Abstract
Our objectives were to describe the antimicrobial susceptibility of Escherichia coli isolates from dogs in the northeastern USA and to identify temporal trends in resistance to selected antimicrobial agents. Data were collected retrospectively for all canine E. coli isolates from clinical samples submitted to Cornell University’s Animal Health Diagnostic Center between January 1, 2004 and December 31, 2011. Antimicrobial susceptibility testing was performed on 3519 canine E. coli isolates; frequency of resistance to each agent ranged from 0.4% (amikacin) to 34.3% (ampicillin). No trends were evident among urinary isolates, but cephalosporin resistance remained consistently high. Among non-urinary isolates, there was evidence of a significantly increasing trend in prevalence of resistance to several agents, including cephalosporins, enrofloxacin, and tetracycline. These data suggest that some of the most commonly used antimicrobial agents in companion animal practice are becoming less effective against canine E. coli infections outside the urinary tract.
Résumé
Tendances de la résistance antimicrobienne parmi les isolats canins d’Escherichia coli obtenus dans des échantillons cliniques dans le nord-est des États-Unis de 2004 à 2011. Nos objectifs consistaient à décrire la susceptibilité des isolats d’Escherichia coli chez des chiens dans le nord-est des États-Unis et à identifier les tendances de résistance temporelles aux agents antimicrobiens sélectionnés. Des données ont été recueillies rétrospectivement pour tous les isolats canins d’E. coli provenant d’échantillons cliniques soumis à l’Animal Health Diagnostic Center de l’Université Cornell entre le 1er janvier 2004 et le 31 décembre 2011. Des épreuves de sensibilité antimicrobienne ont été réalisées sur 3519 isolats canins E. coli; la fréquence de résistance à chaque agent allait de 0,4 % (amikacine) à 34,3 % (ampicilline). Aucunes tendances n’étaient évidentes parmi les isolats urinaires, mais la résistance à la céphalosporine demeurait constamment élevée. Parmi les isolats non urinaires, il y avait des preuves d’une tendance significative à la hausse de la prévalence de la résistance à plusieurs agents, y compris les céphalosporines, l’enrofloxacine et la tétracycline. Ces données suggèrent que certains des agents antimicrobiens les plus communément utilisés en pratique des animaux de compagnie deviennent de moins en moins efficaces contre les infections canines par E. coli à l’extérieur des voies urinaires.
(Traduit par Isabelle Vallières)
Introduction
Antimicrobial resistance among bacteria isolated from companion animals is an emerging problem with implications for patient management and public health (1). In dogs, common bacterial infections that are treated with antimicrobial agents include gastroenteritis, otitis, pyoderma, respiratory infections, urinary tract infections (UTIs), and wound infections (2–4). Antimicrobial resistance limits treatment options and increases the risk of therapeutic failure. In addition, the occurrence of drug-resistant bacteria in dogs represents a potential threat to human health. Evidence suggests that direct contact between companion animals and humans can lead to interspecies transmission of pathogenic bacteria, including strains that demonstrate antimicrobial resistance (5,6).
Escherichia coli is a useful sentinel organism for monitoring antimicrobial susceptibility in dogs and other species (4,7). Some strains are commensal organisms in the mammalian intestinal tract, but E. coli is also one of the most frequently isolated bacterial pathogens in companion animal practice (8,9). Most recognized mechanisms of antimicrobial resistance have been detected in E. coli, and it is an organism that readily acquires resistance in the face of local selection pressure (10,11). Thus, E. coli is a key source of antimicrobial resistance genes that could confer resistance to other bacterial pathogens via horizontal transfer mechanisms (1). However, the scope of our understanding has been constrained by limited surveillance for antimicrobial resistance among E. coli and other bacteria isolated from companion animals.
The objectives of this study were thus to describe the antimicrobial susceptibility of E. coli isolates from dogs in New York and other northeastern states during 2004–2011 and to identify trends in resistance to certain antimicrobial agents over time. This information may help to guide empiric selection of antimicrobial agents when treating canine infections and may serve to further inform the discussion of judicious antimicrobial use in companion animal practice.
Materials and methods
Study design
We collected data retrospectively for all canine E. coli isolates that were obtained from clinical samples submitted to the Cornell University Animal Health Diagnostic Center (AHDC) between January 1, 2004 and December 31, 2011 and that were subsequently tested for antimicrobial susceptibility. Based on AHDC canine sample submission data over an overlapping time frame of comparable duration, we estimate that 65% of samples were submitted by regional veterinary practices and 35% by the Cornell University Hospital for Animals (CUHA). The proportion of samples submitted by the CUHA on an annual basis during this time frame ranged from 33% to 39%. We assume that a history of clinical disease generally prompted sample submission by practitioners. Variables collected from the computerized records database included the date of E. coli isolation, sample source, and susceptibility to each antimicrobial agent. Information concerning disease severity and previous therapy was not available.
Microbiologic procedure for E. coli detection
Personnel at the AHDC used standard bacteriologic culture methods to isolate E. coli from samples. Briefly, sample material was inoculated onto Columbia agar with 5% sheep blood and onto Eosin Methylene Blue (EMB) agar. Individual colonies were then chosen as presumptive for E. coli based upon morphology. Identity of isolates was confirmed as E. coli using the Sensititre Automated Microbiology System (TREK Diagnostic Systems, Cleveland, Ohio, USA). Guidelines established by the Clinical and Laboratory Standards Institute (CLSI) were used throughout the isolation process (12).
Antimicrobial susceptibility testing
Antimicrobial susceptibility of E. coli isolates was determined by use of the broth microdilution method. Minimal inhibitory concentrations (MIC) were established for each isolate against various panels of antimicrobial agents. The antimicrobial agents selected for this study have pharmacologic activity against E. coli and are clinically relevant to canine medicine, either through therapeutic use or as markers for susceptibility to commonly used agents. This list includes amikacin (AMI), amoxicillin/clavulanic acid (AUG), ampicillin (AMP), cefazolin (FAZ), cefoxitin (FOX), cephalothin (CEP; used as a marker for susceptibility to other first-generation cephalosporins including cephalexin and cefadroxil), chloramphenicol (CHL), enrofloxacin (ENRO), gentamicin (GEN), tetracycline (TET), and trimethoprim/sulfamethoxazole (SXT). The CLSI guidelines were used to interpret MIC values (13,14). Regardless of isolation year, all MIC values were interpreted using the same set of current guidelines. Isolates were classified as being resistant or susceptible to each agent; those few isolates with intermediate susceptibility were categorized as being susceptible. Quality control was performed weekly using Escherichia coli ATCC 25922, Staphylococcus aureus 29213, Enterococcus faecalis 29212, and Pseudomonas aeruginosa 27853. The MIC ranges for quality control recommended by the CLSI were used, and results were accepted if the MIC values were within expected ranges for these bacterial strains.
Statistical analysis
Data were imported into a commercial statistical software program (SAS, version 9.2; SAS Institute, Cary, North Carolina, USA) for variable coding and analysis. Descriptive analysis was performed on all variables. Associations between antimicrobial resistance and isolate source were analyzed using separate logistic regression models for each agent, while controlling for year of isolation. Susceptibility status (resistant or not) was used as the dichotomous outcome variable in these models. Temporal trends in the prevalence of resistant E. coli between 2004 and 2011 were investigated for each antimicrobial agent using the Cochran-Armitage trend test. For all analyses, P-values < 0.05 were considered significant.
Results
Between January 1, 2004 and December 31, 2011, the AHDC performed antimicrobial susceptibility testing on 3519 canine E. coli isolates from submitted clinical samples. Among these isolates, 57.8% (2034) were obtained from urine or urinary tract samples, 7.1% (251) from external ear samples, 4.5% (160) from wounds or other integumentary samples, 3.8% (132) from feces or intestinal tract samples, 2.8% (98) from respiratory tract samples, 22.1% (779) from miscellaneous locations (including blood, bone, cerebrospinal fluid, eye, gall bladder, joint, liver, lymph node, reproductive tract, and spleen), and 1.8% (65) from unspecified locations. Antimicrobial agents were used with varying frequency for MIC determinations, with a median of 1713 isolates (range: 1325 to 3480) being tested for susceptibility to each agent.
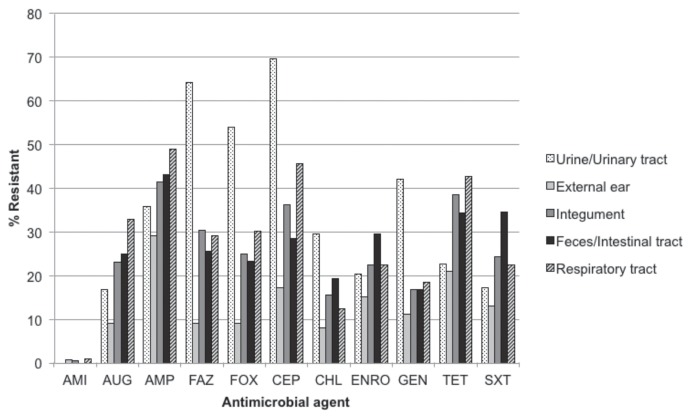
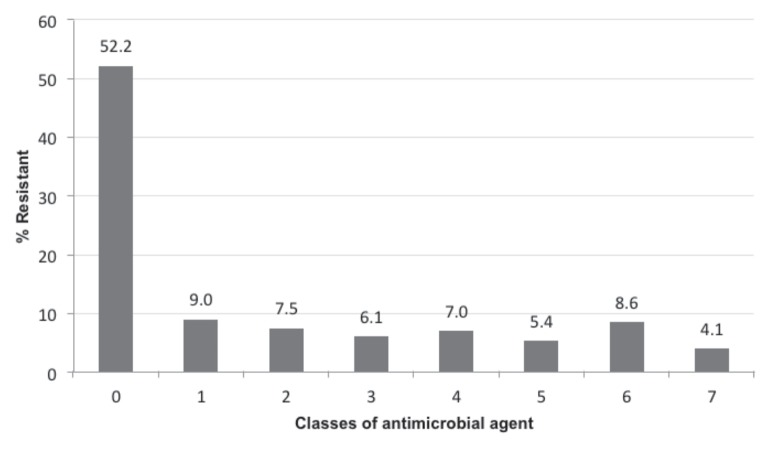
Frequency of resistance to individual antimicrobial agents (Table 1) ranged from 0.4% (amikacin) to 34.3% (ampicillin) of all isolates tested. Urinary isolates displayed a higher frequency of resistance to cephalosporins than to any other class of agent (Figure 1), with more than half being resistant to cefazolin (64.3%), cefoxitin (54.1%), and cephalothin (69.7%). In contrast, resistance to ampicillin was most frequent among isolates from the external ear (29.1%), integument (41.5%), intestinal tract (43.1%), and respiratory tract (49.0%; Figure 1). Among the 1161 isolates that were tested using all antimicrobial agents in this study, the most common resistance patterns (Table 2) were pan-susceptible (52.2%), AUG-AMP-FAZ-FOX-CEP-ENRO-GEN-TET-SXT (4.0%), AMP (3.9%), and AUG-AMP-FAZ-FOX-CEP-CHL-ENRO-GEN-TET-SXT (3.2%). Multidrug resistance, defined here as in vitro resistance to 2 or more classes of antimicrobial agent, was observed in 38.8% (450/1161) of isolates that were tested using all antimicrobial agents in this study. Resistance to a single class of antimicrobial agent was observed in 9.0% (105/1161) of these isolates (Figure 2).
Table 1.
Resistance to individual antimicrobial agents among E. coli isolates from dogs in the northeastern USA, 2004–2011
| Antimicrobial agent | Number of isolates tested | % Resistant |
|---|---|---|
| Ampicillin | 3480 | 34.3 |
| Cephalothin | 1325 | 27.9 |
| Tetracycline | 2974 | 23.6 |
| Cefazolin | 1669 | 22.9 |
| Cefoxitin | 1686 | 19.9 |
| Enrofloxacin | 3473 | 18.1 |
| Trimethoprim/sulfamethoxazole | 3474 | 17.9 |
| Amoxicillin/clavulanic acid | 3476 | 15.7 |
| Gentamicin | 1713 | 15.5 |
| Chloramphenicol | 1711 | 12.6 |
| Amikacin | 1711 | 0.4 |
Figure 1.
Resistance to individual antimicrobial agents among canine E. coli isolates stratified by sample source, 2004–2011.
AMI — amikacin; AUG — amoxicillin/clavulanic acid; AMP — ampicillin; FAZ — cefazolin; FOX — cefoxitin; CEP — cephalothin; CHL — chloramphenicol; ENRO — enrofloxacin; GEN — gentamicin; TET — tetracycline; SXT — trimethoprim/sulfamethoxazole
Table 2.
Most common resistance patterns among 1161 canine E. coli isolates that were tested for susceptibility to all antimicrobial agents used in this study, 2004–2011
| Resistance patterna | Number of isolates | % of isolates |
|---|---|---|
| Pan-susceptible | 606 | 52.2 |
| AUG-AMP-FAZ-FOX-CEP-ENRO-GEN-TET-SXT | 47 | 4.0 |
| AMP | 45 | 3.9 |
| AUG-AMP-FAZ-FOX-CEP-CHL-ENRO-GEN-TET-SXT | 37 | 3.2 |
| TET | 28 | 2.4 |
| AUG-AMP-FAZ-FOX-CEP-CHL-ENRO-TET-SXT | 25 | 2.2 |
| AUG-AMP-FAZ-FOX-CEP | 21 | 1.8 |
| AMP-TET-SXT | 20 | 1.7 |
| AMP-ENRO-GEN-TET-SXT | 14 | 1.2 |
| AUG-AMP-FAZ-FOX-CEP-ENRO-TET | 13 | 1.1 |
| AUG-AMP-FAZ-FOX-CEP-ENRO-TET-SXT | 11 | 0.9 |
| AMP-ENRO-TET-SXT | 11 | 0.9 |
AUG — amoxicillin/clavulanic acid; AMP — ampicillin; FAZ — cefazolin; FOX — cefoxitin; CEP — cephalothin; CHL — chloramphenicol; ENRO — enrofloxacin; GEN — gentamicin; TET — tetracycline; SXT — trimethoprim/sulfamethoxazole.
Figure 2.
Distribution of resistance by number of antimicrobial classes among 1161 canine E. coli isolates that were tested for susceptibility to all antimicrobial agents used in this study, 2004–2011.
Multivariable logistic regression analysis showed that urinary isolates were significantly more likely than non-urinary isolates (i.e., isolates from all other specified sources) to demonstrate in vitro resistance to cefazolin [odds ratio (OR) = 9.7; 95% confidence interval (95% CI) = 7.1 to 13.2; P < 0.0001], cefoxitin (OR = 7.1; 95% CI = 5.2 to 9.5; P < 0.0001), cephalothin (OR = 8.9; 95% CI = 6.3 to 12.5; P < 0.0001), chloramphenicol (OR = 4.2; 95% CI = 3.0 to 5.9; P < 0.0001), and gentamicin (OR = 5.9; 95% CI = 4.3 to 8.0; P < 0.0001), after accounting for year of isolation. Resistance to other antimicrobial agents did not differ significantly by isolate source. Among the isolates that were tested using all antimicrobial agents in this study, urinary isolates were significantly more likely to be multidrug-resistant than were non-urinary isolates (OR = 11.4; 95% CI = 7.4 to 17.6; P < 0.0001), after accounting for year of isolation.
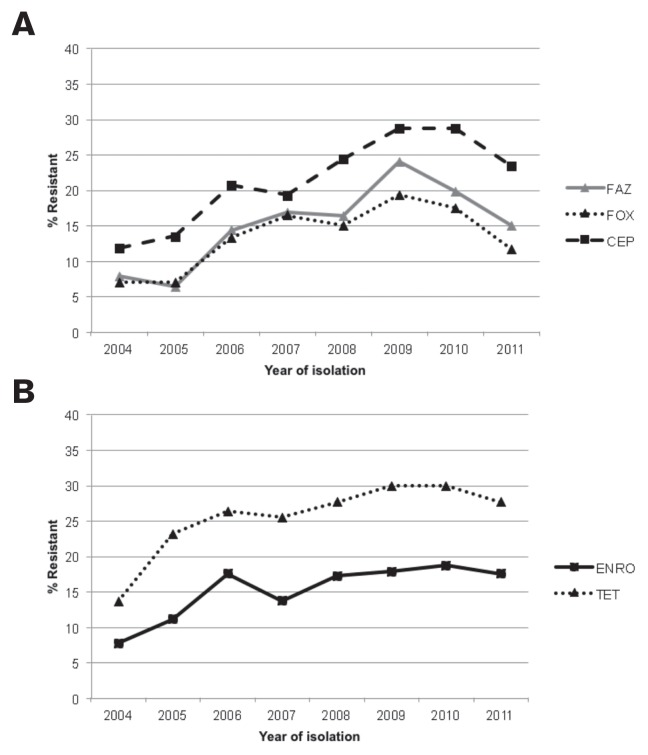
The Cochran-Armitage test revealed no significant temporal trends for antimicrobial resistance among urinary isolates. In contrast, among non-urinary isolates there was evidence of a significantly increasing trend in prevalence of resistance to several antimicrobial agents over time: cefazolin (P = 0.0002), cefoxitin (P = 0.02), cephalothin (P = 0.0003), enrofloxacin (P = 0.04), and tetracycline (P = 0.0007) (Figures 3A, 3B). There were no significant trends for resistance to amikacin, amoxicillin/clavulanic acid, ampicillin, chloramphenicol, gentamicin, or trimethoprim/sulfamethoxazole among non-urinary isolates.
Figure 3.
A — Temporal trends in the prevalence of cephalosporin resistance among non-urinary E. coli isolates from dogs in the northeastern USA, 2004–2011.
FAZ — cefazolin; FOX — cefoxitin; CEP — cephalothin
B — Temporal trends in the prevalence of enrofloxacin and tetracycline resistance among non-urinary E. coli isolates from dogs in the northeastern USA, 2004–2011.
ENRO — enrofloxacin; TET — tetracycline
Discussion
Monitoring antimicrobial resistance trends among bacteria isolated from dogs is useful for guiding antimicrobial use practices in companion animal medicine. Although antimicrobial susceptibility among bacteria isolated from food animals is regularly monitored through various federal programs (15,16), targeted surveillance in companion animals has been limited. This study was based on data collected from the AHDC database over an 8-year period, including 3519 clinical canine E. coli isolates which had been subjected to antimicrobial susceptibility testing. Although not all isolates were tested for susceptibility to each of the 11 antimicrobial agents used in this study, the scope of our sample (in terms of isolate number and time span) and source of our isolates (diagnostic laboratory submissions from a single geographic region) made this a valuable dataset for studying antimicrobial resistance trends.
Escherichia coli was isolated from a diverse array of body sites during the study period, but the predominant source of isolates was the urinary tract. This is in agreement with other studies in which the urinary tract was the most common source of clinical canine E. coli isolates (4,17). Urinary tract infections are observed frequently in dogs, especially older female dogs, and are generally caused by ascending bacteria (18,19). Clinical signs can include stranguria, pollakiuria, and hematuria (9). Canine UTIs are typically uncomplicated and resolve with an appropriate course of oral antimicrobial therapy (20). However, infections can be persistent or recurrent because of pathogen factors or predisposing conditions in the host (21,22). Escherichia coli is the most common pathogen isolated from the canine urinary tract, accounting for 40% to 50% of isolates from clinical cases of UTI (18,21–25).
Urinary E. coli isolates were more likely to be multidrug-resistant than were isolates from other body sites, after accounting for year of isolation. Cephalosporins had the poorest in vitro efficacy against urinary isolates, with resistance frequency ranging from 54% to 70% depending on the agent. Selection pressure associated with prior antimicrobial therapy may be responsible for the relatively high resistance to cephalosporins among urinary isolates in this study. A number of samples, particularly among those submitted by CUHA veterinarians, likely originated from dogs that had received previous antimicrobial therapy, and cephalosporins are commonly recommended for treating canine UTIs (20). Cephalosporins are excreted by the kidneys, and recent work highlights the potential of their metabolites to exert selection pressure for resistant E. coli (26). Alternatively, cephalosporin resistance genes could be physically linked to virulence genes that facilitate colonization and infection of the urinary tract. Various beta-lactamase genes, which are the most important mediators of cephalosporin resistance in E. coli, are increasingly being recognized among uropathogenic and other extraintestinal pathogenic E. coli (27). A number of E. coli sequence types, including the uropathogenic ST131, appear to be experiencing clonal expansion as a result of a combination of antimicrobial resistance [including resistance conferred by extended-spectrum beta-lactamases (ESBL)] and assorted virulence mechanisms (28–31).
Among canine non-urinary E. coli isolates, the prevalence of resistance to several antimicrobial agents, including cefazolin, cefoxitin, cephalothin, enrofloxacin, and tetracycline, increased significantly over the 2004–2011 study period. Interestingly, these increasing trends in resistance were not evident among urinary E. coli isolates; cephalosporin resistance remained consistently high among these isolates during the study period. Furthermore, there were no decreasing trends in resistance to any of the agents in this study, regardless of isolate source. These results suggest that current antimicrobial use practices in canine medicine might be driving an increase in the emergence and dissemination of drug-resistant E. coli in the region served by the laboratory. The scope of our conclusions is limited by our lack of antimicrobial use data from this population of dogs during the time frame of interest. However, cephalosporins, enrofloxacin, and doxycycline were recently found to be among the most commonly prescribed antimicrobial agents at a small animal teaching hospital in the northeastern United States (3). Regardless of the cause, our data suggest that some of the most commonly used agents in companion animal practice are becoming less effective against canine E. coli infections outside the urinary tract. Our data also suggest that cephalosporins are not likely to be effective against the relatively high percentage of canine UTIs that are caused by E. coli. It is important to note, however, that resistance to antimicrobial agents of the beta-lactam class among E. coli isolates in the urinary tract does not necessarily presage treatment failures of those agents at that anatomic site. Resistance is defined by the concentration of antimicrobial agent achievable in the serum. As this class of agents is concentrated highly in urine, they may reach concentrations sufficient to overcome the high resistance levels that we observed and thus remain clinically effective. Nevertheless, our work suggests that they be used with caution in this setting.
Nearly 20% of E. coli isolates were resistant to enrofloxacin, including 30% of isolates from feces or intestinal tract samples. Similarly, 20% of canine E. coli isolates were resistant to enrofloxacin according to a recent study in which investigators evaluated a relatively small number of isolates from various geographic locations in the United States (4). In contrast, investigators evaluating E. coli isolates from dogs between 1990–1998 (17) and from dogs and cats between 1989–1997 (32) found a lower prevalence of enrofloxacin resistance, with estimates ranging from 2% to 8%. Thus, enrofloxacin resistance among canine E. coli isolates might be increasing over a broad temporal scale. Enrofloxacin was approved for the treatment of bacterial infections in dogs in the United States in 1989, and it is commonly used to treat infections occurring in a wide range of body sites (3). Potential mechanisms of quinolone resistance in E. coli include mutations in genes encoding the quinolone target enzymes DNA gyrase and topoisomerase IV (33,34), mutations in genes that regulate the expression of efflux pumps thus resulting in overexpression (35,36), decrease in permeability of the bacterial cell wall (33), and expression of various plasmid-encoded resistance genes such as qnr (protection of target enzyme) and qepA (efflux pump) (34,37–39).
The occurrence of drug-resistant E. coli in dogs represents a potential threat to public health. The role of livestock as a source of pathogen transmission to people has been well-documented, predominantly through foodborne exposure but also via direct contact (40–42). However, dogs generally share the home environment. There are approximately 69.9 million pet dogs in the United States, living in 36.5% of households (43); in Canada, approximately 6.1 million pet dogs live in 32.3% of households (44); 66.7% of people with dogs in the United States consider them to be family members, while another 32.6% consider them to be pets or companions (43). Dogs typically have wide access to the home, including bedrooms and beds (45). Thus, direct contact with dogs is frequent among the human population and could serve as an important route of E. coli transmission to humans. In fact, an increasing body of evidence indicates that transfer of resistant bacteria or mobile resistance determinants can occur between dogs and humans (in either direction) through direct contact (46–52). This further underscores the importance of routine hand hygiene following animal contact, particularly among children under the age of 5 years, elderly adults, and immunocompromised persons. It also emphasizes the key role of the veterinarian in zoonotic disease prevention through client education and preventive veterinary care. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Weese JS. Antimicrobial resistance in companion animals. Anim Health Res Rev. 2008;9:169–176. doi: 10.1017/S1466252308001485. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen K, Pedersen K, Jensen H, Finster K, Jensen VF, Heuer OE. Occurrence of antimicrobial resistance in bacteria from diagnostic samples from dogs. J Antimicrob Chemother. 2007;60:775–781. doi: 10.1093/jac/dkm269. [DOI] [PubMed] [Google Scholar]
- 3.Wayne A, McCarthy R, Lindenmayer J. Therapeutic antibiotic use patterns in dogs: Observations from a veterinary teaching hospital. J Small Anim Pract. 2011;52:310–318. doi: 10.1111/j.1748-5827.2011.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boothe D, Smaha T, Carpenter DM, Shaheen B, Hatchcock T. Antimicrobial resistance and pharmacodynamics of canine and feline pathogenic E. coli in the United States. J Am Anim Hosp Assoc. 2012;48:379–389. doi: 10.5326/JAAHA-MS-5805. [DOI] [PubMed] [Google Scholar]
- 5.Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother. 2004;54:321–332. doi: 10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd DH. Reservoirs of antimicrobial resistance in pet animals. Clin Infect Dis. 2007;45(Suppl 2):S148–52. doi: 10.1086/519254. [DOI] [PubMed] [Google Scholar]
- 7.De Graef EM, Decostere A, Devriese LA, Haesebrouck F. Antibiotic resistance among fecal indicator bacteria from healthy individually owned and kennel dogs. Microb Drug Resist. 2004;10:65–69. doi: 10.1089/107662904323047826. [DOI] [PubMed] [Google Scholar]
- 8.Beutin L. Escherichia coli as a pathogen in dogs and cats. Vet Res. 1999;30:285–298. [PubMed] [Google Scholar]
- 9.Thompson MF, Litster AL, Platell JL, Trott DJ. Canine bacterial urinary tract infections: New developments in old pathogens. Vet J. 2011;190:22–27. doi: 10.1016/j.tvjl.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Aarestrup FM. Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic Clin Pharmacol Toxicol. 2005;96:271–281. doi: 10.1111/j.1742-7843.2005.pto960401.x. [DOI] [PubMed] [Google Scholar]
- 11.Erb A, Sturmer T, Marre R, Brenner H. Prevalence of antibiotic resistance in Escherichia coli: Overview of geographical, temporal, and methodological variations. Eur J Clin Microbiol Infect Dis. 2007;26:83–90. doi: 10.1007/s10096-006-0248-2. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI) CLSI document M35-A2. 2nd ed. 2008. Abbreviated Identification of Bacteria and Yeast; Approved Guideline. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) CLSI document M31-A3. 3rd ed. 2008. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute (CLSI) CLSI document M100-S20. 2010. Performance Standards for Antimicrobial Susceptibility Testing; 20th Informational Supplement. [Google Scholar]
- 15.Centers for Epidemiology and Animal Health, National Animal Health Monitoring System (NAHMS) Dairy. Salmonella and Campylobacter on U.S. Dairy Operations, 1996–2007. United States Department of Agriculture (USDA); Fort Collins, Colorado, USA: 2007. 2009. [Google Scholar]
- 16.Food and Drug Administration (FDA), National Antimicrobial Resistance Monitoring System — Enteric Bacteria (NARMS) 2010 Executive Report. U.S. Department of Health and Human Services, Food and Drug Administration; Rockville, Maryland, USA: 2012. [Google Scholar]
- 17.Oluoch AO, Kim CH, Weisiger RM, et al. Nonenteric Escherichia coli isolates from dogs: 674 cases (1990–1998) J Am Vet Med Assoc. 2001;218:381–384. doi: 10.2460/javma.2001.218.381. [DOI] [PubMed] [Google Scholar]
- 18.Ling GV, Norris CR, Franti CE, et al. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995) J Vet Intern Med. 2001;15:341–347. [PubMed] [Google Scholar]
- 19.Smee N, Loyd K, Grauer G. UTIs in small animal patients: Part 1: Etiology and pathogenesis. J Am Anim Hosp Assoc. 2013;49:1–7. doi: 10.5326/jaaha-ms-5943. [DOI] [PubMed] [Google Scholar]
- 20.Smee N, Loyd K, Grauer GF. UTIs in small animal patients: Part 2: Diagnosis, treatment, and complications. J Am Anim Hosp Assoc. 2013;49:83–94. doi: 10.5326/JAAHA-MS-5944. [DOI] [PubMed] [Google Scholar]
- 21.Norris CR, Williams BJ, Ling GV, Franti CE, Johnson, Ruby AL. Recurrent and persistent urinary tract infections in dogs: 383 cases (1969–1995) J Am Anim Hosp Assoc. 2000;36:484–492. doi: 10.5326/15473317-36-6-484. [DOI] [PubMed] [Google Scholar]
- 22.Seguin MA, Vaden SL, Altier C, Stone E, Levine JF. Persistent urinary tract infections and reinfections in 100 dogs (1989–1999) J Vet Intern Med. 2003;17:622–631. doi: 10.1111/j.1939-1676.2003.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 23.Prescott JF, Hanna WJ, Reid-Smith R, Drost K. Antimicrobial drug use and resistance in dogs. Can Vet J. 2002;43:107–116. [PMC free article] [PubMed] [Google Scholar]
- 24.Ball KR, Rubin JE, Chirino-Trejo M, Dowling PM. Antimicrobial resistance and prevalence of canine uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002–2007. Can Vet J. 2008;49:985–990. [PMC free article] [PubMed] [Google Scholar]
- 25.Hall JL, Holmes MA, Baines SJ. Prevalence and antimicrobial resistance of canine urinary tract pathogens. Vet Rec. 2013;173:549. doi: 10.1136/vr.101482. [DOI] [PubMed] [Google Scholar]
- 26.Subbiah M, Shah DH, Besser TE, Ullman JL, Call DR. Urine from treated cattle drives selection for cephalosporin resistant Escherichia coli in soil. PLoS One. 2012;7:e48919. doi: 10.1371/journal.pone.0048919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitout JD. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front Microbiol. 2012;3:9. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis. 2010;51:286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 29.Guenther S, Bethe A, Fruth A, et al. Frequent combination of antimicrobial multiresistance and extraintestinal pathogenicity in Escherichia coli isolates from urban rats (Rattus norvegicus) in Berlin, Germany. PLoS One. 2012;7:e50331. doi: 10.1371/journal.pone.0050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain A, Ewers C, Nandanwar N, et al. Multiresistant uropathogenic Escherichia coli from a region in India where urinary tract infections are endemic: Genotypic and phenotypic characteristics of sequence type 131 isolates of the CTX-M-15 extended-spectrum-beta-lactamase-producing lineage. Antimicrob Agents Chemother. 2012;56:6358–6365. doi: 10.1128/AAC.01099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Bij AK, Peirano G, Pitondo-Silva A, Pitout JD. The presence of genes encoding for different virulence factors in clonally related Escherichia coli that produce CTX-Ms. Diagn Microbiol Infect Dis. 2012;72:297–302. doi: 10.1016/j.diagmicrobio.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Normand EH, Gibson NR, Taylor DJ, Carmichael S, Reid SW. Trends of antimicrobial resistance in bacterial isolates from a small animal referral hospital. Vet Rec. 2000;146:151–155. doi: 10.1136/vr.146.6.151. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins KL, Davies RH, Threlfall EJ. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int J Antimicrob Agents. 2005;25:358–373. doi: 10.1016/j.ijantimicag.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Shaheen BW, Nayak R, Foley SL, Boothe DM. Chromosomal and plasmid-mediated fluoroquinolone resistance mechanisms among broad-spectrum-cephalosporin-resistant Escherichia coli isolates recovered from companion animals in the USA. J Antimicrob Chemother. 2013;68:1019–1024. doi: 10.1093/jac/dks514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaheen BW, Boothe DM, Oyarzabal OA, Wang C, Johnson CM. Evaluation of the contribution of gyrA mutation and efflux pumps to fluoroquinolone and multidrug resistance in pathogenic Escherichia coli isolates from dogs and cats. Am J Vet Res. 2011;72:25–32. doi: 10.2460/ajvr.72.1.25. [DOI] [PubMed] [Google Scholar]
- 37.Yamane K, Wachino J, Suzuki S, et al. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 2007;51:3354–3360. doi: 10.1128/AAC.00339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin Microbiol Rev. 2009;22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Boothe DM, Thungrat K, Aly S. Mechanisms accounting for fluoroquinolone multidrug resistance Escherichia coli isolated from companion animals. Vet Microbiol. 2012;161:159–168. doi: 10.1016/j.vetmic.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Dechet AM, Scallan E, Gensheimer K, et al. Outbreak of multidrug-resistant Salmonella enterica serotype Typhimurium definitive type 104 infection linked to commercial ground beef, northeastern United States, 2003–2004. Clin Infect Dis. 2006;42:747–752. doi: 10.1086/500320. [DOI] [PubMed] [Google Scholar]
- 41.Varma JK, Marcus R, Stenzel SA, et al. Highly resistant Salmonella Newport-MDRAmpC transmitted through the domestic US food supply: A FoodNet case-control study of sporadic Salmonella Newport infections, 2002–2003. J Infect Dis. 2006;194:222–230. doi: 10.1086/505084. [DOI] [PubMed] [Google Scholar]
- 42.Cummings KJ, Warnick LD, Davis MA, et al. Farm animal contact as risk factor for transmission of bovine-associated Salmonella subtypes. Emerg Infect Dis. 2012;18:1929–1936. doi: 10.3201/eid1812.110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Veterinary Medical Association (AVMA) US Pet Ownership and Demographics Sourcebook. 2013. [Google Scholar]
- 44.Perrin T. The business of urban animals survey: The facts and statistics on companion animals in Canada. Can Vet J. 2009;50:48–52. [PMC free article] [PubMed] [Google Scholar]
- 45.Chomel BB, Sun B. Zoonoses in the bedroom. Emerg Infect Dis. 2011;17:167–172. doi: 10.3201/eid1702.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simjee S, White DG, McDermott PF, et al. Characterization of Tn1546 in vancomycin-resistant Enterococcus faecium isolated from canine urinary tract infections: Evidence of gene exchange between human and animal enterococci. J Clin Microbiol. 2002;40:4659–4665. doi: 10.1128/JCM.40.12.4659-4665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Duijkeren E, Wolfhagen MJ, Box AT, Heck ME, Wannet WJ, Fluit AC. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg Infect Dis. 2004;10:2235–2237. doi: 10.3201/eid1012.040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidjabat HE, Townsend KM, Lorentzen M, et al. Emergence and spread of two distinct clonal groups of multidrug-resistant Escherichia coli in a veterinary teaching hospital in Australia. J Med Microbiol. 2006;55:1125–1134. doi: 10.1099/jmm.0.46598-0. [DOI] [PubMed] [Google Scholar]
- 49.Ishii S, Meyer KP, Sadowsky MJ. Relationship between phylogenetic groups, genotypic clusters, and virulence gene profiles of Escherichia coli strains from diverse human and animal sources. Appl Environ Microbiol. 2007;73:5703–5710. doi: 10.1128/AEM.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson JR, Clabots C, Kuskowski MA. Multiple-host sharing, long-term persistence, and virulence of Escherichia coli clones from human and animal household members. J Clin Microbiol. 2008;46:4078–4082. doi: 10.1128/JCM.00980-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platell JL, Cobbold RN, Johnson JR, et al. Commonality among fluoroquinolone-resistant sequence type ST131 extraintestinal Escherichia coli isolates from humans and companion animals in Australia. Antimicrob Agents Chemother. 2011;55:3782–3787. doi: 10.1128/AAC.00306-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stokholm J, Schjorring S, Pedersen L, et al. Living with cat and dog increases vaginal colonization with E. coli in pregnant women. PLoS One. 2012;7:e46226. doi: 10.1371/journal.pone.0046226. [DOI] [PMC free article] [PubMed] [Google Scholar]