The Long-Term Persistence Substudy of the Shingles Prevention Study (SPS) assessed zoster vaccine efficacy in a subset of the SPS vaccine recipients followed for up to 11 years postvaccination. Vaccine efficacy was no longer statistically significant beyond 8 years postvaccination.
Keywords: herpes zoster, herpes zoster vaccine, herpes zoster burden of illness, postherpetic neuralgia, persistence of vaccine efficacy
Abstract
Background. The Shingles Prevention Study (SPS) demonstrated zoster vaccine efficacy through 4 years postvaccination. A Short-Term Persistence Substudy (STPS) demonstrated persistence of vaccine efficacy for at least 5 years. A Long-Term Persistence Substudy (LTPS) was undertaken to further assess vaccine efficacy in SPS vaccine recipients followed for up to 11 years postvaccination. Study outcomes were assessed for the entire LTPS period and for each year from 7 to 11 years postvaccination.
Methods. Surveillance, case determination, and follow-up were comparable to those in SPS and STPS. Because SPS placebo recipients were offered zoster vaccine before the LTPS began, there were no unvaccinated controls. Instead, SPS and STPS placebo results were used to model reference placebo groups.
Results. The LTPS enrolled 6867 SPS vaccine recipients. Compared to SPS, estimated vaccine efficacy in LTPS decreased from 61.1% to 37.3% for the herpes zoster (HZ) burden of illness (BOI), from 66.5% to 35.4% for incidence of postherpetic neuralgia, and from 51.3% to 21.1% for incidence of HZ, and declined for all 3 outcome measures from 7 through 11 years postvaccination. Vaccine efficacy for the HZ BOI was significantly greater than zero through year 10 postvaccination, whereas vaccine efficacy for incidence of HZ was significantly greater than zero only through year 8.
Conclusions. Estimates of vaccine efficacy decreased over time in the LTPS population compared with modeled control estimates. Statistically significant vaccine efficacy for HZ BOI persisted into year 10 postvaccination, whereas statistically significant vaccine efficacy for incidence of HZ persisted only through year 8.
(See the Editorial Commentary by Whitley on pages 910–11.)
Herpes zoster (HZ) results from the reactivation, multiplication, and spread of varicella zoster virus (VZV) that remained latent in sensory neurons following primary VZV infection [1]. The Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) 403, the Shingles Prevention Study (SPS), demonstrated that live attenuated Oka/Merck VZV vaccine (zoster vaccine) reduced the HZ burden of illness (BOI) (a severity-by-duration measure of HZ pain and discomfort) by 61.1%, incidence of postherpetic neuralgia (PHN) by 66.5%, and incidence of HZ by 51.3%. Zoster vaccine efficacy for all 3 study endpoints persisted through 4 years postvaccination [2, 3]. Following SPS, 14 270 SPS vaccine and placebo recipients from 12 of the original 22 study sites were re-enrolled into a Short-Term Persistence Substudy (STPS) and followed from 3.3 to 7.8 years postvaccination to further assess duration of vaccine efficacy [4]. In STPS, zoster vaccine reduced the HZ BOI by 50.1%, incidence of PHN by 60.1%, and incidence of HZ by 39.6% [4]. Combined results of SPS and STPS demonstrated persistence of vaccine efficacy through year 5 postvaccination [4]. This Long-Term Persistence Substudy (LTPS) further assessed duration of vaccine efficacy by continuing to follow a cohort of SPS vaccine recipients from 5 to as long as 11 years postvaccination.
METHODS
Study Design and Timeline
The design and results of SPS and STPS have been previously published [2–4]. In October 2005, SPS placebo recipients who could be contacted were offered zoster vaccine per SPS protocol, and >80% elected to receive it [5]. Consequently, LTPS had no SPS placebo recipients to serve as unvaccinated controls. Re-enrollment of SPS vaccine recipients into LTPS took place from 9 March 2006 to 6 June 2007. Closeout calls began on 1 July 2010. Surveillance for HZ ended on 30 December 2010 (Supplementary Data).
Study Population and Sites
LTPS was limited to SPS vaccine recipients at the 12 STPS sites (Figure 1). A telephone consent procedure to re-enroll subjects into LTPS was approved by VA CSP, a CSP Human Rights Committee, and local institutional review boards. Subjects with prior HZ were ineligible.
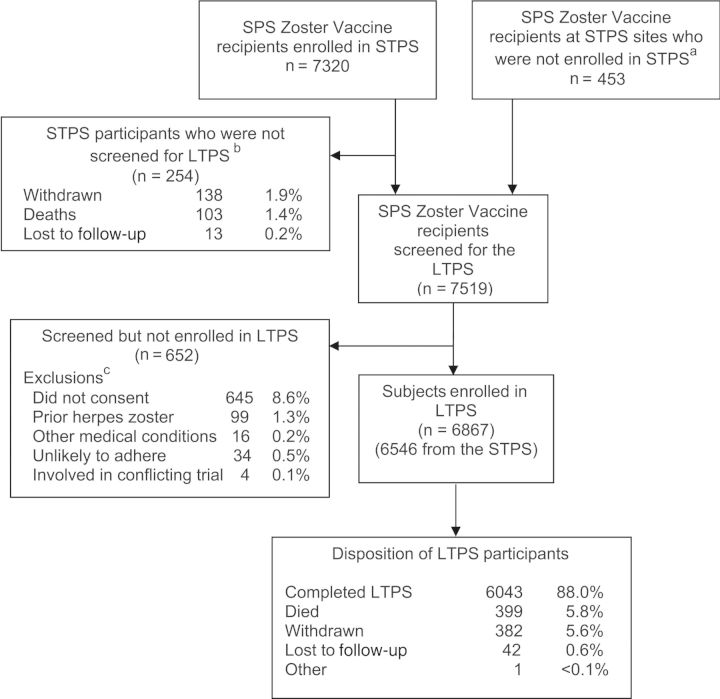
Figure 1.
Screening, enrollment and disposition of participants in the Long-Term Persistence Substudy (LTPS). aDuring LTPS, 453 Shingles Prevention Study (SPS) zoster vaccine recipients who did not enroll in the Short-Term Persistence Substudy (STPS) were screened for LTPS, and 321 were enrolled; bTwo hundred fifty-four participants were withdrawn, died, or were lost to follow-up in STPS before screening in LTPS; cParticipants may have had >1 reason for not enrolling in LTPS. Participants who withdrew from STPS and were not enrolled in LTPS are not counted in the screened population.
Follow-up
Active follow-up, with surveillance for HZ aided by an automated telephone response system, was the same as in SPS [2, 3] except that, as in STPS [4], frequency of contact with subjects with suspected HZ was reduced from weekly to monthly after week 4. As in SPS, the threshold for evaluating suspected cases of HZ was set very low to ensure inclusion of mild, atypical, or vaccine-modified cases of HZ (Supplementary Data).
HZ Case Determination and Endpoint Measurements
Evaluation of suspected cases of HZ, including diagnosis, management, and measurement of HZ-associated pain and/or discomfort, was the same as in SPS [2]. Subjects with suspected HZ were seen as soon as possible after rash onset, again during the first week if the rash was still evolving, and subsequently on days 8, 31, 61, 91, 121, 151 and 183; written consent was obtained to collect clinical data and diagnostic specimens from skin lesions [6]. As in SPS, confirmed cases of HZ were determined using a hierarchical algorithm based on central polymerase chain reaction (PCR) assay results, local virus culture, and adjudication by the Clinical Evaluation Committee (CEC) [2, 6] (Supplementary Data).
The Initial Zoster Impact Questionnaire and Zoster Brief Pain Inventory (ZBPI) were used to record subject-reported HZ pain and/or discomfort (eg, severe pruritus) [2–4, 7, 8]. Responses were used to determine HZ severity of illness scores and the presence or absence of clinically significant PHN (defined as a ZBPI worst pain score of ≥3 on a 0–10 scale persisting or appearing >90 days after HZ rash onset) [2–4, 7, 8]. The HZ severity of illness score for each case of HZ was defined as the area under the curve of the ZBPI worst pain and/or discomfort severity plotted against time during the 182-day period after HZ rash onset [2, 3, 8]. Subjects who did not develop HZ were assigned HZ severity of illness scores of zero [2].
The HZ BOI was a composite measure reflecting incidence of HZ, and severity and duration of HZ pain and/or discomfort in a population of subjects. It was defined as the sum of the HZ severity of illness scores of all evaluable cases of HZ in the group (eg, 60- to 69-year-old zoster vaccine recipients) divided by the person-years of observation.
Statistical Methods
Definition of HZ BOI and methods for calculating vaccine efficacy for study outcomes were previously published [2–4, 8, 9]. Analysis of incidence of HZ and PHN assumed a Poisson distribution for events and used a conditional exact method for calculating rates [9–12]. Data management and statistical analysis employed SAS programming language [13], with exact confidence limits calculated using StatXact [14].
Because there was no concurrent placebo control group, historical control estimates were calculated for HZ BOI, incidence of PHN, and incidence of HZ using data from the placebo groups in SPS and STPS in Poisson regression models for incidence of HZ and PHN and linear regression for HZ severity of illness (Johnson et al, manuscript in preparation; Supplementary Data). A primary analysis and 2 sensitivity analyses with historical control estimates adjusted for age were prespecified in the LTPS statistical analysis plan. Two of the 3 historical control estimates were also adjusted for an increase in the incidence of HZ observed in SPS and STPS placebo recipients over the study period (the “calendar effect”). The 3 resulting models were (1) a conservative placebo control group (sensitivity analysis I) that included data from SPS only and did not include the calendar effect; (2) an intermediate placebo control group (chosen for the primary vaccine efficacy analysis) that included data from SPS only but was adjusted to include the calendar effect; and (3) a contemporary placebo control group (sensitivity analysis II) that included data from SPS and STPS and was adjusted for the calendar effect observed in both studies (Supplementary Tables 1 and 3).
Vaccine effects in LTPS were estimated by calculating vaccine efficacy for HZ BOI and for incidence of HZ and PHN, and estimating 95% confidence intervals (CIs) based on the variance of the observed LTPS population and treating the historical control as constant. Analyses were stratified by age at randomization in SPS into 2 prespecified age groups: 60–69 years of age, and ≥70 years of age.
Supportive analyses assessed the change in vaccine efficacy for the 3 study outcomes over each year of follow-up. To estimate the effect of zoster vaccine on HZ BOI within a specific year postvaccination, the HZ severity of illness for that year was divided by the number of subject-years of follow-up in that year, and vaccine efficacy for HZ BOI was calculated as 1 – (HZ BOIVaccine/HZ BOIHistorical Control). Vaccine efficacy for incidence of PHN and incidence of HZ within a specific year postvaccination were calculated similarly. For analyses by year postvaccination, results from SPS and STPS were pooled for each year after vaccination for years 1 through 6, with methods published previously [4]. For years 7 and 8, STPS and LTPS results were pooled. Only LTPS results existed for years 9–11 postvaccination.
RESULTS
Study Population
Of 7519 screened participants, 6867 (91%) were enrolled into LTPS (Table 1; Figure 1). Main reasons for subjects not enrolling into LTPS (n = 652) are shown in Figure 1. Of the 6867 LTPS subjects, 97.8% were white; 56.3% were men; ages ranged from 64 to 95 years (median, 74 years); 20.8% were >80 years of age. On average, subjects were 6 years older when they enrolled in LTPS (mean age, 74.5 years [standard deviation (SD), 5.8 years]) than when vaccinated in SPS (mean age, 68.3 years [SD, 5.7 years]). LTPS participants were younger when randomized in SPS than subjects screened but not enrolled (mean age at SPS randomization, 68.3 vs 69.6 years, respectively).
Table 1.
Summary of Zoster Vaccine Efficacy for Herpes Zoster (HZ) Burden of Illness, Incidence of Postherpetic Neuralgia, and Incidence of HZ by Age Stratum in the Long-Term Persistence Substudy
| Age Groupa, y | Cases of HZ, No. | Cases of PHN, No. | Subjects in Follow-up, No.b | Follow-up Time, PY | HZ BOI | Zoster Vaccine Efficacyc for HZ BOI, % | Incidence of PHN per 1000 PY | Zoster Vaccine Efficacyc for Incidence of PHN, % | Incidence of HZ per 1000 PY | Zoster Vaccine Efficacyc for Incidence of HZ, % |
|---|---|---|---|---|---|---|---|---|---|---|
| 60–69 | 157 | 18 | 4127 | 15 518 | 1.58 (1.28–1.95) | 32.5 (16.6–45.4) | 1.16 (0.69–1.83) | 17.1 (−31.0 to 50.9) | 10.1 (8.6–11.8) | 20.2 (6.7–32.2) |
| ≥70 | 104 | 14 | 2740 | 9731 | 1.98 (1.57–2.50) | 42.5 (27.5–54.4) | 1.44 (0.79–2.41) | 49.7 (15.6–72.5) | 10.7 (8.7–12.9) | 22.4 (6.0–36.6) |
| All | 261 | 32 | 6867 | 25 250 | 1.74 (1.48–2.03) | 37.3 (26.7–46.4) | 1.27 (0.87–1.79) | 35.4 (8.8–55.8) | 10.3 (9.1–11.7) | 21.1 (10.9–30.4) |
Vaccine efficacy results are presented as % (95% confidence interval) unless otherwise indicated. The LTPS was a prospective study but, because the subjects and study personnel were aware that the subjects had all received zoster vaccine in the SPS, and there were no concomitant placebo groups, it was not a true vaccine efficacy study. Nevertheless, “vaccine efficacy” is still being used as the most appropriate term to describe the estimated reduction in study endpoints observed in the zoster vaccine recipients.
Abbreviations: HZ, herpes zoster; HZ BOI, HZ burden of illness (1 of the 2 primary SPS endpoints); LTPS, Long-Term Persistence Substudy; PHN, postherpetic neuralgia; PY, person-years; SPS, Shingles Prevention Study.
a The LTPS population was stratified by age at the time participants were randomized to receive zoster vaccine or placebo in the SPS: 60–69 years and ≥70 years.
b LTPS participants completed >98% of their monthly contacts: 90% through the automated telephone response system and 8.1% through telephone calls initiated by study site personnel.
c Primary efficacy analysis using the intermediate model control group.
Surveillance and Follow-up
Participants accrued 25 250 subject-years of follow-up during the 58 months of LTPS. Mean follow-up time was 3.74 years (SD, 0.75 years); 88% (6043/6867) completed follow-up per protocol. LTPS participants completed >98% of their monthly contacts; reasons for not completing follow-up are shown in Figure 1.
Suspected Cases of HZ
During LTPS, 978 subjects with rashes and 13 subjects with unilateral pain/discomfort without rash were evaluated as possible cases of HZ. When evaluated by LTPS personnel, 347 (35.0%) were classified as suspected cases. Specimens for central PCR assay were collected from 326 (94%), with valid results obtained from 317 (91%); CEC adjudication was completed for 30 (9%). Of the suspected cases, 76% (263 of 347) were confirmed cases of HZ, 259 (98%) by PCR assay and 4 (2%) by CEC adjudication.
Among the 263 confirmed cases of HZ, primary dermatomes were thoracic (48.7%), cervical (18.3%), trigeminal (14.8%, including 12.1% V1), lumbar (10.5%), and sacral (7.3%)—similar to the distribution of primary dermatomes in SPS [15]. Prodromal pain was reported in 147 (56%) and acute pain in 224 (85%) cases.
Safety
No serious adverse events judged possibly, probably, or definitely related to vaccination occurred during LTPS. The cumulative mortality rate was approximately 1% per year, similar to that in SPS and STPS [2, 16].
Vaccine Efficacy in LTPS (Primary Analysis)
HZ BOI was 1.74 per 1000 person-years: 1.58 among subjects 60–69 years of age and 1.98 among subjects ≥70 years of age at SPS enrollment (Table 1). Incidence of protocol-defined PHN was 1.27 cases per 1000 person-years; 1.16 cases in subjects 60–69 years of age and 1.44 cases in subjects ≥70 years of age at SPS enrollment (Tables 1 and 2). Similarly, the incidence of PHN was greater among older participants with other duration definitions of PHN up to 120 days after rash onset (Table 2). Incidence of HZ was 10.3 cases per 1000 person-years; 10.1 in subjects 60–69 years of age and 10.7 in subjects ≥70 years of age at SPS enrollment (Table 1).
Table 2.
Summary of Incidence of Postherpetic Neuralgia (PHN) in the Long-Term Persistence Substudy by Age Stratum Using Protocol and Alternative Definitions of PHN
| Cutoff Day for Defining PHN as HZ Pain After Rash Onset | Age Group, y | Zoster Vaccine Recipients Enrolled in LTPS (n = 6867) |
||
|---|---|---|---|---|
| Cases of PHN, No. | Incidence of PHN |
|||
| Per 1000 Person-Years | (95% CI) | |||
| 30 days | 60–69 | 42 | 2.71 | (1.95–3.66) |
| ≥70 | 35 | 3.60 | (2.51–5.00) | |
| All | 77 | 3.05 | (2.41–3.81) | |
| 60 days | 60–69 | 23 | 1.48 | (.94–2.22) |
| ≥70 | 20 | 2.06 | (1.26–3.17) | |
| All | 43 | 1.70 | (1.23–2.29) | |
| 90 daysa | 60–69 | 18 | 1.16 | (.69–1.83) |
| ≥70 | 14 | 1.44 | (.79–2.41) | |
| All | 32 | 1.27 | (.87–1.79) | |
| 120 days | 60–69 | 12 | 0.77 | (.40–1.35) |
| ≥70 | 10 | 1.03 | (.49–1.89) | |
| All | 22 | 0.87 | (.55–1.32) | |
| 182 days | 60–69 | 7 | 0.45 | (.18–.93) |
| ≥70 | 4 | 0.41 | (.11–1.05) | |
| All | 11 | 0.44 | (.22–.78) | |
The LTPS population was stratified by age at the time of randomization in the SPS. The number of participants aged 60–69 years was 4127, who were followed for 15 518 person-years; the number of participants aged ≥70 years was 2740, who were followed for 9731 person-years.
Abbreviations: CI, confidence interval; HZ, herpes zoster; LTPS, Long-Term Persistence Substudy; PHN, postherpetic neuralgia; SPS, Shingles Prevention Study; ZBPI, Zoster Brief Pain Inventory.
a The protocol definition of PHN was zoster pain or discomfort with a ZBPI score of ≥3 that persisted beyond 90 days after HZ rash onset.
No “calendar effect” (increase over time) was observed in the incidence of PHN or in the average HZ severity of illness score (Supplementary Table 1).
In the age- and calendar effect-adjusted “intermediate” historical control group used for the primary vaccine efficacy analysis, HZ BOI was 2.77 per 1000 person-years, incidence of PHN was 1.96 cases per 1000 person-years, and incidence of HZ was 13.1 cases per 1000 person-years (Supplementary Table 3). Primary analysis vaccine efficacy in LTPS was 37.3% (95% CI, 26.7%–46.4%) for HZ BOI, 35.4% (95% CI, 8.8%–55.8%) for incidence of PHN, and 21.1% (95% CI, 10.9%–30.4%) for incidence of HZ (Figure 2 and Table 3). Unlike the SPS, vaccine efficacy in the LTPS appears to be greater in the older age cohort for HZ BOI and to be comparable in the 2 age strata for incidence of HZ (Table 1). Vaccine efficacy in the LTPS also appears to be greater in the older age cohort for incidence of PHN.
Figure 2.
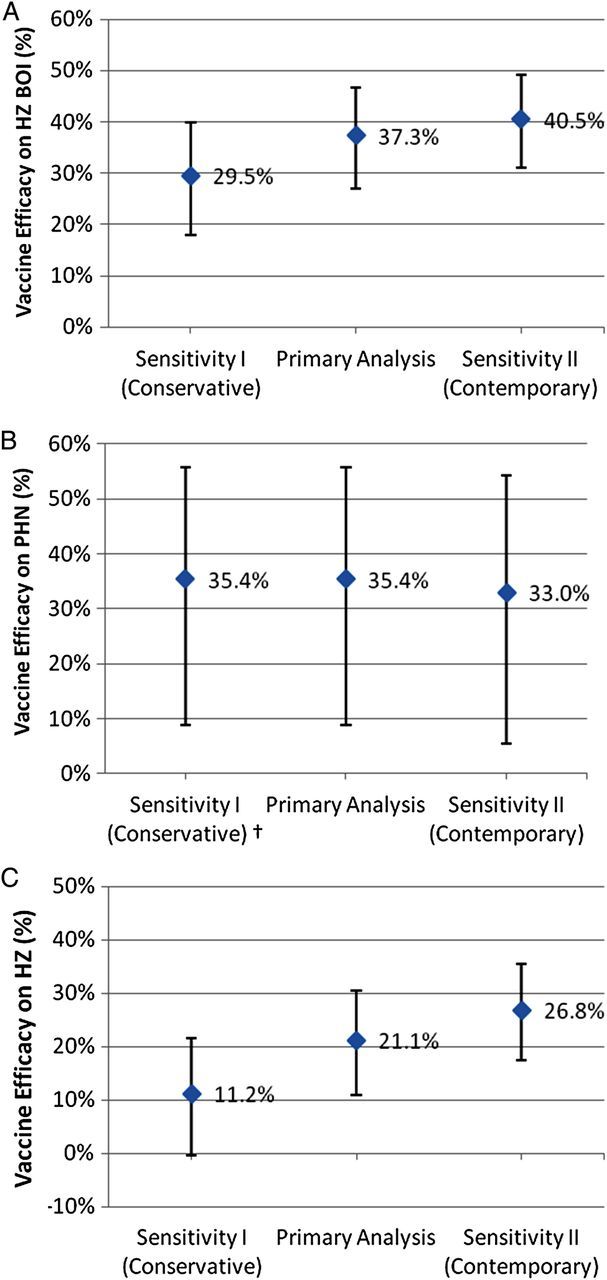
Vaccine efficacy for the 3 study outcomes in the Long-Term Persistence Substudy—primary analysis and sensitivity analyses. A, Vaccine efficacy for herpes zoster (HZ) burden of illness (BOI). B, Vaccine efficacy for the incidence of postherpetic neuralgia (PHN). C, Vaccine efficacy for the incidence of HZ. Estimates of vaccine efficacy are plotted with 95% confidence intervals. For the primary vaccine efficacy analysis, the historical model placebo control group only incorporated data from the Shingles Prevention Study (SPS) and was adjusted to include the calendar effect on the incidence of HZ observed in the placebo group in the SPS. For sensitivity analysis I (conservative assumptions), the historical model placebo control group incorporated only data from the SPS and did not include the calendar effect observed in the SPS. For sensitivity analysis II (contemporary assumptions), the historical model placebo control group incorporated data from both the SPS and the Short-Term Persistence Substudy, and was also adjusted for the calendar effect on the incidence of HZ observed in the placebo groups of the 2 studies. †Sensitivity analysis I for vaccine efficacy for incidence of PHN yielded the same result as the primary analysis, as there was no calendar effect adjustment for the incidence of PHN.
Table 3.
Vaccine Efficacy of Zoster Vaccine Estimated for Years Postvaccination in the Shingles Prevention Study, the Short-Term Persistence Substudy, and the Long-Term Persistence Substudy
| Time Period Since Randomizationa, y | No. of PY | Burden of Illness (Zoster Vaccine Group) | Vaccine Efficacy for HZ BOI Point Estimate (95% CI) | Incidence of PHN (Zoster Vaccine Group) | Vaccine Efficacy for Incidence of PHN Point Estimate (95% CI) | Incidence of HZ (Zoster Vaccine Group) | Vaccine Efficacy for Incidence of HZ Point Estimate (95% CI) |
|---|---|---|---|---|---|---|---|
| SPS + STPSb | |||||||
| Year 1 | 17 584 | 0.43 | 79.2 (66.8–86.9) | 0.28 | 83.4 (56.7–95.0) | 3.9 | 62.0 (49.6–71.6) |
| Year 2 | 18 869 | 0.78 | 54.9 (32.0–70.1) | 0.37 | 69.8 (27.3–89.1) | 5.4 | 48.9 (34.7–60.1) |
| Year 3 | 15 181 | 0.98 | 44.4 (17.6–62.5) | 0.66 | 38.3 (−44.7 to 75.0) | 6.1 | 46.8 (31.1–59.2) |
| Year 4a | 6264 | 0.76 | 66.9 (37.5–82.5) | 0.64 | 60.7 (−36.3 to 91.0) | 7.8 | 44.6 (20.5–61.8) |
| Year 5a | 3180 | 0.68 | 74.9 (48.6–87.7) | 0.63 | 73.8 (−37.8 to 97.3) | 8.2 | 43.1 (5.1–66.5) |
| Year 6a | 4850 | 1.81 | 23.6 (−58.1 to 63.1) | 0.83 | 32.0 (−100.0 to 87.3) | 9.9 | 30.6 (−6.0 to 54.6) |
| LTPS | |||||||
| Year 7c | 6865 | 1.37 | 47.7 (20.9–65.5) | 1.31 | 26.3 (−40.0 to 66.3) | 7.0 | 46.0 (28.4–60.2) |
| Year 8c | 6564 | 1.46 | 46.2 (25.8–61.0) | 1.37 | 27.5 (−37.5 to 66.9) | 9.0 | 31.1 (11.2–47.6) |
| Year 9 | 6280 | 2.04 | 27.6 (4.5–45.1) | 0.80 | 60.5 (7.7–87.2) | 12.3 | 6.8 (−16.5 to 26.4) |
| Year 10 | 5005 | 1.95 | 33.3 (1.5–54.8) | 1.20 | 44.2 (−21.5 to 79.5) | 11.4 | 14.1 (−11.3 to 34.9) |
| Year 11 | 1470 | 2.80 | 7.9 (−48.6 to 42.9) | 2.04 | 11.5 (−100.0 to 81.7) | 13.6 | −1.7 (−57.1 to 37.9) |
| SPS (years 0.0–4.9)b | 58 203 | 0.73 | 61.1 (51.1–69.1) | 0.46 | 66.5 (47.5–79.2) | 5.4 | 51.3 (44.2–57.6) |
| STPS (years 3.3–7.8)b | 9967 | 1.42 | 50.1 (14.1–71.0) | 0.70 | 60.1 (−8.8 to 86.7) | 8.4 | 39.6 (18.2–55.5) |
| LTPS (years 4.7–11.6) | 25 250 | 1.74 | 37.3 (26.7–46.4) | 1.27 | 35.4 (8.8–55.8) | 10.3 | 21.1 (10.9–30.4) |
Results of the primary vaccine efficacy analysis by year postvaccination are reported here for the SPS + STPS (years 1–6), and for the LTPS (years 7–11).
Abbreviations: BOI, burden of illness; CI, confidence interval; HZ, herpes zoster; LTPS, Long-Term Persistence Substudy; PHN, postherpetic neuralgia; PY, person-years; SPS, Shingles Prevention Study (primary efficacy study for the zoster vaccine); STPS, Short-Term Persistence Substudy.
a For the calculation of vaccine efficacy in years 4 and 5 postvaccination, HZ events and PY of follow-up were pooled for the zoster vaccine recipients in the SPS and the STPS. For year 4, PY were 97% from the SPS and 3% from the STPS. For year 5, PY were 16% from the SPS and 84% from the STPS. For year 6, 100% of the events and PY were from STPS subjects.
b Results previously published [4] and shown here for reference.
c For the calculation of vaccine efficacy in years 7 and 8, HZ events and PY of follow-up were pooled for the zoster vaccine group in the STPS and the LTPS, and historical model placebo control groups were determined by incorporating data from the placebo group in the SPS. For year 7, 31% (2136/6861) of PY were from the STPS and 69% (4725/6861) were from the LTPS. For year 8, 8% (542/6577) of PY were from the STPS and 92% (6035/6577) were from the LTPS.
For years 9–11, HZ events and PY of follow-up for the zoster vaccine group were all from LTPS subjects, and historical model placebo control groups were determined by incorporating data from the placebo group in the SPS. There were 37 PY of follow-up from 294 subjects in year 12; these were excluded from the by-year analysis.
Vaccine Efficacy by Year Postvaccination
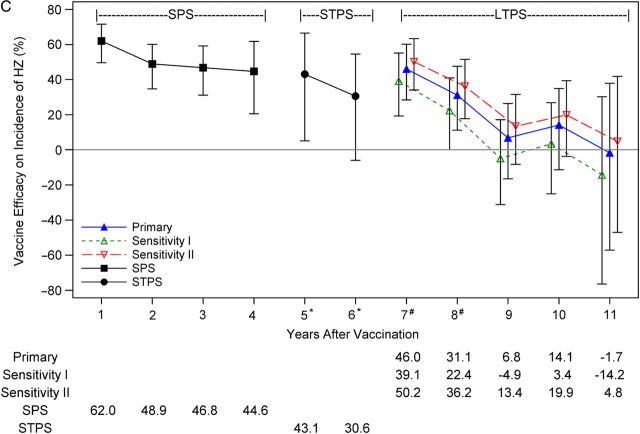
The previously published pooled analysis of SPS and STPS showed that vaccine efficacy for both HZ BOI and incidence of HZ were significantly greater than zero for each year, through year 5 postvaccination [4]. Pooled SPS and STPS results for years 1–6 are presented again (Table 3) for comparison.
The primary analysis for years 7–11 shows decreasing vaccine efficacy over time for HZ BOI and incidence of HZ (Figure 3). Person-years of follow-up ranged from 6865 in year 7 to 5005 in year 10, but were only 1470 in year 11 (Table 3). Vaccine efficacy for HZ BOI declined from 47.7% (95% CI, 20.9%–65.5%) in year 7 to 33.3% (95% CI, 1.5%–54.8%) in year 10, and vaccine efficacy for incidence of HZ declined from 46.0% (95% CI, 28.4%–60.2%) in year 7 to 14.1% (95% CI, −11.3% to 34.9%) in year 10 (Table 3). Vaccine efficacy for incidence of PHN did not decline in LTPS from year 7 (26.3% [95% CI, −40.0% to 66.3%]) through year 10 (44.2% [95% CI, −21.5% to 79.5%]), but CIs were much wider than for the other 2 study endpoints, with only 1 year (year 9) in which the CI excluded zero (Table 3). Although vaccine efficacy for all 3 study endpoints declined with time postvaccination, wide CIs for by-year estimates of vaccine efficacy preclude year-to-year comparisons.
C, Vaccine efficacy for incidence of HZ. Estimates of vaccine efficacy are plotted with 95% confidence intervals. Results from the Shingles Prevention Study (SPS) and Short-Term Persistence Substudy (STPS) were previously published [2, 4]. For the primary vaccine efficacy analysis, the historical model placebo control group only incorporated data from the SPS and was adjusted to include the calendar effect on the incidence of HZ observed in the placebo group in the SPS. For sensitivity analysis I (conservative assumptions), the historical model placebo control group incorporated only data from SPS and did not include the calendar effect on the incidence of HZ observed in the SPS. For sensitivity analysis II (contemporary assumptions), the historical model placebo control group incorporated data from both the SPS and the STPS, and was also adjusted for the calendar effect on the incidence of HZ observed in the placebo groups of the 2 studies. For year 4, person-years were 97% from SPS and 3% from STPS. For year 5, person years were 16% from SPS and 84% from STPS. For years 6, 100% of the events and person-years were from STPS subjects. *Data for years 5–6 from the Long-Term Persistence Substudy (LTPS) are excluded; #For years 7 and 8, both STPS and LTPS contribute vaccine group data. Vaccine efficacy for primary and sensitivity analyses in years 7 to 11 include only data from the LTPS.
Figure 3.
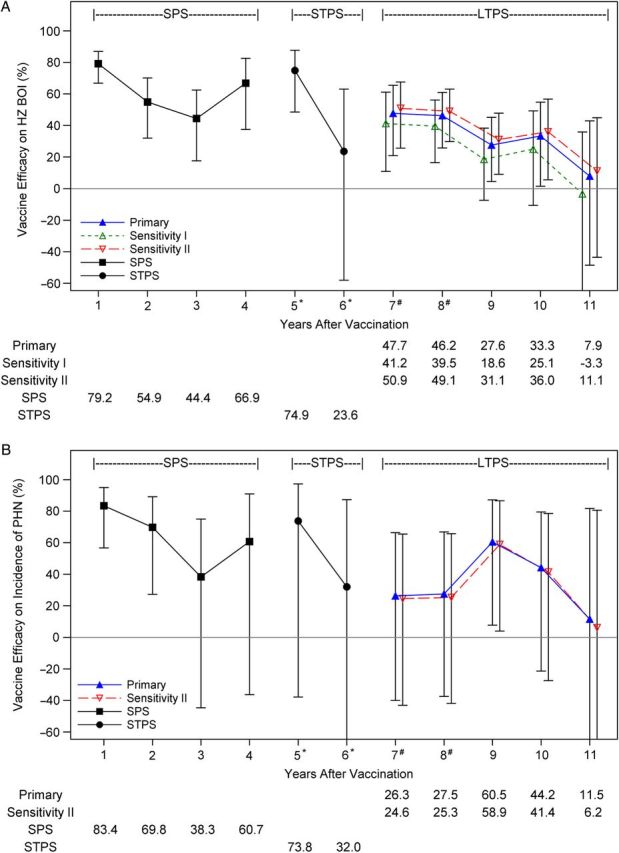
Vaccine efficacy for the 3 study outcomes by year postvaccination. A, Vaccine efficacy for herpes zoster (HZ) burden of illness (BOI). B, Vaccine efficacy for incidence of postherpetic neuralgia (PHN).
DISCUSSION
Estimated vaccine efficacy in LTPS is 39% lower for HZ BOI, 47% lower for incidence of PHN, and 59% lower for incidence of HZ than vaccine efficacy in SPS (Table 3) [2], and 26% lower for HZ BOI, 41% lower for incidence of PHN, and 47% lower for incidence of HZ than vaccine efficacy in STPS (Table 3) [4].
Previous analyses for each year from 1 through 7 years postvaccination in the combined SPS and STPS populations showed a decline in vaccine efficacy after the first year postvaccination for all 3 endpoints, but demonstrated that vaccine efficacy for HZ BOI and incidence of HZ was statistically significant for each year through year 5 [4]. Analysis of vaccine efficacy in LTPS for each year from 7 through 11 years postvaccination showed that vaccine efficacy continued to decline, but remained statistically significant through year 8 postvaccination. However, wide CIs preclude definitive conclusions from year-to-year comparisons.
Absence of a placebo group in LTPS required use of historical controls, based on data from placebo recipients in SPS and STPS, to calculate vaccine efficacy. The calculated vaccine efficacy in LTPS is affected by a temporal increase in the age-specific incidence of HZ observed during SPS and STPS (the “calendar effect”), which was incorporated into 2 of the 3 historical control groups (Supplementary Tables 1 and 3), including that used for the primary vaccine efficacy analysis. No such “calendar effect” was observed for incidence of PHN or average HZ severity of illness scores. However, the “calendar effect” increased the calculated vaccine efficacy for incidence of HZ and, to a lesser degree, for HZ BOI (which incorporates incidence of HZ).
Most [17–20], but not all [21, 22], retrospective epidemiological studies employing medical records or healthcare utilization data indicate that the age-specific incidence of HZ has been increasing, beginning long before, and independent of, the introduction of varicella vaccine. Moreover, absence of exposure to varicella does not appear to increase the age-specific incidence of HZ [20, 23, 24]. These observations indicate that, at least in the short term, elimination of boosting of immunity to VZV by asymptomatic exogenous reinfection of latently infected adults is not responsible for the “calendar effect.”
The prospective nature of SPS and STPS, active follow-up with capture of even mild and atypical cases of HZ, retention of almost all enrolled subjects to the end of the study, and provision of antiviral therapy eliminated most of the potential causes of the “calendar effect” observed in retrospective epidemiologic studies [18–21]. Although the calendar effect was observed for incidence of HZ among placebo recipients in SPS and STPS, there was no comparable increase with time in incidence of PHN or in the average HZ severity of illness scores (Supplementary Table 1). This suggests that mechanisms involved in reactivation of latent VZV and development of HZ may be different from those governing the severity and duration of HZ-associated pain and discomfort and development of the persistent neuropathic pain of PHN.
The comparable mortality rates among vaccine and placebo recipients in SPS and STPS and the absence of additional vaccine-related serious adverse events in LTPS support the long-term safety of zoster vaccine [2, 4, 16].
The LTPS has limitations: It was designed to provide descriptive results with no prespecified hypotheses for vaccine efficacy, there were no concurrent controls, and study participants and investigators were aware of subjects' vaccination status. Consequently, the results reported do not represent true vaccine efficacy. However, for lack of a better descriptor, we have used “vaccine efficacy” to describe the effects of zoster vaccine. The LTPS population was limited in size by resources, permitting enrollment of SPS vaccine recipients only at the 12 SPS sites included in STPS. Thus, the study protocol was approved with sample size estimates with adequate power (>90%) to detect vaccine efficacy for incidence of HZ greater than zero if the vaccine efficacy was as low as 20%, but LTPS was not powered to detect a specific time-point at which vaccine efficacy fell below a prespecified level. The necessity of constructing age- and calendar effect–adjusted placebo control groups to calculate vaccine efficacy because there was no placebo group in LTPS introduced potential bias, as the controls were derived from placebo recipients followed in SPS and STPS, and there were limited clinical data for modeling projected rates. Alternative models were evaluated; 2 were chosen for sensitivity analyses and 1, the “intermediate” placebo control model, was chosen for the primary efficacy analysis (Supplementary Table 3; Johnson et al, manuscript in preparation). Sensitivity analyses support the results of the primary vaccine efficacy analysis (Figures 2 and 3), and LTPS showed a continuation of the temporal decline in vaccine efficacy observed in the STPS.
The declining levels of protection against HZ and PHN with increasing time postvaccination may be due to declining levels of vaccine-induced immunity to VZV with increasing time postvaccination, as well as to declining host immune responses as the SPS vaccinees grow older (ie, to immunosenescence). A better understanding of both phenomena will be important as our population ages and the need for adult vaccines increases.
While statistically significant values for vaccine efficacy are presented, it is clinically significant efficacy that should inform public health policy and vaccine utilization. The decline in efficacy reported here suggests that the clinical efficacy of zoster vaccine becomes increasingly limited beyond 5–8 years postvaccination. Thus, although it is essential to administer zoster vaccine to older adults to protect against HZ and its debilitating complications, new strategies will be needed to maintain protection as vaccine recipients grow older. Our findings support the need for adequately powered and controlled prospective studies to assess long-term protection against HZ and its debilitating complications, as well as the efficacy of revaccinating zoster vaccine recipients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Data management and analysis support were provided at West Haven Cooperative Studies Program Coordinating Center by Karen Dellert and Kathy Newvine. The Long-Term Persistence Substudy was planned and/or administered by an Executive Committee: Michael N. Oxman (Chair), Robert F. Betts, Kathy D. Boardman, Lawrence Gelb, Gary R. Johnson, Myron J. Levin, Peter N. Peduzzi (past), Jane H. Zhang, Kenneth E. Schmader, Vicki A. Morrison, Ruth Harbecke, Kathy M. Neuzil, Heather M. Williams (past), Paula Annunziato, W. B. B. Wang (past), Janie Parrino (past), Xiaoming Li (past), and Ivan S. F. Chan.
This work is dedicated to the memory of Professor Michiaki Takahashi, who developed the attenuated Oka vaccine strain of varicella zoster virus that is now protecting children and adults worldwide from varicella and herpes zoster.
Financial support. The study was conducted by the Cooperative Studies Program, Department of Veterans Affairs, Office of Research and Development through an agreement between Merck & Co, Inc, and the VA Connecticut Research and Education Foundation (VACREF) under which funding was provided to VACREF by Merck & Co. Additional support was provided by the James R. and Jesse V. Scott Fund for Shingles Research, and by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. M. J. L. has received grant support and consulting fees from Merck Sharpe & Dohme and has participated in review activities for GlaxoSmithKline. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
The VA Cooperative Studies Program (CSP) Shingles Prevention Study Investigators include L. E. Davis (Albuquerque, New Mexico), C. A. Kauffman (Ann Arbor, Michigan), S. K. Keay (Baltimore, Maryland), S. E. Straus (deceased), A. R. Marques, N. E. Soto, P. Brunell (Bethesda, Maryland), J. W. Gnann (Birmingham, Alabama), R. Serrao, D. J. Cotton, R. P. Goodman, R. D. Arbeit (Boston, Massachusetts), C. T. Pachucki (Hines, Illinois), M. J. Levin (Denver, Colorado), K. E. Schmader (Durham, North Carolina), W. A. Keitel (Houston, Texas), R. N. Greenberg (Lexington, Kentucky), V. A. Morrison (Minneapolis, Minnesota), P. F. Wright, M. R. Griffin (Nashville, Tennessee), M. S. Simberkoff (New York, New York), S. S. Yeh, Z. Lobo (Northport, New York), M. Holodniy, J. Loutit (Palo Alto, California), R. F. Betts (Rochester, New York), L. D. Gelb (St Louis, Missouri), G. E. Crawford (San Antonio, Texas), J. Guatelli, P. A. Brooks, D. J. Looney (San Diego, California), K. M. Neuzil (Seattle, Washington), and J. F. Toney (Tampa, Florida).
The VA CSP Long-Term Persistence Substudy Investigators include C. A. Kauffman (Ann Arbor), S. K. Keay (Baltimore), A. R. Marques (Bethesda), C. T. Pachucki (Hines), M. J. Levin (Denver), K. E. Schmader (Durham), V. A. Morrison (Minneapolis), P. F. Wright, M. R. Griffin (Nashville), R. F. Betts (Rochester), L. D. Gelb (St Louis), J. Guatelli, D. J. Looney (San Diego), K. M. Neuzil, B. Menzies (Seattle), and J. F. Toney (Tampa).
References
- 1.Hope-Simpson RE. The nature of herpes zoster. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxman MN, Levin MJ, Johnson GR, et al. for the Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 3.Oxman MN, Levin MJ the Shingles Prevention Study Group. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008;197:S228–36. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmader KE, Oxman MN, Levin MJ, et al. for the Shingles Prevention Study Group. Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–8. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison VA, Oxman MN, Levin MJ, et al. for the Shingles Prevention Study Group. Safety of zoster vaccine in elderly adults following documented herpes zoster. J Infect Dis. 2013;208:559–63. doi: 10.1093/infdis/jit182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310–22. doi: 10.1002/jmv.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmader KE, Sloane R, Pieper C, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–6. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 8.Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–56. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Chang MN, Guess HA, Heyse JF. Reduction in burden of illness: a new efficacy measure for prevention trials. Stat Med. 1994;13:1807–14. doi: 10.1002/sim.4780131803. [DOI] [PubMed] [Google Scholar]
- 10.Guess HA, Lydick EG, Small RD, Miller LP. Exact binomial CIs for the relative risk in follow-up studies with sparsely stratified incidence density data. Amer J Epidemiol. 1987;125:340–7. doi: 10.1093/oxfordjournals.aje.a114535. [DOI] [PubMed] [Google Scholar]
- 11.Guess HA, Thomas JE. A rapidly converging algorithm for exact binomial confidence intervals about the relative risk in follow-up studies with stratified incidence-density data. Epidemiol. 1990;1:75–7. doi: 10.1097/00001648-199001000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Martin DO, Austin H. Exact estimates for a rate ratio. Epidemiol. 1996;7:29–33. doi: 10.1097/00001648-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 13.SAS Software Inc. Cary, NC: SAS Institute: 2002–2008. SAS, Version 9.1-9.2. [Google Scholar]
- 14.Cytel Software Inc. Cambridge, MA: Cytel Software Inc: 2001. StatXact, Version 8.0. [Google Scholar]
- 15.Oxman MN, Williams HM, Levin MJ, et al. Efficacy of zoster vaccine according to dermatome region. Presented at Interscience Conference on Antimicrobial Agents and Chemotherapy,; 16–19 December; Washington, DC. 2005. [Google Scholar]
- 16.Simberkoff MS, Arbeit RD, Johnson GR, et al. Shingles Prevention Study Group. Safety of herpes zoster vaccine in the Shingles Prevention Study: a randomized trial. Ann Intern Med. 2010;152:545–54. doi: 10.7326/0003-4819-152-9-201005040-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–40. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 19.Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect. 2007;135:908–13. doi: 10.1017/S0950268807007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Int Med. 2013;159:739–45. doi: 10.7326/0003-4819-159-11-201312030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 22.Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997–2002. Epidemiol Infect. 2005;133:245–53. doi: 10.1017/s095026880400281x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaillat J, Gajdos V, Launay O, et al. Does monastic life predispose to the risk of Saint Anthony's fire (herpes zoster)? Clin Infect Dis. 2011;53:405–10. doi: 10.1093/cid/cir436. [DOI] [PubMed] [Google Scholar]
- 24.Crumpacker CS., II Absence of exposure to varicella does not increase the risk of zoster. Clin Inf Dis. 2014;53:411–2. doi: 10.1093/cid/cir439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.