The prevalence of cryptococcal infection among individuals with advanced AIDS in the United States was 2.9%, above the level of cost-effectiveness for screening, supporting the revision of cryptococcal screening guidelines in AIDS patients with a CD4 count ≤100 cells/µL.
Keywords: cryptococcal meningitis, prevalence, screening, HIV/AIDS
Abstract
Background. Cryptococcal meningitis (CM) is one of the most common causes of AIDS-related mortality worldwide, accounting for 33%–63% of all cases of adult meningitis in sub-Saharan Africa and >500 000 deaths annually. In sub-Saharan Africa, the World Health Organization recommends routinely screening AIDS patients with a CD4 count ≤100 cells/µL for cryptococcal infection. In the United States, there are no recommendations for routine screening. We aimed to determine the prevalence of cryptococcal infection and outcomes of those infected among people living with advanced AIDS in the United States, to inform updates in the prevention and management of CM.
Methods. Using stored sera from participants in the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study from 1986 to 2012, we screened 1872 specimens with CD4 T-cell counts ≤100 cells/µL for cryptococcal antigen (CrAg) using the CrAg lateral flow assay.
Results. The overall prevalence of CrAg positivity within the study population was 2.9% (95% confidence interval, .2%–3.8%). Results from multivariable analysis revealed that a previous diagnosis with CM and a CD4 count ≤50 cells/µL were significantly associated with CrAg positivity. Participants who were CrAg positive had significantly shorter survival (2.8 years) than those who were CrAg negative (3.8 years; P = .03).
Conclusions. The prevalence of cryptococcal infection among advanced AIDS patients in the United States was high and above the published cost-effectiveness threshold for routine screening. We recommend routine CrAg screening among human immunodeficiency virus-infected patients with a CD4 count ≤100 cells/µL to detect and treat early infection.
Cryptococcal meningitis (CM), a central nervous system infection caused by the soil-borne fungus Cryptococcus neoformans, is one of the leading opportunistic infections associated with AIDS worldwide [1–5]. Moreover, in low-resource settings, a high mortality rate follows the diagnosis of CM. In 2008, the global burden of CM was estimated to be 957 900 cases, with approximately 624 700 deaths within 3 months of diagnosis [4, 6]. Sub-Saharan Africa bears the highest burden of CM, with a median incidence of 3.2% among human immunodeficiency virus (HIV)-infected individuals, accounting for between 33% and 63% of all cases of adult meningitis [7–9]. In the United States, early HIV case identification and access to highly active antiretroviral therapy (HAART) have been successful in reducing morbidity and mortality due to CM [10–12]. However, CM continues to be a problem, with an incidence rate among those with AIDS between 2 and 7 cases per 1000 person-years, and a mortality rate as high as 12% in the era of HAART [13]. Further, in a cross-sectional study of hospitalized patients with CM in 18 states from 1997 to 2009, there was an average of 3400 cases of CM annually; the percentage of patients with CM coinfected with HIV was 71% [14].
Early diagnosis and treatment are key in reducing CM-related mortality. Cryptococcal antigen (CrAg) is detectable in serum at least 3 weeks prior to the onset of symptoms, allowing screening to identify cryptococcal-infected persons early and the initiation of effective treatment to prevent serious disease and death [2, 4, 10, 11, 15–18]. The gold standard method of detection of cryptococcal infection has been culture; however, culture requires sophisticated laboratory infrastructure and can take up to several days to weeks for a diagnosis. Antifungal medication such as amphotericin, fluconazole, and flucytosine are used for the treatment of cryptococcal infection. Reoccurring infection is prevented by taking fluconazole for life or until immune reconstitution [15].
In December 2011, the World Health Organization recommended routinely screening AIDS patients with a CD4 T-cell count ≤100 cells/µL for cryptococcal infection and initiating anticryptococcal therapy to prevent CM [15, 19]. In the United States, the Department of Health and Human Services and the Infectious Diseases Society of America do not recommend routine screening for cryptococcal infection, unlike other common opportunistic infections such as tuberculosis and Mycobacterium avium complex, but suggest “consideration” of cryptococcal screening for patients with a CD4 count <50 cells/µL [18, 20]. However, it has been shown that routine screening in populations with a CM prevalence of ≥2% is cost-effective, perhaps cost-saving, and saves lives [4, 7, 21–23]. With the advent of new, less costly, highly accurate tests, such as the CrAg lateral flow assay, the prevalence at which CM screening is cost-effective may be considerably lower [4, 21, 22]. However, there is limited information on the prevalence of cryptococcal infection among HIV-infected individuals in the United States. With the development of new tests, an improved understanding of the epidemiology of cryptococcal infection among patients with AIDS in the United States is needed to guide future updates in the prevention and management of CM.
MATERIALS AND METHODS
Stored sera from the Multicenter AIDS Cohort Study (MACS) and the Women's Interagency HIV Study (WIHS) that met eligibility criteria were randomly selected to be screened for cryptococcal infection. Eligible study specimens were required to be from participants who were HIV-infected, had a CD4 count ≤100 cells/µL, and had ≥0.5 mL of serum available for testing. A total of 1872 unique serum samples from participants were selected to be screened from the MACS and WIHS serum registry. Serum samples from both participants on and off antiretroviral therapy (ART) were eligible for screening. With a total of 1872 unique serum samples, and assuming an α level of 5%, there was >99% power to detect the proportion cryptococcal infected.
Multicenter AIDS Cohort Study
The MACS is a longitudinal study following men since 1983. The study has enrolled 6972 adult, urban, homosexual/bisexual, HIV-infected men from Baltimore, Maryland; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California. There have been 3 enrollment periods, the first in March 1985, in which 4955 men were enrolled. The second enrollment was between April 1987 and September 1991, in which 668 men were enrolled. Finally, between October 2001 and August 2003, 1350 additional men were enrolled in the study. Both questionnaires and laboratory procedures are performed on participants every 3–6 months. Over the 3 decades that the study has been ongoing, the drop-out rate has been <15% [23].
Women's Interagency HIV Study
The WIHS is a longitudinal study comprised of 3772 women who are HIV-infected or at high risk for HIV seroconversion. Demographic and clinical data, as well as specimens, have been collected every 6 months since 1994, making it the largest study in the United States focusing on HIV infection among women. There are a total of 6 study sites throughout the United States: Bronx/Manhattan, New York; Brooklyn, New York; Los Angeles/Southern California/Hawaii; San Francisco/Bay Area, California; Chicago, Illinois; and Washington, District of Columbia. Retention in the study has been high, especially among HIV-infected participants (83% vs 76% for HIV-uninfected participants). The median age of HIV-infected participants is 40 years. The majority of the HIV-infected study population is unemployed (62.3%) and uninsured (65.8%); many participants live below the poverty line (48.9%) and are single mothers (32.7%). Therefore, the cohort of women that makes up WIHS is diverse and representative of the US population of women infected with HIV or at high risk of HIV infection [24].
Laboratory Testing
Selected frozen sera were thawed, and 1- to 2-mL serum aliquots shipped to the central testing laboratory. Sera were tested using the CrAg LFA (IMMY, Inc, Norman, Oklahoma), a US Food and Drug Administration–cleared lateral flow assay for the detection of CrAg. In validation studies, the assay was 100% accurate at detecting true positives and negatives (sensitivity and specificity, respectively) in serum as compared with culture [25–27]. Titers were performed on all CrAg-positive specimens.
Analysis
Data were analyzed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina). All 1872 sera, including those of participants with a history of cryptococcal disease, were included in the analysis. History of cryptococcal disease was self-reported as ever having been diagnosed with CM. The χ2 test with corresponding 95% confidence intervals (CIs) was used to compare categorical variables among patients with a positive and negative CrAg test result, and Student t tests and corresponding 95% CIs were used to test the difference in means for all continuous variables. Univariate and multivariate logistic regression analyses were used to examine correlates of cryptococcal infection. Correlates that were statistically significant (P ≤ .05) after backward elimination, or those known to be associated with cryptococcal infection, were included in the model. Logistic regression yields maximum likelihoods estimates of the odds ratio; however, these estimates are good approximations of the prevalence ratio when the disease is rare [28–32]. Because cryptococcal infection in the United States meets the rare disease assumption needed for the approximation, prevalence ratios can be approximated from the results of the study. Survival analysis was conducted using the Proc Lifetest, with multiple comparisons being made between all pairs of curves and adjusted using the Bonferroni method. Log-rank, Wilcoxon, and Peto test statistics were generated to assess the difference between survival rates in ≥2 groups. Finally, survival rates were calculated with and without patients with a prior history of cryptococcal disease.
RESULTS
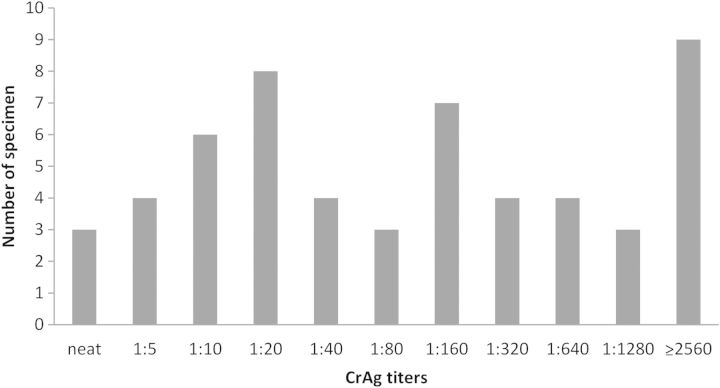
A total of 1872 randomly selected specimens from participants in the WIHS and MACS cohorts were tested; of those, 55 (2.9%; 95% CI, .2%–3.8%) were CrAg positive, 10 of which were from participants who reported a prior history of CM. Thus, the CrAg positivity in those with no history of cryptococcal disease was 2.5% (95% CI, 1.8%–3.2%). The median age of the study population was 39 years (range, 20–70 years). Additional demographic and clinical characteristics of the study population, along with the CrAg prevalence by those characteristics, are shown in Table 1. CD4 counts for participants ranged from 0 to 100 cells/µL. Titers for positive specimen ranged from neat (undiluted) to ≥1:2560. Figure 1 shows the distribution of titers for all positive specimens.
Table 1.
Characteristics of Participants With Selected Specimens and Cryptococcal Antigen Prevalence, Multicenter AIDS Cohort Study and the Women's Interagency HIV Study, 1986–2012
| Characteristics | Total, No. (%) (N = 1872) | Prevalence of CrAg+, % (95% CI) |
|---|---|---|
| Sex | ||
| Male | 989 (53.3) | 2.6 (1.7–3.8) |
| Female | 866 (46.7) | 3.3 (2.3–4.7) |
| Study location | ||
| Baltimore, Maryland | 241 (12.9) | 3.0 (1.5–6.0) |
| Bronx, New York | 183 (9.9) | 4.3 (2.2–8.4) |
| Brooklyn, New York | 162 (8.7) | 1.9 (.6–5.3) |
| Chicago, Illinois | 342 (18.4) | 0.5 (.08–2.6) |
| District of Columbia | 123 (6.6) | 4.0 (1.7–9.1) |
| Los Angeles, California | 514 (27.7) | 3.3 (2.1–5.2) |
| Pittsburgh, Pennsylvania | 187 (10.1) | 4.2 (2.1–8.3) |
| San Francisco, California | 103 (5.6) | 3.9 (1.5–9.5) |
| Race/ethnicity | ||
| White | 849 (45.9) | 2.5 (1.6–3.8) |
| Black | 651 (35.2) | 2.5 (1.5–4.0) |
| Hispanic | 117 (6.3) | 1.7 (.47–6.7) |
| Other | 234 (12.6) | 6.4 (3.9–10.3) |
| Education | ||
| High school or less | 790 (42.9) | 3.8 (2.7–5.4) |
| College | 773 (42.1) | 2.5 (1.6–3.8) |
| Graduate school | 275 (14.9) | 1.5 (.6–3.9) |
| Period of specimen collection | ||
| 1986–1990 | 485 (26.3) | 2.1 (1.1–3.8) |
| 1991–1995 | 620 (33.7) | 3.6 (2.4–5.3) |
| 1996–2000 | 255 (13.9) | 1.6 (.6–4.0) |
| 2001–2005 | 288 (15.6) | 3.5 (1.9–6.3) |
| 2006–2012 | 193 (10.5) | 3.6 (1.8–7.3) |
| Age, y | ||
| 20–30 | 188 (10.0) | 2.1 (.8–5.4) |
| 30–40 | 775 (41.4) | 3.1 (2.1–4.6) |
| 41–50 | 556 (29.7) | 2.5 (1.5–4.2) |
| 51–60 | 142 (7.6) | 4.2 (2.0–8.9) |
| ≥61 | 18 (9.6) | 5.6 (1.0–25.8) |
| CD4 T-cell count | ||
| 51–100 cells/µL | 992 (53.0) | 1.7 (1.1–2.7) |
| ≤50 cells/µL | 881 (47.1) | 4.3 (3.2–5.9) |
| Receiving ART at time of specimen collection | ||
| Yes | 1047 (55.9) | 2.6 (1.0–2.1) |
| No | 743 (39.7) | 3.5 (1.0–2.1) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CrAg, cryptococcal antigen; HIV, human immunodeficiency virus.
Figure 1.
Frequency distribution of positive cryptococcal antigen (CrAg) titers, Multicenter AIDS Cohort Study and the Women's Interagency HIV Study, 1986–2012. Abbreviation: HIV, human immunodeficiency virus.
There were no statistically significant differences by age, sex, study location, race, and education and CrAg test result. However, both previous CM diagnosis and CD4 T-cell count were significantly associated with cryptococcal positivity. Among those CrAg positive, the odds of a CD4 count ≤50 cells/µL was 5.3 (95% CI, 2.4–11.7) times that compared with those who were CrAg negative, and among those who were CrAg positive, the odds of a history of cryptococcal disease was 45.9 (95% CI, 18.6–113.2) times that of those who were CrAg negative.
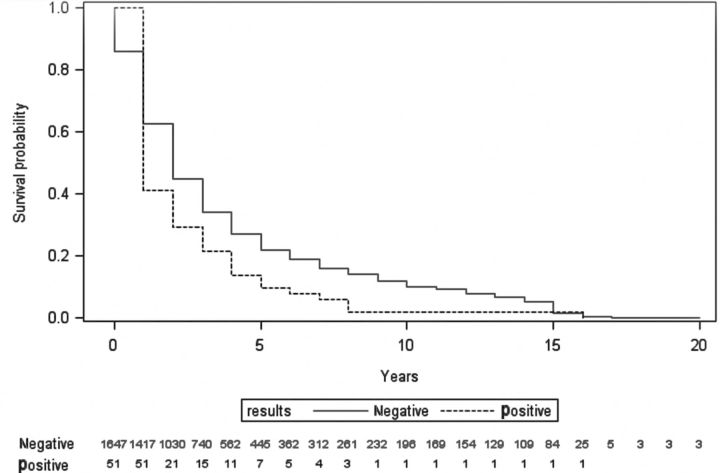
When assessing the time from specimen collection to death among participants without a history of CM, there was a significant difference between mean survival for those with positive CrAg specimens and those with negative CrAg specimens (mean difference, 1.0 year; P = .03). Participants with a CrAg-positive result lived for an average of 2.8 years after the date of specimen collection, whereas those with a negative CrAg result lived on average for 3.8 years after specimen collection. Among those with a reported history of CM, there was a marginally significant difference between mean survival for those with positive CrAg specimens and those with negative specimens (mean difference, 1.2 years; P = .05). Those with a positive CrAg result lived on average 1.4 years after date of specimen collection, whereas those with a negative CrAg result lived on average 2.6 years after specimen collection. The survival curve can be seen in Figure 2. A separate analysis was conducted excluding those with a history of CM; however, there was no significant difference in the survival curves nor the correlates associated with survival and thus, 1 survival curve among all participants is presented. After controlling for age, CD4 T-cell count, prior diagnosis of CM, and year of specimen collection, women had a longer survival than men (P = .02), and blacks had a longer survival than both Hispanics and whites (P = .02). The adjusted probability of 5-year survival for blacks and Hispanics was 30% compared with 10% for whites. Furthermore, the adjusted probability of 5-year survival for women was >30% vs <10% in males.
Figure 2.
Survival analysis by cryptococcal antigen test result, Multicenter AIDS Cohort and the Women's Interagency HIV Study, 1986–2012. Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
Using sera from study participants from both the MACS and WIHS cohorts, participants with a CD4 count ≤100 cells/µL were screened. Screening was performed using CrAg lateral flow assay, which has both a high sensitivity and specificity [25–27]. Of the 1872 specimen tested, 2.9% were positive for CrAg, of which 18% were among participants with a reported history of CM. Lower CD4 T-cell count and prior history of cryptococcal disease were significant correlates of a positive CrAg result. Participants positive for CrAg, with or without a history of CM, had a significantly lower survival than those negative for CrAg. Moreover, sex (female) and race/ethnicity (black/African American) were independent predictors of survival after controlling for age, CD4 T-cell count, prior diagnosis of CM, and year of specimen collection.
The overall prevalence of cryptococcal infection in our study was similar or slightly lower than prevalences observed in studies conducted in low-resource settings [1, 6]. For instance, the prevalence of cryptococcal infection in Kenya was estimated to be 8.2% [2]. Similar prevalences to that of Kenya were also found in Ethiopia, Bangkok, Thailand, and Uganda [1, 5, 18]. However, within certain subgroups of our study, CrAg prevalence was high and similar to those estimates. South Africa, which began implementing CrAg screening and treatment of those with positive CrAg tests in 2012, has observed a CrAg prevalence of 5% in the 12 months following the initiation of that program [33].
Our results are similar to other studies that examined correlates of cryptococcal infection [1, 2, 4, 5, 12, 18, 34, 35]. The majority of studies did not find that cryptococcal infection differed significantly by sex or age. The results from our study suggest that a lower CD4 T-cell count is a significant correlate of CrAg positivity. From an immunological perspective, CM would be expected to be more common at lower rather than higher CD4 T-cell levels because those with lower CD4 T-cell levels are more immunosuppressed and have less ability to control infection [36].
There have been several studies that have examined the cost-effectiveness of cryptococcal screening, mainly in resource-limited settings; however, a few have examined it within the United States [4, 7, 12, 15, 22]. In a study in Uganda conducted by Meya et al, the prevalence threshold for diagnosing cryptococcal infection in which the benefits exceeded the costs was 2% [4, 18, 21]. Yet another study examining the cost-effectiveness of 2 different approaches compared with the standard of care (CrAg screening with targeted treatment of all positive individuals with fluconazole and CrAg screening followed by lumbar puncture in all CrAg-positive individuals) found that either approach was more cost-effective than the standard of care [7]. However, CrAg screening followed by treatment of all positive individuals was considerably less expensive than CrAg screening followed by lumbar puncture [7]. Moreover, the study showed that CrAg screening followed by treatment was more cost-effective than the standard of care (no screening) in prevalences as low as 0.6%, and CrAg screening followed by lumbar puncture was more cost-effective than the standard of care in prevalences as low as 2.5% [7].
An analysis done within the United States found that, compared to the cost of hospitalization due to CM ($50 000 over a 14-day period), cryptococcal screening followed by a 10-day treatment with fluconazole was significantly less costly [21]. Moreover, screening followed by treatment is likely to be more cost-effective in prevalences as low as 0.1%, a prevalence nearly 30-fold lower than what we found in our study; suggesting that cryptococcal screening in the United States coupled with treatment is the more cost-effective option [21].
Compared with other biomedical interventions in HIV/AIDS care such as ART and substance use treatment programs, screening for cryptococcal infection may be more cost-effective [37]. In a cost-effective analysis conducted by Cohen et al in which different HIV/AIDS interventions were analyzed, the individual costs for cryptococcal screening were significantly lower than the cost of ART per person, estimated to be $20 000, and cryptococcal screening was found to be more beneficial [38].
With regard to methods of diagnosis, the lateral flow assay is equally accurate and significantly less costly compared to the latex agglutination test ($5 and $16.75, respectively). Additionally, compared with other forms of diagnostic testing for CM, the lateral flow assay is easier to use, produces rapid results, is stable at room temperature (allowing for a longer shelf life), and is highly sensitive and specific [18, 39]. When compared to latex agglutination, the lateral flow assay is more sensitive to lower levels of CrAg, allowing for earlier detection [39].
To our knowledge, this is the first study and likely the largest in over a decade to examine the prevalence of cryptococcal infection among patients with advanced AIDS in the United States, despite the fact that CM is one of the most common opportunistic infections among HIV-infected individuals, as well as one of the leading causes of AIDS-related mortality [1–5].
There were several limitations with our study. First, our study spanned >2 decades and includes many participants who were diagnosed before the advent of HAART; predictors of cryptococcal infection may be different among those enrolled prior to the advent of HAART than for those enrolled during the HAART era. However, when we compared results by various periods and current ART use, we found no differences. Second, there were no lumbar puncture results available to help determine what proportion of patients with a positive serum CrAg result had central nervous system cryptococcal infection. Third, history of CM was self-reported and might be misclassified. However, excluding individuals with a history of CM did not substantially alter the prevalence estimate or affect survival results. Finally, our study was conducted using serum samples from the MACS and WIHS cohorts, both of which include participants who may not be completely representative of the HIV-infected population in the United States.
The results from our study suggest that the prevalence of cryptococcal infection among patients with advanced AIDS in the United States is substantial, clinically important, and above the published cost-effectiveness threshold for routine screening and treatment in those with a CD4 count ≤100 cells/μL. Current screening recommendations warrant revision.
Notes
Acknowledgments. The authors thank the Multicenter AIDS Cohort Study and Women's Interagency Health Study for providing specimens and support, and IMMY Inc for providing testing of the specimens.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Alemu A, Kempker R, Tenna A, et al. High prevalence of cryptococcal antigenemia among HIV-infected patient receiving antiretroviral therapy in Ethiopia. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0058377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer A, Kendi C, Penner J, et al. The impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in Kenya. Trop Med Int Health. 2013;18:495–505. doi: 10.1111/tmi.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warkentien T, Crum-Cianflone NF. An update on Cryptococcus among HIV-infected patients. Int J STD AIDS. 2010;21:679–84. doi: 10.1258/ijsa.2010.010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meya D, Manabe Y, Castelnuovo B, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count <= 100 cells/μl who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51:448–55. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pongsai P, Atamasirikul K, Sungkanuparph S. The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell counts. J Infect. 2010;60:474–7. doi: 10.1016/j.jinf.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Park B, Wannemuehler K, Marston B, et al. Estimation of current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis J, Harrison T, Lawn S, et al. Cost-effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0069288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:526. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 9.Jarvis J, Meintjes G, Harrison T. Outcomes of cryptococcal meningitis in antiretroviral naïve and experienced patients in South Africa. J Infect. 2010;60:496–8. doi: 10.1016/j.jinf.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayanja-Kizza H, Oishi K, Mitarai S, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Uganda patients with AIDS. Clin Infect Dis. 1998;26:1362–6. doi: 10.1086/516372. [DOI] [PubMed] [Google Scholar]
- 11.Kambugu A, Meya D, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of high active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis J, Lawn S, Vogt M, et al. Screening for cryptococcal antigenemia in patients accessing antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–61. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmquist L, Russo A, Elixhauser A. Meningitis-related hospitalizations in the United States, 2006. 2008. Statistical brief 57, Healthcare and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality. [PubMed]
- 14.Pyrogos V, Sietz A, Steiner C, et al. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One. 2013;8:e56269. doi: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajasingham R, Meya D, Boulware D. Integrating cryptococcal antigen screening and preemptive treatment into routine HIV care. J Acquir Immune Defic Syndr. 2012;59:85–91. doi: 10.1097/QAI.0b013e31824c837e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 17.Hajjeh R, Conn L, Stephens D, et al. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. J Infect Dis. 1999;179:449–54. doi: 10.1086/314606. [DOI] [PubMed] [Google Scholar]
- 18.Liechty C, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents, and children. Geneva, Switzerland: WHO; 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241502979_eng.pdf . Accessed January 2014. [PubMed] [Google Scholar]
- 20.Aberg J, Gallant J, Ghanem K, et al. Primary care guidelines for management of persons infected with HIV: 2013 updated by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2013:1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 21.Rajasingham R, Boulware D. Reconsidering cryptococcal antigen screening in the U.S. among persons with CD4 <100 cells/mcl. Clin Infect Dis. 2012;55:1742–4. doi: 10.1093/cid/cis725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis J, Lawn S, Wood R, Harrison T. Cryptococcal antigen screening for patients initiating antiretroviral therapy: time for action. Clin Infect Dis. 2010;51:1463–5. doi: 10.1086/657405. [DOI] [PubMed] [Google Scholar]
- 23.Multicenter AIDS Cohort Study. Available at: http://www.statepi.jhsph.edu/macs/history.html . Accessed October 2013.
- 24.Bacon M, Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsley M, Mekha N, Baggett H, et al. Evaluation of newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis. 2011;53:321–5. doi: 10.1093/cid/cir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis J, Percival A, Bauman S, et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011;53:1019–23. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IMMY, Inc. Package insert for cryptococcal antigen lateral flow assay. Available at: http://www.immy.com/wp-content/uploads/2013/02/MSDS-CR2003.pdf . Accessed January 2014.
- 28.Peterson M, Deddens J. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008;8:1–9. doi: 10.1186/1471-2288-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deddens J, Peterson M. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65:501–6. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 30.Coutinho L, Scazufca M, Menezes P. Methods for estimating prevalence ratios in cross-sectional studies. Revista de Salude Publica. 2008;42:1–6. [PubMed] [Google Scholar]
- 31.Behrens T, Taeger D, Wellman J, Keil U. Different methods to calculate effect estimates in cross-sectional studies. Methods Inf Med. 2004;43:505–9. [PubMed] [Google Scholar]
- 32.Barros A, Hirakata V. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:1–13. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute for Communicable Diseases, South Africa. Monthly surveillance report: January 1–31, 2014. Available at: http://www.nicd.ac.za/assets/files/Monthly%20NICD%20Surveillance%20Report%20-%20January%202013.pdf . Accessed March 2014.
- 34.Oursler K, Moore R, Chaisson R. Risk factors for cryptococcal meningitis in HIV-infected patients. AIDS Res Hum Retroviruses. 1999;15:625–31. doi: 10.1089/088922299310926. [DOI] [PubMed] [Google Scholar]
- 35.Micol D, Lortholary O, Sar B, et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr. 2007;45:555–9. doi: 10.1097/QAI.0b013e31811ed32c. [DOI] [PubMed] [Google Scholar]
- 36.AIDS.gov. Opportunistic infections. Available at: http://aids.gov/hiv-aids-basics/staying-healthy-with-hiv-aids/potential-related-health-problems/opportunistic-infections/ Accessed February 2014.
- 37.Lomborg B. Rethink HIV: smarter ways to invest in ending HIV in sub-Saharan Africa. Cambridge University Press; 2012. Cambridge, UK. [Google Scholar]
- 38.Cohen D, Wu S, Farley T. Comparing the cost-effectiveness of HIV prevention interventions. J Acquir Immune Defic Syndr. 2004;37:1404–14. doi: 10.1097/01.qai.0000123271.76723.96. [DOI] [PubMed] [Google Scholar]
- 39.Boulware D, Rolfes M, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis. 2014;20:43–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]