Human immunodeficiency virus-infected individuals have benefited from improved viral suppression, but a discrepancy in end-stage renal disease risk between black and nonblack HIV-infected persons remains, in part due to continued disparities in antiretroviral use and viral suppression, and higher rates of comorbidities.
Keywords: end-stage renal disease (ESRD), chronic kidney disease (CKD), HIV infection/AIDS, HIV/AIDS, glomerular filtration rate (GFR)
Abstract
Background. Human immunodeficiency virus (HIV)-infected adults, particularly those of black race, are at high-risk for end-stage renal disease (ESRD), but contributing factors are evolving. We hypothesized that improvements in HIV treatment have led to declines in risk of ESRD, particularly among HIV-infected blacks.
Methods. Using data from the North American AIDS Cohort Collaboration for Research and Design from January 2000 to December 2009, we validated 286 incident ESRD cases using abstracted medical evidence of dialysis (lasting >6 months) or renal transplant. A total of 38 354 HIV-infected adults aged 18–80 years contributed 159 825 person-years (PYs). Age- and sex-standardized incidence ratios (SIRs) were estimated by race. Poisson regression was used to identify predictors of ESRD.
Results. HIV-infected ESRD cases were more likely to be of black race, have diabetes mellitus or hypertension, inject drugs, and/or have a prior AIDS-defining illness. The overall SIR was 3.2 (95% confidence interval [CI], 2.8–3.6) but was significantly higher among black patients (4.5 [95% CI, 3.9–5.2]). ESRD incidence declined from 532 to 303 per 100 000 PYs and 138 to 34 per 100 000 PYs over the time period for blacks and nonblacks, respectively, coincident with notable increases in both the prevalence of viral suppression and the prevalence of ESRD risk factors including diabetes mellitus, hypertension, and hepatitis C virus coinfection.
Conclusions. The risk of ESRD remains high among HIV-infected individuals in care but is declining with improvements in virologic suppression. HIV-infected black persons continue to comprise the majority of cases, as a result of higher viral loads, comorbidities, and genetic susceptibility.
Since the human immunodeficiency virus (HIV) epidemic began, several renal complications resulting from HIV infection of renal cells, immune dysregulation, and specific medications have been noted among individuals infected with HIV [1, 2]. The most severe of these, HIV-associated nephropathy (HIVAN), caused an estimated 35% of kidney lesions among HIV-infected patients biopsied during 1995–2004 [3] and was the fourth leading cause of end-stage renal disease (ESRD) among black individuals aged 20–64 years in 1994–1998 [4].
The incidence of HIVAN has declined with increased use of potent antiretroviral therapy (ART) [5–7]; however, there are other potential causes of ESRD in HIV-infected persons. Antiretroviral medications have renal effects including nephrotoxicity and stone formation [8]. Comorbidities such as diabetes mellitus, hypertension, and hepatitis C virus (HCV) infection are risk factors for progression to ESRD and are more common among HIV-infected persons [9–11]. Illicit drug use, which is more common among HIV-infected populations, also increases the risk of ESRD [12, 13]. Studies suggest that HIV-infected persons are more likely to experience ESRD than HIV-uninfected persons, with estimated incidence rates (IRs) in the US and Europe of 3–10 per 1000 person-years [14, 15] and 0.5 per 1000 PYs [16, 17], respectively, corresponding to a 2- to 20-fold greater risk compared with the general population.
A large racial discrepancy has been noted in the burden of ESRD among HIV-infected and -uninfected individuals, with disproportionately higher risk borne by black individuals [17, 18]. Data from the United States Renal Data System (USRDS) indicated that the age- and sex-adjusted incidence of ESRD was approximately 3.4 times higher among blacks in the general population compared with whites in 2010 [19]. Whether reductions in the incidence of HIVAN have impacted the magnitude of the racial disparity in rates of ESRD among HIV-infected individuals is unknown.
There is sparse information on the evolving face of ESRD in the HIV-infected population despite the significant burden of morbidity and mortality that ESRD presents [20–23]. Given the many potential contributors to ESRD risk, the goal of the present study was to assess the relative contributions of clinical and demographic factors to ESRD incidence and describe recent trends in ESRD risk in a cohort of HIV-infected individuals who are representative of HIV patients in care [24]. Our data, which include ESRD cases validated through an extensive medical record review of HIV-infected patients in clinical care through 2009, allowed us to capture changes in ESRD incidence concurrent with, and likely reflective of, modern therapy improvements. We hypothesized that well-documented improvements in HIV treatment in the modern therapy era have led to declines in risk of ESRD over this period, particularly among HIV-infected black individuals in care, given the diminishing role of HIVAN.
METHODS
Study Sample
The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) is a consortium of clinical and interval HIV cohorts from Canada and the United States [25]. It is one of 7 regional collaborations of the International Epidemiologic Databases to Evaluate AIDS supported by the National Institutes of Health and draws participants from >100 clinical sites to represent HIV-infected persons engaged in clinical care. In brief, each contributing cohort uses standardized cohort-specific methods of data collection. At scheduled intervals, cohorts securely transfer demographic, medication, laboratory, diagnosis, and vital status information to the NA-ACCORD central Data Management Core (University of Washington), where data undergo quality control for completeness and accuracy before they are combined into harmonized data files. The human subject activities of the NA-ACCORD and each participating cohort study have been reviewed and approved by their respective local institutional review boards and the Johns Hopkins School of Medicine. Eleven clinic-based cohorts (9 in the United States and 2 in Canada) within the NA-ACCORD used medical record evidence to validate cases of ESRD.
ESRD Validation
A screening algorithm identified potential ESRD cases as those subjects who had 2 estimated glomerular filtration rates (eGFRs; calculated from the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation [26]) <30 mL/minute/1.73 m2 measured >90 days apart with no intervening eGFR ≥30 mL/minute/1.73 m2, or a diagnosis of ESRD, kidney transplant, or dialysis. Each potential case identified between January 2000 and December 2009 underwent validation for ESRD by review of all available laboratory and clinical records for medical evidence of (1) hemodialysis or peritoneal dialysis; (2) renal transplant; or (3) arteriovenous fistula (AVF) placement with additional evidence of dialysis, using a standardized protocol. Dates of renal transplant, dialysis initiation or AVF placement, confirmation source, kidney biopsy reports, medication use, and substance use were all abstracted from medical records as part of the validation process and recorded in a centralized Web-based data entry application. Dialysis delivered temporarily (renal replacement therapy lasting <6 months) for acute kidney injury in hospitalized subjects was not considered ESRD. ESRD date was defined as the earliest confirmed date of chronic renal replacement therapy or, when not available, the date of AVF placement.
Covariate Definitions
ART was defined as a combination of 3 antiretroviral agents from at least 2 classes or a triple nucleoside/nucleotide reverse transcriptase inhibitor regimen containing abacavir or tenofovir. Self-reported race was categorized as black, white, or other/unknown, and ethnicity was categorized as Hispanic or non-Hispanic. For most analyses, the race/ethnicity categories were collapsed to black and nonblack. CD4+ cell count was categorized as <200, 200–349, 350–499, or ≥500 cells/µL. Plasma HIV RNA level ≤400 copies/mL was considered suppressed. For analyses, HIV RNA level was combined with any antiretroviral drug (ARV) use to form a 3-category variable: (1) off ARVs, (2) on ARVs and HIV RNA >400 copies/mL (unsuppressed), or (3) on ARVs and HIV RNA ≤400 copies/mL (suppressed). History of AIDS-defining illness was based on clinical diagnoses. History of injection drug use (IDU) was based on self-report of HIV transmission risk factors. Hypertension was defined as the use of antihypertensive medication, diagnosis of hypertension, ≥2 systolic blood pressure readings >140 mm Hg, or ≥2 diastolic blood pressure readings >100 mm Hg. Diabetes was defined as the use of insulin or oral hypoglycemic medication, diagnosis of diabetes, fasting blood glucose >200 mg/dL, or glycosylated hemoglobin >6.5%. HCV-positive serostatus was defined based on a reactive HCV antibody or detectable HCV RNA level. The Kidney Disease: Improving Global Outcomes (KDIGO) classifications based on eGFR thresholds were used to define eGFR categories representing severity of chronic kidney disease (CKD) [27].
Statistical Analysis
Person-time accrued from study enrollment on or after 1 January 2000 to the end of the study period on 31 December 2009, death, ESRD, or 1 year after the last serum creatinine measurement. Age- and sex-standardized (directly standardized) IRs were calculated overall and by calendar year using the Centers for Disease Control and Prevention's Bridged Race Intercensal Estimates Dataset, which uses year 2000 census data [28]. Consistent with USRDS methods [19], a 3-year rolling average was used to present a smoothed trend over calendar time. Trends in comorbidity prevalence were also estimated using the proportion of participants with any past occurrence of hypertension, diabetes, or HCV infection according to the aforementioned definitions. The trend in viral suppression was estimated using the proportion in a given year with all reported viral load measurements ≤400 copies/mL. Age-, sex-, and race-standardized (indirectly standardized) sex-standardized incidence ratios (SIRs) were estimated using rates from the USRDS [19]. To avoid possible bias resulting from higher prevalence of IDU among the NA-ACCORD HIV-infected participants, only the subset of cases without reported IDU were used in the SIR analysis.
Adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were estimated using multivariate Poisson regression models that included the following covariates: age, CD4+ count, HIV RNA, and ARV use as time-varying; female sex, black race, IDU, AIDS, hypertension, diabetes mellitus, and HCV seropositivity as time-fixed using the measurement closest to entry into the present study (within 6 months).
Lastly, to assess the predictors and correlates of ESRD in virally-suppressed participants, a separate analysis looked at the incidence of ESRD among the subset of well-suppressed individuals. A well-suppressed individual was defined as one whose HIV RNA measurements were ≤400 copies/mL for 90% of the observed follow-up time.
All analyses were performed using SAS software version 9.1 (SAS Institute).
RESULTS
Case Validation
The screening algorithm identified 822 potential cases of ESRD. Of these, 616 were validated as having ESRD for a positive predictive value of 75%, with only 4 of the 822 not validated due to missing medical records. After removing 15 cases with missing year of diagnosis and 9 cases where AVF placement was the only evidence of ESRD, there were 592 cases. Of these cases, 286 were incident ESRD cases used in the analysis (Supplementary Appendix Figure A1). In 62 (22%) of these, a missing month and/or day of ESRD was imputed as January and/or the 1st, respectively. Date of AVF placement was used in 44 (15%) cases, in which the start date of dialysis was unknown.
Characteristics of HIV-Infected Adults With ESRD
A total of 38 354 HIV-infected adults aged 18–80 years contributed 159 825 PYs to this analysis. A cohort-level description of ESRD cases and PYs contributed is provided in the Supplementary Appendix. The median follow-up time was 3.6 years (interquartile range [IQR], 1.6–6 years). At study entry, HIV-infected participants had a median age of 41 years (IQR, 34–47 years). HIV-infected adults who later developed ESRD were more likely to be female and of black race, have a history of diabetes, hypertension, IDU, or AIDS-defining illnesses, and have an unsuppressed HIV RNA load while on any ARVs, compared with participants who did not experience ESRD during the period of observation (Table 1). The median baseline CD4+ cell counts were 236 and 346 cells/µL in ESRD cases and noncases, respectively. The proportion prescribed ART at study entry was 57% in ESRD cases and 49% in noncases, and no differences were seen in use of tenofovir, atazanavir, or boosted protease inhibitor regimens (Table 1).??
Table 1.
Baseline Characteristics for HIV-Infected Study Participants With a Comparison to End-Stage Renal Disease Cases Reported in the US Renal Database System
| Characteristic | ESRD, NA-ACCORDa (N = 286) | No ESRD, NA-ACCORDa (N = 38 068) | ESRD Cases in the Population Data From 2000–2009 USRDS [19]b |
|---|---|---|---|
| Male sex, % | 69 | 80 | 55 |
| Race, % | |||
| White | 11 | 43 | 64 |
| Black | 80 | 32 | 30 |
| Hispanic ethnicity, % | 6 | 13 | 13 |
| Age, y | 43 (36–49) | 40 (34–47) | 63 |
| Body mass index, kg/m2 | 25 (22–29) | 25 (22–28) | 28 |
| Diabetes, % | 20 | 4 | 47 |
| Hypertension, % | 50 | 17 | 25 |
| Hepatitis C, % | 11 | 5 | |
| eGFR category, %, mL/minc | |||
| ≥90 | 28 | 78 | |
| 60–89 | 21 | 19 | |
| 45–59 | 13 | 2 | |
| 30–44 | 14 | 1 | |
| 15–29 | 12 | 0 | |
| <15 | 12 | 0 | |
| Follow-up time, y | 2.9 (1.1–5) | 3.6 (1.6–6) | |
| HIV-related factors | |||
| CD4+ count, cells/µL | 236 (118–432) | 346 (178–536) | |
| Viral load, copies/mL | 13 262 (405–66 417) | 3244 (<400–46 171) | |
| On ART | 57 | 49 | |
| ARV/viremia status, % | |||
| On ARV, suppressed | 18 | 27 | |
| On ARV, unsuppressed | 36 | 20 | |
| Off ARV | 35 | 43 | |
| AIDS | 35 | 21 | |
| Injection drug use, % | 19 | 13 | |
| History of tenofovir use, % | 2.8 | 5.2 | |
| History of atazanavir use, % | 1.4 | 2.6 | |
| History of boosted PI use, % | 13.6 | 11.3 | |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral drugs; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; NA-ACCORD, North American AIDS Cohort Collaboration on Research and Design; PI, protease inhibitor; USRDS, US Renal Disease System.
a Statistically significant differences were assessed P < .05 by χ2 test or Wilcoxon rank-sum test comparing ESRD cases in NA-ACCORD with noncases in NA-ACCORD. All factors but body mass index, tenofovir use, atazanavir use, boosted PI use, and follow-up time (not tested) were significantly different.
b Comorbidity prevalence based on primary diagnosis; mean age from 2011 incident cases.
Comparison of HIV-Infected ESRD Cases With the General Population
Using USRDS data as reference, the overall SIR contrasting the expected number of ESRD cases in HIV-infected non-IDU participants by age-, race-, and sex-specific strata to the observed was 3.2 (95% CI, 2.8–3.6). When stratified by race/ethnicity, the SIR was 4.5 (95% CI, 3.9–5.2) for blacks, 1.5 (95% CI, 1.0–2.2) for whites, and 1.7 (95% CI, 1.1–2.5) for Hispanics, compared with race/ethnicity-specific USRDS reference data. By calendar period, the overall SIR was 3.9 (95% CI, 3.3–4.6) in 2000–2004 and 2.8 (95% CI, 2.4–3.3) in 2005–2009.
Trends in ESRD Rates and Risk Factors Over Time
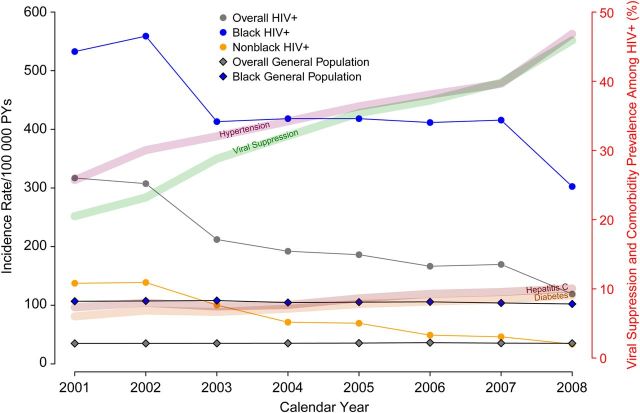
Figure 1 shows the age- and sex-standardized incidences over time in the NA-ACCORD sample, using a 3-year moving average to smooth the trend (eg, the reported incidence in 2001 is the average of the standardized incidence estimated from 2000 to 2002). We also present the age- and sex-standardized incidence over time in the general population as reported in the 2013 USRDS annual report [19]. Overall, HIV-infected participants experienced higher rates of ESRD compared with the general population across all calendar years, with evidence of a declining temporal trend (from 317 to 119 per 100 000 PYs in 2001 and 2008, respectively, Ptrend < .001), coincident with overall improvements in median CD4+ cell count and viral suppression. When stratified by race/ethnicity, the increased ESRD rate noted overall was largely driven by very high rates in HIV-infected black individuals, though there was evidence of a decline in ESRD incidence over calendar time among HIV-infected black patients. The ESRD incidence appeared to decline annually by 28 per 100 000 PYs per year (95% CI, −40 to −16) in black individuals from a high of 532 per 100 000 PYs, and by 16 per 100 000 PYs per year (95% CI, −19 to −13) in nonblack patients from a high of 138 per 100 000 PYs.??
Figure 1.
Age- and sex-standardized incidence of end-stage renal disease among human immunodeficiency virus (HIV)-infected adults stratified by race and compared with age- and sex-standardized rates in the general population (US Renal Database System). Incidence rates are 3-year rolling averages. Abbreviation: PY, person-years.
The decline in ESRD incidence contrasted with an increase in the prevalence of comorbidities that are known risk factors for ESRD (Figure 1; Table 2). ESRD cases in both black and nonblack patients diagnosed from 2005 to 2009 were more likely to have a history of hypertension compared with cases diagnosed from 2000 to 2004 (P = .018 and P = .030, respectively). Among ESRD cases in black patients, there was a higher prevalence of HCV infection in the later calendar period (P = .03). Among nonblack ESRD cases, there was a nonsignificant increase in the prevalence of diabetes (P = .30). One marked difference between ESRD cases in black and nonblack patients was the rate of viral suppression while on ART (Table 2). Twenty-six percent and 41% of black patients with ESRD were treated with ARVs and suppressed in the early and later calendar periods, respectively, vs 48% and 73% of nonblack patients with ESRD (P = .02 and P = .003, respectively) (Table 2). The striking trend in improved viral load suppression aligned with the noted decline in ESRD incidence in both black and nonblack patients.??
Table 2.
Characteristics of Cases at the Time of Diagnosis (±6 Months) by Calendar Period
| Characteristic | Early ESRD 2000–2004 (n = 145) |
Late ESRD 2005–2009 (n = 141) |
||||
|---|---|---|---|---|---|---|
| Overall | Black (n = 114) | Nonblack (n = 31) | Overall | Black (n= 115) | Nonblack (n = 26) | |
| Male sex, % | 67 | 63 | 81 | 71 | 69 | 81 |
| Age, y | 44 (38–51) | 44 (38–50) | 47 (39–56) | 47 (41–54) | 47 (42–54) | 49 (40–61) |
| Diabetes, % | 25 | 25 | 26 | 28 | 26 | 39 |
| Hypertension, % | 68 | 70 | 58 | 84 | 84 | 85 |
| Hepatitis C, % | 10 | 11 | 10 | 18 | 21 | 8 |
| HIV-related factors | ||||||
| CD4+ count, cells/µL | 176 (36–320) | 176 (26–344) | 177 (107–264) | 246 (118–448) | 248 (112–448) | 229 (138–457) |
| Viral load, copies/mL | 6093 (400–70 104) | 8009 (400–85 557) | 637 (<400–21 083) | 400 (<400–28 224) | 789 (<400–34 428) | <400 (<400–400) |
| ARV/viremia status, % | ||||||
| On ARV, suppressed | 31 | 26 | 48 | 47 | 41 | 73 |
| On ARV, unsuppressed | 53 | 56 | 42 | 35 | 40 | 15 |
| Off ARV | 12 | 12 | 10 | 12 | 13 | 8 |
| AIDS, % | 57 | 61 | 45 | 42 | 43 | 39 |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: ARV, antiretroviral drugs; ESRD, end-stage renal disease; HIV, human immunodeficiency virus.
Risk Factors for ESRD Among HIV-Infected Persons
A higher incidence of ESRD was observed among HIV-infected adults of black race (vs white), of female sex, of older age, with a history of IDU, and in lower baseline eGFR category (Table 3). Within the subset who maintained suppressed HIV RNA loads (n = 22 cases occurring over 32 222 PYs), risk factors remained consistent, but absolute rates of ESRD were much lower (68 per 100 000 PYs overall) and approached that seen in the general population (35 per 100 000 PYs) [19].??
Table 3.
Unadjusted Incidence Rates of End-Stage Renal Disease Among HIV-Infected Patients in Care
| Characteristic | Incidence Rate per 100 000 PY (95% CI) |
|
|---|---|---|
| Overall (N = 286) | Well-Suppresseda (n = 22) | |
| Overall | 179 (160–201) | 68 (45–104) |
| Sex | ||
| Male | 157 (136–180) | 56 (34–93) |
| Female | 259 (211–320) | 127 (61–269) |
| Race | ||
| White | 45 (32–64) | 13 (3–51) |
| Black | 437 (384–498) | 204 (121–345) |
| Ethnicity | ||
| Hispanic | 80 (49–130) | 65 (21–202) |
| Risk behavior | ||
| IDU | 263 (202–343) | 161 (60–428) |
| Non-IDU | 166 (146–189) | 61 (38–96) |
| Age, y | ||
| 18–39 | 142 (114–177) | 45 (17–120) |
| 40–49 | 172 (143–206) | 52 (25–109) |
| 50–59 | 234 (185–295) | 83 (37–184) |
| ≥60 | 285 (190–429) | 195 (81–469) |
| eGFR category, mL/min | ||
| ≥90 | 73 (57–93) | 11 (3–42) |
| 60–89 | 233 (176–308) | 51 (17–159) |
| 45–59 | 1875 (1340–2625) | 772 (290–2056) |
| 30–44 | 4778 (3396–6720) | 2008 (647–6225) |
| 15–29 | 14 674 (10 197–21 117) | 3193 (449–22 668) |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; IDU, injection drug use; PY, person-years [26].
a Defined as HIV RNA ≤400 copies/mL for 90% of the observed follow-up time.
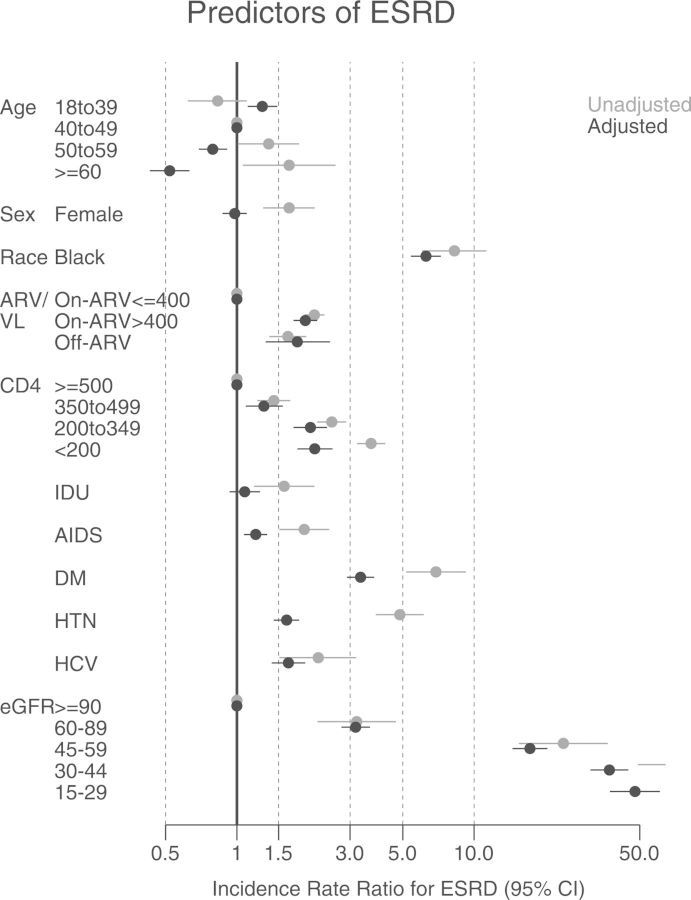
Figure 2 shows the results of the unadjusted and adjusted Poisson regression. The adjusted IRRs were significantly higher for black patients, those with unsuppressed viral loads while on ARVs, those with lower CD4+ counts, and those with a history of AIDS. Three well-established risk factors for ESRD in the general population—diabetes mellitus, hypertension, and HCV infection—were also highly related to the risk of ESRD. As expected, eGFR category at baseline was strongly associated with the risk of ESRD, with adjusted IRRs that increased stepwise from 3.2 to 48 with increasing CKD severity, compared to participants with eGFR >90 mL/minute/1.73 m2. Older HIV-infected persons were at higher risk of ESRD in unadjusted analyses; however, with adjustment for comorbidities and baseline kidney function, that trend reversed, consistent with selective survival of healthier individuals, such that for any given level of baseline kidney dysfunction, older age represents a better risk profile and slower CKD progression. Estimates are also presented in Supplementary Appendix Table A2.??
Figure 2.
Unadjusted and adjusted incidence rate ratios for end-stage renal disease (ESRD) risk factors. Adjusted estimates are from multivariate Poisson models adjusting for all factors presented. All factors were assessed at baseline except for age, CD4+ count, human immunodeficiency virus (HIV) RNA, and antiretroviral drugs (ARV) use, which were a time-varying factors. Age is shown in years. Abbreviations: CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate (mL/min) [26]; HCV, hepatitis C virus; HTN, hypertension; IDU, injection drug use; VL, viral load (copies/mL).
DISCUSSION
Over the past decade, HIV care has dramatically improved, as evidenced by increasing rates of viral suppression and declining numbers of new AIDS diagnoses and deaths in the United States and Canada [24, 29, 30]. Whereas rates of HIVAN—which is directly linked to HIV viremia among black individuals [31]—have fallen since their peak in the pre-ART era [5–7], HIV-infected blacks in care remain at high risk for ESRD. In this large collaborative cohort of HIV-infected persons in care, we observed that ESRD risk was approximately 6 times higher in black HIV-infected adults compared to their white counterparts, a disparity in risk that remained after accounting for sex, IDU, HIV severity, ART use, baseline eGFR, comorbidity history, and age differences. Although ESRD IRs are declining in the HIV-infected population coincident with overall improvements in viral suppression, HIV-infected black patients with incident ESRD in our study continue to exhibit higher viral loads than their nonblack counterparts. This may result from previously reported lower ART utilization and delayed entry into care seen among HIV-infected black patients [32]; differential access to care is unlikely to underlie our observations, as all NA-ACCORD participants are engaged in clinical care. Unsuppressed viral load and prior AIDS diagnoses were important risk factors for ESRD in our study, which is consistent with some but not all prior studies [14–16, 23]. Thus, further reductions in ESRD risk among HIV-infected blacks may be attained by eliminating racial differences in treatment while in care.
Racial disparities in the risk of ESRD would likely persist even given similar rates of viral suppression as a result of genetic susceptibility to more aggressive CKD progression through APOL1 risk variants [33, 34]. Innate immune activation leads to potentially damaging overexpression of APOL1 protein among carriers of APOL1 high-risk alleles, who are almost exclusively black and comprise 13% of the US black population [35, 36]. Thus, HIV-associated immune activation, which continues even after immune restoration and viral suppression [37], may disproportionately increase ESRD risk among HIV-infected black individuals carrying APOL1 risk alleles. Such a mechanism could explain the elevated risk noted in well-suppressed HIV-infected black patients in NA-ACCORD and would suggest that earlier entry into care, earlier ART initiation, and long-term viral suppression to attenuate immune activation may be particularly important for renal protection in blacks.
Immune activation is an important ESRD risk factor, even in HIV-infected individuals who do not carry APOL1 risk variants, through its impact on age-related comorbidity risk [37]. Kidney disease resulting from comorbid risk factors may be supplanting that caused by HIV; trends in comorbidity prevalence demonstrate the increasing presence of hypertension, diabetes mellitus, and HCV infection among our cohort. Diabetes and hypertension have been previously found to be strong predictors of ESRD risk in HIV-infected adults [14]. A recent study by Ryom et al found effect estimates similar to the present study [23]. Lower CD4+ counts and higher viral loads characterized early ESRD cases, whereas those occurring after 2005 were less advanced in their HIV disease but had more comorbidity. Prevention and treatment of comorbidities in HIV-infected populations may be the future of ESRD prevention in the modern ART era.
This study describing ESRD trends and risk factors was strengthened by the extensive follow-up of a large population of HIV-infected individuals from >100 clinical sites across North America. It should be noted this breadth of representation also results in heterogeneity in terms of individual cohort ESRD risk, as shown in the Supplementary Appendix Table A1 where cohort IR estimates range from 0 to 550 per 100 000 PYs. The rigorous validation process for ESRD diagnoses assured high specificity of the case definition. GFR was estimated using the CKD-EPI equation, a serum creatinine–based estimating formula, which has been recently validated in an HIV-infected population [38]. The study is limited, however, in the degree to which we can explore myriad potential contributing factors such as smoking, recreational drug use, and non-ARV nephrotoxic medication use as well as kidney disease biomarkers such as urine protein, as these data were not consistently collected across the cohorts at the time of the ESRD validation. Socioeconomic factors that may drive discrepancies in viral load suppression rates between blacks and nonblack patients were not collected.
In conclusion, our findings suggest that HIV-infected individuals have benefited from improved viral suppression in terms of ESRD risk, despite an apparent rising trend in the prevalence of comorbid risk factors for kidney disease. However, there remains a large racial disparity in ESRD risk resulting from several potential mechanisms: (1) continued disparities in ARV use and viral suppression; (2) higher rates of comorbid risk factors for kidney disease; and (3) differences in genetic or gene–environment risk profiles. These racial differences in ESRD risk have important implications. They highlight the need for continued efforts to close racial gaps in early access to ART to achieve durable viral suppression as well as early interventional strategies for comorbid risk factors to slow kidney disease progression in HIV-infected black individuals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Rosemary McKaig for her input and guidance.
Disclaimer. The US population data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government.
Financial support. This work was supported by the National Institutes of Health (grant numbers U01-AI069918, U01-AA013566, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-HD32632, U01-AI42590, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI38855, U01-AI38858, U01-AI68634, U01-AI68636, U01-AI69432, U01-AI69434, U10-EY08057, U10-EY08052, U10- EY08067, UL1-RR024131, MO1-RR-00052, M01-RR000071, M01-RR000079, M01-RR000083, M01- RR025747, N02-CP55504, P30-AI094189, P30-AI27757, P30-AI27767, P30-AI50410, P30-AI54999, P30-AI036219, R01-AA16893, R01-DA04334, R01-DA12568, R01-DA11602, R01-CA165937, R24-AI067039, Z01-CP010176, U54MD007587, G12MD007583, and K01-AI071725 [to M. J. S.], K01-AI071754 [to B. R. L.], K01-AI093197 [to K. N. A.], K23-DK081317 [to M. M. E.], K24-00432, K23-EY013707, and F31-DA30254 [to D. H.], and K24 DA035684 and R01 DA026770 [to G. M. L.]); the Centers for Disease Control and Prevention (contract number CDC200-2011-41872); the Agency for Healthcare Research and Quality (contract number HHSA 290-2011-00007C); the Health Resources and Services Administration (contract number HHSA 250-2012-00008C); the Canadian Institutes of Health Research (grant numbers KRS-86251, TGF-96118, HCP-97105, CBR-86906, CBR-94036, and 169621); the Canadian HIV Trials Network (project 242); and the government of British Columbia, Canada. G. M. L. was supported by R01 DA026770/DA/NIDA NIH HHS/United States.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
Cohorts and representatives of the NA-ACCORD: AIDS Link to the IntraVenous Experience: Gregory D. Kirk; AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson, Ronald J. Bosch, and Ann C. Collier; Fenway Health HIV Cohort: Stephen Boswell, Chris Grasso, and Ken Mayer; HAART Observational Medical Evaluation and Research: Robert S. Hogg, Richard Harrigan, Julio Montaner, and Angela Cescon; HIV Outpatient Study: John T. Brooks and Kate Buchacz; HIV Research Network: Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort: Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez; Kaiser Permanente Mid-Atlantic States: Michael A. Horberg; Kaiser Permanente Northern California: Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne; Multicenter Hemophilia Cohort Study–II: James J. Goedert; Multicenter AIDS Cohort Study: Lisa P. Jacobson; Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein.
Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann Burchell, and Anita R. Rachlis; Retrovirus Research Center, Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor; Southern Alberta Clinic Cohort: M. John Gill; Studies of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin; University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig; University of North Carolina, Chapel Hill, HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane; Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin; Vanderbilt-Meharry Centers for AIDS Research Cohort: Timothy R. Sterling, David Haas, Sally Bebawy, and Megan Turner; Women's Interagency HIV Study: Stephen J. Gange and Kathryn Anastos.
NA-ACCORD Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Keri N. Althoff, Mari M. Kitahata, Rosemary G. McKaig, Amy C. Justice, and Aimee M. Freeman.
NA-ACCORD Administrative Core: Richard D. Moore, Aimee M. Freeman, and Carol Lent.
NA-ACCORD Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Eric Webster, Liz Morton, and Brenda Simon.
NA-ACCORD Epidemiology and Biostatistics Core: Stephen J. Gange, Keri N. Althoff, Alison G. Abraham, Bryan Lau, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Shari Modur, David B. Hanna, Peter Rebeiro, Cherise Wong, and Adell Mendes.
References
- 1.Bruggeman LA, Bark C, Kalayjian RC. HIV and the kidney. Curr Infect Dis Rep. 2009;11:479–85. doi: 10.1007/s11908-009-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimmel PL, Barisoni L, Kopp JB. Pathogenesis and treatment of HIV-associated renal diseases: lessons from clinical and animal studies, molecular pathologic correlations, and genetic investigations. Ann Intern Med. 2003;139:214–26. [PubMed] [Google Scholar]
- 3.Berliner AR, Fine DM, Lucas GM, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28:478–86. doi: 10.1159/000112851. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2000. USRDS 2000 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, Table A.18. [Google Scholar]
- 5.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–6. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 6.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–52. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt CM. The kidney in HIV infection: beyond HIV-associated nephropathy. Top Antivir Med. 2012;20:106–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Izzedine H, Harris M, Perazella MA. The nephrotoxic effects of HAART. Nat Rev Nephrol. 2009;5:563–73. doi: 10.1038/nrneph.2009.142. [DOI] [PubMed] [Google Scholar]
- 9.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS Cohort Study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JT, Anderson HL, Jr, Markowitz GS, Appel GB, Pogue VA, D'Agati VD. Hepatitis C virus–associated glomerular disease in patients with human immunodeficiency virus coinfection. J Am Soc Nephrol. 1999;10:1566–74. doi: 10.1681/ASN.V1071566. [DOI] [PubMed] [Google Scholar]
- 11.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–60. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 12.Dettmeyer R, Wessling B, Madea B. Heroin associated nephropathy—a post-mortem study. Forensic Sci Int. 1998;95:109–16. doi: 10.1016/s0379-0738(98)00082-6. [DOI] [PubMed] [Google Scholar]
- 13.Norris KC, Thornhill-Joynes M, Robinson C, et al. Cocaine use, hypertension, and end-stage renal disease. Am J Kidney Dis. 2001;38:523–8. doi: 10.1053/ajkd.2001.26845. [DOI] [PubMed] [Google Scholar]
- 14.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis. 2012;59:628–35. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas GM, Mehta SH, Atta MG, et al. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21:2435–43. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- 16.Bansi L, Hughes A, Bhagani S, et al. Clinical epidemiology of HIV-associated end-stage renal failure in the UK. AIDS. 2009;23:2517–21. doi: 10.1097/QAD.0b013e3283320e12. [DOI] [PubMed] [Google Scholar]
- 17.Bickel M, Marben W, Betz C, et al. End-stage renal disease and dialysis in HIV-positive patients: observations from a long-term cohort study with a follow-up of 22 years. HIV Med. 2013;14:127–35. doi: 10.1111/j.1468-1293.2012.01045.x. [DOI] [PubMed] [Google Scholar]
- 18.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–57. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. [Google Scholar]
- 20.Ahuja TS, Grady J, Khan S. Changing trends in the survival of dialysis patients with human immunodeficiency virus in the United States. J Am Soc Nephrol. 2002;13:1889–93. doi: 10.1097/01.asn.0000019773.43765.bf. [DOI] [PubMed] [Google Scholar]
- 21.Atta MG, Fine DM, Kirk GD, Mehta SH, Moore RD, Lucas GM. Survival during renal replacement therapy among African Americans infected with HIV type 1 in urban Baltimore, Maryland. Clin Infect Dis. 2007;45:1625–32. doi: 10.1086/523728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryom L, Kirk O, Lundgren J, et al. Advanced chronic kidney disease, end-stage renal disease and renal death among HIV-positive individuals in Europe. HIV Med. 2013;14:503–8. doi: 10.1111/hiv.12038. [DOI] [PubMed] [Google Scholar]
- 23.Ryom L, Mocroft A, Kirk O, et al. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. AIDS. 2014;28:187–99. doi: 10.1097/QAD.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 24.Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–35. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 28.National Center for Health Statistics. Bridged-race intercensal estimates of the resident population of the United States for July 1, 2000–July 1, 2009, by year, county, single-year of age (0, 1, 2, …, 85 years and over), bridged race, Hispanic origin, and sex. Prepared under a collaborative arrangement with the U.S. Census Bureau. Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm . Accessed 26 October 2012.
- 29.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006;41:194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 30.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 31.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84–92. doi: 10.1172/JCI119525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–8. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24:1484–91. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–96. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int. 2014 doi: 10.1038/ki.2014.270. doi:10.1038/ki.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor HE, Khatua AK, Popik W. The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol. 2014;88:592–603. doi: 10.1128/JVI.02828-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inker LA, Wyatt C, Creamer R, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. J Acquir Immune Defic Syndr. 2012;61:302–9. doi: 10.1097/QAI.0b013e31826a6c4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.