Abstract
Background and Aims:
The effect of perineural dexmedetomidine on the time to onset, quality and duration of motor block with ropivacaine has been equivocal and its interaction with general anaesthesia (GA) has not been reported. We assessed the influence of dexmedetomidine added to 0.5% ropivacaine on the characteristics of supraclavicular brachial plexus block and its interaction with GA.
Methods:
In a randomised, double blind study, 36 patients scheduled for orthopaedic surgery on the upper limb under supraclavicular block and GA were divided into either R group (35 ml of 0.5% ropivacaine with 0.5 ml of normal saline [n - 18]) or RD group (35 mL of 0.5% ropivacaine with 50 μg dexmedetomidine [n - 18]). The onset time and duration of motor and sensory blockade were noted. The requirement of general anaesthetics was recorded.
Results:
Both the groups were comparable in demographic characteristics. The time of onset of sensory block was not significantly different. The proportion of patients who achieved complete motor blockade was more in the RD group. The onset of motor block was earlier in group RD than group R (P < 0.05). The durations of analgesia, sensory and motor blockade were significantly prolonged in group RD (P < 0.00). The requirement of entropy guided anaesthetic agents was not different in both groups.
Conclusions:
The addition of dexmedetomidine to 0.5% ropivacaine improved the time of onset, quality and duration of supraclavicular brachial plexus block but did not decrease the requirement of anaesthetic agents during GA.
Keywords: Dexmedetomidine, brachial plexus block, requirement of general anaesthetics
INTRODUCTION
Ropivacaine is a local anaesthetic (LA) effective for both intraoperative anaesthesia and post-operative analgesia. For peripheral nerve blockade, ropivacaine is comparable to bupivacaine and levobupivacaine.[1,2] However, the lower lipid solubility of ropivacaine causes greater sensory and motor differential blockade.[3] Though this is an advantage when analgesia with minimal motor block is required, it may be a drawback in orthopedic procedures where reduction of fracture is dependent on good muscle relaxation. Dexemedetomidine, an α2 adrenoreceptor agonist, has evinced a lot of interest of late as a useful additive to local anesthetics in peripheral nerve blocks.[4,5,6,7,8] The combination of dexmedetomidine and ropivacaine has been associated with significant prolongation of the duration of sensory blockade and post-operative pain relief.[5,7] However, its effect on the time of onset and the quality of motor block with ropivacaine has been equivocal.[5,7] Regional anaesthesia is often supplemented with general anaesthesia (GA). Drugs added as adjuvants to LAs may be systemically absorbed and interact with general anaesthetics. Though intravenous (IV) dexmedetomidine may decrease the requirement of anaesthetic agents during GA,[9] the interaction of perineural dexmedetomidine with GA has not been evaluated.
In this study, we investigated whether the combination of dexmedetomidine and 0.5% ropivacaine improved the quality, time to onset and duration of the supraclavicular brachial plexus block. We also evaluated whether perineural dexmedetomidine reduced the requirements of general anaesthetics.
METHODS
After obtaining the informed written consent from patients and institutional ethics committee clearance (No. EC/NIMS/1445/2013), 36 patients of American Society of Anaesthesiologists [ASA] physical status I-II scheduled for surgery of fractures of shaft of humerus, elbow and forearm under supraclavicular block and GA were enrolled for prospective, double blind randomised controlled trial. Patients receiving beta blockers, with difficult airway, ischemic heart disease, head injury and pregnant women were excluded. Patients were randomly allocated using a computer generated randomisation sequence to receive either 35 mL of ropivacaine 0.5% with 0.5 mL of isotonic sodium chloride solution (group R, n - 18), or 35 mL of ropivacaine 0.5% with 0.5 ml (50 μg) of dexmedetomidine (group RD, n - 18). The person who prepared the drug solutions was different from the person who administered the block and the person who monitored the quality and duration of block and the haemodynamics post-operatively. The anaesthetic plan was administration of supraclavicular brachial plexus block followed by induction of GA 45 min later. The combination of GA and block was planned to study the interaction of perineural dexmedetomidine with general anaesthetics as earlier studies reported sedation with its use. Also, surgeries on shaft of humerus and elbow are performed in the lateral position, hence, supplemental GA was preferred to just supraclavicular block alone.
In the operating room, an IV cannula was inserted in the contralateral upper limb and standard ASA monitoring was applied. Heart rate (HR), systolic arterial pressure (SAP) and diastolic arterial pressure (DAP), oxygen saturation (SpO2) and sedation score according to Ramsay sedation scale (RSS) [Appendix] were recorded before the block was performed. Under strict aseptic precautions and after infiltration of 2 ml lidocaine 2% locally, supraclavicular brachial plexus was located with a peripheral nerve stimulator (Stimuplex®, Braun, Germany) connected to a 21-gauge, 10-mm-long needle (Vygon, France) by the classical Kulenkampff method. The localisation of the plexus was considered optimal when an output current <0.5 mA caused contraction of the muscles of the hand or forearm. Sensory block in the territories of median, ulnar, radial and musculocutaneous nerves was assessed by pinprick test using a 3-point scale: 0 - normal sensation, 1 - loss of sensation of pinprick (analgesia), 2 - loss of sensation of touch (anaesthesia). Motor block was evaluated by thumb abduction (radial nerve), thumb adduction (ulnar nerve), thumb opposition (median nerve) and flexion at the elbow (musculocutaneous nerve) on a 3-point scale for motor function: 0 - normal motor function, 1 - reduced motor strength but able to move fingers, 2 - complete motor block.
The onset time of the sensory and motor blocks were recorded by assessment of the block every 3 min for 45 min after injection of the LA. The HR, SAP, and DAP were documented at every 5 min for 45 min. Sedation was assessed by RSS every 5 min for 45 min after giving the block.
A successful block was defined as a grade 2 sensory block in 3 or more nerve territories. The time of onset of sensory block was defined as the time between the administration of the LA and complete sensory block. Duration of sensory block was defined as the time taken from the administration of LA to complete recovery of anaesthesia on all nerves. The duration of analgesia was defined as the time to attain a visual analogue score (VAS) of >4 after the LA administration.
A grade 2 motor block was defined as complete motor block. Motor block was designated incomplete when there is grade 1 motor block in the presence of complete sensory block. When both sensory and motor blocks were incomplete, this was termed as failure of block, and the patients were excluded from the study. Onset time of motor block was defined as the time interval between the completion of the LA administration and complete motor block. In the case of an incomplete motor block at the induction of GA, which progressed to complete motor block at extubation, the time for the onset of motor block was taken as 45 min. Duration of motor block was defined as the time to the recovery of complete motor function of the hand and forearm after the administration of LA.
General anaesthesia was induced 45 min after institution of the block. The patient was pre-medicated with 1 μg/kg fentanyl and 0.2 mg glycopyrrolate IV. Electroencephalogram (EEG) entropy was measured with a special electrode applied to the forehead and connected to the entropy module (M-Entropy® plug in module, S/5: Datex-Ohmeda, Finland). After 5 min, the patient was induced with 30 mg boluses of thiopentone sodium (TPS) given IV every 30 s, while monitoring the verbal response and both state entropy (SE) and response entropy (RE). The induction was terminated when entropy reduced to 60. The dose of TPS required to induce loss of verbal response (TPS - LOVR) and for the RE to decrease to below 60 (TPS-entropy) were noted. The patient was ventilated with face mask at a respiratory rate of 14/min with 50% O2 and 50% N2 O and the trachea was intubated 2 min after giving 0.6 mg/kg rocuronium by a single experienced anaesthesiologist. The HR, SAP and DAP were recorded after induction, during laryngoscopy and endotracheal intubation, 1, 2, 3, 4, 5, 15, 30, 45, 60, 90 and 120 min after intubation and 5 min after extubation. The anaesthesia was maintained with 50% N2 O: 50% O2 and isoflurane. RE was maintained between 50 and 60 with isoflurane. The end tidal concentration of isoflurane was noted as iso - et at every 10 min interval, and the average was taken at the end of surgery. Additional doses of 0.5 μg/kg fentanyl were administered if the difference in RE and SE >10. The muscle relaxation was maintained with boluses of 10 mg of inj. rocuronium IV if there was clinical sign of recovery from muscle relaxation (evidenced as spontaneous breathing on capnography). The total dose of rocuronium and fentanyl given were noted. Adverse perioperative events comprising of hypoxemia (SpO2 < 90%), a decrease in SAP > 20% from the baseline value), bradycardia (HR < 50 beats/min), or nausea and vomiting were noted from the time of institution of block to conclusion of the study.
Post-operatively HR, SAP, DAP and sedation score (Ramsay score 1-6) were recorded every 30 min for 2 h. The sensory and motor block were assessed every 30 min after surgery until they resolved. Pain was assessed using the VAS every 30 min, and when the VAS was > 4, the patient received inj tramadol 2 mg/kg as rescue analgesic and the study was discontinued.
Eighteen patients in each group were enrolled. Sample size calculation was performed using PASS from the data of a recently published study,[5] which reported a significantly earlier onset of motor blockade (21 min in RD group and 47 min in R group). Twelve patients were needed in each group to achieve 75% power with significance of 0.05 using two sided t-test to detect a difference of 26 min. Statistical analysis was performed with Statistical package for Social Sciences 17.0 developed by IBM Corporation for Windows. Mean, median, standard deviation and interquartile range were given as definitive statistics. The intergroup comparison was done with Mann–Whitney test and intragroup comparisons were done with Freidman test. P value <0.05 was considered to be statistically significant.
RESULTS
Eighteen patients in each group were enrolled for the study. Due to inability to localise the brachial plexus, one patient in each group was excluded. Two patients in R group and one patient in RD group were excluded due to failure of block. Thirty-one patients (15 in the R and 16 in the RD group) were included in the final analysis of the results.
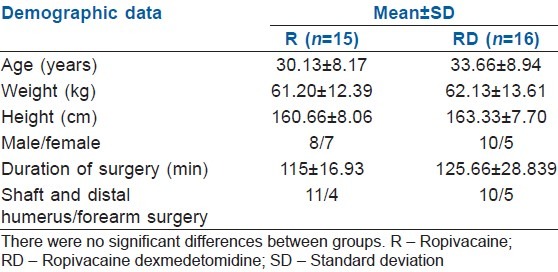
The demographic data and surgical characteristics were comparable in both groups [Table 1].
Table 1.
The demographic data and surgical characteristics of both groups
The onset of sensory blockade was faster in the RD group. However, there was no statistical significance (P = 0.133). The duration of sensory blockade and analgesia was significantly prolonged in group RD (P < 0.05) [Table 2].
Table 2.
Onset of sensory and motor blockade; duration of sensory, motor block and analgesia
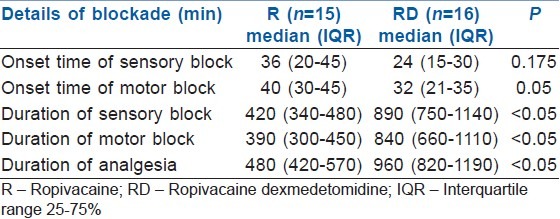
The proportion of patients who achieved complete motor blockade was more in the RD group. Five out of 15 (33%) patients in R group and 2 out of 16 (12%) in RD group had incomplete motor blockade and complete sensory block before induction of GA. In these 7 patients, 2 patients in the R group and 1 in the RD group had complete motor blockade at extubation. In these 3 patients, the time for the onset of motor blockade was taken as 45 min. In the remaining patients (three patients in R and 1 patient in RD group), the time to the highest grade of block achieved was noted as the onset time. The onset of motor block was significantly faster in group RD [Table 2]. In the patients who did not have complete motor blockade, time to recover from the highest degree of block achieved was recorded as the duration of motor blockade. The duration of motor block was prolonged in group RD (P < 0.05) [Table 2].
The HR was significantly lower at 30, 35, 40, 45 min after administration of block, at induction, intubation, 1, 2, 3, 4, 5, 15, min after intubation and at 5 min after extubation in RD group (P < 0.05) [Figure 1]. However, the incidence of bradycardia (HR < 50/min) was similar in both RD and R group. Two patients in each group had bradycardia. The SAP was significantly lower in the RD group at 30, 35, 45 min after the institution of block and at 4 and 5 min after intubation. The DAP was significantly lower in the patients in RD group at 5 min after intubation (P < 0.05) [Figure 2]. Transient hypotension (>20% fall in baseline systolic blood pressure) was seen in 3 of the 16 patients in the RD group and none in the R group after addition of isoflurane for maintenance of anaesthesia which responded to IV fluids and 6mg bolus of IV mephentermine.
Figure 1.
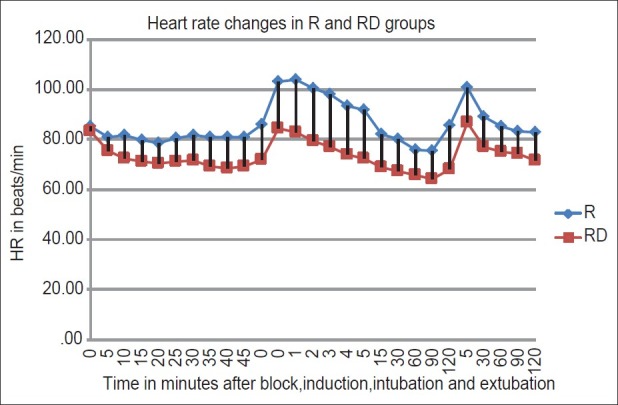
Comparison of heart rate changes in the R and RD group. R – Ropivacaine; RD – Ropivacaine Dexmedetomidine
Figure 2.
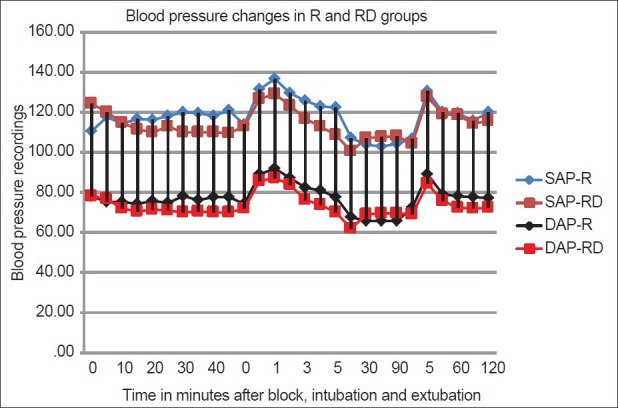
Comparison of blood pressure changes in the R and RD group. R – Ropivacaine; RD – Ropivacaine Dexmedetomidine; SAP – systolic aortic pressure, DAP – diastolic aortic pressure
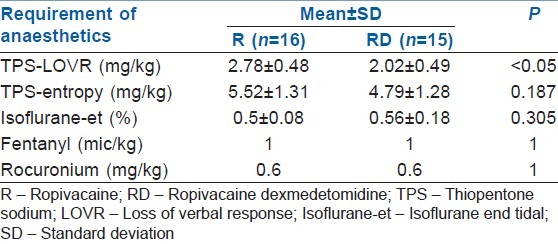
Pre-induction, the patients in RD group had significantly higher scores on RSS (P - 0.00) [Figure 3]. The onset of the sedation was noticed about 10 min after the injection of the drug, and the RSS continued to be higher in these patients up to the time of induction of GA. The dose of thiopentone for LOVR was significantly lower in the RD group in comparison to R group (P - 0.00). However, there was no difference in the requirement of thiopentone in either group when the end point of induction was taken as entropy <60. Also, the requirement of isoflurane to maintain entropy between 40 and 60 was not different in either group. None of the patients in either group needed additional doses of fentanyl and rocuronium [Table 3].
Figure 3.
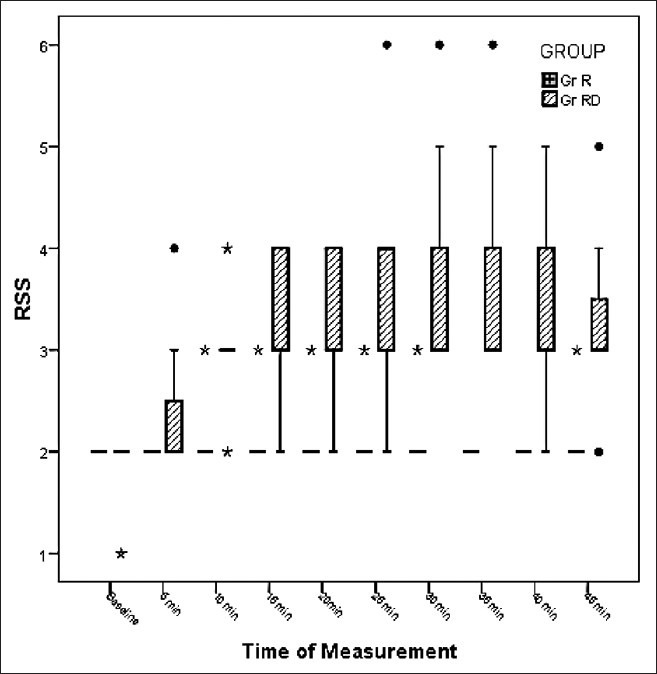
Ramsay sedation scores in the R and RD group. R – Ropivacaine, RD – Ropivacaine Dexmedetomidine (P < 0.05)
Table 3.
Requirements of anaesthetic agents
There was no significant difference in the incidence of nausea and vomiting in either group. There was no incidence of persistent paraesthesias or residual weakness of the operated upper limb in any patient before discharge from the hospital.
DISCUSSION
Brachial plexus block is commonly used as a sole anaesthetic technique or may be supplemented with GA for surgeries on the upper limb. Many adjuvants are added to LAs to hasten the onset and prolong the duration of the block.[10,11,12] Dexmedetomidine, an alpha 2 agonist, has been used with LAs in regional blocks and IV regional anaesthesia and has been found to significantly reduce the onset time and prolong the duration of sensory block and analgesia.[4,5,6,7,10,11,13] The action of dexmedetomidine in peripheral nerve blockade seems to be due to increase in hyperpolarization activated cation current that prevents the nerve from returning to resting membrane potential.[14] It has been used in combination with various LAs like levobupivacaine 0.5%,[4] bupivacaine 0.25%[6] and ropivacaine 0.75% and 0.5%[5,7] and in various plexus and nerve blocks like axillary,[4] posterior tibial,[5] supraclavicular[6] and ulnar nerve.[7]
Good muscle relaxation is a prerequisite in orthopaedic procedures. Though lignocaine causes profound muscle relaxation, it may be associated with significant local nerve toxicity. Bupivacaine not only causes motor sparing but also has serious cardiac complications if accidental intravascular injection occurs. Ropivacaine is a newer LA which has less systemic cardiac and local neurological complications. However, ropivacaine causes a greater sensory and motor differential blockade than bupivacaine[3] which is dose dependent, with higher concentrations (1%) causing greater degree of motor blockade than lower concentration (0.5% and 0.75%).[15] Combination of dexmedetomidine and ropivacaine increases the duration of sensory blockade and analgesia,[5,7] but its efficacy in improving the onset time, duration and the quality of motor blockade has been equivocal. Rancourt et al. evaluated the effect of 1 μg/kg dexmedetomidine added to 0.5% ropivacaine in posterior tibial nerve block.[7] In their study, although the duration of sensory block was significantly prolonged (by 5.3 h), the motor blockade was incomplete with ropivacaine and dexmedetomidine did not affect the quality of motor block. In another recent study by Marhofer et al. the effect of dexmedetomidine on 0.75% ropivacaine for ulnar nerve block was studied.[5] They compared three drug regimes with ropivacaine 0.75% (R), interaction of ropivacaine 0.75% with systemic dexmedetomidine or peineural dexmedetomidine on ulnar nerve block. Onset of motor block was faster, and the duration of motor block was significantly prolonged by the perineural administration of dexmedetomidine.
This study was undertaken to elucidate the influence of dexmedetomidine added to 0.5% ropivacaine for supraclavicular brachial plexus block on the characteristics of sensory and motor block with particular emphasis on the quality of motor blockade. Addition of dexmedetomidine to ropivacaine 0.5% shortened the onset time of motor block and significantly extended the duration of sensory and motor blocks and the duration of analgesia. Though the difference in the onset time between the groups was statistically significant, the sample size was not sufficiently powered to exclude an alpha error. The discrepancy in the onset time in the earlier study could have been as a result of this.[5] The duration of motor blockade was prolonged by 8.06 h in the RD group. The study had a power of 87% to detect this difference with an alpha error of 0.00001. We conclude that dexemedetomidine causes faster onset, extends the duration and improves the quality of motor blockade.
Intravenous dexmedetomidine acts on locus coeruleus and causes sedation[16] that mimics non-rapid eye movement sleep. This may generate falsely high values on the sedation scores. Significant sedation with the use of perineural dexmedetomidine was observed in our study and was thought to be due to systemic absorption of dexmedetomidine. Ramsay sedation score was at least one point higher in the RD group when compared to R group. But unlike Rancourt et al. who observed the effect between 60 and 120 min after the injection, the onset of sedation was seen as early as 10 min and patients in RD group continued to have a higher RSS until induction of GA.[7] This variation could be explained by the fact that the site of peripheral nerve block in both the studies was different and systemic absorption is faster with supraclavicular than posterior tibial nerve block. Due to the sedation, the dose of thiopentone required for LOVR was significantly less in the RD group. However, when objective measure of depth of anaesthesia like entropy was used neither the dose of thiopentone for induction nor the MAC of isoflurane for maintainence of anaesthesia was decreased in the RD group. From this, we hypothesize that though perineural dexmedetomidine reaches the systemic circulation in concentrations to cause sedation, the blood levels are not sufficiently high to influence the EEG entropy. We need further studies on the pharmacokinetics of perineural dexmedetomidine to confirm this hypothesis.
Perineural dexmedetomidine causes significant side effects like bradycardia and hypotension.[4] The incidence of these side effects increases with higher doses. Esmaoglu et al. in their study reported that the addition of 100 μg of dexmedetomidine to levobupivacaine for axillary block caused a high incidence of bradycardia.[4] But when lower doses (1 μg/kg, an average of 50–60 μg total) were used, the incidence of bradycardia was not significant.[5,6,7] The incidence of bradycardia was similar in both the groups in the present study. Three patients in RD group had transient hypotension, the incidence of which is similar to that reported in earlier studies.[7] Hypotension occurred between 15 and 60 min after GA was induced and was treated with IV fluids and mephenteramine. Therefore, we conclude that the interaction of perineural dexmedetomidine with GA does not seem to increase the incidence of bradycardia and hypotension.
This study has some limitations. Firstly, the fixed dose of 50 μg of dexmedetomidine was chosen empirically as earlier studies of higher doses of the drug showed significant bradycardia.[4] We wanted to ascertain whether the lower dose of the drug was effective in improving the quality of block without causing significant side effects. Secondly, a total of 7 patients in the study (5 in RD and 2 in R group) did not achieve complete blockade at the time of induction. Among the 7, in 3 patients complete motor block was observed at extubation. In these patients, the onset time of motor blockade was taken as 45 min. In fact, the onset time could have been much longer.
CONCLUSION
The addition of 50 μg of dexmedetomidine to 0.5% ropivacaine for supraclavicular brachial plexus block extended the duration of the sensory and motor blockade and post-operative analgesia. It improved the time of onset and the quality of motor blockade. Though perineural dexemedetomidine causes sedation, it does not reduce the requirement of anaesthetic agents when used in conjunction with GA. Further research is needed to study the pharmacokinetics of perineural dexmedetomidine and the effects of its systemic absorption.
Appendix 1: Ramsay sedation score

Footnotes
Source of Support: This work was undertaken by the department of Anaesthesiology and Critical care, Nizams Institute of Medical Sciences, Hyderabad, India and was funded by the Nizams Institute of Medical Sciences as a part of ongoing academic research
Conflict of Interest: None declared.
REFERENCES
- 1.Hickey R, Hoffman J, Ramamurthy S. A comparison of ropivacaine 0.5% and bupivacaine 0.5% for brachial plexus block. Anesthesiology. 1991;74:639–42. doi: 10.1097/00000542-199104000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hickey R, Rowley CL, Candido KD, Hoffman J, Ramamurthy S, Winnie AP. A comparative study of 0.25% ropivacaine and 0.25% bupivacaine for brachial plexus block. Anesth Analg. 1992;75:602–6. doi: 10.1213/00000539-199210000-00024. [DOI] [PubMed] [Google Scholar]
- 3.McClure JH. Ropivacaine. Br J Anaesth. 1996;76:300–7. doi: 10.1093/bja/76.2.300. [DOI] [PubMed] [Google Scholar]
- 4.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 5.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 6.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (a2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 8.Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: A single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39:37–47. doi: 10.1097/AAP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 9.Ghodki PS, Thombre SK, Sardesai SP, Harnagle KD. Dexmedetomidine as an anesthetic adjuvant in laparoscopic surgery: An observational study using entropy monitoring. J Anaesthesiol Clin Pharmacol. 2012;28:334–8. doi: 10.4103/0970-9185.98329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 11.Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ, Gwak MS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012;59:21–7. doi: 10.1007/s12630-011-9604-5. [DOI] [PubMed] [Google Scholar]
- 12.Sindjelic RP, Vlajkovic GP, Davidovic LB, Markovic DZ, Markovic MD. The addition of fentanyl to local anesthetics affects the quality and duration of cervical plexus block: A randomized, controlled trial. Anesth Analg. 2010;111:234–7. doi: 10.1213/ANE.0b013e3181e1e9ab. [DOI] [PubMed] [Google Scholar]
- 13.Esmaoglu A, Mizrak A, Akin A, Turk Y, Boyaci A. Addition of dexmedetomidine to lidocaine for intravenous regional anaesthesia. Eur J Anaesthesiol. 2005;22:447–51. doi: 10.1017/s0265021505000761. [DOI] [PubMed] [Google Scholar]
- 14.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finucane BT, Sandler AN, McKenna J, Reid D, Milner AL, Friedlander M, et al. A double-blind comparison of ropivacaine 0.5%, 0.75%, 1.0% and bupivacaine 0.5%, injected epidurally, in patients undergoing abdominal hysterectomy. Can J Anaesth. 1996;43:442–9. doi: 10.1007/BF03018104. [DOI] [PubMed] [Google Scholar]
- 16.Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993;71:447–9. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]


