Abstract
Multiwalled carbon nanotube (MWCNT) length is suggested to critically determine their pulmonary toxicity. This stems from in vitro and in vivo rodent studies and in vitro human studies using cell lines (typically cancerous). There is little data using primary human lung cells. We addressed this knowledge gap, using highly relevant, primary human alveolar cell models exposed to precisely synthesized and thoroughly characterized MWCNTs. In this work, transformed human alveolar type-I-like epithelial cells (TT1), primary human alveolar type-II epithelial cells (ATII) and alveolar macrophages (AM) were treated with increasing concentrations of MWCNTs before measuring cytotoxicity, inflammatory mediator release and MAP kinase signalling. Strikingly, we observed that short MWCNTs (~0.6 µm in length) induced significantly greater responses from the epithelial cells, whilst AM were particularly susceptible to long MWCNTs (~20 µm). These differences in the pattern of mediator release were associated with alternative profiles of JNK, p38 and ERK1/2 MAP kinase signal transduction within each cell type. This study, using highly relevant target human alveolar cells and well defined and characterized MWCNTs, shows marked cellular responses to the MWCNTs that vary according to the target cell type, as well as the aspect ratio of the MWCNT.
1. Introduction
Carbon nanotubes (CNT) are receiving ever-increasing attention due to their unique structural characteristics offering high strength to weight ratios, improved conductivity and thermal stability e.g. CNT fillers in polymers result in CNT/polymer composites with significantly improved thermal or electrical conductivity compared to the polymer alone [1,2]. Typically occurring with diameters in the 10 – 100 nm range and with lengths often extending much greater than 1 µm, CNTs are being applied in areas such as electronics, automotive, aerospace and medical industries [3]. The application of these materials to consumer products is certain to expand significantly and the risk of exposure to humans is therefore also set to significantly increase. Human exposure to CNTs may occur at the initial synthesis stage of the material, during the industrial application and incorporation of the material into products and subsequently throughout the life cycle of the CNT composite material/products. The biological reactivity of CNTs is likely to depend on their physicochemical properties, including their structure (single-walled or multi-walled; SWCNT or MWCNT respectively), their length, diameter, surface charge, aggregative state and synthesis catalyst impurities.
The inhalation of some engineered nanomaterials, such as MWCNTs, is potentially a significant human health concern. Evidence for this from existing studies shows the adverse effects of inhaled air pollution particles (which contains an ultrafine component that is analogous in size to engineered nanomaterials) on the cardiopulmonary system [4]. A large portion of inhaled nano-sized particles deposit in the alveolar unit [5] where the active gas-blood interface has a tight-junctional cellular barrier composed of type-I (ATI) and type-II (ATII) epithelial cells abutting the capillary endothelial cells for effective gas exchange. Although there are approximately equal numbers of ATI and ATII cells within the alveolar unit, the thin, attenuated ATI epithelial cells cover 95% of the alveolar surface [6]. Cuboidal ATII cells synthesise, secrete and recycle surfactant, modulate both fluid balance and host defense and can terminally differentiate into ATI cells, where gas exchange occurs by diffusion. Resident alveolar macrophages (AM) police the airspaces, removing inhaled particles, pathogens and cellular debris.
In early MWCNT studies by Shvedova et al (mouse pharyngeal aspiration exposure to SWCNTs) and Muller et al (rat intratracheal instillation exposure to MWCNTs), MWCNTs were found to persist in the lung for up to 60 days post exposure, elicit dose-dependent increases in lung cell inflammation, stimulate oxidative stress markers in lung tissue and induce early onset of lung fibrosis [7,8]. Significantly, the biopersistent, fibrous nature of MWCNTs renders them somewhat comparable to the well-studied lung toxicant, asbestos. Evidence suggests that long, thin, needle-like and biopersistent fibers (e.g. amphibole asbestos) are involved in the development of pulmonary mesothelioma (an incurable cancer largely due to asbestos exposure), while both long and short asbestos fibers contribute to fibrosis and the development of asbestosis (a chronic inflammatory and fibrotic pathology) [9].
MWCNT length may play a significant role in the nature of any MWCNT-induced lung cell bioreactivity. Indeed, recent studies by Mühlfeld et al and Murphy et al have shown that the MWCNT length can determine the inflammatory responses and morphological characteristics of mouse lung parenchyma post treatment, while MWCNT length is also critical in THP-1-derived macrophage activation and subsequent pro-inflammatory responses from adjacent Met5A mesothelial cells [10–12]. While these studies highlight MWCNT length as a potential determinant of pulmonary toxicity, significant challenges in precisely synthesising MWCNTs, so that just one physicochemical property is changed while all others remain identical, make it difficult to determine the role of a single, specific physicochemical property in bioreactivity of MWCNTs.
Furthermore, while the ever-expanding research effort into MWCNT pulmonary toxicity is beginning to reveal some important mechanisms by which these materials may induce cellular bioreactivity in the lung (recently reviewed by Donaldson et al [13]), there is a paucity of experimental data using primary human lung cells. This is a critical knowledge gap, as it is well known, for example, that cellular processes and mediators differ between species and thus the relevance of some in vitro screening studies, which utilize non-human material and cell lines, is unclear. There is therefore a crucial need for more studies of relevant human pulmonary cell models. Additionally, human ATI epithelial cells remain very poorly studied, despite their significant relevance in nanomaterial deposition in the respiratory units after inhalation of nanosized materials. Therefore, in this study, we chose to study the bioreactivity of MWCNT length using highly relevant primary human ATII and AMs (from the same subjects) and human alveolar type-I-like epithelial cell line (TT1) generated in-house [14]. We hypothesized that three thoroughly-characterised MWCNTs formats (each with a different length but, importantly, maintaining a comparable diameter, surface charge and carbon purity i.e. % atomic carbon) would exhibit adverse effects on primary human alveolar epithelium and alveolar macrophages, such that the long MWCNTs, with similar lengths to those of amphibole asbestos implicated in the development of mesothelioma, would exert the most deleterious response, whilst shorter MWCNTs would be less bioreactive.
2. Experimental
2.1 Physicochemical characterisation of carbon nanotubes
Non-functionalised, MWCNTs (synthesised by chemical vapor deposition), denoted MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm, were purchased from Nanothinx, Greece. MWCNT surface charge (as zeta potential) was determined by zeta-meter using a Zetasizer Nano by Malvern Instruments (Worcestershire, UK). All MWCNT samples were suspended in deionised water and DCCM-1 culture medium to determine any alteration of physiochemical characteristics. The respective length and diameter distribution of each MWCNT sample was determined by transmission electron microscopy using a Hitachi 7100 TEM, while the respective agglomerative state of each MWCNT format was determined by scanning electron microscopy using a Carl Zeiss Ultra Plus Field emission SEM. The suspension stability was estimated by zeta potential measurements, using Malvern ZetaSizer Nano. Elemental mapping, to determine the nature and degree of MWCNT impurities, was also performed using a Carl Zeiss Ultra Plus Field emission SEM in energy-dispersive mode for X-ray spectroscopy (EDX). All three MWCNT samples were assayed for endotoxin contamination, using a Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, UK) and all three were below the 0.1 EU/mL limit of detection. BET surface area measurements were performed on Beckman Coulter SA3100 (Beckman Coulter Inc., USA).
2.2 Ethics Statement
The human lung tissue used in this study was surplus tissue obtained following resection for lung carcinoma. Written informed consent was obtained for all samples and the study was carried out with the approval of the Royal Brompton and Harefield Ethical Committee (Ref: 08/H0708/73).
2.3 Cell models
Primary human ATII cells and AMs were isolated from human lung of grossly normal appearance obtained following resection for lung carcinoma, as described by Thorley et al [15]. Cells were isolated from a minimum of three subjects per experiment, with a total of fifteen subjects used in this study. Briefly, lung tissue sections were perfused by injection of 0.15 M sterile sodium chloride saline solution until the draining lavage ran clear and the cell count was < 1×104 cells/ml. The saline perfusate was collected and centrifuged at 1300rpm for 10 minutes at 20°C. The cell pellet was re-suspended in serum-free RPMI cell culture medium containing 1% penicillin/streptomycin/L-glutamine (PSG) and plated on 96 well culture plates at 0.2 ×106 AMs/well. After AM adherence (approximately three hours), culture medium was removed and non-adherent cells were removed by phosphate buffered saline (PBS) washing. AM cultures were then maintained in serum-free RPMI cell culture medium containing 1% PSG.
The tissue was then prepared for ATII cell isolation by perfusing with trypsin (0.25% in Hanks balanced salt solution) and incubating at 37°C for 45 minutes; trypsin was replaced twice during the incubation period. The tissue was then finely chopped in the presence of newborn calf serum (NCS), shaken for five minutes with DNase (250 µg/ml) at room temperature and passed through a 400 µm filter followed by a 40 µm filter to remove large tissue debris. The cell-containing filtrate was then centrifuged at 1300 rpm for 10 minutes and the resulting pellet was re-suspended in DCCM-1 culture medium containing 50 µg/ml DNase. Cells were then incubated in tissue culture flasks for two hours (humidified incubator, 5% CO2/95% air at 37°C) to allow differential adherence and removal of contaminating mononuclear cells. Non-adherent ATII cells were removed after 2 hours and the cell suspension was centrifuged at 1300 rpm for 10 minutes. The resulting pellet was re-suspended in a DCCM-1 culture medium containing 10% NCS and 1% PSG and cells were seeded on 96 well culture plates at 0.1 ×106 cells/well. The 96 well culture plates were previously coated with 50 µl of 1% PureCol collagen solution (Leimuiden, Netherlands; Type I collagen) and allowed to air dry. Cells reached confluence after 48 hours. These cells have been thoroughly characterised using electron microscopy, which shows that the cells are cuboidal in morphology, have surfactant-containing lamellar bodies, tight junctions and microvilli. Furthermore they stain positively for the ATII cell marker alkaline phosphatase and express surfactant proteins A and C and maintain their phenotype for up to six days [16,17].
This laboratory has previously immortalised human ATI-like cells (derived from freshly isolated and cultured primary human ATII cells) using transduction with the catalytic subunit of telomerase (human telomerase reverse transcriptase; hTERT) and a temperature sensitive mutant of simian virus 40 large-tumour antigen [14]. These ATI-like (transformed type-I-like; TT1) cells, the first human ATI-like cell line to be produced, are negative for the ATII cell markers SP-C, alkaline phosphatase and thyroid transcription factor-1. Moreover, TT1s do not contain lamellar bodies. They display a thin, attenuated morphology containing vesicles, being caveolae positive. TT1 cells were cultured to confluence in 96-well culture plates using DCCM-1 tissue culture medium with 10 % FCS new-born calf serum, 0.05% G418 and 10% PSG. An initial cell seeding of 0.1 × 106/well yielded a confluent monolayer after 48 hours. Growth medium was replaced with serum-free DCCM-1 24 hours prior to MWCNT exposure. Cells were incubated in humidified 5% CO2/95% air at 37°C.
TT1 and ATII cells possess the ability to spread and divide, unlike AMs. Consequently, the final cell numbers in confluent TT1 and ATII monolayers could not be determined from the initial number of seeded cells. Similarly, some of the plated AMs may not adhere. Such a potential discrepancy in seeded and actual final cell numbers, when significant, would influence the determination cytokine and chemokine activity when comparing responses between cell types. Confluent monolayers of each cell type were therefore imaged under light microscope (100× magnification) and counted manually. We determined that final TT1, ATII and AM cell numbers per well were comparable (data not shown).
2.4 Treatment of TT1 cells, ATII cells and AMs with MWCNTs
Respective MWCNT format doses were prepared in fresh serum-free DCCM-1 or RPMI culture medium for subsequent TT1 cell, ATII and AM exposure. It was favorable to freshly prepare the MWCNT doses immediately prior to cell exposure. Each MWCNT dose preparation was sonicated in a sonicating water bath (135W, 42 kHz; VWR) for 30 seconds prior to cell culture exposure.
2.5 Choice of dose metric
The choice of dose metric in nanomaterial toxicology studies (e.g. particle mass, particle number, particle surface area) is a contentious issue [18–21]. While we appreciate that there are relative merits to each of these, we have chosen to present our data with a mass dose metric, using a wide concentration range; this facilitates a useful comparison of our study with similar in vitro studies evaluating MWCNT length in cellular reactivity and where mass is used as a dose metric (e.g. [12,22,23]).
2.6 Measurement of cell viability
The effect of each of the three MWCNT formats on AM, TT1 and ATII cell viability, at 24 hours, was assessed using an MTT and an LDH assay of respective cell cultures. Briefly, post MWCNT exposure, culture medium was removed to a new 96 well plate for lactate dehydrogenase (LDH) analysis. This plate was centrifuged at 3000 rpm for 20 minutes at 4°C to remove any MWCNTs that might have interfered with subsequent optical absorbance readings. The supernatant was assayed using the LDH assay (Roche Diagnostics, Germany) according to the manufacturer's protocol. For the MTT assay, treated cells were washed three times with PBS to remove residual MWCNT particles. Cells were then incubated with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) prepared at 50 µg/ml in fresh serum-free DCCM-1 or RPMI culture medium, for 2 hours at 37°C. The culture medium/MTT solution was then removed and replaced with 200 µl DMSO. After gently pipetting this solution up and down to aid solubilisation of mitochondria associated formazan, the contents of each well were transferred to a corresponding 96 well (V bottomed) centrifuging plate. This plate was then centrifuged at 3000 rpm for 20 minutes at 4°C to remove any MWCNTs that might have interfered with subsequent optical absorbance readings. Supernatant absorbance at 570nm was measured in a SPECTRA Fluor Plus microplate spectrophotometer (TECAN, Italy). Cell viability of treated cells was calculated as a percentage of the vehicle control (cell culture medium). Exposures for MTT and LDH assays were repeated in triplicate using three separate TT1 cell passages and matched ATII cells and AMs from four subjects. We have established that the MWCNTs used in this study do not interfere with the solubilisation of formazan and therefore do not interfere with the MTT cell viability assay (data not shown).
2.7 Measurement of cytokines and chemokines by ELISA
Exposures were repeated in triplicate using three separate TT1 cell passages and ATII cells and AMs from four subjects. Mediator-conditioned medium from MWCNT-exposed TT1 cell, ATII cell and AM cultures was assayed for concentrations of inflammatory cytokines (human IL-6) and inflammatory chemokines (human IL-8 and MCP-1) using sandwich enzyme-linked immunosorbent assays (ELISA). The assays were performed using DuoSet® antibody kits. The manufacturer’s directions (R&D systems, USA) were followed.
2.8 Inhibition of the MAP kinases JNK, p38 and ERK 1/2 in TT1 cells, ATII cells and AMs
To assess the involvement of individual MAP kinases in MWCNT-mediated inflammatory mediator secretion, cultures of TT1 cells, ATII cells and AMs were incubated for 30 minutes with 10 µM of commercially available inhibitors to: p38 (SB203580), ERK 1/2 (PD98059) (New England Biolabs, Hitchin UK) and JNK (SP600125) (Sigma-Aldrich, Poole UK), prior to exposure to the 50 µg/ml of MWCNT-0.6 µm, MWCNT-3 µm or MWCNT-20 µm formats for 24 hours. Exposures were repeated in triplicate using three separate TT1 cell passages and ATII cells and AMs from four subjects. Mediator-conditioned medium, from each of the MAP-kinase-inhibition exposure conditions, was assayed for concentrations of the inflammatory mediators human IL-6, IL-8 and MCP-1 using ELISA as previously described. Western blot analysis confirmed successful inhibition of respective MAP kinases in all three cell types (data not shown).
2.9 Statistical Analysis
Data from MTT, LDH, ELISA, and MAP kinase experiments is presented as the mean ± standard error (where three independent experiments were performed using three separate TT1 passage generations and where four independent experiments were performed using four separate donor tissues for ATII and AM studies). Significant differences in MWCNT (format/dose) treatment on cell viability, cytokine/chemokine release and significant effects of MAP kinase inhibition on cellular responses were determined using a parametric one-way analysis of variance with Bonferroni comparisons (for TT1 cell line experiments) and using a non-parametric Kruskal–Wallis test followed by a Mann–Whitney test where appropriate (for ATII and AM primary cell experiments). In all analyses, a P value <0.05 was considered significant.
3. Results and Discussion
3.1 Physicochemical properties of selected carbon nanotubes
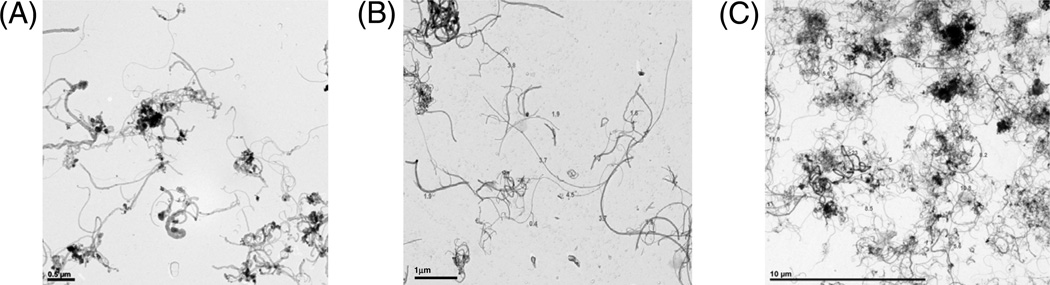
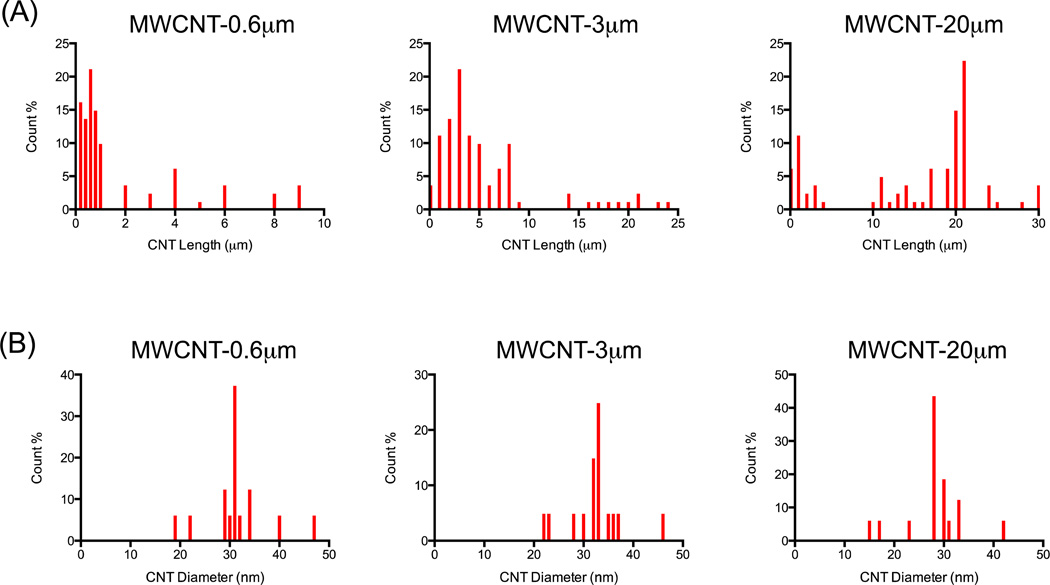
Transmission electron microscopy (TEM) characterisation of the three non-functionalised MWCNT formats, MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm (as labeled by the manufacturer, Nanothinx Greece, and as denoted throughout the article), revealed MWCNTs with median lengths of 1.1 µm (0.23 – 9.7 µm), 4.3 µm (0.5 – 24.4 µm) and 19.3 µm (0.6 – 30.8 µm) respectively (length ranges in parenthesis). Each MWCNT format had comparable median diameters (ranges in parenthesis): 30.6 nm (19.5 – 47.6 nm), 33.3 nm (22.6 – 45.8 nm) and 27.8 nm (15.6 – 41.9 nm) for MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm respectively (Figures 1 and 2). Brunauer, Emmett and Teller (BET) measurements indicated that MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm had surface areas of 255.24, 213.64 and 204.8 m2/g respectively. Personal communication with the manufacturer revealed that the 95% purity of MWCNT-20 µm is achieved “as synthesised” with no post-synthesis chemical purification treatment. The manufacturer also detailed that MWCNT-20 µm was shortened using a proprietary sonication method to produce MWCNT-0.6 µm and MWCNT-3 µm (although the manufacturer did not disclose the sonication medium, the comparable surface area and relatively low oxygen variation among samples suggests minimal surface modification).
Figure 1.
TEM micrographs of (A) MWCNT-0.6 µm, (B) MWCNT-3 µm and (C) MWCNT-20 µm.
Figure 2.
Length and diameter distribution of MWCNTs used in the present study. Length distributions were characterised for each MWCNT format, based on a total of 100 counts per format (A). Diameter distributions were characterised for each MWCNT format, based on a total of 100 counts per format (B).
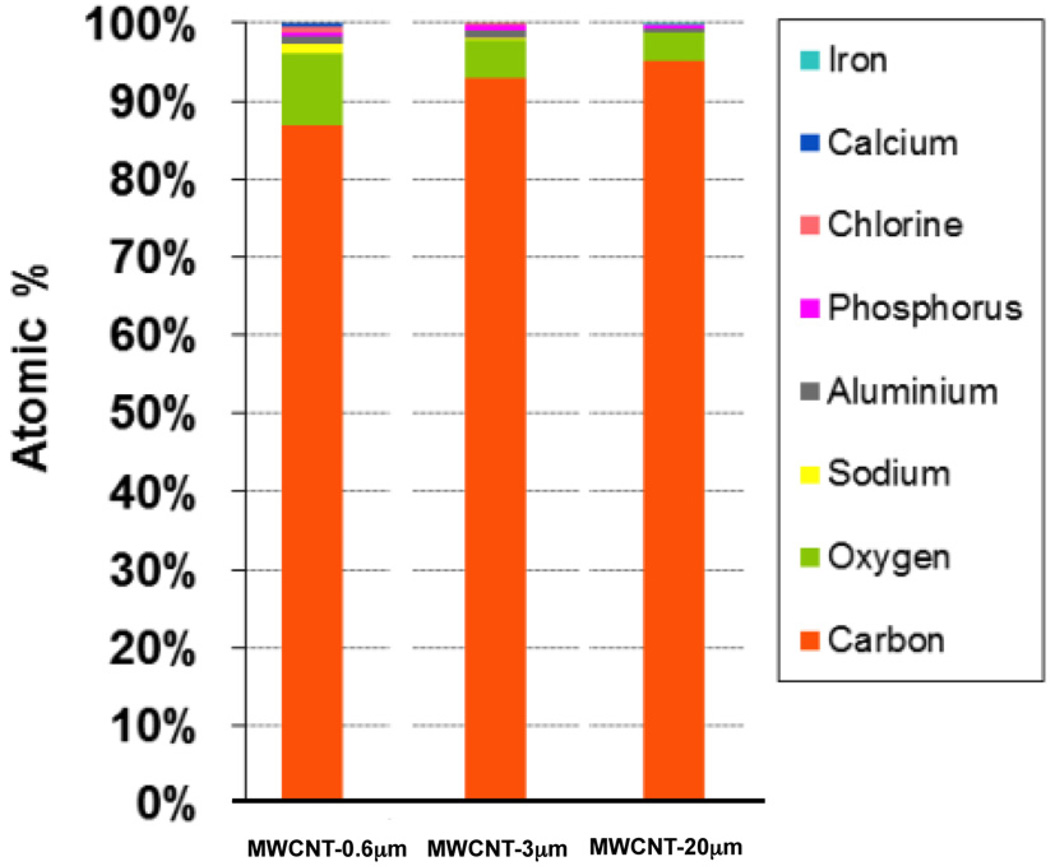
Each MWCNT format had reasonably comparable carbon purities (i.e the % atomic carbon) of 87, 93 and 95% for MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm respectively as determined by EDX spectroscopy (Figure 3). While we did observe a difference in oxygen content between the MWCNTs, the atomic % variation on oxygen content was below 10% for all MWCNTs, as determined by EDX spectroscopy. The metal synthesis catalysts for these MWCNTs was not disclosed by the manufacturer, however we believe these are likely to be aluminum (accounting for 0.6, 0.5 and 0.3% of total atomic content for MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm respectively) and iron (<0.1% for any of the MWCNT formats), as determined by EDX spectroscopy. The surface charge of all three MWCNTs was also comparable at -13.2, -15.7 and -12 mV for MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm respectively. A summary of the physicochemical characterisation in non-biological medium (deionised water) and DCCM-1 cell culture medium, for all three MWCNT formats, is shown in Table 1.
Figure 3.
Measurement of MWCNT purity. The elemental composition of each MWCNT format was analysed by EDX spectroscopy, the observations of which are presented here using atomic % representation.
Table 1.
Characterisation of MWCNTs.
| CNT format* | Length# (TEM) |
Diameter# (TEM) |
Surface Area (BET) |
Carbon purity (EDX) |
Zeta Potential (Zetasizer Nano) |
|---|---|---|---|---|---|
| MWCNT-0.6 µm in DW | 1.1 µm (0.23 – 9.7 µm) | 30.6 nm (19.5 – 47.6 nm) | 255.24 m2/g | 87% | −19.0 mV |
| MWCNT-0.6 µm in DCCM-1 | NA | NA | NA | NA | −13.2 mV |
| MWCNT-3 µm in DW | 4.3 µm (0.5 – 24.4 µm) | 33.3 nm (22.6 – 45.8 nm) | 213.64 m2/g | 93% | −13.4 mV |
| MWCNT-3 µm in DCCM-1 | NA | NA | NA | NA | −15.7 mV |
| MWCNT-20 µm in DW | 19.3 µm (0.6 – 30.8 µm) | 27.8 nm (15.6 – 41.9 nm) | 204.8 m2/g | 95% | −18.7 mV |
| MWCNT-20 µm in DCCM-1 | NA | NA | NA | NA | −12.0 mV |
DW: Deionised water
DCCM-1: Cell culture medium
TEM: Transmission electron microscopy
BET: Brunauer, Emmett and Teller analysis
EDX: Energy-dispersive x-ray spectroscopy
NA: Not applicable
There was no physical difference between MWCNTs suspended in DW or DCCM-1
Means were calculated from lengths with count frequencies greater than 5% of the total counts
The nominal length is that is provided by the manufacturer and is that used throughout the article.
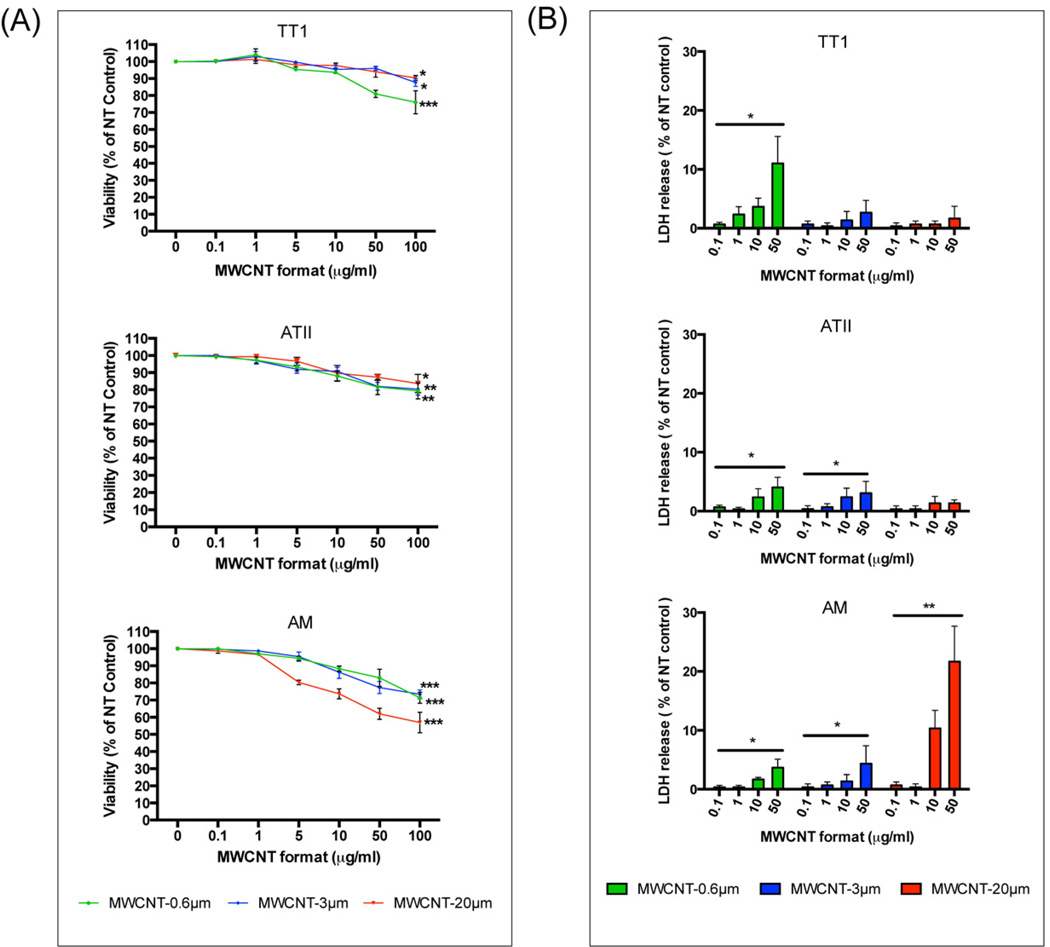
3.2 Cell viability
The effect of MWCNT length on TT1, ATII and AM cell viability (from 0.1 to 100 µg/ml) was assessed over a 24-hour exposure period (Figure 4; viability was also assessed at 4 hours, however no significant changes were observed; data not shown). Because certain MWCNTs have been shown to interfere in some cell viability assays [24], we performed two experimentally distinct assays (MTT and LDH assays) to validate our cell viability conclusions. In the MTT assay, TT1 cell viability remained unchanged up to 10 µg/ml, regardless of MWCNT length (Figure 4A). Small but significant decreases in TT1 cell viability (up to 20%) did occur at higher MWCNT doses (50 and 100 µg/ml), however these doses are unlikely to be physiologically relevant to human health. As with TT1 cells, ATII cell viability remained unchanged for all MWCNT format exposures at low doses that are more likely to be physiologically relevant (up to 10 µg/ml; Figure 4A), only falling at the highest doses, by 15–20%. Of all three lung cell types, AMs displayed the greatest reduction in cell viability following treatment with MWCNTs of all test lengths (Figure 4A). Even at the relatively low dose of 5 µg/ml, MWCNT-20 µm caused 20% AM cell death (P < 0.05). Striking decreases in AM viability were found following exposure to higher doses of MWCNT-20 µm (up to 40%). This may be due to the primarily phagocytic nature of AMs, rendering them vulnerable to complicated interactions with MWCNTs with a high aspect ratio equal to, or greater than their own diameter. While beyond the aims of the present study, this phenomenon, termed ‘frustrated phagocytosis’, has been observed by Murphy et al following treatment of THP-1 macrophage cell line with high aspect ratio MWCNTs; MWCNTs of 13 – 36 µm in length and 85 – 165 nm in diameter stimulated the release of the acute phase inflammatory mediators IL-1β, TNFα, IL-6 and IL-8 from treated THP-1 macrophages while shorter MWCNTs had no such effect [12]. Additionally, using mouse AMs, Hirano et al reported that MWCNTs associate with the plasma membrane of AM and induce cell death by rupturing the cell membrane [25] while Cheng et al have shown that MWCNTs pierce the membranes of human macrophages [26]; this may be one mechanism for the MWCNT-induced AM cell death seen in the present study. The reduction in AM viability in response to shorter MWCNT formats (MWCNT-0.6 µm and MWCNT-3 µm), while still significant at doses from 10 to 100 µg/ml, and relatively greater than that seen in epithelial cells, was significantly less than (~15%) responses seen following exposure to the longer MWCNTs.
Figure 4.
Viability of TT1 and ATII epithelial cells and AMs following 24 hours treatment with MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm, as determined by (A) MTT assay and (B) LDH assay. Data is presented as a % of the untreated control; significance is shown where *P < 0.05, **P < 0.01 ***P < 0.001.
Using the LDH release cytotoxicity assay, the trend in the degree of damage and reduction in cell viability reflected that observed in the MTT assay, where short MWCNTs caused more LDH release from epithelial cells and long MWCNTs caused most release from AMs (Figure 4B). However, the magnitude of cytotoxicity as measured by LDH release was less than that measured using the MTT assay. For example, an MTT-determined 38% reduction in AM cell viability following treatment with 50 µg/ml of MWCNT-20 µm, measured as a 22% reduction when the LDH release assay was used for the same treatment. Nevertheless, the agreement between these two distinct assays, in terms of the trend in cell viability reduction, verifies our cell viability observations.
3.3 Inflammatory cytokine and chemokine release
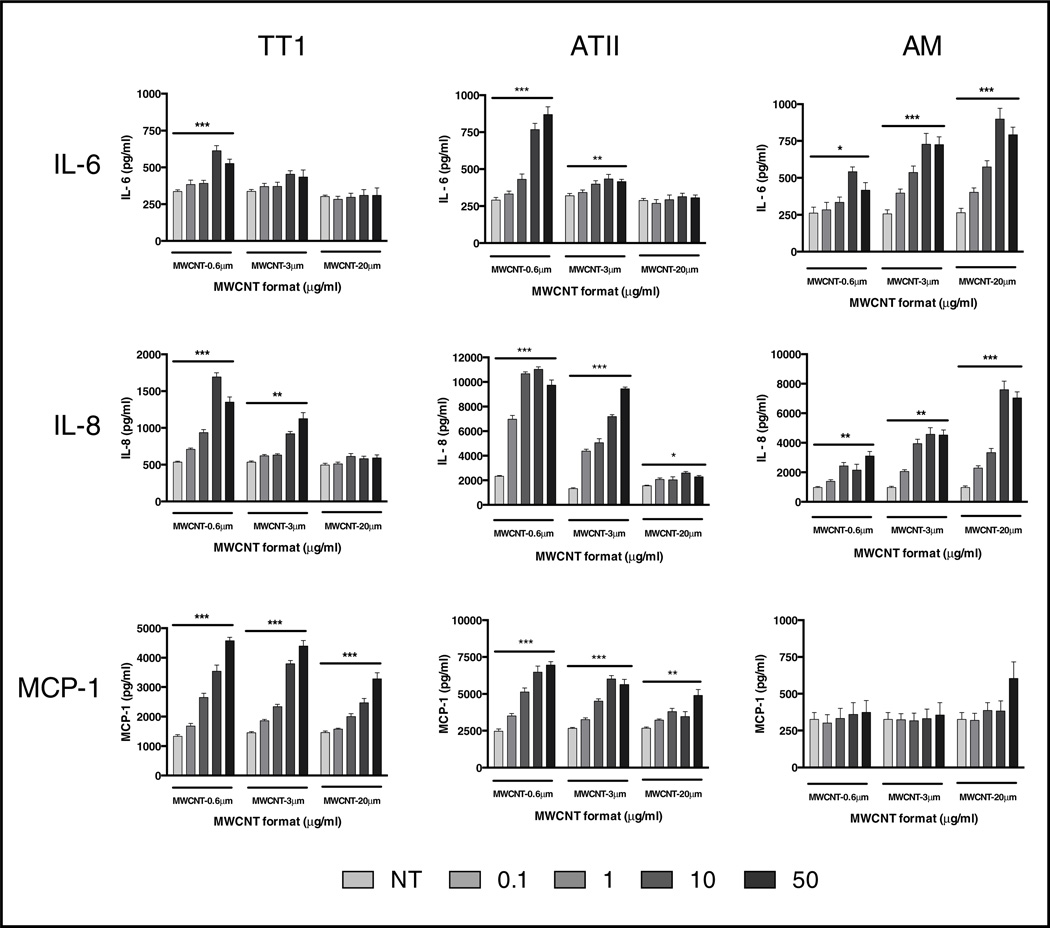
Based on cell viability data, a dose range of 0 – 50 µg/ml was chosen for inflammatory mediator release studies. Following 24-hour treatment (inflammatory mediators were also assessed at 4 hours treatment, however no significant changes were observed; data not shown) we found that differential responses to MWCNT lengths were critically dependent on the cell type being exposed. The TT1 and ATII epithelial cells were significantly more responsive to the shorter MWCNTs (MWCNT-0.6 µm and MWCNT-3 µm) than to the long MWCNT-20 µm formats following 24 hour treatment (Figure 5). For example, 10 µg/ml of MWCNT-0.6 µm induced a significant 4.7-fold increase in interleukin-8 (IL-8) release from ATII cells (P < 0.05) while the same dose of MWCNT-20 µm induced only a 1.6-fold increase in IL-8 release. The profile of ATII IL-8 release, following treatment with MWCNT-3 µm, was similar to that of MWCNT-0.6 µm. Even very low doses, 0.1 and 1.0 µg/ml, of MWCNT-0.6 µm and MWCNT-3 µm (0.1 and 1 µg/ml) were sufficient to evoke significant increases in interleukin-6 (IL-6) and IL-8 release from treated ATII cells e.g. more than a two-fold increase in IL-8 release following MWCNT-0.6 µm treatment at these low doses. TT1 and ATII monocyte chemoattractant protein-1 (MCP-1) release was also increased; again, the greatest increases were seen following treatment with the shortest MWCNTs (Figure 5); at very low doses, where there were striking increases in MCP-1 release by both TT1 and ATII cells. IL-8 and MCP-1 are proinflammatory chemokines, functioning in neutrophil and monocyte attraction to sites of cellular inflammation. Notably, increased release of IL-6 (a pleiotropic cytokine important in inflammation) is associated with the pathogenesis of numerous lung diseases such as asthma [27], chronic obstructive pulmonary disease [28] and idiopathic pulmonary fibrosis [29]. IL-6 has been shown to readily translocate from the lung to systemic circulation upon exposure to particulate matter [30] and it is also a robust predictor of cardiovascular disease in humans [31]. Therefore, the finding of a concentration-dependent increase in IL-6 release by TT1 and ATII cells is significant. Thus, in the present study we have shown that inhalation of very small amounts of short MWCNT and deposition in the respiratory units, would be would be very likely to induce inflammation within the human alveolar unit, even in the absence of cell death.
Figure 5.
Inflammatory mediator release from TT1 and ATII epithelial cells and AMs following treatment with MWCNTs for 24 hours. Release of IL-6, IL-8 and MCP-1 from MWCNT-exposed (1–50 µg/ml) TT1, ATII cells and AMs. Mediator release is presented as pg/ml; significant differences are indicated where * P < 0.05, ** P < 0.01 and *** P < 0.001.
In contrast to the findings with the alveolar epithelial cells, the longest (MWCNT-20 µm) format was the most bioreactive with AMs, inducing the greatest IL-6 and IL-8 release following 24 hour treatment (Figure 5). The differential response was most marked for IL-8 release, where 10 µg/ml of MWCNT-20 µm induced a striking 7.8-fold increase in IL-8 release from AMs (P < 0.05) while the same dose of MWCNT-0.6 µm induced only a 2.2-fold increase (P < 0.05). Even at very low doses of MWCNT-20 µm (0.1 and 1.0 µg/ml), AM release of IL-6 and IL-8 was significantly increased. Thus, for very long MWCNTs that reach the human peripheral lung, our data indicates that even very low amounts would be capable of inducing substantial proinflammatory mediator release from resident AMs.
There was a similar, but less marked, profile of response for AM IL-6 release, and both the shorter MWCNT formats induced IL-6 and IL-8, though not to the same extent as the long MWCNTs. However, in contrast to the IL-6 and IL-8 release profiles, there was almost no effect on MCP-1 release (Figure 5). Interestingly, this is very different from the TT1 and ATII epithelial cells, where even the long MWCNT-20 µm format induced increases in MCP-1 release. IL-6 and IL-8 have important roles in injured tissue with respect to the balance between inflammation and repair. The observed increase in AM release of IL-6 and IL-8 may be a result of extracellular receptor interactions or alternatively it may be in response to attempted internalisation of the long MWCNTs or incomplete phagocytosis. These AM observations complement the work of Murphy et al [12] who have described a similar response from a MWCNT-exposed THP-1 macrophage cell line. Indeed Palomaki et al have recently shown that long, needle-like MWCNTs activate the inflammasome complex in the cytoplasm of human monocyte-derived macrophages via engagement of toll-like receptors (TLR) [23]. Previous studies by us have shown that primary human AMs (and indeed primary human ATII cells and TT1 cells) abundantly express TLRs including TLR-2 and TLR-4 [32,33]. Considering this, and the well-established downstream TLR signaling connection with MAP kinases [34] (which were activated in the present study; discussed below), these extracellular receptors are potentially important for both short and long MWCNT interactions with alveolar epithelial cells and AMs.
The phenomenon of differential MWCNT activity, based on length, has also been described by Liu et al [35]. In their study, they showed that treatment of RAW264.7 murine macrophages with long MWCNT (12.5 – 200 µg/ml; 3 – 14 µm in length, 40 – 60 nm in diameter (nominal values, material characterisation was not performed)) reduced cell viability to a greater extent than short MWCNTs (12.5 – 200 µg/ml; 1.5 µm in length, 40 – 60 nm in diameter (nominal values, material characterisation was not performed by the authors). However the same study reported that the shorter MWCNTs induced greater inflammatory mediator release (TNFα and IL-12) when compared to the longer MWCNTs; the greater number of viable cells may account for this however. Bussy et al recently reported on the effects of surface oxidation of MWCNTs (resulting from length shortening) on cellular reactivity in murine macrophages [22]. Here, the authors found that shortened MWCNTs (~5 µm in length; produced by sonicating MWCNTs of ~10 µm in length) had increased surface oxygen and were more bioreactive than their parent MWCNTs. We also acknowledge that increased oxygen content may play some part in MWCNT bioreactivity. However, in the present study, while we did observe a small difference in oxygen content between the parent and shortened MWCNTs, the atomic percent variation on oxygen content was below 10% for all MWCNTs and unlikely to contribute, particularly since the short (9% oxygen) were most bioreactive with epithelial cells, whilst long (~4% oxygen) were most bioreactive with AMs, indicating that oxygen is unlikely to be a driving factor (Figure 3). Considering this, it is much more likely that MWCNT length is the critical determinant in human lung cell bioreactivity. The observations in the present study are also important as they highlight that the use of single cell type in vitro models, often employed in other studies and in industrial safety assessments, may not be sufficient to understand the risk of MWCNTs on human pulmonary health. For example, the alveolar epithelial cell study alone would deceptively indicate that long MWCNTs are not as important in bioreactivity within the human alveolar unit. Yet, our finding that treating human AMs with short MWCNTs had a lesser effect on mediator release when compared with long MWCNTs, suggests that particle length is an important regulatory factor in human AM activation. Similarly, the AM study alone would mislead us into thinking that induced MCP-1 release, and consequential monocyte recruitment, is not important; however this conclusion would neglect the substantial amount of MCP-1 produced by the intimately neighbouring alveolar epithelial cells following MWCNT treatment.
3.4 Role of MAP kinase signalling in MWCNT bioreactivity with TT1 and ATII cells and AMs
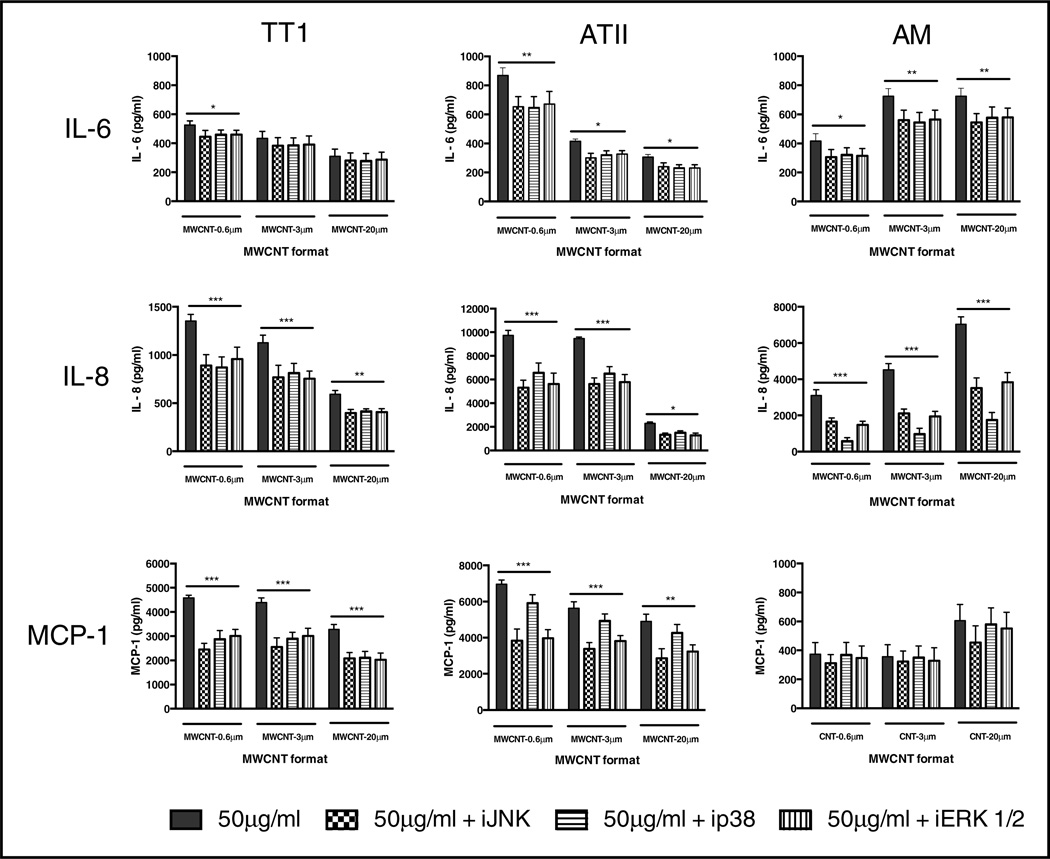
To further investigate the cellular processes leading to pro-inflammatory mediator release from TT1 and ATII epithelial cells and AMs upon exposure to MWCNTs of different lengths, we studied the potential involvement of MAP kinase signalling. Specific inhibition of JNK, p38 and ERK 1/2, prior to MWCNT treatment, was achieved using 10 µM of each respective inhibitor (as previously optimised by Thorley et al [33]). Their effect on pro-inflammatory mediator release was then measured following treatment with each of the MWCNT formats (a 50 µg/ml dose was chosen as it typically induced the greatest magnitude of mediator release, thus it would give us the best chance of seeing any effects of the MAP kinase inhibitors on reducing mediator release).
SP600125 is a cell-permeable, selective inhibitor of JNK phosphorylation [36], SB203580 inhibits p38 catalytic activity but does not inhibit the phosphorylation of p38 by upstream kinases [37] and PD98059 binds to the inactive forms of ERK 1/2, preventing activation by upstream activators [38]. In TT1 and ATII epithelial cells, inhibition of MAP kinases significantly reduced the magnitude of the cytokine and chemokine response to MWCNTs (Figure 6), with the exception of TT1 cell IL-6 release, which was minimally affected and significant only for MWCNT-0.6 µm, where all three inhibitors caused a similar reduction (~15%).
Figure 6.
Effect of MAP kinase inhibitors on inflammatory mediator release from MWCNT-treated TT1 and ATII epithelial cells and AMs. To examine the effect of MAP kinase signaling in MWCNT reactivity with TT1 and ATII epithelial cells and AMs, we inhibited JNK, p38 and ERK 1/2 with SP600125, SB203580 and PD98059, respectively, prior to TT1, ATII and AM treatment with 50 µg/ml of each MWCNT format. IL-6, IL-8 and MCP-1 releases are expressed with respect to releases by TT1 and ATII cells and AMs in the absence of MAP kinase inhibitors. Significance is indicated where *P < 0.05, **P < 0.01 ***P < 0.001.
The greatest magnitude of change following TT1 treatment with MAP kinase inhibitors was observed for MWCNT-induced MCP-1 release. Inhibition of JNK, p38 and ERK 1/2 reduced the TT1 MCP-1 response to 50 µg/ml of short MWCNT-0.6 µm by 48%, 40% and 38% respectively. A generally similar inhibitory effect of MAP kinase inhibitors on MCP-1 release was found following MWCNT-3 µm exposure, whilst the effect on MWCNT-20 µm MWCNT exposure responses was less marked, but still highly significant. IL-8 release was also inhibited by all three MAP kinase inhibitors, but to a lesser degree (~30%). Unlike TT1 cells, ATII cell release of IL-6 fell significantly, to a similar extent (~20%; P < 0.05) for all three MWCNT formats, following treatment with each inhibitor. ATII cell MAP kinase inhibition was greater for IL-8 release (~40%; P < 0.05, for all three inhibitors. However, the effect of ATII treatment with MAP kinase inhibitors on MCP-1 release varied between the three: inhibition of JNK and ERK 1/2 caused a significantly greater reduction when compared with inhibition of p38. Indeed, MAP kinase inhibition was most marked in the ATII response to short, 0.6 µm MWCNTs, where inhibition of JNK and ERK 1/2 reduced MCP-1 release by 51% and 49%, respectively. There was a similar trend in ATII cell MAP kinase inhibition of MCP-1 with both the MWCNT-3 µm and MWCNT-20 µm formats, albeit less marked. Critically, these data indicate that different pathways may be involved in the TT1 and ATII epithelial cell responses to MWCNT exposure, and that ATII release of MCP-1 is via a different pathway to that of TT1, not involving p38.
AMs displayed the greatest reduction in IL-8 release as a result of treatment with MAP kinase inhibitors (Figure 6). Inhibition of JNK, p38 and ERK 1/2 reduced the AM IL-8 response to 50 µg/ml of MWCNT-0.6 µm by 49%, 86% and 53% respectively. The profile of IL-8 inhibition was generally similar following exposure to MWCNT-3 µm and MWCNT-20 µm. Notably however, and unlike ATII cells, p38 was found to be the most important MAP kinase in the AM IL-8 release response to MWCNT exposure, irrespective of MWCNT length. There was a small but significant effect of each inhibitor on AM IL-6 release (~20%), regardless of MWCNT length; there was no effect on AM MCP-1 release, again in contrast to the epithelial cell responses, likely reflecting the lack of impact of these MWCNTs on AM MCP-1 release. The role of MAP kinases in MWCNT bioreactivity with lung cells has been studied by others, with variable conclusions across other species. For example, Hirano et al showed that MWCNTs (10 – 100 µg/ml) did not activate ERK 1/2, JNK or p38 in J774.1 mouse monocyte macrophages [25]. It is possible that the biointeraction of MWCNTs with mouse blood monocytes is different to that with human alveolar macrophages, thus we see contrasting observations in the present study. Furthermore, while the diameter of MWCNTs in the Hirano et al study was reported as 67 nm, their length was not determined. In agreement with the present study, Lee et al showed that MWCNTs (10 – 100 µg/ml; 10–30 µm in length, 20–30 nm in diameter) activate ERK 1/2 in RAW264.7 macrophages [39]. Additionally, an interesting co-culture study by Snyder-Talkington et al showed that MWCNTs (1.2 µg/ml; ~4 µm in length, 50 nm in diameter) activate human small airway epithelial cells which in turn activate p38 in human microvascular endothelial cells [40]. The present study therefore highlights the importance of using representative human lung cell models to determine MWCNT bioreactivity, while it also reveals the possibility of intercellular responses by virtue of target alveolar epithelial cells/AMs being activated and subsequently activating other types of neighbouring lung cells. The latter is currently under investigation in our laboratory.
4. Conclusion
Exposure to MWCNTs through inhalation could activate the pulmonary alveolar epithelium and alveolar macrophages, resulting in a pro-inflammatory response and possibly longer term, chronic pathology, depending on the exposure load. We studied the effect of well-characterised MWCNTs using highly relevant and primary human lung cell models, to address the hypothesis that increased aspect ratio would be a critical determinant of any cytotoxicity and bioreactivity. We used three MWCNT formats with different lengths (MWCNT-0.6 µm, MWCNT-3 µm and MWCNT-20 µm), but comparable diameters (~30nm), surface charges (negative) and carbon purities (~92%). We have shown that all three MWCNTs caused adverse effects, where bioreactivity depended on MWCNT length. Unexpectedly, and in contrast to our original hypothesis, the severity of the effect also depended on the cell type exposed. Shorter MWCNTs were more reactive with the TT1 and ATII epithelial cells, even at very low concentrations, for all inflammatory mediators. However, long MWCNTs had virtually no effect on IL-6 and IL-8 release from the epithelial cells, and a reduced effect on MCP-1 release. In direct contrast, the longer MWCNTs were more reactive with the AMs, causing significant cell death and stimulating significant increases in IL-6 and IL-8. Nevertheless, the shorter MWCNTs did induce AM IL-6 and IL-8 release, however this was relatively lower and decreased in relation to decreasing MWCNT length. The inability of MWCNTs to induce MCP-1 release by AMs is also interesting. The pro-inflammatory effect of these MWCNT formats was found to correspond to MAP kinase signalling, the relative role differing according to cell type and mediator released. Our discovery that the short MWCNTs induce the greatest responses from alveolar epithelial cells, without significant cell death, opens up a number of possibilities, that, following inhalation of short MWCNTs, these cells (particularly ATII cells, which release higher levels of mediators under the same experimental conditions) might orchestrate the inflammatory response and subsequent interstitial/cardiovascular events.
MWCNTs of different lengths have numerous commercial applications and can therefore all be considered a potential respiratory hazard. This unique study, comparing responses between a human alveolar type-I-like epithelial cell line, and primary human ATII epithelial cells and AMs from the same donor subjects, allows us to draw strong conclusions as to the lung-cell-specific pro-inflammatory response to MWCNTs of different lengths and furthermore, highlights the inadequacy of using a single cell model for nanomaterial safety screening.
Acknowledgments
Financial support for SS from Unilever, UK is gratefully acknowledged. We would like to thank Dr Katerina Kouravelou at Nanothinx, Greece, for her generous advice throughout the study. This research was supported by the Medical Research Council and Public Health England, Centre for Environment and Health. The project was also supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winey KI, Kashiwagi T, Mu M. Improving electrical conductivity and thermal properties of polymers by the addition of carbon nanotubes as fillers. Mrs Bulletin. 2007;32:348–353. [Google Scholar]
- 2.Park JG, Cheng Q, Lu J, Bao J, Li S, Tian Y, et al. Thermal conductivity of MWCNT/epoxy composites: The effects of length, alignment and functionalization. Carbon. 2012;50:2083–2090. [Google Scholar]
- 3.Schnorr JM, Swager TM. Emerging Applications of Carbon Nanotubes. Chem Mater. 2011;23:646–657. [Google Scholar]
- 4.Stone V, Johnston H, Clift MJD. Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE Trans Nanobioscience. 2007;6:331–340. doi: 10.1109/tnb.2007.909005. [DOI] [PubMed] [Google Scholar]
- 5.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crapo J, Barry B, Gehr P, Bachofen M. Cell number and cell characteristics of the normal human lung. - Abstract - UK PubMed Central. Am Rev Respir Dis. 1982;125:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 7.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 8.Muller J, Huaux F, Moreau N, Misson P, Heilier J-F, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson K, Tran CL. An introduction to the short-term toxicology of respirable industrial fibres. Mutat Res. 2004;553:5–9. doi: 10.1016/j.mrfmmm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Mühlfeld C, Poland CA, Duffin R, Brandenberger C, Murphy FA, Rothen-Rutishauser B, et al. Differential effects of long and short carbon nanotubes on the gas-exchange region of the mouse lung. Nanotoxicology. 2012;6:867–879. doi: 10.3109/17435390.2011.626533. [DOI] [PubMed] [Google Scholar]
- 11.Murphy FA, Poland CA, Duffin R, Jamal AlKT, Ali-Boucetta H, Nunes A, et al. Length-Dependent Retention of Carbon Nanotubes in the Pleural Space of Mice Initiates Sustained Inflammation and Progressive Fibrosis on the Parietal Pleura. Ajpa. 2011;178:2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy FA, Schinwald A, Poland CA, Donaldson K. The mechanism of pleural inflammation by long carbon nanotubes: interaction of long fibres with macrophages stimulates them to amplify pro-inflammatory responses in mesothelial cells. Particle and Fibre Toxicology. 2012;9:8. doi: 10.1186/1743-8977-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson K, Poland C, Duffin R, Bonner J. The Toxicology of Carbon Nanotubes. Cambridge University Press; 2012. [Google Scholar]
- 14.Kemp SJ, Thorley AJ, Gorelik J, Seckl MJ, O'Hare MJ, Arcaro A, et al. Immortalization of Human Alveolar Epithelial Cells to Investigate Nanoparticle Uptake. Am J Respir Cell Mol Biol. 2008;39:591–597. doi: 10.1165/rcmb.2007-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol. 2005;32:262–267. doi: 10.1165/rcmb.2004-0196OC. [DOI] [PubMed] [Google Scholar]
- 16.Witherden IR, Tetley TD. Isolation and Culture of Human Alveolar Type II Pneumocytes. Methods Mol Med. 2001;56:137–146. doi: 10.1385/1-59259-151-5:137. [DOI] [PubMed] [Google Scholar]
- 17.Witherden I, Vanden Bon E, Goldstraw P, Ratcliffe C, Pastorino U, Tetley T. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol. 2004;30:500–509. doi: 10.1165/rcmb.4890. [DOI] [PubMed] [Google Scholar]
- 18.Murray AR, Kisin ER, Tkach AV, Yanamala N, Mercer R, Young S-H, et al. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part Fibre Toxicol. 2012;9:1–19. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006;91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- 20.Wittmaack K. In Search of the Most Relevant Parameter for Quantifying Lung Inflammatory Response to Nanoparticle Exposure: Particle Number, Surface Area, or What? Environ Health Perspect. 2006;115:187–194. doi: 10.1289/ehp.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2010;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 22.Bussy C, Pinault M, Cambedouzou J, Landry MJ, Jegou P, Mayne-L'hermite M, et al. Critical role of surface chemical modifications induced by length shortening on multi-walled carbon nanotubes-induced toxicity. Particle and Fibre Toxicology. 2012;9:1–1. doi: 10.1186/1743-8977-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palomäki J, Välimäki E, Sund J, Vippola M, Clausen PA, Jensen KA, et al. Long, Needle-like Carbon Nanotubes and Asbestos Activate the NLRP3 Inflammasome through a Similar Mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- 24.Wörle-Knirsch JM, Pulskamp K, Krug HF. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Lett. 2006;6:1261–1268. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 25.Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol. 2008;232:244–251. doi: 10.1016/j.taap.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Cheng C, Müller KH, Koziol KKK, Skepper JN, Midgley PA, Welland ME, et al. Toxicity and imaging of multi-walled carbon nanotubes in human macrophage cells. Biomaterials. 2009;30:4152–4160. doi: 10.1016/j.biomaterials.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Tillie-Leblond I, Pugin J, Marquette C-H, Lamblin C, Saulnier F, Brichet A, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 28.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, et al. Inflammatory Biomarkers Improve Clinical Prediction of Mortality in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 29.Pantelidis P, Fanning GC, Wells AU, Welsh KI, Bois Du RM. Analysis of tumor necrosis factor-, lymphotoxin-, tumor necrosis factor receptor II, and interleukin-6 polymorphisms in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;163:1432–1436. doi: 10.1164/ajrccm.163.6.2006064. [DOI] [PubMed] [Google Scholar]
- 30.Kido T, Tamagawa E, Bai N, Suda K, Yang H-HC, Li Y, et al. Particulate Matter Induces Translocation of IL-6 from the Lung to the Systemic Circulation. Am J Respir Cell Mol Biol. 2011;44:197–204. doi: 10.1165/rcmb.2009-0427OC. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma Concentration of Interleukin-6 the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong L, Medford ARL, Uppington KM, Robertson J, Witherden IR, Tetley TD, et al. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:241–245. doi: 10.1165/rcmb.2004-0078OC. [DOI] [PubMed] [Google Scholar]
- 33.Thorley AJ, Grandolfo D, Lim E, Goldstraw P, Young A, Tetley TD. Innate immune responses to bacterial ligands in the peripheral human lung--role of alveolar epithelial TLR expression and signalling. PLoS ONE. 2011;6:e21827. doi: 10.1371/journal.pone.0021827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 35.Liu D, Wang L, Wang Z, Cuschieri A. Different cellular response mechanisms contribute to the length-dependent cytotoxicity of multi-walled carbon nanotubes. Nanoscale Res Lett. 2012;7:361–361. doi: 10.1186/1556-276X-7-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USa. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar S, Han J, Sinsimer KS, Liao B, Foster RL, Brewer G, et al. RNA-binding protein AUF1 regulates lipopolysaccharide-induced IL10 expression by activating IkappaB kinase complex in monocytes. Mol Cell Biol. 2011;31:602–615. doi: 10.1128/MCB.00835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cotrim CZ, Amado FL, Helguero LA. Estrogenic effect of the MEK1 inhibitor PD98059 on endogenous estrogen receptor alpha and beta. J Steroid Biochem Mol Biol. 2011;124:25–30. doi: 10.1016/j.jsbmb.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Lee JK, Sayers BC, Chun K-S, Lao H-C, Shipley-Phillips JK, Bonner JC, et al. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP Kinase-dependent and -independent mechanisms in mouse RAW264.7 macrophages. Particle and Fibre Toxicology. 2012;9:1–1. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder-Talkington BN, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotubes induce human microvascular endothelial cellular effects in an alveolar-capillary co-culture with small airway epithelial cells. Particle and Fibre Toxicology. 2013;10:1–1. doi: 10.1186/1743-8977-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]