Abstract
An 8-year-old, spayed female, bichon frisé dog had incidental nodules within its falciform ligament identified on routine abdominal ultrasonography. A laparoscopic-assisted technique provided both a diagnostic and a therapeutic treatment option. A histopathological diagnosis of hemangiosarcoma was made. This is the second case reporting hemangiosarcoma of the falciform fat.
Résumé
Extraction assistée par laparascopie d’un hémangiosarcome du ligament falciforme chez un chien. Une chienne Bichon frisé stérilisée âgée de 8 ans avait des nodules secondaires dans le ligament falciforme qui ont été identifiés lors d’une échographie abdominale de routine. Une technique assistée par laparascopie a fourni un diagnostic et une option de traitement thérapeutique. Un diagnostic d’hémangiosarcome a été posé à l’histopathologie. Il s’agit du deuxième cas d’hémangiosarcome du gras falciforme signalé.
(Traduit par Isabelle Vallières)
An 8-year-old, spayed female bichon frisé dog was presented for further evaluation of recurrent urinary tract infections. These occurred multiple times annually, with increasing frequency.
Case description
Upon presentation to the Ontario Veterinary College the dog was bright, alert, and responsive, with normal vital parameters. Thoracic auscultation revealed a grade III/VI left sided systolic heart murmur. The remainder of the physical examination was unremarkable. For complete evaluation of the reported urinary tract signs, a complete blood (cell) count (CBC), serum biochemistry profile, urinalysis, urine bacterial culture, and abdominal ultrasonography were performed.
The CBC revealed mild hemoconcentration [0.56 L/L; reference range (RR): 0.39 to 0.56 L/L] and mild hyperproteinemia (84 g/L; RR: 55 to 75 g/L). The serum biochemistry profile revealed mild elevations in alanine aminotransferase (122 U/L; RR: 19 to 107 U/L), amylase (1408 U/L; RR: 299 to 947 U/L), and lipase (833 U/L; RR: 25 to 353 U/L). A urine sample obtained by cystocentesis revealed hematuria (25 to 35 red blood cells/400× field), pyuria (80 to 100 leukocytes/400× field), bacteriuria (3+) and triple phosphate crystalluria (2+). Enterococcus faecalis and Staphylococcus pseudintermedius were cultured from the urine and both organisms were susceptible to amoxicillin-clavulanate (Clavamox; Zoetis, Kirkland, Quebec).
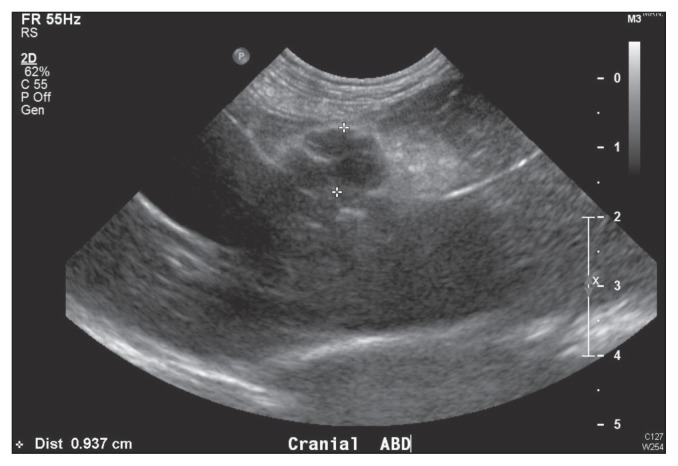
Abdominal ultrasonography identified urinary cystic calculi (< 1 mm), bilateral mild central renal mineralization, and bilateral mild pyelectasia. Additionally, a 1-cm hypoechoic nodule was visualized within the hyperechoic fat of the falciform ligament in the cranioventral abdomen (Figure 1). Cytological evaluation of an ultrasound-guided fine-needle aspirate of the focal hypoechoic nodule revealed extramedullary hematopoeisis and blood contamination.
Figure 1.
Focal hypoechoic nodule, demarcated by the markers, within the hyperechoic falciform fat.
The dog was treated with amoxicillin-clavulanate (Zoetis) for 1 mo prior to a repeat urine bacterial culture and abdominal ultrasound. Urine bacterial culture was negative and abdominal ultrasonography revealed mild left-sided pyelectasia, and several hypoechoic nodules within the hyperechoic fat of the falciform ligament compared with the single nodule identified previously. Differential diagnoses for the hypoechoic falciform ligament nodules included: vascular malformation, granuloma, neoplasia, or less likely abscesses or hematomas. Since previous cytological examination was non-diagnostic, histological biopsies were recommended for definitive diagnosis.
Upon further discussion with the owner, laparoscopy was recommended for a superficial exploration of the abdomen with concurrent removal of the falciform ligament, as an excisional biopsy. This was performed 1 wk later. The dog was anesthetized and exploratory laparoscopy was performed with the dog in dorsal recumbency. The assistant surgeon stood on the left side of the dog viewing a video monitor placed on the right side of the dog, while the primary surgeon stood at the caudal aspect of the dog and viewed a second monitor placed at the cranial aspect of the dog. A 3-portal technique was used. A modified Hasson technique was used to obtain abdominal access by placing a 6 mm, non-threaded, trocar-cannula assembly (KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) on the ventral midline, 1 cm caudal to the umbilicus. The abdomen was mechanically insufflated with carbon dioxide, to a maximum pressure of 12 mmHg. A 5 mm × 29 cm, 0°, laparoscope (KARL STORZ GmbH & Co. KG) was inserted and used for superficial exploration. No gross abnormalities were identified upon visualization of the liver, spleen, kidneys, urinary bladder, stomach, and readily visualized intestinal tract. The first instrument portal was established 5 cm lateral and 3 cm cranial to the umbilicus on the left side, with a 6-mm threadless trocar-cannula assembly (KARL STORZ GmbH & Co. KG) using laparoscopic observation. A second instrument portal was established on the midline 7 cm caudal to the subumbilical portal with a 12-mm trocar-cannula assembly (KARL STORZ GmbH & Co. KG). The laparoscope was then removed from the subumbilical portal and inserted into the craniolateral paramedian portal to visualize the falciform ligament. No gross abnormalities were seen on direct visualization of the falciform ligament, confirming the interior location of the nodules.
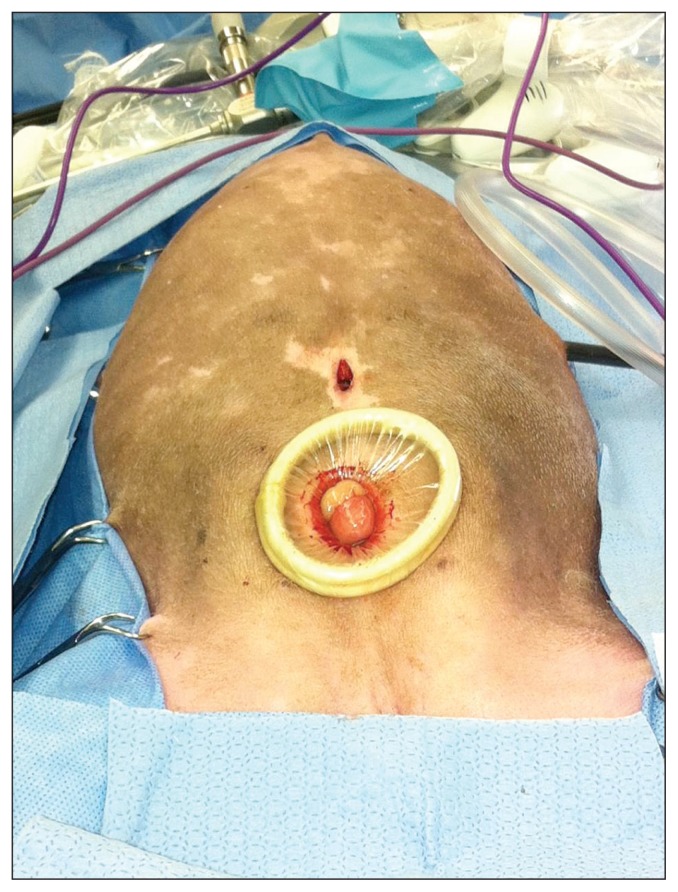
The falciform ligament was extirpated using a 10 mm × 20 cm vessel sealing device (Ligasure; Covidien Surgical, Boulder, Colorado) introduced through the caudal portal. The falciform fat was excised along its length from the ventral abdominal wall in a caudal to cranial direction. The caudal portal was converted to a 3 cm mini-celiotomy by incising the skin, subcutaneous tissues, and linea alba, thereby allowing abdominal desufflation. A 2- to 4-cm wound retractor (Alexis wound retractor; Applied Medical, Rancho Santa Margarita, California, USA) was placed in the mini-celiotomy (Figure 2). The intact falciform ligament was exteriorized through the wound retractor. The mini-celiotomy was closed routinely in 3 layers. After recreating a pneumoperitoneum, laparoscopic examination confirmed no active bleeding and complete resection of the falciform ligament. The remaining portals were removed and the portal incision sites were closed routinely in 3 layers. Recovery was unremarkable and the dog was discharged from the hospital the next day.
Figure 2.
Alexis wound retractor™ placed in a caudal mini-celiotomy incision.
Histopathological evaluation of the falciform ligament identified a central well-demarcated mass, with additional masses in the surrounding adipose tissue. The central mass consisted mainly of erythrocytes that were present in channels, separated by bundles of well-differentiated spindyloid cells, on columns of collagen. The smaller masses displayed the same channels. All masses were considered to be hemangiosarcoma.
Based on the histopathological diagnosis, 3 view thoracic radiographs were taken and did not reveal any abnormalities. The dog received chemotherapy treatment with a doxorubicin-based protocol (Adriamycin; Zoetis). The initial dose was 1 mg/kg body weight (BW), and treatments were administered every 3 wk for a total of 12 wk (4 treatments). Due to persistent urinary tract infections cystoscopy was performed 4 mo after surgery and revealed a urethral stricture. Seven months after surgery, abdominal ultrasonography identified a thickened bladder wall and several hyperechoic nodules within the mesenteric fat, the largest of which was 6 mm in diameter. Cytologic or histopathologic diagnosis of these nodules was not performed. With the presence of the mesenteric nodules metastatic disease was suspected and metronomic chemotherapy with chlorambucil (Leukeran; GlaxoSmithKline, Research Triangle Park, North Carolina, USA), 0.2 mg/kg BW, was initiated 9 mo after surgery. No further staging has been performed since initiation of metronomic chemotherapy.
Discussion
In dogs, the falciform ligament is a remnant of the embryonic ventral mesentery. It consists of a fold of peritoneum that passes from the umbilicus to the diaphragm, and has an attachment between the left medial and quadrate lobes of the liver. The round ligament of the liver, previously the umbilical vein, may be identified on the free edge of the falciform ligament in young dogs and consists mainly of adipose tissue. The left and right peritoneal sheets of the falciform ligament become an extension of the left portion of the coronary ligament and the ventral portion of the coronary ligament respectively (1). There is limited information in the veterinary literature regarding the blood supply of the falciform ligament. In a human anatomical study (2), the blood vessels of the falciform ligament were identified to anastamose with multiple vessels including the left inferior phrenic artery and the middle segment artery to form collateral circulation of the liver. The falciform veins were identified to drain into the left inferior phrenic vein.
Hemangiosarcoma (HSA) is a highly malignant tumor arising from vascular endothelium (3). This tumor occurs more frequently in dogs than in any other species, with the German shepherd, golden retriever, and Labrador retriever being over-represented (4). Most common sites for primary HSA are the spleen, right atrium, skin, subcutis, and liver (5). Less commonly HSAs have been reported in the lung, kidney, oral cavity, muscle, bone, urinary bladder, left ventricle, uterus, tongue, digit, and retroperitoneum (5). With splenic HSA, the most frequent sites of distant metastasis include the liver, omentum, and lungs (5). Spread of disease may occur via a hematogenous route or by seeding of local tissues upon tumor rupture (5). It is therefore pertinent that complete staging be performed to rule out a site of primary HSA when a diagnosis is made in an uncommon location, such as in the dog in this report, which documents the second reported case of HSA of the falciform ligament. The dog in the initial report remained tumor-free 4 y following excision of the falciform ligament, with no evidence of metastasis on thoracic and abdominal radiographs (6).
Diseases of the falciform ligament are extremely rare in the veterinary literature. One report (7) describes a sterile nodular panniculitis and pansteatitis in the subcutaneous, mesenteric and falciform fat of 3 unrelated Weimaraner dogs which presented with pyrexia and subcutaneous nodules. It was presumed that this represented an idiopathic sterile disease due to lack of microbial growth, and was speculated to be immune-mediated. Diseases of the falciform ligament are similarly sporadic in the human literature. Internal herniation through the falciform ligament has been described (8). It is most commonly associated with congenital defects, although it has been reported in association with trauma, pregnancy, or iatrogenic defects from previous surgery (8). Other diseases reported include falciform ligament abscess and hematoma formation (9,10).
Several reports exist in the human literature of falciform ligament primary tumors, including fibromyxoid sarcoma and lymphangioma (11,12). In humans, patients with diseases of the falciform ligament most commonly present with abdominal pain, anorexia, or vomiting, unlike the dog of this report (8–12). Although disease of the falciform ligament remains uncommon, diagnostic imaging such as abdominal radiographs, ultrasonography, and computed tomography are performed based on the presenting complaint of abdominal pain (8–12). A computed tomography (CT) scan is considered essential prior to surgical treatment of these diseases (13). However, CT scan may not always offer a definitive diagnosis of a falciform-related disease as was demonstrated when diagnostic laparoscopy confirmed a mass associated with the falciform ligament, and not the liver as was suspected on CT scan imaging (11).
In dogs, biopsy samples of the falciform ligament may be obtained by ultrasound-guided Tru-Cut biopsy, laparoscopy, or laparotomy. Percutaneous ultrasound-guided biopsy is a minimally invasive biopsy technique which may provide an equivalent histologic sample when compared to surgically acquired samples. However, there is a risk of seeding of the needle tract or the abdominal cavity with neoplastic cells from the biopsy site with this procedure (14). Laparoscopy offers a minimally invasive technique for biopsy, which allows for superior visualization, easy sample collection, and reduced morbidity compared with laparotomy (15). Conversion to laparotomy may be performed if deemed necessary based on findings during exploratory laparoscopy (16). We decided to perform a laparoscopic-assisted technique in the dog of this report as it was a minimally invasive technique for extirpation of the falciform ligament to obtain a tissue sample for histopathological evaluation. Laparoscopy provides improved illumination, reduced post-operative pain and hospital stay (16). Additionally, laparoscopic-assisted extirpation prevented inadvertent trauma or compromise to the falciform ligament that may have occurred during creation of the laparotomy incision that could result in tumor seeding. Finally, this technique allowed for removal of the falciform ligament by use of the vessel sealing device which allowed for resection of the ligament in close proximity to the body wall.
We used a three-portal technique for laparosopic-assisted extirpation of the falciform ligament. The subumbilical portal was only used to obtain access to the abdomen and superficial exploration. The laparoscope was then removed from the subumbilical portal and inserted through the left paramedian portal to guide resection of the falciform ligament using the vessel-sealing device in a caudal to cranial direction. Because of the concern for port site metastasis, we recommend not placing the subumbilical portal in future cases of laparoscopic-assisted extirpation of the falciform. Further recommendations include the use of a 30° laparoscope to allow for improved visualization and inspection of the falciform ligament and consideration of a 2-portal technique in which the initial laparoscopic portal can be placed in a paramedian location. A 3-portal technique can be considered where bilateral paramedian portals are placed, in addition to a caudal abdominal instrument portal. Bilateral paramedian portals allow for complete visualization and inspection of the falciform ligament which may improve resection depending on lesion location. The vessel-sealing device used for falciform resection was essential to the surgical technique as it limited hemorrhage and allowed for accurate resection of the falciform ligament proximal to the body wall. A final consideration in future cases is removal of the falciform ligament by using a specimen retrieval device, making it an entirely laparoscopic procedure compared with the laparoscopic-assisted approach performed in the dog herein.
The wound retractor used in the dog in this report is made of self-expanding polyurethane, which exerts a radial force when inserted into a small abdominal wall incision (17). This atraumatic force allows expansion of a small incision and protects the body wall from contact with exteriorized viscera (17). The wound retractor also reduces the risk of surgical site infection by preventing bacteria from contaminating the abdominal wall during surgery (18). An additional benefit of using the wound retractor was to reduce the risk of seeding the abdominal wall with neoplastic cells (port site metastasis) upon specimen retrieval.
In a retrospective study, treatment of stage I and stage II splenic hemangiosarcoma with surgery alone identified a lack of correlation between disease stage and survival times (19). Due to the rapid growth and metastasis of these tumors, surgery alone does not often result in improved survival times (19). Doxorubicin-based chemotherapy protocols have shown the greatest efficacy and improvement in survival times, with acceptable morbidity (19,20). The dog in this report received doxorubicin every 3 wk for 4 treatments. Re-staging performed at the time of the third chemotherapy session revealed no evidence of abdominal re-growth or thoracic metastatic disease.
This is the second report describing a case of hemangiosarcoma of the falciform ligament in a dog. Laparoscopy, in combination with the use of a wound retraction device, offers an excellent diagnostic and therapeutic option with minimal morbidity for extirpation of the falciform ligament if histopathological evaluation is required. Complete resection of the falciform ligament, in combination with doxorubicin chemotherapy provided a good short-term outcome for this dog. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Evans HE, de Lahunta A. Miller’s Anatomy of the Dog. 4th ed. St. Louis, Missouri: WB Saunders; 2013. p. 330. [Google Scholar]
- 2.Li XP, Xu DC, Tan HY, Li CL. Anatomical study on the morphology and blood supply of the falciform ligament and its clinical significance. Surg Radiol Anat. 2004;26:106–109. doi: 10.1007/s00276-003-0184-0. [DOI] [PubMed] [Google Scholar]
- 3.Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006;34:870–878. doi: 10.1016/j.exphem.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Brown NO. Hemangiosarcomas. Vet Clin North Am Small Anim Pract. 1985;15:569–575. doi: 10.1016/s0195-5616(85)50058-3. [DOI] [PubMed] [Google Scholar]
- 5.Thamm DH. Miscellaneous tumors. In: Withrow SJ, Vail DM, Page RL, editors. Small Animal Clinical Oncology. 5th ed. St. Louis, Missouri: Elsevier Saunders; 2013. pp. 679–688. [Google Scholar]
- 6.Triolo J, Breu BA. What is your diagnosis? Hemangiosarcoma of the falciform ligament. J Am Vet Med Assoc. 1993;202:133–134. [PubMed] [Google Scholar]
- 7.German AJ, Foster AP, Holden D, Hotston Moore A, Day MJ, Hall EJ. Sterile nodular panniculitis and pansteatitis in three weimaraners. J Small Anim Pract. 2003;44:449–455. doi: 10.1111/j.1748-5827.2003.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 8.Egle J, Gupta A, Mittal V, Orfanou P, Silapaswan S. Internal hernias through the falciform ligament: A case series and comprehensive literature review of an increasingly common pathology. Hernia. 2013;17:95–100. doi: 10.1007/s10029-012-0990-6. [DOI] [PubMed] [Google Scholar]
- 9.Warren LR, Chandrasegaram MD, Madigan DJ, Dolan PM, Neo EL, Worthley CS. Falciform ligament abscess from left sided portal pyaemia following malignant obstructive cholangitis. World J Surg Oncol. 2012;10:278–281. doi: 10.1186/1477-7819-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sari S, Ersöz F, Güneş ME, Paşaoğlu E, Arikan S. Hematoma of the falciform ligament: A rare cause of acute abdomen. Turk J Gastroenterol. 2011;22:213–215. doi: 10.4318/tjg.2011.0196. [DOI] [PubMed] [Google Scholar]
- 11.Harish K, Ashok AC, Alva NK. Low grade fibromyxoid sarcoma of the falciform ligament: A case report. BMC Surg. 2004;3:7. doi: 10.1186/1471-2482-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan K, Ricketts RR. Lymphangioma of the falciform ligament — A case report. J Pediatr Surg. 2004;39:1276–1279. doi: 10.1016/j.jpedsurg.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Brock JS, Pachter HL, Schreiber J, Hofstetter SR. Surgical diseases of the falciform ligament. Am J Gastroenterol. 1992;87:757–758. [PubMed] [Google Scholar]
- 14.Trochsler MI, Ralph Q, Bridgewater F, Kanhere H, Maddern GJ. Technical note: Facilitating laparoscopic liver biopsy by the use of a single-handed disposable core biopsy needle. HPB Surg. 2013;2013:462–498. doi: 10.1155/2013/462498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lansdowne JL, Mehler SJ, Bouré LP. Minimally invasive abdominal and thoracic surgery: Principles and instrumentation. Compend Contin Educ Vet. 2012;34:E1. [PubMed] [Google Scholar]
- 16.Karateke F, Özdoğan M, Özyazıcı S, et al. The management of penetrating abdominal trauma by diagnostic laparoscopy: A prospective non-randomized study. Ulus Travma Acil Cerrahi Derg. 2013;19:53–57. doi: 10.5505/tjtes.2013.40799. [DOI] [PubMed] [Google Scholar]
- 17.Gower SB, Mayhew PD. A wound retraction device for laparoscopic-assisted intestinal surgery in dogs and cats. Vet Surg. 2011;40:485–488. doi: 10.1111/j.1532-950X.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 18.Horiuchi T, Tanishima H, Tamagawa K, et al. Randomized, controlled investigation of the anti-infective properties of the Alexis retractor/protector of incision sites. J Trauma. 2007;62:212–215. doi: 10.1097/01.ta.0000196704.78785.ae. [DOI] [PubMed] [Google Scholar]
- 19.Wood CA, Moore AS, Gliatto JM, Ablin LA, Berg RJ, Rand WM. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991–1993) J Am Anim Hosp Assoc. 1998;34:417–421. doi: 10.5326/15473317-34-5-417. [DOI] [PubMed] [Google Scholar]
- 20.Kim SE, Liptak JM, Gall TT, Monteith GJ, Woods JP. Epirubicin in the adjuvant treatment of splenic hemangiosarcoma in dogs: 59 cases (1997–2004) J Am Vet Med Assoc. 2007;231:1550–1557. doi: 10.2460/javma.231.10.1550. [DOI] [PubMed] [Google Scholar]