Abstract
Background and aims
Several smaller studies reported interactions between dietary factors and apolipoprotein A5 (APOA5) gene polymorphisms in determination of plasma lipids. We tested interactions between APOA5 haplotypes and dietary intake in determination of plasma triglycerides (TG) and other lipids.
Methods and results
Participants (5487 males and females aged 45–69) were classified according to the number (0, 1, 2+) of minor APOA5 alleles (using T-1131 > C; rs662799 and Ser19 > Trp; rs3135506 polymorphisms) and into three groups of low (bottom 25%), medium (26th–75th percentile) and high (top 25%) of intake of total energy and total, saturated and polyunsaturated fats, assessed by food frequency questionnaire. The age-sex adjusted geometric means of plasma TG increased with the number of minor alleles, from 1.57 (standard error 0.01), to 1.79 (0.02) to 2.29 (0.10) mmol/L (p < 0.00001) but TG did not differ between groups with low, medium and high total energy intake (p = 0.251). TG concentrations were highest in subjects with the combination of 2+ minor alleles and the highest energy intake (mean 2.59 [0.19], compared with 1.62 [0.03] in subjects with lowest energy intake and no minor allele) but the interaction between energy intake and APOA5 haplotypes was not statistically significant (p = 0.186). Analogous analyses with total, saturated and polyunsaturated fat intake yielded similar nonsignificant results. Effects of APOA5 and dietary intakes on total and HDL cholesterol were weaker and no interactions were significant.
Conclusion
In this Slavic Caucasian population sample, we did not detect the hypothesized interaction between common SNPs within the APOA5 gene and diet in determination of blood lipids.
Keywords: Apolipoprotein A5, Triglycerides, Polymorphism, Total energy intake, Interaction
Abbreviations: APOA5, apolipoprotein A5; FFQ, food frequency questionnaire; HAPIEE, Health Alcohol and Psychosocial factors In Eastern Europe; HDL, high density lipoprotein; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; TG, triglycerides; VLDL, very low density lipoproteins
Introduction
Plasma levels of triglycerides (TG) are independent risk factor of cardiovascular disease development [1]. The plasma levels of TG are significantly genetically determined. Probably the most important environmental factor that may interact with genetic polymorphisms in determination of the plasma TG levels is diet. There is growing interest in effect modification between genes and environment because such interactions could explain a number of discrepancies, such as differences in results between association studies in different populations or inconsistent effects of dietary interventions. However, the number of studies addressing gene–environment interactions on sufficient number of individuals remains modest.
The most significant impact on plasma TG levels seems to be associated with apolipoprotein A5 gene (APOA5, gene ID 116519, OMIM accession number 606368) variants [2], [3]. ApoA5 is located on TG-rich and high density lipoprotein (HDL) particles, enhances the activity of lipoprotein lipase [4], [5], and recombinant apoA5 binds to the LDL receptor family members [6]. Minor alleles of two tagging APOA5 SNPs T-1131 > C [rs662799] and Ser19 > Trp [C56 > G, rs3135506] were associated, although with different strengths, with elevated plasma TG levels, regardless of ethnicity and sex [3], [7], [8], [9], [10].
Several studies explored interactions of the effects of APOA5 variants on different biochemical traits with dietary factors. The results suggest that the APOA5 genotypes modify the effects of dietary interventions (e.g. low/high fat diet) [11], [12], [13], [14], intake of fat [15], [16] or alcohol intake [17] on triglycerides (and less consistently on other lipids). Since previous studies have been relatively small, used different designs, selected patients and ethnically mixed populations, the results remain inconclusive.
In this study, we have investigated the potential interaction of APOA5 with energy and fat intake in a large sample of a general Slavonic Caucasian population. Our working hypothesis was that plasma levels of TG are highest in the combination of high energy/fat intake and two or more minor alleles of APOA5 haplotypes (based on the analysis of two polymorphisms (T-1131 > C and Ser19 > Trp).
Methods
Study population and study subjects
The study sample comes from the Czech part of the HAPIEE (Health, Alcohol and Psychosocial factors In Eastern Europe) project. The study examined random samples of men and women aged 45–69 years in seven Czech towns: Jihlava, Havirov, Hradec Kralove, Karvina, Kromeriz, Liberec and Usti nad Labem. Details of the study have been described elsewhere [18]. Briefly, of the 8856 individuals recruited (response rate 55%), 6681 (3079 males and 3602 females) had DNA samples available. Of these, 5847 people with non-missing data on all variables of interest are included in the analyses reported here. The subjects completed an extensive questionnaire on medical history, health status, life style, diet and socioeconomic and psychosocial factors, underwent a short examination, including anthropometry, and provided a fasting blood sample. The study was approved by the Local Ethics Committees at both Czech National Institute of Public Health and University College London, UK.
Laboratory analyses
DNA was extracted using salting out method, and APOA5 SNPs rs662799 and rs3135506 were genotyped using PCR – RFLP as described in details elsewhere [9]. Subjects were classified according to the presence of minor APOA5 alleles (C-1131 and Trp19) into three groups – 0, 1, 2 and more minor alleles present.
Plasma levels of TG, total cholesterol and HDL cholesterol were analysed enzymatically using autoanalyzers and conventional methods with reagents from Boehringer Mannheim Diagnostics and Hoffmann-La Roche. The laboratory (IKEM, Prague) is accredited by CDC, Atlanta.
Assessment of diet
Diet was assessed by a 143-item food frequency questionnaire (FFQ) with specified portion sizes adapted from FFQ previously used in the US [19] and the UK [20]. The intakes of total energy and fats (and other nutrients) were estimated from the FFQ data using the McCance and Widdowson's The Composition of Foods [21], with correction for differences in the composition of principal foods and adding composition of local foods and recipes [22]. For the present analyses, subjects were classified into three groups according to low (bottom 25%), medium (25th–75th percentile) and high (top 25%) intakes of total energy and total, saturated and polyunsaturated fat (as proportion of total energy); sex-specific cut-off points were used to create these categories.
Statistical analysis
After excluding subjects with unreliable dietary data and missing data for covariates, 5847 individuals with valid APOA5 genotype were included in the analysis. The associations of TG, total cholesterol and HDL cholesterol with energy intake and APO5 haplotype was evaluate by linear regression for males and females separately, controlling for age. Interactions were assessed by adding interaction terms to the linear regression models. Results are reported as means and standard errors (SE); two-sided p-values are also given. Since the distribution of TG was skewed, TG values were logarithmically transformed. STATA statistical software (version 12; College Station, TX) was used for all statistical analyses.
Results
Descriptive characteristics of the individuals included in the analysis are summarized in Table 1. The genotype frequencies of both polymorphisms did not differ significantly from the previously described distributions in Caucasian populations. In the entire study sample, 4322 (73.9%) subjects were carriers of the common alleles only; 1406 (24.0%) were carriers of one minor allele; and 119 (2.0%) were carriers of at least two less common alleles.
Table 1.
Descriptive characteristics of the study sample.
| Males | Females | All | |
|---|---|---|---|
| N | 2690 | 3157 | 5847 |
| Age (years), mean (SE) | 58.0 (6.9) | 57.2 (6.9) | 57.6 (7.0) |
| TG (mmol/L), mean (SE) | 2.14 (1.65) | 1.70 (0.96) | 1.90 (1.34) |
| TC (mmol/L), mean (SE) | 5.61 (1.04) | 5.84 (1.03) | 5.73 (1.04) |
| HDL-C (mmol/L), mean (SE) | 1.25 (0.34) | 1.51 (0.40) | 1.39 (0.39) |
| BMI, (kg/m2), mean (SE) | 28.2 (3.9) | 28.1 (5.0) | 28.2 (4.6) |
| Energy intake (kcal/day), mean (SE) | 2096 (698) | 1979 (709) | 2033 (706) |
| Total fat (% of energy), mean (SE) | 36.4 (0.1) | 36.2 (0.2) | 36.3 (0.1) |
| Saturated fats (% of energy), mean (SE) | 13.1 (0.05) | 13.0 (0.04) | 13.1 (0.03) |
| Polyunsaturated fats (% of energy), mean (SE) | 6.5 (0.03) | 6.7 (0.03) | 6.6 (0.02) |
| APOA5 minor alleles, n (%) | |||
| 0 | 1999 (74.3) | 2323 (73.6) | 4322 (73.9) |
| 1 | 636 (23.6) | 770 (24.4) | 1406 (24.1) |
| 2 and more | 55 (2.0) | 64 (2.0) | 119 (2.0) |
As expected, both variants had a significant effect on plasma TG levels (results not shown). When the two variants were combined into one variable indicating the number of minor alleles, the geometric means of TG increased with the number of minor APOA5 alleles, from 1.57 (SE 0.01) mmo/L over 1.79 (0.02) mmo/L to 2.29 (0.10) mmo/L, p < 0.00001 (Table 2). Total cholesterol (p < 0.001) increased linearly and HDL-cholesterol values decreased (p < 0.001) with the number of minor APOA5 alleles, and intakes of energy and fats were not associated with the number of the APOA5 minor alleles (Table 2).
Table 2.
Means (standard errors) of lipid parameters (in mmol/L), BMI (in kg/m2) and intake of energy (in kcal) and fat (as % of energy) with APOA5 haplotype, adjusted for age and sex.
| Number of the APOA5 minor alleles |
p | |||
|---|---|---|---|---|
| 0 | 1 | 2 And more | ||
| N | 4322 | 1406 | 119 | |
| Triglyceridesa | 1.57 (0.01) | 1.79 (0.02) | 2.29 (0.10) | 0.00001 |
| Total cholesterol | 5.70 (0.07) | 5.79 (0.03) | 6.01 (0.09) | 0.001 |
| HDL-cholesterol | 1.40 (0.01) | 1.36 (0.01) | 1.32 (0.03) | 0.001 |
| BMI | 28.2 (0.07) | 28.2 (0.12) | 28.0 (0.41) | 0.892 |
| Energy intake | 2036 (11) | 2013 (19) | 2083 (64) | 0.545 |
| Total fat | 36.3 (0.1) | 36.2 (0.2) | 36.7 (0.6) | 0.906 |
| Saturated fats | 13.1 (0.04) | 13.0 (0.06) | 13.2 (0.22) | 0.499 |
| Polyunsaturated fats | 6.6 (0.02) | 6.6 (0.04) | 6.7 (0.16) | 0.350 |
Geometric mean.
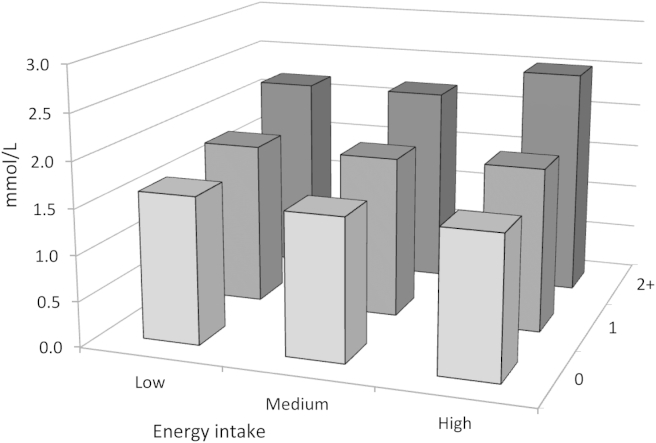
Plasma TG levels did not differ significantly between groups with low, medium and high total energy intake; the geometric means were 1.66 (0.02), 1.62 (0.02) and 1.63 (0.02), respectively, p for trend 0.251. There were no differences in lipids by intakes of total fat, saturated fat or polyunsaturated fat (not shown in table). The geometric means of TG by the combination of energy intake category and the number of minor alleles of APOA5 are shown in Table 3. There is a suggestion that the combination of high energy intake and 2 or more minor alleles produces the highest TG levels (Fig. 1) but the interaction between total energy intake and APOA5 haplotypes was not statistically significant (p = 0.186).
Table 3.
Mean concentrations (SE) of triglycerides and total and HDL cholesterol (all in mmol/L) with the number of APOA5 minor alleles by total energy intake category, adjusted for age and sex.
| Energy intake | Number of APOA5 minor alleles |
p for trenda | ||
|---|---|---|---|---|
| 0 | 1 | 2 And more | ||
| TG | ||||
| <25% | 1.62 (0.03) | 1.78 (0.05) | 2.19 (0.21) | <0.001 |
| >25–75% | 1.56 (0.02) | 1.78 (0.03) | 2.19 (0.14) | <0.001 |
| >75% | 1.55 (0.02) | 1.80 (0.05) | 2.52 (0.19) | <0.001 |
| p for interactionb = 0.186 | ||||
| TC | ||||
| <25% | 5.76 (0.03) | 5.70 (0.06) | 6.02 (0.21) | 0.843 |
| >25–75% | 5.68 (0.02) | 5.81 (0.04) | 5.92 (0.14) | 0.003 |
| >75% | 5.71 (0.03) | 5.84 (0.06) | 6.12 (0.17) | 0.004 |
| p for interactionb = 0.090 | ||||
| HDL | ||||
| <25% | 1.41(0.01) | 1.36 (0.02) | 1.34 (0.07) | 0.033 |
| >25%–75% | 1.40(0.01) | 1.36 (0.01) | 1.35 (0.05) | 0.003 |
| >75% | 1.41(0.01) | 1.37 (0.02) | 1.22 (0.06) | 0.004 |
| p for interactionb = 0.401 | ||||
p-value for trend by the number of APOA5 minor alleles.
p-values for differences in slope between 0 and 2 and more minor alleles (interaction between energy intake and APOA5).
Figure 1.
Geometric means of TG by the combination of energy intake and the number of APOA5 minor alleles.
Similarly, interactions between total energy intake and APOA5 haplotype were not significant in determination of concentrations of total and HDL cholesterol (Table 3).
We also examined interactions with dietary intakes of total fat, saturated fat or polyunsaturated fat. None of the fat intake variables acted as effect modifiers of the association between APOA5 haplotypes and plasma lipids (all p vales > 0.3, detailed results available on request). Finally, there were no interactions between dietary intakes and the individual APOA5 polymorphisms.
We conducted additional analyses using other metabolic syndrome variables: systolic and diastolic blood pressure and blood glucose. While all these variables were associated with TG as expected (all p-values <0.001), none of them was significantly associated with the APOEA5 haplotype (all p-values >0.4), and stratification for dietary intake of energy or fat did not identify any association with APOA5 in any subgroup (not shown in table).
Discussion
This study examined the potential interaction between APOA5 haplotypes and dietary intake of energy and fats in a large general Slavic Caucasian population sample in relation to plasma lipids. We found that lipids were associated with both individual polymorphisms in APOA5 and with their combination but not with dietary intakes of energy or saturated, unsaturated or polyunsaturated fats. Although there was a suggestion that TG are highest in carriers of at least two minor APOA5 alleles who also had high energy intake, the interaction between APOA5 haplotype and any of the dietary indicators were not statistically significant.
Limitations and strengths
The common/main problem of nutrigenetic studies is insufficient statistical power. The combination of small sample size, heterogeneity of dietary assessments' and measurement inaccuracies reduce the ability of nutrigenetic studies to reliably detect interactions between genetic and dietary factors. Despite the large sample size, only 2% of subjects in our study had 2 or more minor APOA5 alleles; this would allow detection of only relatively strong effect modifications. This may be one of the reasons why none of the tested interactions in this study were significant.
Second, as mentioned above, most methods of dietary assessment, including food frequency questionnaires, are inaccurate. This misclassification would further reduce the ability of the study to detect main effects and interactions. We have attempted to minimise this issue by comparing groups with contrasting intakes (highest and bottom 25%) but this may have not been sufficient to completely overcome the measurement bias.
Third, the subjects in this study were relatively old, and the negative findings may not be generalised to younger subjects with different life style than older persons.
However, the study also has several strengths. The study was perhaps the largest so far published on the potential APOA5-nutrition interaction and lipids. Second, we have combined two tagging polymorphisms to create a variable with a strong effect on plasma TG levels. Both these aspects would increase the probability of detecting an interaction with diet, if it exists. Finally, this study examined a general population sample, unselected by medical history, and the study population is ethnically homogenous.
Interpretation
The need for the nutrigenetic analyses is widely acknowledged by numerous authors [23], [24], [25]. However, studies with sufficient numbers of well phenotypically characterised individuals and sufficient power remain scarce. So far, there is some, but limited, evidence that variants within the APOA5 gene may interact with environmental, mainly dietary, factors.
Several experimental studies assessed whether changes in blood lipids depended on the combination of diet and APOA5 genotype. In a dietary trial in Korean patients with hypertriglyceridemia, APOA5 T-1131T homozygotes showed larger decrease in plasma TG after combined dietary and exercise intervention [11]. In a Chinese study, the changes in TG after high carbohydrate/low fat diet interact with APOA5-1131 TT genotype in women but not in men [12]. Another Koran study of low/high fat diet suggested that postprandial TG elevation differed by APOA5 T-1131T genotype [13]. In the Pounds Lost trial, another APOA5 variant, rs964184, influences reduction in total and LDL cholesterol in the low fat intake group [14].
In addition to experimental studies, there have also been studies in general population samples. Among 117 Czech males whose dietary composition markedly changed over an 8-year period, decrease in total cholesterol (but not TG) was associated with the Ser19 > Trp polymorphism [26], [27]. Results from the Framingham Heart study have shown that individuals consuming the diet rich in polyunsaturated fatty acids had higher fasting TG levels, if they carried at least one C-1131 allele; this interaction was specific for the omega-6 fatty acids only [15]. Interestingly, the second common APOA5 variant (Ser19 > Trp) did not interact with high polyunsaturated fat intake. In the Boston Puerto Rican Health Study, carriers of the C-1131 allele had higher levels of TG and total cholesterol if they also had high total fat intake [16]. By contrast, no gene–diet interaction was observed in as study of 250 elderly Brazilian women in Ref. [28]. Finally, a study of a Spanish population sample reported an interaction between APOA5-1131, fat intake and TG but the lowest TG levels were found in the combination of minor allele with high fat intake [29]. Among studies focused on the same polymorphism as this investigation, all studies, except Sanchez-Moreno et al., found highest TG levels in subjects with the minor allele and high fat intake. This general pattern is consistent with our findings, but in our study, despite being several times larger than the others, the interaction did not reach statistical significance.
Overall, the non-significant tendency of TG being highest among subjects with the combination 2+ minor alleles and the highest energy intake, together with studies reported in the literature may be consistent with the hypothesis that common SNPs within the APOA5 gene interact with diet in determination of blood lipids. However, the interaction is likely to be weak and a conclusive study would require a very large sample size to confirm or reject this hypothesis.
Acknowledgement
The study was funded by grants from the Welcome Trust (064947 and 081081), the US National Institute on Aging (R01 AG23522-01), and the Ministry of Health of the Czech Republic (grant 00023001 to IKEM, Prague).
References
- 1.Forrester J.S. Triglycerides: risk factor or fellow traveler? Curr Opin Cardiol. 2001;16:261–264. doi: 10.1097/00001573-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Pennacchio L.A., Olivier M., Hubacek J.A., Cohen J.C., Cox D.R., Fruchart J.C., et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 3.Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Sarwar N., Sandhu M.S., Ricketts S.L., Butterworth A.S., Di Angelantonio E., Boekholdt S.M., et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruchart-Najib J., Bauge E., Niculescu L.S., Pham T., Thomas B., Rommens C., et al. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun. 2004;319:397–404. doi: 10.1016/j.bbrc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Schaap F.G., Rensen P.C., Voshol P.J., Vrins C., van der Vliet H.N., Chamuleau R.A., et al. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J Biol Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 6.Dorfmeister B., Zeng W.W., Dichlberger A., Nilsson S.K., Schaap F.G., Hubacek J.A., et al. Effects of six APOA5 variants, identified in patients with severe hypertriglyceridemia, on in vitro lipoprotein lipase activity and receptor binding. Arterioscler Thromb Vasc Biol. 2008;28:1866–1871. doi: 10.1161/ATVBAHA.108.172866. [DOI] [PubMed] [Google Scholar]
- 7.Pennacchio L.A., Olivier M., Hubacek J.A., Krauss R.M., Rubin E.M., Cohen J.C. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet. 2002;11:3031–3038. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 8.Hubacek J.A. Apolipoprotein A5 and triglyceridemia. Focus on the effects of the common variants. Clin Chem Lab Med. 2005;43:897–902. doi: 10.1515/CCLM.2005.153. [DOI] [PubMed] [Google Scholar]
- 9.Hubacek J.A., Skodova Z., Adamkova V., Lanska V., Poledne R. The influence of APOAV polymorphisms (T-1131 > C and S19 > W) on plasma triglyceride levels and risk of myocardial infarction. Clin Genet. 2004;65:126–130. doi: 10.1111/j.0009-9163.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 10.Tai E.S., Ordovas J.M. Clinical significance of apolipoprotein A5. Curr Opin Lipidol. 2008;19:349–354. doi: 10.1097/MOL.0b013e328304b681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang Y., Chae J.S., Kim O.Y., Park H.J., Kim J.Y., Paik J.K., et al. APOA5-1131T > C genotype effects on apolipoprotein A5 and triglyceride levels in response to dietary intervention and regular exercise (DIRE) in hypertriglyceridemic subjects. Atherosclerosis. 2010;211:512–519. doi: 10.1016/j.atherosclerosis.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Lin J., Fang D.Z., Du J., Shigdar S., Xiao L.Y., Zhou X.D., et al. Elevated levels of triglyceride and triglyceride-rich lipoprotein triglyceride induced by a high-carbohydrate diet is associated with polymorphisms of APOA5-1131T > C and APOC3-482C > T in Chinese healthy young adults. Ann Nutr Metab. 2011;58:150–157. doi: 10.1159/000327913. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.Y., Kim O.Y., Koh S.J., Jang Y., Yun S.S., Ordovas J.M., et al. Comparison of low-fat meal and high-fat meal on postprandial lipemic response in non-obese men according to the -1131T > C polymorphism of the apolipoprotein A5 (APOA5) gene (randomized cross-over design) J Am Coll Nutr. 2006;25:340–347. doi: 10.1080/07315724.2006.10719544. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X., Qi Q., Bray G.A., Hu F.B., Sacks F.M., Qi L. APOA5 genotype modulates 2-y changes in lipid profile in response to weight-loss diet intervention: the Pounds Lost Trial. Am J Clin Nutr. 2012;96:917–922. doi: 10.3945/ajcn.112.040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai C.Q., Corella D., Demissie S., Cupples L.A., Adiconis X., Zhu Y., et al. Dietary intake of n-6 fatty acids modulates effect of apolipoprotein A5 gene on plasma fasting triglycerides, remnant lipoprotein concentrations, and lipoprotein particle size: the Framingham Heart Study. Circulation. 2006;113:2062–2070. doi: 10.1161/CIRCULATIONAHA.105.577296. [DOI] [PubMed] [Google Scholar]
- 16.Mattei J., Demissie S., Tucker K.L., Ordovas J.M. Apolipoprotein A5 polymorphisms interact with total dietary fat intake in association with markers of metabolic syndrome in Puerto Rican older adults. J Nutr. 2009;139:2301–2308. doi: 10.3945/jn.109.109900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques-Vidal P., Arveiler D., Evans A., Amouyel P., Ferrieres J., Ducimetiere P. Different alcohol drinking and blood pressure relationships in France and Northern Ireland: the PRIME Study. Hypertension. 2001;38:1361–1366. doi: 10.1161/hy1101.095328. [DOI] [PubMed] [Google Scholar]
- 18.Peasey A., Bobak M., Kubinova R., Malyutina S., Pajak A., Tamosiunas A., et al. Determinants of cardiovascular disease and other non-communicable diseases in Central and Eastern Europe: rationale and design of the HAPIEE study. BMC Public Health. 2006;18:255. doi: 10.1186/1471-2458-6-255. www.biomedcentral.com/1471-2458/6/255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett W.C., Sampson L., Stampfer M.J., Rosner B., Bain C., Witschi J., et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 20.Brunner E., Stallone D., Juneja M., Bingham S., Marmot M. Dietary assessment of Whitehall II: comparison of 7-day diet diary and food frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;85:405–414. doi: 10.1079/bjn2001414. [DOI] [PubMed] [Google Scholar]
- 21.McCance R., Widdowson E. Royal Statistical Society; Cambridge: 2002. McCance & Widdowson's the composition of foods: summary edition. [Google Scholar]
- 22.Boylan S., Welch A., Pikhart H., Malyutina S., Pajak A., Kubinova R., et al. Dietary habits in three Central and Eastern European countries: the HAPIEE study. BMC Public Health. 2009;9:439. doi: 10.1186/1471-2458-9-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauss R.M. Dietary and genetic probes of atherogenic dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:2265–2272. doi: 10.1161/01.ATV.0000186365.73973.f0. [DOI] [PubMed] [Google Scholar]
- 24.Fenech M., El-Sohemy A., Cahill L., Ferguson L.R., French T.A., Tai E.S., et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69–89. doi: 10.1159/000327772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Rios A., Perez-Martinez P., Delgado-Lista J., Lopez-Miranda J., Perez-Jimenez F. Nutrigenetics of the lipoprotein metabolism. Mol Nutr Food Res. 2012;56:171–183. doi: 10.1002/mnfr.201100513. [DOI] [PubMed] [Google Scholar]
- 26.Hubacek J.A., Skodova Z., Adamkova V., Lanska V., Pitha J. APOA5 variant Ser19Trp influences a decrease of the total cholesterol in a male 8 year cohort. Clin Biochem. 2006;39:133–136. doi: 10.1016/j.clinbiochem.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Hubacek J.A., Bohuslavova R., Skodova Z., Pitha J., Bobkova D., Poledne R. Polymorphisms in the APOA1/C3/A4/A5 gene cluster and cholesterol responsiveness to dietary change. Clin Chem Lab Med. 2007;45:316–320. doi: 10.1515/CCLM.2007.056. [DOI] [PubMed] [Google Scholar]
- 28.Paula R.S., Souza V.C., Benedet A.L., Souza E.R., Toledo J.O., Moraes C.F., et al. Dietary fat and apolipoprotein genotypes modulate plasma lipoprotein levels in Brazilian elderly women. Mol Cell Biochem. 2010;337:307–315. doi: 10.1007/s11010-009-0313-0. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Moreno C., Ordovas J.M., Smith C.E., Baraza J.C., Lee Y.C., Garaulet M. APOA5 gene variation interacts with dietary fat intake to modulate obesity and circulating triglycerides in a Mediterranean population. J Nutr. 2011;141:380–385. doi: 10.3945/jn.110.130344. [DOI] [PMC free article] [PubMed] [Google Scholar]