Abstract
Background
Appropriate use of highly active antiretroviral therapy (HAART) can markedly decrease the risk of progression to acquired immunodeficiency syndrome (AIDS) and of premature mortality. We aimed to characterize the trends between 1981 and 2013 in AIDS-defining illnesses (ADIs) and in the number AIDS-related deaths in British Columbia (BC), Canada.
Methods
We included data of 3550 HIV-positive individuals, aged 19 years or older, from different administrative databases in BC. We estimated the relative risk of developing an ADI over time using a Negative Binomial model, and we investigated trends in the percentage of all deaths associated with AIDS using generalized additive models.
Findings
The number of ADIs has decreased dramatically to its lowest level in 2013. The peak of the AIDS epidemic in BC happened in 1994 with 696 ADIs being reported (rate 42 ADIs per 100 person-years). Since 1997, the number of ADIs decreased from 253 (rate 7 per 100 person-years) to 84 cases in 2013 (rate 1 per 100 person-years) (p-value equals to zero for the trend in the number of ADIs). We have also shown that out of 22 ADIs considered, only PCP maintained its prominent ranking (albeit with much reduced overall prevalence). Finally, we observed that over time very few deaths were related to AIDS-related causes, especially in the most recent years.
Interpretation
We showed that the number of new ADIs and AIDS-related mortality have been decreasing rapidly over time in BC. These results provide further evidence that integrated comprehensive free programs that facilitate testing, and deliver treatment and care to this population can be effective in markedly decreasing AIDS-related morbidity and mortality, thus suggesting that controlling and eventually ending AIDS is possible.
Funding
The British Columbia Ministry of Health, the US National Institutes of Health, the US National Institute on Drug Abuse, the Canadian Institutes of Health Research, and the Michael Institute for Health Research.
Introduction
First introduced in 1996, highly active antiretroviral therapy (HAART) has had a dramatic impact on the natural history of human immunodeficiency virus (HIV)-related diseases, including the acquired immunodeficiency syndrome (AIDS).1 Appropriate use of HAART can markedly decrease the risk of progression to AIDS and of premature mortality.2–4 Worldwide, the expansion in the number of individuals accessing HAART since 2005 has been associated with a 30% decrease in AIDS-related mortality.5 More recently, an association has been described between the expansion of HAART coverage and a decrease in the incidence of AIDS and AIDS-related mortality, as well as a decrease in estimated HIV incidence.4,6–9 As a result, there has been growing optimism regarding the possibility of ending the AIDS epidemic.10–13 However, this remains a matter of significant controversy.10–13
BC provides a unique environment to assess to what extent currently available tools can control the AIDS epidemic, and whether the end of AIDS represents a realistic goal. BC’s HIV/AIDS epidemic is highly concentrated around urban centres. HIV/AIDS initially affected men who have sex with men (MSM) with a peak in 1994/1995. In 1996/1997 a rapid increase in cases among people who inject drugs (PWID) was seen.14 Currently, the homeless, individuals with mental health issues, individuals of Aboriginal ancestry and women through sex work are overrepresented within the BC epidemic.14 In addition, vertical transmission has been virtually eliminated in the province - only two infants were born to women who did not receive antenatal HAART prior to delivery in the last decade.14 HAART and related medical and laboratory monitoring have been fully subsidized in BC since 1996, and eligibility for HAART has been consistent with the IAS-USA guidelines.1 Since 2003, BC has had mandatory (nominal or non-nominal) HIV reporting legislation. Additionally, the availability of unique personal health numbers for all BC residents provides a great opportunity to perform anonymized data linkages between administrative datasets to address our research question. Therefore, in this paper we focused on assessing the population impact of the expansion of HAART coverage on changes in AIDS incidence and mortality since the beginning of the HIV epidemic in BC in 1981. Specifically, we aimed to characterize the trends between 1981 and 2013 in AIDS-defining illnesses (ADIs) and in the number AIDS-related deaths.
Methods
Data
Data for these analyses came from: (1) the BC-Centre for Excellence in HIV/AIDS (BC-CfE), which provided the list of eligible individuals for this study;15 (2) St. Paul’s Hospital, which is the main HIV/AIDS care provider in BC, where the BC-CfE is located and it provides real-time clinical data updates for all eligible individuals; (3) the BC Vital Statistics Agency, which provides mortality data that are monthly linked with the BC-CfE database;15 and (4) the BC Cancer Agency, which is the provincial agency responsible for providing all cancer-related care in BC, which also provides data that are yearly linked to the BC-CfE database.15 ADI case-reports were obtained from the BC-CfE, enriched with clinical records from St. Paul’s Hospital, the BC Cancer Agency, and the BC Vital Statistics Agency. All these agencies use passive reporting systems to collect data on ADIs. The complete list of ADIs can be found in Table 1.16 Mortality data and causes of deaths were provided by BC Vital Statistics Agency and BC Cancer Agency.
Table 1.
List of AIDS-defining illnesses used in this study.
| AIDS defining illnesses | Abbreviation |
|---|---|
| Candidiasis of esophageal, bronchi, trachea, or lungs | CANDIDA |
| Invasive cervical cancer | CERVCA |
| Cytomegalovirus disease (site not specified) | CMV |
| Coccidiomycosis disseminated or extrapulmonary | COCCID |
| Cryptococcosis extrapulmonary | CRYPTOCO |
| Cryptosporidiosis chronic intestinal | CRYPTOSP |
| HIV encephalopathy | DEMENT |
| Histoplasmosis disseminated or extrapulmonary | HISTO |
| Herpes simplex infection, chronic mucocutaneous | HSV |
| lsosporiasis chronic intestinal | ISOSPOR |
| Kaposi's sarcoma AIDS-defining unspecified | KS |
| Lymphoma primary of brain | LYMPHOMA |
| Mycobacterium Avium Complex (MAC) or Mycobacterium kansasii disseminated or extrapulmonary | MAC |
| Tuberculosis AIDS-defining unspecified | MTB |
| Mycobacterium other or unspecified species disseminated or extrapulmonary | MYCO |
| Lymphoma, non-Hodgkins, AIDS defining unspecified | NHL |
| Pneumocystis jiroveci pneumonia | PCP |
| Progressive multifocal leukoencephalopathy | PML |
| Recurrent bacterial pneumonia | REC PNEUM |
| Recurrent salmonella septicemia | SALM SEP |
| Toxoplasmosis of brain | TOXO |
| Wasting syndrome due to HIV | WASTING |
In BC, antiretroviral treatment, HIV care, and laboratory monitoring are fully subsidized by the provincial government without any co-payments or deductibles since 1992 and under the aegis of the BC-CfE. Antiretroviral treatment is distributed to eligible individuals based on the BC-CfE HIV therapeutic guidelines, which have remained consistent with those put forward by the International AIDS Society-USA (more recently renamed International Antiviral Society-USA) since 1996.1,3 For these analyses, we obtained data for the number and type of ADIs and for the number of people followed in the pre-HAART and post-HAART era (i.e. HAART available as of July 1st, 1996) from the BC-CfE. In addition, ADI case-reports were allocated according to the calendar year when an individual was diagnosed with the first episode for each type of ADI.
Analyses
All analyses were restricted to HIV-positive individuals 19 years or older. These individuals were followed from January 1st, 1981 until December 31st, 2013, the last contact date, or until they died, whichever came first. The first analysis focused on characterizing the number of ADIs over time. For this purpose, we obtained the number of ADIs from 1981 until 2013 for individuals who have ever had an ADI diagnosed in BC. We stratified these data by gender, age at the time the ADI was diagnosed (grouped as <30, 30 – 39, 40 – 49 and ≥ 50 years), and by calendar year (grouped as 1981 – 1996, 1997 – 1999, 2000 – 2003, 2004 – 2012). The first period, from 1981 to 1996, relates to the era prior to the initial rollout of HAART in BC. The second period, from 1996 to 1999, relates to the initial rollout of HAART. The third, from 2000 to 2003, relates to a steady-state phase of HAART use. The fourth, from 2004 to 2012, relates to the second HAART expansion in BC. We divided the calendar years this way to control for the cohort effect in our analyses and to accommodate the changes in HIV treatment guidelines during the study period. Based on these factors, we estimated the relative risk of developing an ADI through a Negative Binomial regression analysis.17,18 In this case, we assessed the effect of gender and age group, by calendar period, on the number of ADIs. The criteria for testing the goodness of fit of this statistical model and for testing for overdispersion are presented in the Supplementary Material. Note that in this analysis, we used the statistical software R© version 3.0.2 libraries MASS, AER, pcsl and lmtest. In a sub-analysis, we investigated trends in the most common ADIs during the pre-HAART and post-HAART era.
We then characterized AIDS-related mortality over time. For this purpose, an AIDS-related mortality was ascertained using either of the following criteria: (1) having at least one ADI within three months from the death date (see Table 1 for the list of ADIs); or (2) having at least one HIV-related diagnosis code for any cause of death. The codes for HIV-related death were based on the International Classification of Disease (ICD) codes versions 9 and 10. The HIV-related ICD-9 codes considered were 795·71 and those starting with 042, 043, 044, V08 and 795·8. The HIV-related ICD-10 codes considered were those starting with B20, B21, B22, B23, B24, R75 and Z21. We modeled the trend in the proportion of deaths associated with an ADI using generalized additive models due to the non-linear trend in this outcome. 19 The criteria for assessing goodness of fit of this model is also presented in the Supplementary Material. In this model, the outcome variable was the proportion of deaths associated with an ADI and the explanatory variable was calendar year. Note that for these analyses, we used the statistical software R© version 3.0.2 libraries mgcv and lmtest. In all analyses all p-values were two-sided and significance level was set at 5%.
The BC-CFE received approval for this study from the University of British Columbia ethics review committee at the St Paul’s Hospital, Providence Health Care site (P05–123). The study complies with the BC’s Freedom of Information and Protection of Privacy Act. The study was conducted primarily using anonymized administrative databases, and therefore informed consent was not required.
Role of funding source
The funding sources had no role in the choice of methods, the contents or form of this work, or the decision to submit the results for publication. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Data based on HIV-positive individuals ever diagnosed with an ADI in BC
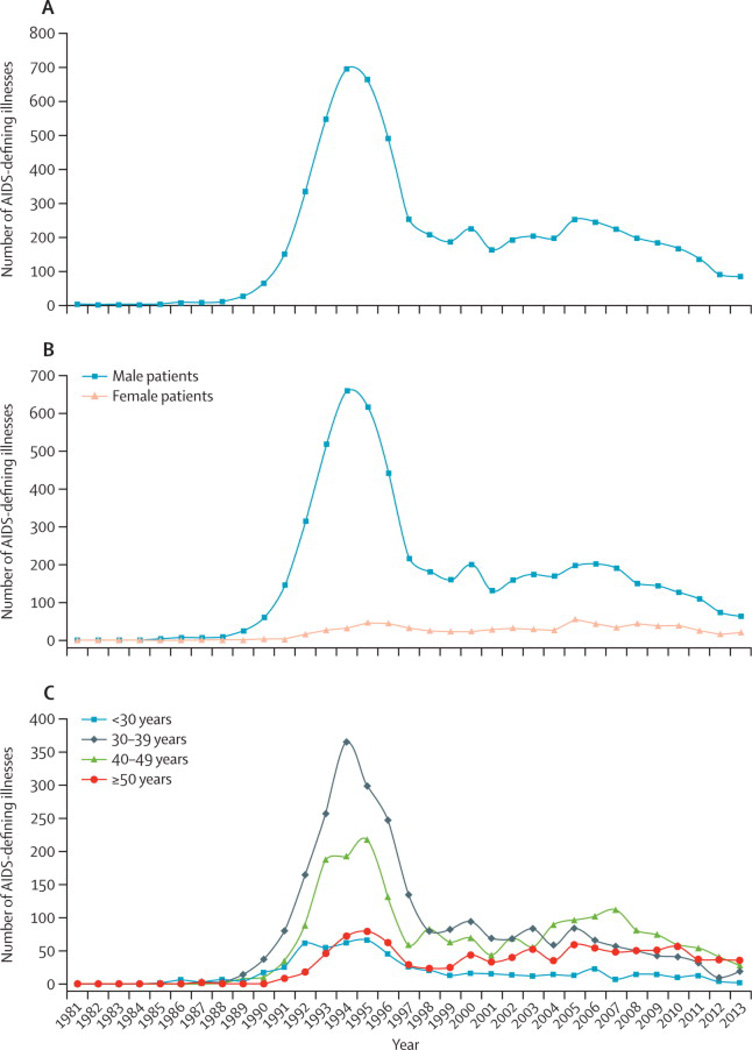
In this study, we included data on ADIs of 3097 males and 453 females reported between 1981 and 2013. The mean age (at the first ADI of each type) was 41 years (standard deviation 9·7 years). The mean number of ADIs per person was 2 ADIs (standard deviation: 1·1; Maximum 7). The number of ADIs over time are reported in Figure 1A. The peak of the AIDS epidemic in BC happened in 1994 with 696 ADIs being reported for a rate of 42 ADIs per 100 person-years. Since 1997, the number of ADIs decreased from 253 (rate 7 per 100 person-years) to 84 cases in 2013 (rate 1 per 100 person-years) (p-value equals to zero for the trend in the number of ADIs).
Figure 1.
Overall trend in the number of AIDS-defining illnesses from January 1st, 1981 to December 31st, 2013 in British Columbia.
(A) Overall
(B) By gender
(C) By age group
Figure 1B shows the trend in ADIs stratified by gender. Males throughout the study period had consistently higher ADI cases than females, with the highest male to female ratios obtained from 1989 to 1995 (ranging from 13:1 to 49:1). Since the introduction of HAART, this ratio between males and females has been decreasing from 10:1 in 1996 to as low as 3:1 in 2013. The consistent higher trend in males was due to the epidemic among MSM, which reached its peak in 1994. The second peak of the epidemic was in 1997 among PWID.
The number of ADIs by age and calendar years are highlighted in Figure 1C. In terms of age, the AIDS epidemic in BC has been concentrated among those aged 30 – 39 years. This trend has been changing since 2004 and individuals in the age group 49 – 49 years, and more recently, those in the age group ≥ 50 years have now the highest number of AIDs in the province. For this latest age group, the ADIs most frequent in 2013 (total=35) were WASTING (N=8; 23%), NHL (N=7; 20%), MTB (N=4; 11%), CANDIDA (N=4; 11%) and DEMENT (N=3; 9%).
As noted in Table 2, we fitted a Negative Binomial model for each period of time 1981 – 1996 (period 1), 1997 – 1999 (period 2), 2000 – 2003 (period 3), and 2004 – 2013 (period 4), considering as the outcome the total number of ADIs in these periods and as covariates gender and age group. In this model, we demonstrated that the relative risk of developing an ADI for females was consistently lower than that for males ranging from 0.10 (95% Confidence Interval (CI): 0·07 – 0·14) (period 1) to 0·27 (95%CI 0·22 – 0·33) (period 4). For example, the relative risk for period 1 means that being female was associated with a 90% reduction in the risk of developing an ADI relative to males. The relative risk of developing an ADI for individuals <30 years was consistently lower than the age group 30 – 39 years (the reference group) ranging from 0·25 (95%CI 0·15 – 0·42) (period 1) to 0·29 (95%CI 0·20 – 0·41) (period 4). For those in the age group 40 – 49 years, the relative risk of developing an ADI, in relation to the age group 30 – 39 years, only reached statistical significance in the last period of observation and it was equal to 1·47 (95%CI 1·11 – 1·94) (period 4). Those aged ≥ 50 years also had a lower relative risk of developing and ADI than those in the age group 30 – 39 years during the follow-up, ranging from 0·27 (95%CI 0·15 – 0·47) (period 1) to 0·35 (95%CI 0·16 – 0·80) (period 2). The relative risk in this last age group seems to be increasing since 2000, but it did not reach statistical significance probably due to the limited sample size of these groups.
Table 2.
Negative Binomial regression multivariable results for the effect of gender and age group on the number of AIDS-defining illnesses.
| Variables | Relative Risk (95% Confidence Interval) |
|||
|---|---|---|---|---|
| Period | ||||
| 1981 – 1996 Total ADIs = 77 |
1997 – 1999 Total ADIs = 31 |
2000 – 2003 Total ADIs = 23 |
2004 – 2013 Total ADIs = 77 |
|
| Gender | ||||
| Male | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Female | 0.10 (0.07 – 0.14) | 0.15 (0.09 – 0.24) | 0.21 (0.13 – 0.33) | 0.27 (0.22 – 0.33) |
| Age Group | ||||
| < 30 years | 0.25 (0.15 – 0.42) | 0.19 (0.09 – 0.38) | 0.19 (0.10 – 0.35) | 0.29 (0.20 – 0.41) |
| 30 – 39 years | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| 40 – 49 years | 0.62 (0.34 – 1.14) | 0.71 (0.33 – 1.53) | 0.76 (0.34 – 1.72) | 1.47 (1.11 – 1.94) |
| ≥ 50 years | 0.27 (0.15 – 0.47) | 0.35 (0.16 – 0.80) | 0.68 (0.30 – 1.55) | 0.87 (0.67 – 1.13) |
Footnote: ADI is the number of AIDS-defining illnesses.
Next we stratified the distribution of ADIs by the pre- and post-HAART eras. Focusing on the data in the pre-HAART era, the ADIs accounting for more than 60% of the total number of ADIs in this period were Pneumocystis Jiroveci Pneumonia (PCP; 24%), Kaposi's sarcoma (KS; 12%), Mycobacterium Avium Complex (MAC; 11%), Candidiasis of Esophageal, Bronchi, Trachea, or Lungs (CANDIDA; 10%), and Wasting Syndrome due to HIV (WASTING; 9%). In the post-HAART era, the ADIs accounting for more than 60% of the total number of ADIs in this period were PCP (21%), Wasting Syndrome due to HIV (WASTING; 12%), Non-Hodgkins Lymphoma, including Burkitt's, or immunoblastic, or other lymphomas (NHL; 8%), KS (8%), CANDIDA (7%), and Recurrent Bacterial Pneumonia (REC_PNEUM; 7%).
Data based on all HIV-positive individuals in BC
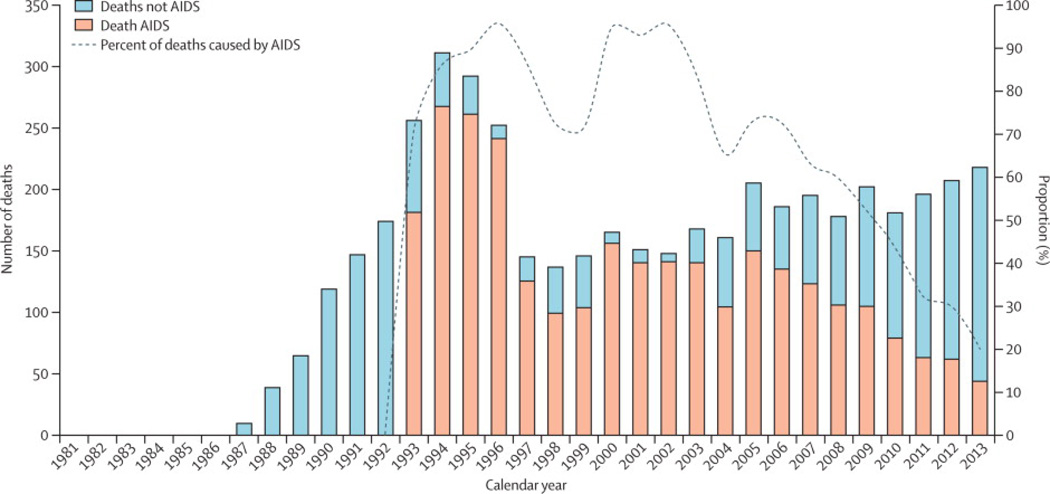
During the study period, we observed 4654 deaths (all-causes) (rate 4 per 100 person-years), in which 2828 (61%) were AIDS-related (Figure 2). Due to data constraints, we were not able to ascertain whether the mortality cases were AIDS-related prior to 1992. Thus after 1992, most notably, the overall mortality rate has been decreasing consistently from the highest observed rate in 1994 (rate 19 per 100 person-years) to its lowest in 2013 (rate 3 per 100 person-years). There were two peaks in which an AIDS-related cause was the main cause of mortality (over 80% of cases); the first peak was between 1994 and 1996 and the second one was between 2000 and 2003. Since 2005, although the number of mortality cases has been somewhat constant, the deaths attributed to an AIDS-related cause have been declining (p-value equals to zero for the trend in the proportion deaths attributed to an ADI), that is from 150 out of 205 (73%; rate 2 per 100 person-years) in 2005 to 44 out of 218 (20%; rate 0·6 per 100 person-years) in 2013. Note that in the same period, the proportion of deaths attributed to non-AIDS related causes has steadily increased.
Figure 2.
Deaths from AIDS and other causes in British Columbia from January 1st, 1981 to December 31 st, 2013.
Discussion
In BC, since the inception of HAART, we observed that the number of individuals developing an ADI and dying of AIDS has decreased dramatically to its lowest level in 2013. Current ADI rates are comparable to those in the early 80’s, at the very beginning of the AIDS epidemic in BC.
We observed two peaks in AIDS-related mortality. The first one between 1994 and 1997, which can be readily explained as part of the pre-HAART era (i.e., less potent regimens). The second one, between 2000 and 2003, can be attributable to the contemporary change in guidelines favoring deferral of HAART initiation,20 as well as the high prevalence of structured treatment interruptions, which came to an end with the results of the SMART trial.21 New guidelines published since 2004 strongly recommended against these practices, and this remains the case at the present time.3,22,23
We showed that males consistently had the largest number of ADIs. There was also an interaction of age and calendar year, since until 2003 the age group with the highest incidence of ADIs was the 30–39 years, and since 2004 the age groups 40–49 and ≥50 years started to have the highest incidence of ADIs. It is important to mention that only in 2013 the number of ADIs in the age group ≥50 years surpassed that of all other groups. We should also mention that given the small numbers, it is important to exercise caution to infer that this trend will continue in the future. We have also shown that out of 22 ADIs considered, only PCP maintained its prominent ranking (albeit with much reduced overall prevalence) with relative increases in the ranking of NHL (including Burkitt's, or immunoblastic, or other lymphomas), WASTING and REC_PNEUM, especially since 2005. These results are consistent with those published in other settings.24–28
Our results strengthen the evidence that programs that aggressively distribute free HAART to infected individuals have a great potential to markedly decrease morbidity and mortality caused by AIDS.29 Our data supports the proposition that expanded access to HAART, in the presence of evidence-based guidelines and systems to ensure early HIV diagnosis, timely HAART initiation and sustained virologic response, can transform AIDS from an epidemic to an endemic disease, and eventually may lead to the elimination of AIDS. However, given that there is no curative therapy available and that life-long suppressive HAART requires high levels of adherence, in order for this effect to be sustained, HAART programs and related support must be strengthened on a long-term basis.
In any setting, the emergence of ADIs and AIDS-related mortality depend on how comprehensive HIV testing is among the population at risk, how effective linkage and retention in care structures are, how accessible HAART is to those in need, and on which strategies to promote adherence and viral suppression are in place. In 2014, we published the HIV continuum of care for BC and a longitudinal ecological study showing the population-level effectiveness and sustainability of a strategy to enhance HIV case finding and to expand the number of HIV-positive individuals accessing and being retained in care, and aimed at reducing the morbidity and mortality associated with HIV/AIDS in BC.7,15,30 In these studies we demonstrated significant improvements in the engagement of individuals along the HIV continuum of care and in treatment outcomes. However, we also identified that there still exists gaps related to HIV testing and retention in care in the province. Therefore, we believe that the results in this paper provide further evidence of the positive effect that HAART can have in reducing the incidence of ADIs and AIDS-related mortality. However, it also highlights that there is room for improvement since the number of AIDS cases is still not zero.
Our findings are relevant within the context of the ongoing optimism regarding the End of AIDS. However, this has been used rather liberally in various media, and warrants further exploration.5,11,12,31–34 It should be emphasized that ending AIDS and HIV represents two different issues, with substantially different levels of complexity. Our report specifically analyzed AIDS incidence rather than HIV. Ending AIDS could be achieved with the identification of all HIV infected individuals in a given jurisdiction before they develop AIDS, and intervening with effective HAART on a sustained basis. Ending HIV would imply stopping HIV transmission, which in all likelihood would not be feasible without a cure and or a vaccine, or both. Additionally, it is important to clarify the definitions for disease control, elimination, eradication and extinction and please refer to the report from the Dahlem Workshop for a formal definition of each of these epidemic outcomes.35 Based on the definitions in this report, our results demonstrate that HAART can lead to AIDS control. Whether this is truly possible with currently available bio-behavioural strategies remains to be demonstrated.
There are some limitations in this study. First, it was based on an ecological study design, and therefore, our results cannot be taken as definitive proof of causality. Second, mandatory (nominal or non-nominal) reporting was implemented in the BC since 2003, which resulted in better case ascertainment. Third, the ADI reporting relies on a passive surveillance system, and although we conducted multiple data linkages to minimize this source of bias, there is still an expected reporting delay to receive notification of ADIs in some of our sources of data, and the potential bias for underreporting. In order to reduce the effect of this potential bias, we restricted the analysis to the end of 2013. Fourth, due to data constraints, we were not able to ascertain whether the mortality cases were AIDS-related prior to 1992. Finally, estimating trends in ADIs and ADI-related mortality in some population groups (e.g. by risk category) was not possible due to data incompleteness.
In conclusion, we have shown that the expanded HAART coverage has led to the control of AIDS and AIDS-related mortality in BC. These results are encouraging since they provide strong evidence that integrated comprehensive free programs that facilitate testing, and deliver treatment and care to this population can be effective in markedly decreasing AIDS-related morbidity and mortality, thus suggesting that controlling and eventually ending AIDS may be possible.
Supplementary Material
Panel: Research in context.
Systematic Review
We reviewed articles and reports about the end of the AIDS epidemic in different countries. These articles were obtained through a search using PubMed using the individual keywords and their combination including: “AIDS”, “acquired immunodeficiency syndrome”, “end of AIDS”, “epidemic”, “trends”, “AIDS-related deaths”, “ADI”, and “mortality”. We also looked at related citations of each paper that was relevant to this work. Reports were obtained through a search using the Google search engine for documents containing the same keywords as described above.
Interpretation
Diminishing the impact of AIDS amongst those living with HIV will require not only lifetime access to HAART, but also a combination of different strategies to minimize leakage along the HIV continuum of care. This study was conducted in an environment where different strategies along the HIV continuum of care are in place to minimize both morbidity and mortality associated with HIV/AIDS. Our results showed that although ADI incidence and AIDS-related mortality have been steadily decreasing over time, in 2013 we still had 84 new cases of ADIs (most due to PCP; incidence rate 1.2 per 100 person-years), and 44 AIDS-related deaths (rate 0·6 per 100 person-years). Thus, this study provides further evidence that controlling the AIDS epidemic is possible and there are still challenges ahead to reach the goal of an AIDS-free generation. It also highlights the importance of securing a long-term commitment from the government and different stakeholders in the healthcare system to provide timely and adequate care to this population.
Acknowledgements
JSGM is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01DA036307).VDL is funded by a grant from the US National Institute on Drug Abuse (R03DA033851), by a grant from the Canadian Institutes of Health Research (MOP-125948) and by a Scholar Award from the Michael Institute for Health Research and a New Investigator award from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
VDL, and JSGM conceived and designed the study. BY extracted the data. VDL analysed the data. VDL drafted the report. All authors revised drafts and approved the final version.
Conflict of interest
We have the following conflicts of interest: JSGM has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. VDL has received limited unrestricted funding, paid to her institution, from GlaxoSmithKline. The remaining authors do not have conflicts to declare.
REFERENCES
- 1.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA : the journal of the American Medical Association. 1996;276(2):146–154. [PubMed] [Google Scholar]
- 2.Lima VD, Le A, Nosyk B, et al. Development and validation of a composite programmatic assessment tool for HIV therapy. PloS one. 2012;7(11):e47859. doi: 10.1371/journal.pone.0047859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA : the journal of the American Medical Association. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 4.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS one. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2013 [Online] Geneva: UNAIDS; 2013. [cited 2014 September 1]. [updated 2013 November;]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 6.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. The Lancet infectious diseases. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montaner JS, Lima VD, Harrigan PR, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the "HIV Treatment as Prevention" experience in a Canadian setting. PloS one. 2014;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havlir D, Beyrer C. The beginning of the end of AIDS? The New England journal of medicine. 2012;367(8):685–687. doi: 10.1056/NEJMp1207138. [DOI] [PubMed] [Google Scholar]
- 12.The Lancet. The beginning of the end of AIDS? Lancet. 2012;380(9858):1967. doi: 10.1016/S0140-6736(12)62137-0. [DOI] [PubMed] [Google Scholar]
- 13.ONE. The Beginning of The end? Tracking Global Commitments on AIDS Volume 2 [Online] ONE; 2013. [cited 2014 September 1]. [updated 2013 November 25;].Available from: http://www.one.org/international/policy/the-beginning-of-the-end-the-2013-one-aids-report/?source=blogUSDC102611262013. [Google Scholar]
- 14.British Columbia Centre for Disease Control (BCCDC) HIV in British Columbia: Annual Surveillance Report 2012 [Online] BCCDC; 2013. [cited 2014 September 1]. Available from: http://www.bccdc.ca/util/about/annreport/default.htm. [Google Scholar]
- 15.Lourenco L, Lima VD, Heath K, et al. Process monitoring of an HIV treatment as prevention program in British Columbia, Canada. Journal of acquired immune deficiency syndromes. 2014;67(3):e94–e109. doi: 10.1097/QAI.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider E, Whitmore S, Glynn KM, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years--United States, 2008. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2008;57(RR-10):1–12. [PubMed] [Google Scholar]
- 17.Cameron AC, Trivedi PK. Regression analysis of count data. UK: Cambridge University Press; 1998. [Google Scholar]
- 18.Cameron AC, Trivedi PK. Microeconometrics: Methods and Applications. UK: Cambridge University Press; 2005. [Google Scholar]
- 19.Wood SN. An Introduction with R. US: Chapman & Hall/CRC; 2006. Generalized Additive Models. [Google Scholar]
- 20.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA : the journal of the American Medical Association. 2000;283(3):381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 21.Strategies for Management of Antiretroviral Therapy Study Group. El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. The New England journal of medicine. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health and Human Services (DHHS) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Online] United States: DHHS; 2014. [cited 2014 September 1]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 23.World Health Organization (WHO) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach June 2013 [Online] Geneva: WHO; 2013. [cited 2014 September 1]. Available from: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed] [Google Scholar]
- 24.Buchacz K, Baker RK, Palella FJ, Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. Aids. 2010;24(10):1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 25.Cobucci RN, Lima PH, de Souza PC, et al. Assessing the impact of HAART on the incidence of defining and non-defining AIDS cancers among patients with HIV/AIDS: A systematic review. Journal of infection and public health. 2014 doi: 10.1016/j.jiph.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Fenner L, Reid SE, Fox MP, et al. Tuberculosis and the risk of opportunistic infections and cancers in HIV-infected patients starting ART in Southern Africa. Tropical medicine & international health : TM & IH. 2013;18(2):194–198. doi: 10.1111/tmi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. Aids. 2013;27(4):597–605. doi: 10.1097/QAD.0b013e32835b0fa2. [DOI] [PubMed] [Google Scholar]
- 28.Grinsztejn B, Luz PM, Pacheco AG, et al. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: shifting from AIDS to non-AIDS related conditions in the HAART era. PloS one. 2013;8(4):e59768. doi: 10.1371/journal.pone.0059768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones A, Cremin I, Abdullah F, et al. Transformation of HIV from pandemic to low-endemic levels: a public health approach to combination prevention. Lancet. 2014 doi: 10.1016/S0140-6736(13)62230-8. [DOI] [PubMed] [Google Scholar]
- 30.Nosyk B, Montaner JS, Colley G, et al. The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. The Lancet infectious diseases. 2014;14(1):40–49. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joint United Nations Programme on HIV/AIDS (UNAIDS) Fast track: Ending the AIDS epidemic by 2030 [Online] Geneva: UNAIDS; 2014. [cited 2014 September 1]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/20140925_Fast_Track_Brochure.pdf, 2014. [Google Scholar]
- 32.Joint United Nations Programme on HIV/AIDS (UNAIDS) Fast-Track - Ending the AIDS epidemic by 2030[Online] Geneva: UNAIDS; 2014. [cited 2014 September 1]. Available from: http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. [Google Scholar]
- 33.San Francisco AIDS Foundation. The end of AIDS is within our grasp [Online] San Francisco: San Francisco AIDS Foundation; 2014. [cited 2014 September 1]. Available from: http://sfaf.org/hiv-info/hot-topics/from-the-experts/end-of-aids-is-within-our-grasp.html. [Google Scholar]
- 34.The Economist. The 20th International AIDS Conference: Is the end in sight? [Online] Melbourne: The Economist; 2014. [cited 2014 September 1]. Available from: http://www.economist.com/node/21608568/print. [Google Scholar]
- 35.Dowdle WR. The Principles of Disease Elimination and Eradication. Morbidity and Mortality Weekly Report (MMWR) 1999;48(SU01):23–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.