not included in our statistical models.
Table 1.
Microsatellite loci assayed from adult female Xiphophorus birchmanni
| Locus | Temp (°C) | San Pedro population | Coacuilco population | F ST | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Range (bp) | A | PHW | N | Range (bp) | A | PHW | |||
| Msd-029 | 58 | 27 | 140–284 | 14 | 0.334 | 10 | 136–154 | 12 | 0.483 | 0.046 |
| Msd-036 | 58 | 26 | 137–206 | 12 | 0.498 | 12 | 145–201 | 10 | 0.753 | 0.098 |
| Msd-049 | 60 | 31 | 193–238 | 10 | 0.336 | 12 | 201–238 | 6 | 0.619 | 0.286 |
| Msd-072 | 60 | 31 | 145–267 | 23 | 0.865 | 12 | 136–252 | 17 | 1.000 | −0.009 |
Name of locus, PCR re-annealing temperature (°C), pairwise F ST values are reported for each locus. Number of females assayed (N), range of PCR product sizes (bp), number of alleles (A), and the P value of the Hardy–Weinberg exact test (PHW) are reported for each population at each locus.
We also used samples collected at another site in a geographically distinct watershed, Coacuilco (N = 12 in 2010, abbreviated “CU”), to consider how multiple mating may vary. Coacuilco is a population of X. birchmanni with an unknown level of introgression from X. malinche populations; preliminary data show that 1 individual of 7 carried 1 X. malinche allele (Z. Culumber, unpublished data). Females were measured, and embryos were dissected, counted, and preserved with a female fin clip in 95% ethanol for DNA extraction. Three embryos from each female were dried to obtain offspring dry mass (a proxy for maternal investment), and so were excluded from the genetic analysis. For additional details on sample collection and the measurement of embryo mass see Kindsvater et al. (2012). In total, 43 females and their broods (N = 590) were included in our analysis.
Genetic and Statistical Analysis
For our microsatellite analysis, we optimized 4 highly variable tetranucleotide microsatellite loci from a database developed for a congeneric species, X. maculatus (Walter et al. 2004). DNA was extracted using the DNeasy kit (Qiagen Inc.) and amplified using primers designed for X. maculatus in modified PCR conditions (Table 1). PCR products were analyzed on an Applied BioSystems 3730xl DNA Analyzer at the Cornell University Life Sciences Core Laboratories Center. Two authors manually scored genotypes independently using PeakScanner v1.0. GERUD2.0 was used to assign the minimum number of fathers to each female’s brood and to reconstruct paternal microsatellite genotypes (Jones 2005). Post hoc review of the GERUD2.0 output shows that within each family any given full-sib group is differentiated from all other full-sib groups within that family by alleles at 2 or more loci. FST and Fisher’s Exact Test for Hardy–Weinberg Equilibrium were calculated using Genepop 4.2 (Raymond and Rousset 1995; Rousset 2008). We then calculated each female’s reproductive skew using the index of monopolization (Ruzzante et al. 1996; Kokko et al. 1999). This index measures the observed variance in number of fertilizations per male as a fraction of the maximum possible variance, with both corrected by an estimate of the variance expected under random distribution of fertilization success (Ruzzante et al. 1996). As this index was non-normal, a permutation test of significance was used in analyses of this variable. We used PrDM (Neff and Pitcher 2002) to measure the probability of detecting multiple mating given the allele frequencies observed in each population. We repeated the simulation with a range of brood sizes, number of mates, and reproductive skew representing the average combinations of multiple paternity and reproductive skew for each population in our data (See Supplementary Table S1).
To investigate the relationship between within-brood reproductive skew and multiple mating, we subsampled 10 offspring from every brood with 10 or more offspring (N = 24) 1000 times and calculated the average number of sires represented in the subsample. We expect that the number of fathers identified increases with brood size as each additional embryo represents an increased chance to capture unique paternal alleles. We controlled for this ascertainment bias by subsampling the broods and then examining the pattern of multiple mating in broods of fixed size. We chose to subsample 10 embryos per brood because it maximized the number of broods included in the analysis while still providing a brood large enough to detect multiple mating. If the relationship between brood size and number of fathers is the result of a biological phenomenon then the relationship between number of subsampled fathers and brood size will be significant. If, however, the relationship is no longer significant, then we can conclude that the relationship is most likely due to an ascertainment bias.
San Pedro females and up to 6 offspring from each full-sib group (as defined by the GERUD2.0 analysis of microsatellites) were also genotyped using TaqMan SNP Genotyping Assays (Applied Biosystems) at 3 SNP markers (DNA Ligase 1, DNA Polymerase Beta, and Tumor Protein 53) previously characterized by Culumber et al. (2011) that show fixed differences between X. birchmanni and X. malinche individuals across 39 populations. Offspring SNP genotypes were used to reconstruct paternal SNP genotypes using COLONY, version 2.0.2.3 (Jones and Wang 2010). COLONY uses a full likelihood approach for building sibships (Wang 2004, 2012) and calculates the probability of a reconstructed genotype at each locus, given the distribution of offspring genotypes. As our interest lies in comparing parental X. birchmanni individuals to those with at least 1 introgressed X. malinche allele, we used COLONY to calculate the probability that a given individual carried at least 1 introgressed allele at 1 of the loci. Using a combination of female SNP genotype, offspring SNP genotype, and inferred full-sib groups (based on microsatellite analysis in GERUD2.0), COLONY calculated the probability of every possible SNP genotype at each locus for each reconstructed male. We then used these data to calculate the probability that the inferred male was homozygous for X. birchmanni alleles across all 3 loci (e.g. parental or nonintrogressed). Individuals assigned a probability value greater than 0.5 were scored as parental and those scored less than 0.5 were scored as introgressed. We were unable to assign SNP genotypes to 9 fathers. In these cases, we could not successfully amplify the SNP alleles in the offspring. Failure to assign SNP genotypes seems largely to be a function of full-sib group size, as males with unassigned SNP genotypes tended to have fewer offspring (mean = 2.1, ranging from 1 to 4) than males with assigned SNP genotypes (mean = 6.3). As male SNP genotypes are reconstructed from their offspring’s SNP genotypes, such small full-sib groups did not provide sufficient information to make a confident assignment in most cases. In 9 cases, female SNP genotype was incomplete (i.e., missing data at 1 or more SNP loci). In these cases female genotype was reconstructed in the same way as male genotype.
We categorized each female’s brood into 1 of 3 “mate categories”: only parental X. birchmanni fathers (N = 8), mixed parental and introgressed fathers (N = 8), and only introgressed fathers (N = 14); males with unassigned genotypes were excluded. In 1 brood, we were not able to amplify the SNP markers in the embryos and so were not able to reconstruct paternal SNP genotypes. This brood was excluded from any statistical analyses that included paternal introgression as an effect. Statistical analyses were performed in JMP (JMP, Version 9.0, SAS Institute Inc., Cary, NC, 2010) and in the R statistical language (R version 2.15.2; 2013). JMP was used to produce partial residual plots to illustrate the effect of each term included in least squares multiple regression models. Partial residual plots highlight the behavior of individual variables in a multivariate model by showing the relationship between a given independent variable and the dependent variable, after statistically controlling for all other independent variables (Gunst and Mason 1980). When standard deviations of groups were unequal, we used Welch’s approximate t-test. Brood size was log-transformed and proportion variables were arcsine-square root transformed. We have deposited these data with Dryad in compliance with the data archiving guidelines of this journal (Baker 2013).
Results
The 4 microsatellite markers optimized for this study from X. maculatus were highly variable with 11–27 alleles per locus identified in the pool of gravid females (Table 1). Genotypic variation was high within populations; no 2 females shared the same multilocus genotype. Similarly, none of the male genotypes inferred from the full-sib offspring groups were shared across broods. The probability of detecting multiple mating is high in both populations; only simulations of broods with fewer than 8 embryos showed a detection probability less than 0.96 (PrDM, Supplementary Table 1). Simulations similar to mean brood size and reproductive skew showed a probability of detection greater than 0.99 in San Pedro (mean = 14.6, N simulation = 15) and approximately 0.97 in Coacuilco (mean = 11.33, N simulation = 10, see Supplementary Table S1 for more detail). We observed no significant departure from Hardy–Weinberg equilibrium in any of the 4 loci within each population (Table 1). The mean F ST value (0.107) suggested moderate differentiation between the San Pedro and Coacuilco populations. F ST estimates varied greatly among loci (Table 1), as expected in cases with low gene flow (Baer 1999) or with low sample sizes. We found a high degree of genic differentiation between populations at Msd-029 (exact G test, P = 0.00198), Msd-036 (exact G test, P < 0.0001), and Msd-049 (exact G test, P < 0.0001), as a result of the high number of private alleles in each population at these loci (14 of 20 in Msd-029, 12 of 17 in Msd-036, and 6 of 11 in Msd-049).
Patterns of Multiple Mating and Reproductive Skew Within Broods
San Pedro Population
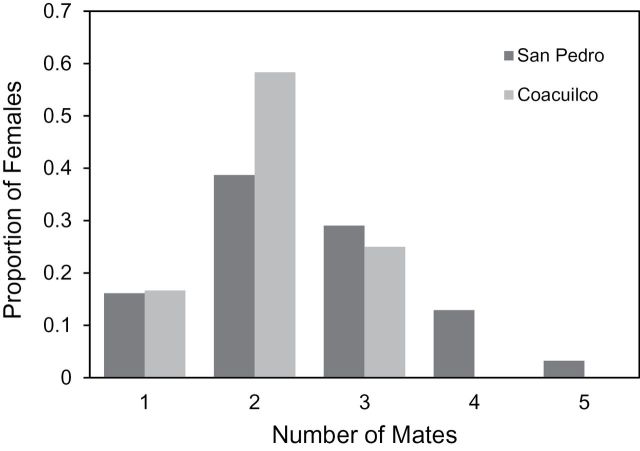
We first examined multiple mating in our focal population, San Pedro. We observed multiple mating in 26 of our 31 females (Table 2, 84%). Females in our sample mated with between 1 and 5 males (mean= 2.48, Figure 1, Table 2). In San Pedro, females in our collection varied from 37.00 to 58.59mm in length (meanSP = 46.00) and carried between 3 and 30 embryos (meanSP = 14.60, Table 2).
Table 2.
Multiple mating in our data and previously published data, including 2 congeneric species
| Group | N | % multiply sired | Mean # mates (SD) | Mean brood size (SD) | % by primary sired |
|---|---|---|---|---|---|
| X. birchmanni, San Pedro | 31 | 84 | 2.48 (0.67) | 14.6 (6.9) | 75 |
| X. birchmanni, Coacuilco | 12 | 83 | 2.08 (1.03) | 11.3 (6.5) | 69 |
| X. multilineatus a | 18 | 27.8 | 1.39 | 13.5 | 74 |
| X. hellerii b | 27 | 64 | 1.80 (0.7) | 23.3 (15.4) | 80 |
| X. nigrensis c | 31 | 61 | 1.87 (0.85) | 8.6 (3.6) | 70 |
| Female-pregnant fishes (by spp) d | 12 | 71 | 2.49 |
Data from a Luo et al. (2005), b Tatarenkov et al. (2008), c Smith (2014), d Avise and Liu (2010).
Figure 1.
Shows the distribution of multiple mating in Coacuilco (light gray) and San Pedro (dark gray) populations. Multiple mating is prevalent in X. birchmanni and the distribution of multiple mating does not differ between populations.
Number of fathers increased with brood size, with an average gain of 1 father per 14.10 additional offspring, but did not vary with female size (Figure 2a and Supplementary Figure S1a–d, least squares multiple regression, overall model: R 2= 0.44, F 3,26 = 6.71, P = 0.0017; female size: F 1,26 = 2.75, b′ = 0.30, P = 0.11, brood size: F 1,26 = 6.14, b′ = 0.48, P = 0.02; female size*brood size: F 1,26 = 0.03, b′ = −0.03, P = 0.85). When broods were subsampled to control for ascertainment bias, large females and females with large broods did not have more mates (Figure 2b and Supplementary Figure S1e–h, least squares multiple regression, overall model: R 2= 0.26, F 3,20 = 2.31, P = 0.11, female size: F 1,20 = 1.63, b′ = 0.29, P = 0.22; brood size: F 1,20 =1.48, b′ = 0.28, P = 0.24; female size*brood size; F 1,20 = 0.52, b′ = −0.15, P = 0.48). Of the 24 broods subsampled, 8 successfully subsampled all fathers in all replicates and 16 broods lost 1 father in at least 1 of the 1000 replicates. Large broods were more likely than small broods to show a reduction in subsampled mates relative to number of mates identified using the whole brood (linear regression, R 2 = 0.52, F 1,22 = 23.65, P < 0.0001); similarly, highly skewed broods were more likely than non-skewed broods to lose a mate in some of the subsample replicates (linear regression, R 2 = 0.40, F 1,22 = 14.66, P = 0.0009).
Figure 2.
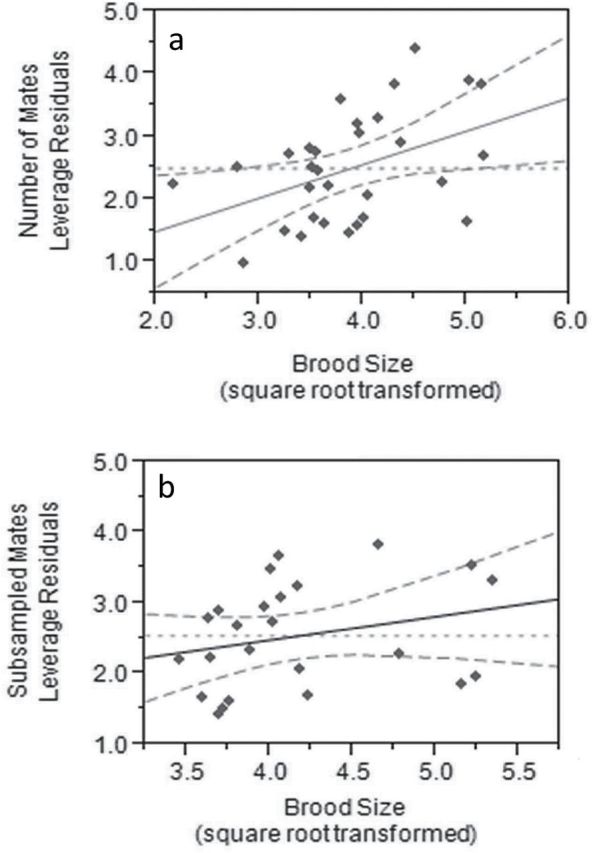
(a) A partial residual plot (or leverage plot) showing the positive effect of brood size on number of fathers, given female size. The dotted line shows the predicted effect of the model of number of fathers without the effect of brood size. The solid line shows the effect of brood size, while the dashed lines show the 95% confidence interval around the line of fit. Subsampling the broods to control for ascertainment bias (as in the partial regression plot of b) removes the statistical significance of this relationship. The partial residual plots of all effects in these models are found in Supplementary Figure S1.
In multiply-sired broods, primary sires (those producing the greatest number of offspring in a brood) fathered on average 64% of the offspring (Table 2, range 37%–95%). Secondary sires (those producing the second greatest number of offspring in a brood) fathered an average of 26% of the offspring (range 5%–43%) and subsequent males (3–5) fathered on average 2.4% of the offspring (range 7%–25%).
Broods with more fathers also had higher reproductive skew (linear regression with permutation test: R 2 = 0.26, F 1,29 = 11.60, b′ = 0.33, P = 0.002). Broods with 2 fathers had, on average, 12.36 offspring and an index of monopolization of −0.06. This value of reproductive skew can arise in a brood of 12 if, for example, 1 male contributes 8 offspring and the other contributes 4 offspring. Skew increased to −0.02 in broods with 3 fathers. This value for the index of monopolization occurs in a brood of 16 offspring (meanbrood size, 3 fathers = 16.44) when, for instance, the 3 fathers sire 11, 4, and 2 offspring apiece. The relationship between multiple paternity and skew was not maintained when the offspring were subsampled as before (linear regression with permutation test: R 2 = 0.008, F 1,22 = 0.19, b′ = 0.04, P = 0.67).
We next investigated the effect on reproductive skew of female size, number of offspring, embryo dry mass, and the interaction between these variables. Reproductive skew increased with number of offspring and the interaction between number of offspring and female size (Figure 3, Supplementary Figure S2, linear regression with a permutation test of slope, full model R 2 = 0.72, F 7,22 = 7.98, P < 0.0001; number of offspring, b′ = 0.83, P < 0.0001; female size, b′ = 0.02, P = 0.96; embryo dry mass, b′ = −0.04, P = 0.84; number of offspring*female size, b′ = 0.55, P = 0.003; number of offspring*embryo dry mass, b′ = 0.32, P = 0.06; female size*embryo dry mass, b′ = 0.17, P = 0.11; number of offspring*female size*embryo dry mass, b′ = 0.005, P = 1.0). A brood of 10 offspring has an average skew value of −0.09, which corresponds to 3 fathers contributing 4, 3, and 3 offspring each, while skew increases to an average value of −0.01 in broods of 20 offspring, as would occur if the fathers sired 12, 5, and 3 offspring per male.
Figure 3.
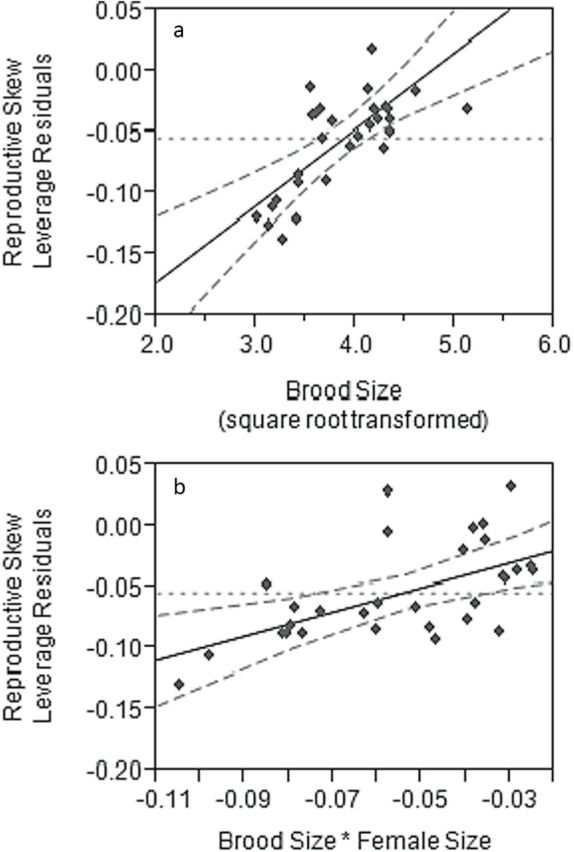
Shows partial residual plots of the positive effect of (a) brood size and (b) the interaction between female size and brood size in the model of reproductive skew, given all other effects in the model. The dotted line shows the predicted effect of the model without this interaction term, the solid line shows the effect of the interaction term and the dashed lines show the 95% confidence interval around the fitted line. Partial residual plots of all effects in this model are depicted in Supplementary Figure S2.
Coacuilco Population
We quantified multiple mating in an additional 12 X. birchmanni females from our second population, Coacuilco, to address whether patterns of multiple mating vary between populations. In Coacuilco, female size varied from 35.9 to 51.00mm (meanCU = 43.60) and brood size varied between 4 and 23 offspring (meanCU = 11.33). Coacuilco females mated with between 1 and 3 males (meanCU = 2.08, 83% multiply mated). The distribution of multiple mating did not differ between San Pedro and Coacuilco (Figure 1, Kolmogorov–Smirnov test, D = 0.20, P = 0.87), although our power to detect a difference is limited by sample sizes.
We found no evidence that the patterns of multiple mating and within-brood reproductive skew in Coacuilco differed from the results we observed in the San Pedro population. We repeated our previous analysis of number of mates (# fathers~ female size + brood size + female size*brood size) and reproductive skew (reproductive skew~ female size + brood size + embryo weight + female size*brood size + female size*embryo weight + brood size*embryo weight + female size*brood size*embryo weight) with data from both populations and included “population” as an effect. Adding population identity as a covariate did not improve the explanatory power of these models (least squares multiple regression on number of mates, AICcwithout population = 100.25, AICcwith population = 103.00, ΔAICc = 2.75, population, P = 0.87; least squares multiple regression on reproductive skew, AICcwithout population = −129.16, AICcwith population = −126.40, ΔAICc = 2.73, population, P = 0.40). Additionally, a discriminant analysis using brood size, female size, number of fathers, and reproductive skew failed to distinguish between the populations (Wilks’ Lambda=0.87, F 4,29 = 1.08, P = 0.38), misclassifying 32.30% of females (11 out of 34).
Introgression of X. malinche Alleles and Within-Brood Reproductive Skew
Introgression in Females
We identified 9 parental X. birchmanni females and 22 introgressed females in our collection from the San Pedro population. Introgressed females did not differ from parental females in body size (Welch’s t-test, P = 0.69, 95% CI, Lb: −4.48, Ub: 7.12), brood size (Welch’s t-test, P = 0.61, 95% CI, Lb: −0.56 Ub: 0.86), offspring size (Welch’s t-test, P = 0.56, 95% CI, Lb: −0.0012, Ub: 0.00061), number of mates (after subsampling, Welch’s t-test, P = 0.72, 95% CI, Lb: −0.64, Ub: 0.91), or reproductive skew (Welch’s t-test, P = 0.35, 95% CI, Lb: −0.03, Ub: 0.07). Female genotype did not vary annually (Fisher’s exact test, P = 0.66).
Introgression in Males
We were able to infer SNP genotypes for 31 nonintrogressed fathers and 34 introgressed fathers using the SNP genotypes of the female and up to 6 offspring in each full-sib group (see Methods). Nonintrogressed X. birchmanni males cumulatively sired 230 offspring while the introgressed males fathered 177. Individual nonintrogressed males did not differ from introgressed males in greater number of offspring (Welch’s t-test P = 0.72, 95% CI, Lb: −2.01, Ub: 2.90) or percentage of their brood (t-test P = 0.20, 95% CI, Lb: −0.05, Ub: 0.23). Finally, we found no evidence that any male mated with more than 1 female in our collection, as none of the male microsatellite genotypes inferred from the full-sib offspring groups were shared across broods.
Reproductive Investment in Females with Parental and Introgressed Mates
We next asked whether a female’s reproductive allocation may be related to the genotypes of her mates, which we summarized as the mate category: all parental mates, all introgressed mates, or mixed parental and introgressed mates. Reproductive skew and embryo dry mass did not vary according to mate category (Tukey–Kramer HSD α = 0.05).
Females with only parental mates tended to have, on average, 8 more offspring than females with only introgressed mates (Figure 4, Tukey–Kramer HSD, P = 0.03); however, this pattern is not robust if we include the effect of female size in the analysis of variance (ANCOVA, full model: brood size ~ female size + mate category, F 3,25 = 6.04, P = 0.003, female size F 1, 25 = 9.49, P = 0.005, mate category F 2,25 = 1.74, P = 0.20).
Figure 4.
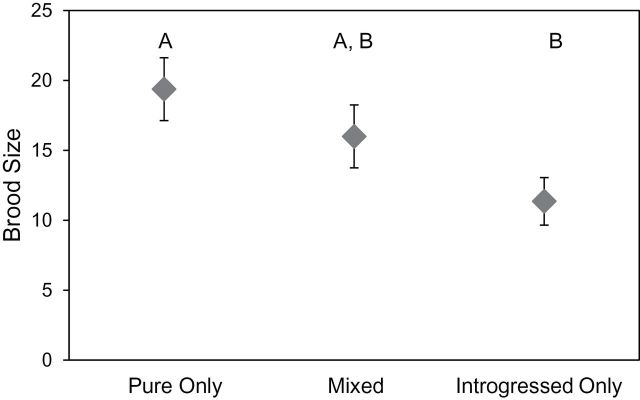
Depicts mean brood size (±SEM) as a function of male genotype. Females that mated with only parental X. birchmanni males (“Parental Only” group) have larger broods than females that mated with only introgressed X. birchmanni males (“Introgressed Only” group, Tukey–Kramer HSD). Neither of these groups is different from the broods of females that mated with both parental and introgressed X. birchmanni males.
Females mating with both parental and introgressed males had, on average, 1.08 more mates than females mated with only introgressed mates (Tukey–Kramer HSD, P = 0.02) and 0.90 more mates than females with only parental mates (Tukey–Kramer HSD, P = 0.076) when we use the subsampling method to control for the effect of brood size. This pattern is robust if we further exclude singly mated females, which could not be included in the mixed mates category; females with mixed mates had on average 1.00 more mates than females with only introgressed mates and 0.5 more mates than females with only parental mates (Tukey–Kramer HSD, p mixed-introgressed = 0.03, p mixed-parental = 0.41).
Interactions among Introgression in Males and Females, and Female Allocation
Female genotype did not predict patterns of mating and reproductive allocation towards parental and introgressed males. Parental females were no more likely than introgressed females to have more parental mates (Welch’s t-test, by count P = 0.21, by proportion P = 0.20), to have a parental male father the largest proportion of their brood (Fisher’s Exact Test, P = 0.12), or have parental males father a greater total number of offspring (Welch’s t-test, P = 0.36) or proportion (Welch’s t-test, P = 0.16) in her brood. Similarly, a contingency table shows that parental and introgressed females were equally likely to mate with only parental, only introgressed, or both parental and introgressed males (female genotype by mate category, Fisher’s exact test, P = 0.67).
Discussion
Understanding the evolutionary causes and consequences of mating system variation is a fundamental question in evolutionary biology, because these processes can play a major role in species diversification and extinction as well as in the evolution of complex traits. Although the mating preferences of female Xiphophorus have been studied extensively, few studies have measured variance in male mating success in these species. Our results suggest that mate preferences of females, the introgression of hybrid alleles, and female life-history traits may play a major role in determining fertilization success of male swordtails.
Our initial objective was to determine if multiple mating occurred in our focal species, X. birchmanni. We did find that a large proportion of females in our collection mated multiply (Table 2, 84%), suggesting that multiple mating is the norm in this species, and that the incidence of multiple mating in X. birchmanni appears to be higher than that observed in other species of Xiphophorus (Table 2, Luo et al. 2005; Simmons et al. 2008; Tatarenkov et al. 2008). However, the pattern of multiple mating we observed is not unexpectedly high, as the frequency of multiple mating and the mean number of mates per female appear to be similar to the level of multiple mating observed across poeciliids and female-pregnant fishes generally (Table 2, Avise and Liu 2010; Coleman and Jones 2011). We also found this pattern of multiple mating to be relatively consistent across the 2 populations we studied, or across years at San Pedro; however, our power to detect a difference may be constrained due to our limited sample size. Further investigation of populations across the X. birchmanni–X. malinche hybrid zone and across environmental gradients may provide a clearer picture of how the mating system varies and how the mating system acts to maintain this hybrid zone.
We did not detect an effect of introgression in males or females on the rate of multiple mating when we compared parental and introgressed individuals from the San Pedro population. Our results suggest that the low level of introgression found in this population (introgressed individuals carry 1 or 2 out of 7 possible X. malinche SNP alleles) does not result in detectable variation in mating rate between introgressed and non-introgressed females. Populations with higher average introgression or greater diversity in the introgression of individuals may show more substantial variation in mating rate between these groups.
We observed substantial reproductive skew within broods in X. birchmanni, indicating that some males have much greater fertilization success than others. We found that larger females with more offspring (larger broods) had more highly skewed broods. Reproductive skew has also been documented in other swordtails and live-bearing fishes, suggesting that within-brood skew is a general phenomenon (Luo et al. 2005; Simmons et al. 2008; Tatarenkov et al. 2008). The mechanisms producing reproductive skew in swordtails is currently unknown, but potential mechanisms include the use of fresh and stored sperm in fertilization (Potter and Kramer 2000), cryptic female choice in favor of some male phenotypes (Eberhard 1996), or sperm competition (Birkhead and Moller 1998).
Although we did not find a relationship between female introgression and reproductive tactics, we did find a relationship between female reproductive traits and male introgression. Our results suggest that fecund females more frequently mate with parental X. birchmanni males than with introgressed males (Figure 4). This tendency does not appear to result in increased paternity for nonintrogressed males, despite the fact that these large females are more fecund, because large females also have more mates and greater reproductive skew. However, males that preferentially target large, fecund females may gain indirect benefits by fathering more successful offspring, as a previous study on a larger collection from this population showed that large females also produce heavier offspring (Kindsvater et al. 2012). The relationship between female fecundity and male genotype may be the result of either a male or female effect. For example, large females may have greater control over mating (i.e. they are less likely to be coerced). There is a robust relationship between size and age in females (Kindsvater et al. 2012), so this pattern may be the result of a learned preference by older, more experienced females for nonintrogressed males, as learned preferences have been shown in X. birchmanni in previous studies (Verzijden and Rosenthal 2011; Verzijden et al. 2012). Alternatively, females may be allocating reproductive effort in favor of non-introgressed mates. An experimental lab study on X. birchmanni showed that female experience also influenced reproductive allocation (Kindsvater et al. 2013). Increased reproductive investment, or differential allocation, would be expected to evolve if some male genotypes offer benefits to offspring fitness (Reynolds and Gross 1992; Sheldon 2000; Kindsvater et al. 2013). The patterns we observed may also arise if nonintrogressed males preferentially court or harass the largest females in the population or if nonintrogressed males are more successful at male–male competition.
Our results suggest that hybrid inviability does not affect the reproductive success of parental and introgressed X. birchmanni. First, we did not see a direct effect of either male or female genotype on fecundity. Female fecundity is related to mate category (Figure 4); however, this relationship is not maintained when female size is included in the model. Similarly, individual parental males did not father more offspring than introgressed males, on average. Additionally, we found no interaction effect between male and female genotype on reproductive skew, brood size, or embryo size, which would be expected if cross-type mating events experienced reduced genetic compatibility. Rather, it is more likely that large females have more offspring and some other factors, either male or female, bias the mating events and reproductive allocation of large females towards nonintrogressed males.
Our data are the beginning of a promising line of research detailing variation in mating systems of X. birchmanni and X. malinche across multiple natural hybrid zones. However, additional information is required to answer many of the more intriguing questions raised by our results. Our methods do not capture phenotypic variation among males that may be fundamental to explaining female mate choice and reproductive allocation. By categorizing inferred males as either “parental” or “introgressed” genotypes, our data do not reflect the extensive phenotypic variation present within hybrid and introgressed male genotypic categories. Such data could not be collected from a wild population without nearly exhaustive sampling, which would permit genotypes of collected males to be matched to the paternal genotypes reconstructed from full-sib groups. Furthermore, several of our inferences are based on comparisons between relatively small samples and could reflect a lack of power to detect an effect. Similarly, our assay of 3 neutral SNP markers provides a relatively low resolution look at genome-wide levels of introgression. On average, individuals carrying all X. birchmanni alleles are likely to have lower levels of introgression than individuals carrying many X. malinche alleles; however, the utility of these markers in measuring individual genome-wide introgression is yet untested. In future studies, the use of high-throughput techniques such as RAD-tag or multiplexed shotgun genotyping (MSG) where thousands of markers are simultaneously assayed to produce a fine-scale measure of individual genomic introgression (Hohenlohe et al. 2011; Schumer et al. 2013) may allow us to revisit the patterns observed here.
Previous studies have characterized the precopulatory mechanisms of sexual selection across Xiphophorus in depth, as they are an excellent system in which to study how mate preference, sensory drive, and hybrid mating affect species diversity. Our study demonstrates that both pre- and postmating processes including multiple mating and the coevolution of male and female traits could be important contributors to patterns of reproductive success in X. birchmanni.
Supplementary Material
Supplementary material can be found at http://www.jhered.oxfordjournals.org/.
Funding
National Science Foundation IOS-0923825; American Livebearer Association (G.G.R); NSF DEB-0909843 (H.K.K.).
Supplementary Material
Acknowledgments
We would like to thank the UBC Whitlock lab for comments on the manuscript, as well as Zachary Culumber, Lauren McMahon, and Andy Marshall for their assistance in completing this study. The Mexican federal government provided permission to collect fish.
References
- Alonzo SH. 2010. Social and coevolutionary feedbacks between mating and parental investment. Trends Ecol Evol. 25:99–108. [DOI] [PubMed] [Google Scholar]
- Avise JC, Liu JX. 2010. Multiple mating and its relationship to alternative modes of gestation in male-pregnant versus female-pregnant fish species. Proc Natl Acad Sci U S A. 107:18915–18920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer CF. 1999. Among-locus variation in Fst: fish, allozymes and the Lewontin-Krakauer test revisited. Genetics. 152:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CS. 2013. Journal of heredity adopts joint data archiving policy. J Hered. 104:1. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Moller AP. 1998. Sperm competition and sexual selection. London: Academic Press. [Google Scholar]
- Birkhead TR, Pizzari T. 2002. Evolution of sex: postcopulatory sexual selection. Nat Rev Genet. 3:262–273. [DOI] [PubMed] [Google Scholar]
- Coleman SW, Jones AG. 2011. Patterns of multiple paternity and maternity in fishes. Biol J Linn Soc. 103:735–760. [Google Scholar]
- Cui R, Schumer M, Kruesi K, Walter R, Andolfatto P, Rosenthal GG. 2013. Phylogenomics reveals extensive reticulate evolution in Xiphophorus fishes. Evolution. 67:2166–2179. [DOI] [PubMed] [Google Scholar]
- Culumber ZW, Fisher HS, Tobler M, Mateos M, Barber PH, Sorenson MD, Rosenthal GG. 2011. Replicated hybrid zones of Xiphophorus swordtails along an elevational gradient. Mol Ecol. 20:342–356. [DOI] [PubMed] [Google Scholar]
- Culumber ZW, Shepard DB, Coleman SW, Rosenthal GG, Tobler M. 2012. Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: Xiphophorus). J Evol Biol. 25:1800–1814. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. 1996. Female control: sexual selection by cryptic female choice, Princeton: (NJ: ): Princeton University Press. [Google Scholar]
- Evans JP, Zane L, Francescato S, Pilastro A. 2003. Directional postcopulatory sexual selection revealed by artificial insemination. Nature. 421:360–363. [DOI] [PubMed] [Google Scholar]
- Evans JP. 2010. Quantitative genetic evidence that males trade attractiveness for ejaculate quality in guppies. Proc Biol Sci. 277:3195–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher HS, Mascuch S, Rosenthal GG. 2009. Multivariate male traits misalign with multivariate female preferences in the swordtail fish, Xiphophorus birchmanni . Anim Behav. 78:265–269. [Google Scholar]
- Gunst RF, Mason RL. 1980. Regression Analysis and its Application: A Data-Oriented Approach. New York: Marcel Dekker, Inc. [Google Scholar]
- Herdman EJE, Kelly CD, Godin JJ. 2004. Male mate choice in the guppy (Poecilia reticulata): Do males prefer larger females as mates? Ethology 110:97–111. [Google Scholar]
- Hildemann WH, Wagner ED. 1954. Intraspecific sperm competition in Lebistes reticulatus . Am Nat. 88:87–91 [Google Scholar]
- Hohenlohe PA, Amish SJ, Catchen JM, Allendorf FW, Luikart G. 2011. Next-generation RAD sequencing identifies thousands of SNPs for assessing hybridization between rainbow and westslope cutthroat trout. Mol Ecol Resour. 11:117–122. [DOI] [PubMed] [Google Scholar]
- Joly D, Bazin C, Zeng LW, Singh RS. 1997. Genetic basis of sperm and testis length differences and epistatic effect on hybrid inviability and sperm motility between Drosophila simulans and D. sechellia. Heredity (Edinb). 78:354–362. [DOI] [PubMed] [Google Scholar]
- Jones AG. 2005. GERUD2.0: A computer program for the reconstruction of paternal genotypes from half-sib progeny arrays with known or unknown parents. Mol Ecol Notes. 5:708–711. [Google Scholar]
- Jones AG, Ratterman NL. 2009. Mate choice and sexual selection: what have we learned since Darwin? Proc Natl Acad Sci U S A. 106:10001–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OR, Wang J. 2010. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Resour. 10:551–555. [DOI] [PubMed] [Google Scholar]
- Kelly CD, Godin JJ, Wright JM. 1999. Geographic variation in multiple paternity within natural populations of the guppy (Poecilia reticulata). Proc R Soc Lond B. 266:2403–2408. [Google Scholar]
- Kindsvater HK, Rosenthal GG, Alonzo SH. 2012. Maternal size and age shape offspring size in a live-bearing fish, Xiphophorus birchmanni. PLoS One. 7:e48473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindsvater HK, Simpson SE, Rosenthal GG, Alonzo SH. 2013. Male diet, female experience, and female size influence maternal investment in swordtails. Behav Ecol. 24:691–697. [Google Scholar]
- Kokko H, Mackenzie A, Reynolds JD, Lindström J, Sutherland WJ. 1999. Measures of inequality are not equal. Am Nat. 154:358–382. [DOI] [PubMed] [Google Scholar]
- Kuijper B, Pen I, Weissing FJ. 2012. A guide to sexual selection theory. Annu Rev Ecol Evol Syst. 43:287–311. [Google Scholar]
- Luo J, Sanetra M, Schartl M, Meyer A. 2005. Strong reproductive skew among males in the multiply mated swordtail Xiphophorus multilineatus (Teleostei). J Hered. 96:346–355. [DOI] [PubMed] [Google Scholar]
- Neff BD, Pitcher TE. 2002. Assessing the statistical power of genetic analyses to detect multiple mating in fishes. J Fish Biol. 61:739–750. [Google Scholar]
- Panhuis TM, Butlin R, Zuk M, Tregenza T. 2001. Sexual selection and speciation. Trends Ecol Evol. 16:364–371. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Olson SL, Braun MJ. 1993. Unidirectional spread of secondary sexual plumage traits across an avian hybrid zone. Science. 260:1643–1646. [DOI] [PubMed] [Google Scholar]
- Potter H, Kramer CR. 2000. Ultrastructural observations on sperm storage in the ovary of the platyfish, Xiphophorus maculatus (Teleostei: poeciliidae): the role of the duct epithelium. J Morphol. 245:110–129. [DOI] [PubMed] [Google Scholar]
- Rauchenberger M, Kallman KD, Morizot DC. 1990. Monophyly and geography of the Río Panuco basin swordtails (genus Xiphophorus) with descriptions of four new species. Am Mus Novit. 2975:1–41. [Google Scholar]
- Raymond M, Rousset F. 1995. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered. 86:248–249 [Google Scholar]
- Reynolds JD, Gross MR. 1992. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulate. P Roy Soc Lond B Bio. 250:57–62. [Google Scholar]
- Rose E, Paczolt KA, Jones AG. 2013. The contributions of premating and postmating selection episodes to total selection in sex-role-reversed Gulf pipefish. Am Nat. 182:410–420. [DOI] [PubMed] [Google Scholar]
- Rosenfield JA, Kodric-Brown A. 2003. Sexual selection promotes hybridization between Pecos pupfish, Cyprinodon pecosensis and sheepshead minnow, C. variegatus. J Evol Biol. 16:595–606. [DOI] [PubMed] [Google Scholar]
- Rosenthal GG. 2013. Individual mating decisions and hybridization. J Evol Biol. 26:252–255. [DOI] [PubMed] [Google Scholar]
- Rosenthal GG, de la Rosa Reyna XF, Kazianis S, Stephens MJ, Morizot DC, Ryan MJ, García de León FJ. 2003. Dissolution of sexual signal complexes in a hybrid zone between the swordtails Xiphophorus birchmanni and Xiphophorus malinche (Poeciliidae). Copeia. 2003:299–307. [Google Scholar]
- Rosenthal GG, Fitzsimmons JN, Woods KU, Gerlach G, Fisher HS. 2011. Tactical release of a sexually-selected pheromone in a swordtail fish. PLoS One. 6:e16994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F. 2008. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 8:103–106. [DOI] [PubMed] [Google Scholar]
- Ruzzante DE, Hamilton DC, Kramer DL, Grant JWA. 1996. Scaling of the variance and the quanitification of resource monopolization. Behav. Ecol. 7:199–207. [Google Scholar]
- Ryan M J, Rosenthal GG. 2001. Variation and selection in swordtails. In: Dugatkin L. A., editor. Model systems in behavioral ecology. Princeton (NJ): Princeton University Press; p. 133–148. [Google Scholar]
- Schumer M, Cui R, Boussau B, Walter R, Rosenthal G, Andolfatto P. 2013. An evaluation of the hybrid speciation hypothesis for Xiphophorus clemenciae based on whole genome sequences. Evolution. 67:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol Evol. 15:397–402. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Beveridge M, Evans JP. 2008. Molecular evidence for multiple paternity in a feral population of green swordtails. J Hered. 99:610–615. [DOI] [PubMed] [Google Scholar]
- Soucy S, Travis J. 2003. Multiple paternity and population genetic structure in natural populations of the poeciliid fish, Heterandria formosa . J Evol Biol. 16:1328–1336. [DOI] [PubMed] [Google Scholar]
- Smith CC. 2014. Polyandry and paternity in a wild population of the swordtail Xiphophorus nigrensis . Behav Ecol Sociobiol. 68:415–424. [Google Scholar]
- Tatarenkov A, Healey CI, Grether GF, Avise JC. 2008. Pronounced reproductive skew in a natural population of green swordtails, Xiphophorus helleri . Mol Ecol. 17:4522–4534. [DOI] [PubMed] [Google Scholar]
- Travis J, Trexler JC, Mulvey M. 1990. Multiple paternity and its correlates in female Poecilia latipinna. Copeia. 1990:722–729. [Google Scholar]
- Trexler JC, Travis J, Dinep A. 1997. Variation among populations of the sailfin molly in the rate of concurrent multiple paternity and its implications for mating-system evolution. Behav Ecol Sociobiol. 40:297–305. [Google Scholar]
- Verzijden MN, Rosenthal GG. 2011. Effects of sensory modality on learned mate preferences in female swordtails. Anim Behav. 82:557–562. [Google Scholar]
- Verzijden MN, Culumber ZW, Rosenthal GG. 2012. Opposite effects of learning cause asymmetric mate preferences in hybridizing species. Behav Ecol. 23:1140–1145. [Google Scholar]
- Walter RB, Rains JD, Russell JE, Guerra TM, Daniels C, Johnston DA, Kumar J, Wheeler A, Kelnar K, Khanolkar VA, et al. 2004. A microsatellite genetic linkage map for Xiphophorus. Genetics. 168:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. 2004. Sibship reconstruction from genetic data with typing errors. Genetics. 166:1963–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. 2012. Computationally efficient sibship and parentage assignment from multilocus marker data. Genetics. 191:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis PM, Ryan MJ, Rosenthal GG. 2011. Encounter rates with conspecific males influence female mate choice in a naturally hybridizing fish. Behav Ecol. 22:1234–1240. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.