Abstract
This study compared needle-free and needle-based injection devices for vaccination of calves against Clostridium chauvoei in warm and cold conditions. Both devices elicited comparable antibody responses in calves. Needle-free injection devices can be used to vaccinate calves provided appropriate precautions are taken in cold weather.
Résumé
Efficacité de l’injection sans seringue sur la production d’anticorps contre Clostridium chauvoei chez les veaux de boucherie dans des conditions sur le terrain. Cette étude a comparé les dispositifs à injection sans seringue et avec seringue pour la vaccination des veaux contre Clostridium chauvoei dans des conditions par temps chaud et froid. Les deux dispositifs ont provoqué des réponses comparables des anticorps chez les veaux. Des dispositifs d’injection sans seringue peuvent être utilisés pour vacciner les veaux pourvu que des précautions appropriées soient prises par temps froid.
(Traduit par Isabelle Vallières)
Vaccines are routinely administered using needle-based injection devices (NS), which may result in accidental needle-stick injuries to operators and broken needle fragments in meat (1). Furthermore, blood-borne diseases such as bovine leukosis and anaplasmosis can be transmitted between animals when using a single needle to inject multiple animals (2,3). Use of needle-free (NF) injection devices to vaccinate animals reduces disease transfer among animals and eliminates the need for needle disposal (3). The purpose of this study was to compare needle-free and needle-based injection devices used to vaccinate calves against Clostridium chauvoei. To test their utility under climatic conditions in western Canada, the devices were compared in temperate and cold conditions.
Two trials were conducted in separate commercial cow-calf beef herds in Manitoba. The first trial was conducted from July to November with 86 spring-born crossbred beef calves (106.2 ± 16.8 kg). The second trial, which ran from October to March, was conducted with 88 fall-born beef calves (101.5 ± 16.6 kg). Calves were initially vaccinated at approximately 2 mo of age (day 0) and re-vaccinated 21 d later. Initial and booster vaccinations were administered on July 6 and 27, respectively, in the first trial and October 19 and November 9, respectively, in the second trial. The calves were vaccinated with a commercial clostridial vaccine (Clostri Shield 7; Novartis Animal Health Canada, Mississauga, Ontario) containing C. chauvoei, C. septicum, C. novyi, C. sordelli, C. perfringens types B, C, and D. Needle-free vaccination was administered with a NF injection device (Pulse 250 NeedleFree Injection System; Pulse NeedleFree Systems, Lexena, Kansas, USA) using a skin-tenting technique. Compressed CO2 was used as the power source for the NF vaccinations in the first trial and on day 0 of the second trial. Sub-zero temperatures (−2.3°C) made it necessary to use compressed N2 as the power source for the booster NF vaccinations on day 21 (November 9) of the second trial. Using guidelines from Pulse NeedleFree Systems, the NF was set at pressures of 45 to 55 PSI and 60 to 65 PSI to administer the vaccine on days 0 and 21, respectively, in order to deliver a SC injection. Needle-based vaccination was administered SC with a multi-dose, pistol-grip syringe (Kane Veterinary Supplies, Edmonton, Alberta) fitted with an 18-gauge, 1-inch needle (Partnar Animal Health, Ilderton, Ontario), using the same skin-tenting technique. Presence of vaccine residue at the skin surface was recorded immediately following vaccination. In the spring and fall, calves were divided into 2 groups and calves in each group were vaccinated with either NF or NS. During vaccination, calves were restrained in a squeeze chute with a head gate.
Clostridium chauvoei antibody levels in 30 randomly selected serum samples from calves in each vaccination group were examined using an indirect immunofluorescence technique (4,5). All slides from the immunofluorescence assays were independently assessed by 2 trained technicians. Assessment was done without knowledge of animal or treatment. The use of negative and positive internal controls allowed monitoring of variation between tests due to reagents and operators. The positive control serum was obtained from a Holstein dairy heifer that had been immunized with 2 doses of the same clostridial vaccine, with a 21-day interval between vaccinations. Serum was collected from this heifer 15 d after the second immunization. The negative control was phosphate-buffered saline.
Calves were visually scored for the presence of post-vaccination skin reactions on days 21, 42, 119, and 140. Any raised surfaces observed at the injection site were considered skin reactions occurring as a result of vaccination.
Animal care and handling procedures were approved by the University of Manitoba Animal Care Committee, in compliance with the guidelines of the Canadian Council of Animal Care (6).
Data for spring-born and fall-born calves were analyzed separately. Antibody titers and presence of visible vaccine residue were analyzed using the MIXED procedure of SAS (7) for repeated measures with calf as the experimental unit. The fixed effects in the model were treatment group (NF and NS) and days post-vaccination. The effects of calf within treatment group were considered random. Antibody titers were analyzed on a log scale with base 2 (log2). Confidence limits (95%) of the estimated frequencies of NF and NS animals having at least 1 skin reaction at the site of vaccine administration were calculated and used to determine significant differences between groups.
Approximately 29% and 19% of NF-vaccinated calves had visible vaccine residue following primary and booster vaccinations, respectively. Residual vaccine on the skin surface following NF use has been reported elsewhere (8). Residual vaccine on the skin following NF vaccination may be unavoidable and may hinder or delay acceptance of the technology (8).
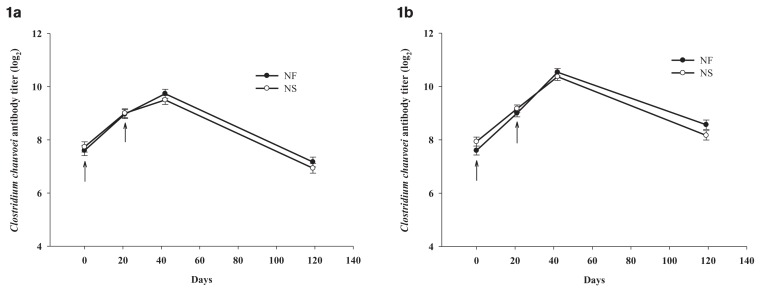
Although the vaccine used in this study contained several Clostridium spp., analysis concentrated on C. chauvoei only. Clostridium chauvoei, a soil-borne anaerobic bacterium, causes blackleg in cattle and other ruminants (9). Blackleg occurs in all areas of Manitoba, and new outbreaks are reported every year (10). The trends in antibody concentrations following NF and NS vaccination were similar. In both spring-born (Figure 1a) and fall-born (Figure 1b) calves, C. chauvoei antibody concentrations in NF- and NS-vaccinated calves increased following primary and booster vaccinations. This immune response is in agreement with research in cattle vaccinated against infectious bovine rhinotracheitis virus (2), Mannheimia haemolytica bacterin toxoid, and Leptospira pomona bacterin (2), bovine herpesvirus-1 (11), and foot-and-mouth disease virus (12), which showed comparable and sometimes enhanced immune response following vaccination with a NF when compared to NS. Enhanced immune response following NF vaccination could be due to wider vaccine dispersion into tissues and greater penetration through the skin (13).
Figure 1.
Antibody levels in spring-born (Figure 1a) and fall-born (Figure 1b) calves vaccinated against C. chauvoei using needle-free (NF) and needle-based (NS) injection devices. Arrows (↑) indicate the day calves were vaccinated.
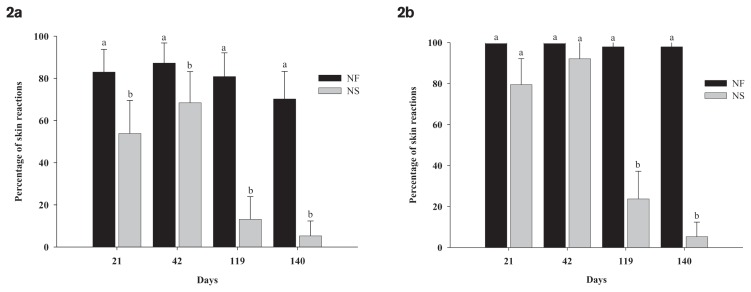
Post-vaccination skin reactions may be caused by several factors, including pressure trauma associated with vaccination or contamination by transmitting microorganisms from the skin/hair surface into the tissue surrounding the injection site (14). In both spring-born (Figure 2a) and fall-born (Figure 2b) calves, NF-vaccinated calves had a greater proportion of post-vaccination skin reactions compared to NS-vaccinated calves (P < 0.05). Post-vaccination skin reactions may impact carcass quality, but this aspect was not explored in the current study.
Figure 2.
Percentage of post-vaccination skin reactions (mean ± 95% confidence limits) in spring-born (Figure 2a) and fall-born (Figure 2b) calves vaccinated against C. chauvoei using needle-free (NF) and needle-based (NS) injection devices. Means within day with a different letter differ (P < 0.05).
For a device to be useful under climatic conditions in western Canada, it should be effective in both warm and cold conditions. Although a direct comparison of the immune response stimulated between the 2 seasons of vaccine administration could not be completed due to differences in animals, feed and environmental conditions between herds, similar trends in immune response between the 2 seasons suggest that the needle-free device can be used in the cold temperatures of western Canada. An appropriate power source during cold conditions is important to ensure the device is in proper working order.
In conclusion, NF and NS injection devices were equally effective in vaccinating spring- and fall-born calves. The greater percentage of post-vaccination skin reactions and higher frequency of vaccine residue in NF-vaccinated calves did not influence immune response.
Acknowledgments
The authors thank Growing Forward — Manitoba Agriculture, Food and Rural Initiatives for financial support. Special thanks are due to Pulse NeedleFree Systems for providing the needle-free injection device. The authors are indebted to Betty Green and family at G7 Ranch and Henry Rosing and staff at EUR Ranch for use of their cattle for the trial. Technical assistance by Terri Garner, Deanne Fulawka, Dana Gardiner, Carson Callum, Nicole Ireland, Mateo Remonda, Romina Livolsi, Ashley Rawluk, Sean Thompson, Harold Echeverry, and Janice Haines is gratefully acknowledged. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Weese JS, Jack DC. Needlestick injuries in veterinary medicine. Can Vet J. 2008;49:780–784. [PMC free article] [PubMed] [Google Scholar]
- 2.Hollis LC, Smith JE, Johnson BJ, Kapil S, Mosier DA. A comparison of serological responses when modified-live infectious bovine rhinotracheitis virus vaccine, Mannheimia haemolytica bacterin toxoid and Leptospira pomona bacterin are administered with needle-free versus conventional needle-based injection in Holstein dairy calves. Bov Pract. 2005;39:110–114. [Google Scholar]
- 3.Reinbold JB, Coetzee JF, Hollis LC, et al. Comparison of iatrogenic transmission of Anaplasma marginale in Holstein steers via needle and needle-free injection techniques. Am J Vet Res. 2010;71:1178–1188. doi: 10.2460/ajvr.71.10.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaoka T, Terakado N. Demonstration of common antigens on cell surface of Clostridium chauvoei and C. septicum by indirect-immunofluorescence assay. J Vet Med Sci. 1994;56:371–373. doi: 10.1292/jvms.56.371. [DOI] [PubMed] [Google Scholar]
- 5.Rahimi MK, Hashemi M, Tayebi Z, Adimi P, Masoumi M, Boroumandi Sh. Evaluation of indirect immunofluorescence assay for diagnosis of Listeria monocytogenes in abortion. Adv Environ Biol. 2011;5:1335–1338. [Google Scholar]
- 6.Olfert ED, Cross BM, McWilliam AA, editors. Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals. 1 and 2. Canadian Council on Animal Care; Ottawa, Canada: 1993. [Google Scholar]
- 7.SAS Institute Inc. SAS Users’ Guide. 4th ed. Gary; North Carolina, USA: 2008. [Google Scholar]
- 8.Jones GF, Rapp-Gabrielson V, Wilke R, et al. Intradermal vaccination for Mycoplasma hypopneumoniae. J Swine Health Prod. 2005;13:19–27. [Google Scholar]
- 9.Kijima-Tanaka M, Ogikubo Y, Kojima A, Tamura Y. Development of a two-site enzyme-linked immunosorbent assay for quantification of the flagellar antigen in blackleg vaccines. J Microbiol Methods. 1997;31:83–88. [Google Scholar]
- 10.German M, Whiting T. Blackleg. Manitoba Agriculture, Food, and Rural Initiatives. Government of Manitoba; Winnipeg, Manitoba: [Google Scholar]
- 11.van Drunen Littel-van den Hurk S. Rationale and perspectives on the success of vaccination against bovine herpesvirus-1. Vet Microbiol. 2006;113:275–282. doi: 10.1016/j.vetmic.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Pandya M, Pacheco JM, Bishop E, et al. An alternate delivery system improves vaccine performance against foot-and-mouth disease virus (FMDV) Vaccine. 2012;30:3106–3111. doi: 10.1016/j.vaccine.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CR, Mundell RD, Monheim LM. Studies on tissue penetration characteristics produced by jet injection. J Am Dental Assoc. 1971;83:625–629. doi: 10.14219/jada.archive.1971.0352. [DOI] [PubMed] [Google Scholar]
- 14.Troxel TR, Burke GL, Wallace WT, et al. Clostridial vaccination efficacy on stimulating and maintaining an immune response in beef cows and calves. J Anim Sci. 1997;75:19–25. doi: 10.2527/1997.75119x. [DOI] [PubMed] [Google Scholar]