Abstract
Many stinkbugs (Insecta: Hemiptera: Heteroptera) are associated with bacterial symbionts in a posterior region of the midgut. In these stinkbugs, adult females excrete symbiont-containing materials from the anus for transmission of the beneficial symbionts to their offspring. For ensuring the vertical symbiont transmission, a variety of female-specific elaborate traits at the cellular, morphological, developmental, and behavioral levels have been reported from diverse stinkbugs of the families Plataspidae, Urostylididae, Parastrachiidae, etc. Meanwhile, such elaborate female-specific traits for vertical symbiont transmission have been poorly characterized for the largest and economically important stinkbug family Pentatomidae. Here, we investigated the midgut symbiotic system of a pentatomid stinkbug, Plautia splendens. A specific gammaproteobacterial symbiont was consistently present extracellularly in the cavity of numerous crypts arranged in four rows on the midgut fourth section. The symbiont was smeared on the egg surface upon oviposition by adult females, orally acquired by newborn nymphs, and thereby transmitted vertically to the next generation and important for growth and survival of the host insects. We found that, specifically in adult females, several rows of crypts at the posterior end region of the symbiotic midgut were morphologically differentiated and conspicuously enlarged, often discharging the symbiotic bacteria from the crypt cavity to the main tract of the symbiotic midgut. The female-specific enlarged end crypts were also found in other pentatomid stinkbugs Plautia stali and Carbula crassiventris. These results suggest that the enlarged end crypts represent a female-specific specialized morphological trait for vertical symbiont transmission commonly found among stinkbugs of the family Pentatomidae.
INTRODUCTION
Many insects are associated with microbial mutualists in the gut lumen, inside the body cavity, or within the cells (1, 2). For ecological and evolutionary persistence of such mutualistic associations, vertical symbiont transmission through host generations is among the pivotal processes, although nonvertical (i.e., horizontal or environmental) symbiont transmission may also be prevalent in marine invertebrates, plants, and some insects (3–5). In intracellular associations such as Buchnera in aphids and Wigglesworthia in tsetse flies, vertical symbiont transmission usually occurs prenatally to developing eggs or embryos within maternal bodies (1, 6–8). In extracellular associations such as gut microbes in termites and stinkbugs, by contrast, vertical symbiont transmission tends to occur postnatally outside the maternal bodies via nymphal feeding of symbiont-containing excrements (1, 9, 10).
The majority of plant-sucking stinkbugs (order Hemiptera: suborder Heteroptera: infraorder Pentatomomorpha: superfamily Pentatomoidea) are associated with bacterial symbionts of a beneficial nature in a posterior region of the midgut, where numerous crypts develop and harbor specific symbiotic bacteria therein (1, 10, 11). In these stinkbugs, adult females excrete symbiont-containing materials from the anus, which are orally acquired by their offspring for vertical symbiont transmission. Most commonly, as observed in the families Pentatomidae, Scutelleridae, Acanthosomatidae, and Cydnidae, vertical symbiont transmission is set up upon oviposition by egg surface contamination with symbiont-containing materials and establishes upon egg hatching by nymphal oral exploitation of the egg surface (1, 12–21). In several presocial stinkbugs of the families Parastrachiidae and Cydnidae, adult females continuously attend and take care of their eggs and excrete symbiont-containing materials just before or after egg hatching, which newborns consume and establish vertical symbiont transmission (22, 23). In the family Plataspidae, uniquely, adult females produce brown-colored particles encasing symbiont-containing materials, so-called symbiont capsules, upon oviposition, and hatchlings suck the content of the capsules for establishing vertical transmission of the symbiont (24, 25). In the family Urostylididae, also uniquely, adult females produce egg masses covered with voluminous, nutritious, symbiont-supplemented, and galactose-based polysaccharide gels, and nymphs feed on the symbiont-containing jelly for growth and symbiont acquisition (26).
In these stinkbugs, a variety of elaborate cellular, morphological, developmental, and behavioral traits specialized for vertical symbiont transmission have been identified. In plataspid stinkbugs, for example, a posterior end region of the symbiotic midgut develops several specialized organs for capsule production in a female-specific manner (27), adult females strictly control the number of capsules as well as the symbiont quantity in each capsule (28), and newborn nymphs are behaviorally programmed to exploit the capsule content (29). In urostylidid stinkbugs, the basal region of the ovarioles is enlarged and distended for jelly production, and novel paired organs for symbiont transmission are formed in association with the female genital chamber (26). In a parastrachiid stinkbug, upon egg hatching, behaviors of adult females and their newborn nymphs are finely synchronized for vertical symbiont transmission, and a posterior region of the symbiotic midgut is hypertrophied in a female-specific manner for production of a copious amount of symbiont-containing materials (22). In acanthosomatid stinkbugs, novel paired organs for symbiont transmission, which are similar to but anatomically distinct from those of urostylidid stinkbugs, are present near the female genital chamber (14). On the other hand, such female-specific traits for vertical symbiont transmission have been poorly described for stinkbugs of the family Pentatomidae, except for a few early morphological observations (1, 30, 31), although members of the largest stinkbug group, embracing over 900 genera and 4,500 species and economically the most important (32, 33), are generally associated with midgut symbiotic bacteria that are vertically transmitted via egg surface contamination (1, 12, 12, 13, 15, 17, 18, 20, 34).
In this study, we investigated in detail the midgut symbiotic system of a pentatomid stinkbug Plautia splendens and found that several rows of crypts at the posterior end region of the symbiotic midgut are morphologically differentiated and conspicuously enlarged only in adult females. Inspection of several other pentatomid species identified similarly specialized midgut formation only in female insects, suggesting that this structure represents a female-specific morphological trait specialized for vertical symbiont transmission in stinkbugs of the family Pentatomidae.
MATERIALS AND METHODS
Insects.
Adult insects of P. splendens were collected at southwestern islands in Okinawa Prefecture, Japan, from the wild thistle Cirsium brevicaule. Some insects were preserved in acetone for DNA analysis (35), whereas other insects were brought to the laboratory and reared in plastic petri dishes (90 mm in diameter, 15 mm high) or plastic cases (150 mm in diameter, 60 mm high) supplied with a wet cotton ball, raw peanuts, and dried soybeans at 25°C under a 16-h light and 8-h dark photoperiod. Plautia stali collected in Tsukuba, Japan, and Carbula crassiventris collected in Okinawa, Japan, were maintained in the laboratory using the same rearing systems (Table 1).
TABLE 1.
Stinkbug samples examined in this study
| Localitya; postfix no. | Date; collectorb | Accession no.c |
|---|---|---|
| Plautia splendens | ||
| Ishigaki Is.; 1 | April 2014; Ho | LC012481 |
| Ishigaki Is.; 2 | April 2014; Ho | LC012482 |
| Hamahiga Is.; 1 | April 2014; Ho | LC012471 |
| Hamahiga Is.; 2 | April 2014; Ho | LC012472 |
| Hamahiga Is.; 3 | April 2014; Ho | LC012473 |
| Hamahiga Is.; 4 | April 2014; Ho | LC012474 |
| Hamahiga Is.; 5 | April 2014; Ho | LC012475 |
| Ikei Is.; 1 | May 2014; Ha | LC012476 |
| Ikei Is.; 2 | May 2014; Ha | LC012477 |
| Ikei Is.; 3 | May 2014; Ha | LC012478 |
| Ikei Is.; 4 | May 2014; Ha | LC012479 |
| Ikei Is.; 5 | May 2014; Ha | LC012480 |
| Itoman; 1 | May 2014; Ha | LC012483 |
| Itoman; 2 | May 2014; Ha | LC012484 |
| Itoman; 3 | May 2014; Ha | LC012485 |
| Itoman; 4 | May 2014; Ha | LC012486 |
| Itoman; 5 | May 2014; Ha | LC012487 |
| Miyagi Is.; 1 | May 2014; Ha | LC012488 |
| Miyagi Is.; 2 | May 2014; Ha | LC012489 |
| Miyagi Is.; 3 | May 2014; Ha | LC012490 |
| Miyagi Is.; 4 | May 2014; Ha | LC012491 |
| Miyagi Is.; 5 | May 2014; Ha | LC012492 |
| Nago; 1 | May 2014; Fu | LC012493 |
| Nago; 2 | May 2014; Fu | LC012494 |
| Nago; 3 | May 2014; Fu | LC012495 |
| Nago; 4 | May 2014; Fu | LC012496 |
| Plautia stali | ||
| Tsukuba | September 2012; Mo | |
| Carbula crassiventris | ||
| Hamahiga Is. | April 2014; Ho |
All collection localities are in Okinawa Prefecture, Japan, except for P. stali, which is from Tsukuba in Ibaraki Prefecture, Japan.
Fu, Takema Fukatsu; Ha, Toshinari Hayashi; Ho, Takahiro Hosokawa; Mo, Minoru Moriyama.
Accession numbers for 16S rRNA gene sequences of the gut symbiont of P. splendens.
DNA analysis.
The insects were dissected to obtain the symbiotic organ (midgut fourth section with symbiont-harboring crypts) and the reproductive organ (ovary or testis) in a plastic petri dish filled with phosphate-buffered saline (PBS; 137 mM NaCl, 8.10 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4 [pH 7.4]) under a dissection microscope using a pair of fine forceps. The dissected tissues were individually subjected to DNA extraction using a QIAamp DNA minikit (Qiagen). A 1.5-kb segment of a bacterial 16S rRNA gene was amplified by PCR with primers 10F (5′-AGT TTG ATC ATG GCT CAG ATT-3′) (36) and 16SB1 (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) (37). The PCR product was treated with exonuclease I (TaKaRa) and shrimp alkaline phosphatase (TaKaRa) and subjected to cycle sequencing with primers 10F, 16SB1, and 16SA2 (5′-GTG CCA GCA GCC GCG GTA ATA C-3′) (37) by using BigDye Terminator v3.1 ready reaction mix (Life Technologies). A 1.2-kb segment of a bacterial gyrB gene was also amplified by PCR with primers gyr320F (5′-TAA RTT YGA YGA YAA CTC YTA YAA AGT-3′) and gyr1470R (5′-TGA TAG CGC AGT TTG TCC-3′) and sequenced as described above. A 1.5-kb segment of a mitochondrial 16S rRNA gene of P. splendens was amplified by PCR with primers mt16SA1 (5′-AAW AAA CTA GGA TTA GAT ACC CTA-3′) (25) and mt16SB1 (5′-TCT TAA TYC AAC ATC GAG GTC GCA A-3′) (25). The PCR product was sequenced with primers mt16SA1, mt16SB1, mt16S390f (5′-ACA YAT CGC CCG TCR CTC YC-3′), and mt16S1290r (5′-GRT TAT GCT ACC TTN GYA CAG T-3′), as described above.
Molecular phylogenetic analysis.
The bacterial 16S rRNA gene sequences were analyzed with 16S rRNA gene sequences of gammaproteobacterial representatives retrieved from the DNA databases. Multiple alignment was conducted using MUSCLE (38), and then aligned nucleotide sites containing gaps were manually removed. Molecular phylogenetic analyses were conducted by the maximum likelihood method, neighbor-joining method, and Bayesian inference. Maximum likelihood and neighbor-joining phylogenies were constructed using MEGA 6.06 (39), for which the GTR+G+I model was selected as the best-fit substitution model using MEGA 6.06. Bootstrap values were calculated with 500 replications. Bayesian phylogeny was inferred using MrBayes (40), for which the GTR+G model was selected as the best-fit substitution model using Kakusan4 (41). Runs were carried out with 3 million generations, from which the first 1 million generations were discarded as burn-in. Consensus trees were constructed based on the 50% majority rule.
In situ hybridization.
A fluorochrome-labeled oligonucleotide probe Pspsym-16S169 (5′-Alexa 555-CCA CTT TGG TCC GAA GAC GT-3′) was used for in situ hybridization targeting 16S rRNA of the gut symbiont of P. splendens. For whole-mount in situ hybridization, dissected tissues were fixed in Carnoy's solution (ethanol-chloroform-acetic acid [6:3:1]) overnight, washed with 95% ethanol, and incubated in 6% hydrogen peroxide for several days to quench autofluorescence (42). After thorough washing with 95% ethanol, the tissue samples were washed and equilibrated with hybridization buffer (20 mM Tris-HCl [pH 8.0], 0.9 M NaCl, 0.01% sodium dodecyl sulfate, 30% formamide) and then incubated with the hybridization buffer containing 100 nM probe and 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) at room temperature overnight. After the incubation, the samples were thoroughly washed with PBS containing 0.1% Tween 20 (PBST), mounted with SlowFade antifade solution (Life Technologies), and observed under a fluorescence stereomicroscope (M165FC; Leica). For in situ hybridization of tissue sections, dissected tissues were fixed in 4% paraformaldehyde phosphate buffer solution (Wako) at 4°C for 1 h, dehydrated through a water-ethanol series, embedded in Technovit 8100 (Heraeus Kulzer), cut into 2-μm sections on a microtome (RM2165; Leica), and mounted on glass slides. Then, the sections on the slides were incubated overnight with the hybridization buffer containing 100 nM probe and 1 μg/ml DAPI in a humidified chamber at room temperature. After thorough washing with Tris-buffered saline (TBS; 137 mM NaCl, 2.68 mM KCl, 25 mM Tris [pH 7.4]), the sections were mounted in Prolong antifade reagent (Life Technologies) with a coverslip and observed under an epifluorescence microscope (Axiophoto; Carl Zeiss). To confirm specificity of the hybridization signals, the following control experiments were conducted: no-probe control, RNase treatment control, and competitive suppression control with excess unlabeled probe.
Transmission electron microscopy.
The dissected tissues were prefixed in 0.1 M sodium phosphate buffer (pH 7.2) containing 2.5% glutaraldehyde at 4°C overnight and postfixed in 2% osmium tetroxide at 4°C for 1 h. After dehydration using an ethanol series, the tissues were embedded in Spurr resin and cut into ultrathin sections (75 nm thick). The sections were double-stained with uranyl acetate and lead citrate and observed with a transmission electron microscope (H-7600; Hitachi).
Diagnostic PCR.
A 0.5-kb segment of a bacterial gyrB gene of the symbiont was amplified by PCR with primers Psp-gyrF (5′-TTT CAG AGA GCG CCG GGT CG-3′) and dPCR-Psp-gyrR (5′-ATC GGC GTG GAA GTC GCA TT-3′) by using Ex Taq DNA polymerase (TaKaRa) and its supplied buffer system under a temperature profile of 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 65°C for 1 min, and 72°C for 2 min. A 1.5-kb segment of an insect mitochondrial 16S rRNA gene of the host insect was amplified by PCR to check the DNA quality with primers hime-mtA1 (5′-AAT CAT AAA AGA GGA ACC TGT T-3′) and hime-mtB1 (5′-TYA TCG ATA AGA ACT CTC CAA-3′) under a temperature profile of 95°C for 5 min followed by 35 cycles of 95°C for 30 s, 58°C for 1 min, and 72°C for 2 min.
Symbiont elimination and fitness measurement.
Newly laid egg masses of P. splendens were allocated to two experimental groups. In the sterilized group, the egg masses were treated with 4% formalin for 10 min, rinsed with sterile water, and dried in air. In the control group, the egg masses were kept untreated. Then, each egg mass was placed in a sterile plastic petri dish, provided with a cotton ball soaked with sterile water containing 0.05% ascorbic acid and sterile raw peanuts that were soaked in 100% ethanol for 30 min and then dried. Nymphs from the egg masses were reared at 25°C under a 16-h light and 8-h dark photoperiod. For inspecting vertical transmission rates, the nymphs were preserved in acetone at the second instar. For fitness measurements, the nymphs were reared for 36 days, during which their molting and survival were recorded every third day.
Quantitative PCR.
First-, second-, third-, fourth-, and fifth-instar nymphs and adults of P. splendens were collected 3 days after hatching or molting. The whole nymphs were preserved in acetone, whereas the adults were dissected and their midgut fourth section was individually preserved in acetone. These samples were individually subjected to DNA extraction using a QIAamp DNA minikit (Qiagen) and quantitative PCR essentially as described previously (43). A 0.1-kb segment of a bacterial gyrB gene of the symbiont was targeted with primers Psp-gyrF (5′-TTT CAG AGA GCG CCG GGT CG-3′) and Psp-gyrR (5′-TCG CGC ACG TGA AAT GAC GC-3′). The PCR mixture was composed of 1× GeneAmp PCR buffer I (Life Technologies), 0.09 mM MgCl2, 3 mM deoxynucleoside triphosphates (dNTPs), 400 nM (each) primers, 0.5 μl of SYBR green (1/1,000 diluted solution) (TaKaRa), 0.04 U/μl AmpliTaq Gold DNA polymerase (Life Technologies), 5 μl of temperate DNA, and sterile water in a total of 25 μl. A standard curve was drawn using PCR product samples containing 102, 103, 104, 105, 106, and 107 gyrB gene copies.
Nucleotide sequence accession numbers.
The nucleotide sequences of the bacterial 16S rRNA gene, the bacterial gyrB gene, and the mitochondrial 16S rRNA gene of P. splendens have been deposited in DDBJ, EMBL, and GenBank under accession numbers LC012471 to LC012496, LC012595, and LC012497, respectively.
RESULTS
General observation of P. splendens and its symbiotic organ.
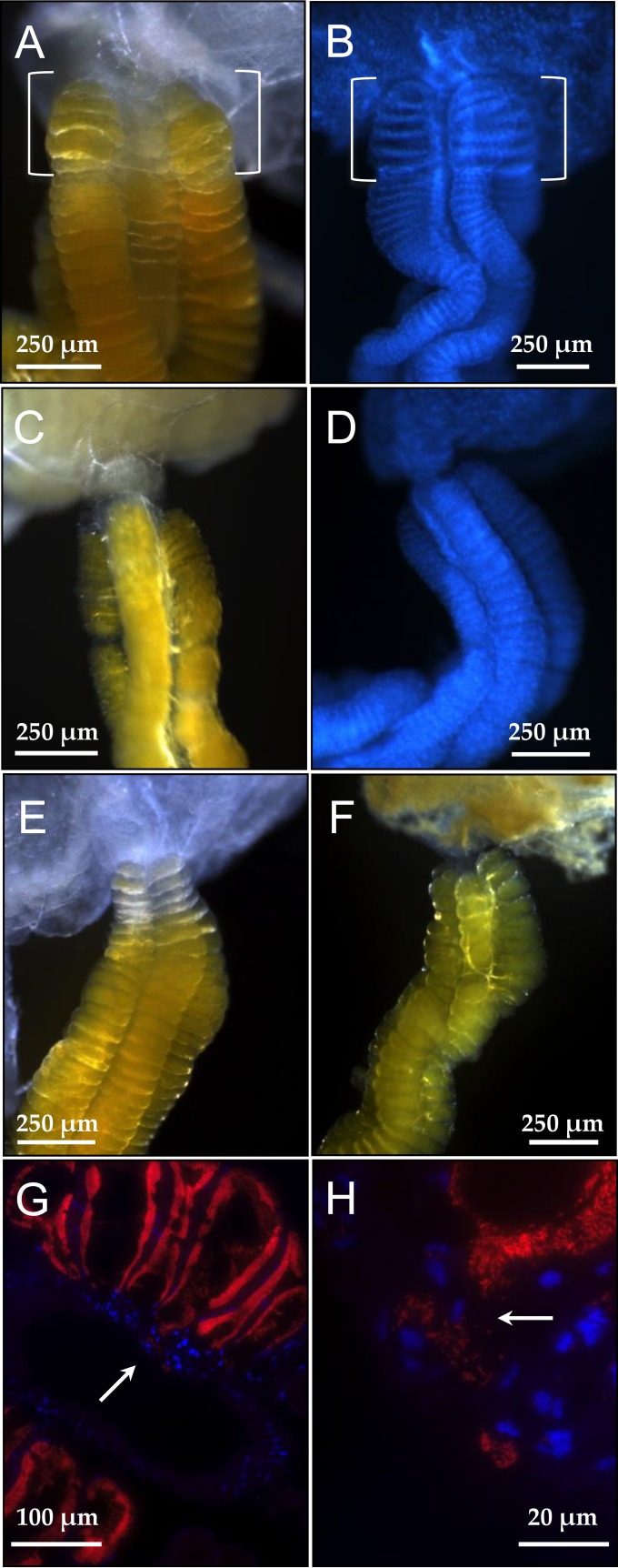
Adult females of P. splendens (Fig. 1A) laid about 14 eggs as an egg mass, from which nymphs hatched within a week or so. The newborn nymphs immediately started exploiting the eggshell with their mouthparts for a while (Fig. 1B), stayed immobile on the eggshell or around the egg mass for about 6 days without feeding, molted to the second instar, and then started feeding. As in other stinkbugs in general (1, 10, 11), the dissected alimentary tract of P. splendens consisted of several morphologically distinct regions. From mouth to anus, following the foregut, the midgut first section was enlarged and sac-like, the midgut second section was long and tubular, the midgut third section was soft and moderately swollen, and the midgut fourth section was yellow and twisted, connecting to the hindgut with Malpighian tubules (Fig. 1C). Along the midgut fourth section, a number of crypts were arranged in four rows (Fig. 1D), in which numerous bacterial cells were present (Fig. 1E).
FIG 1.
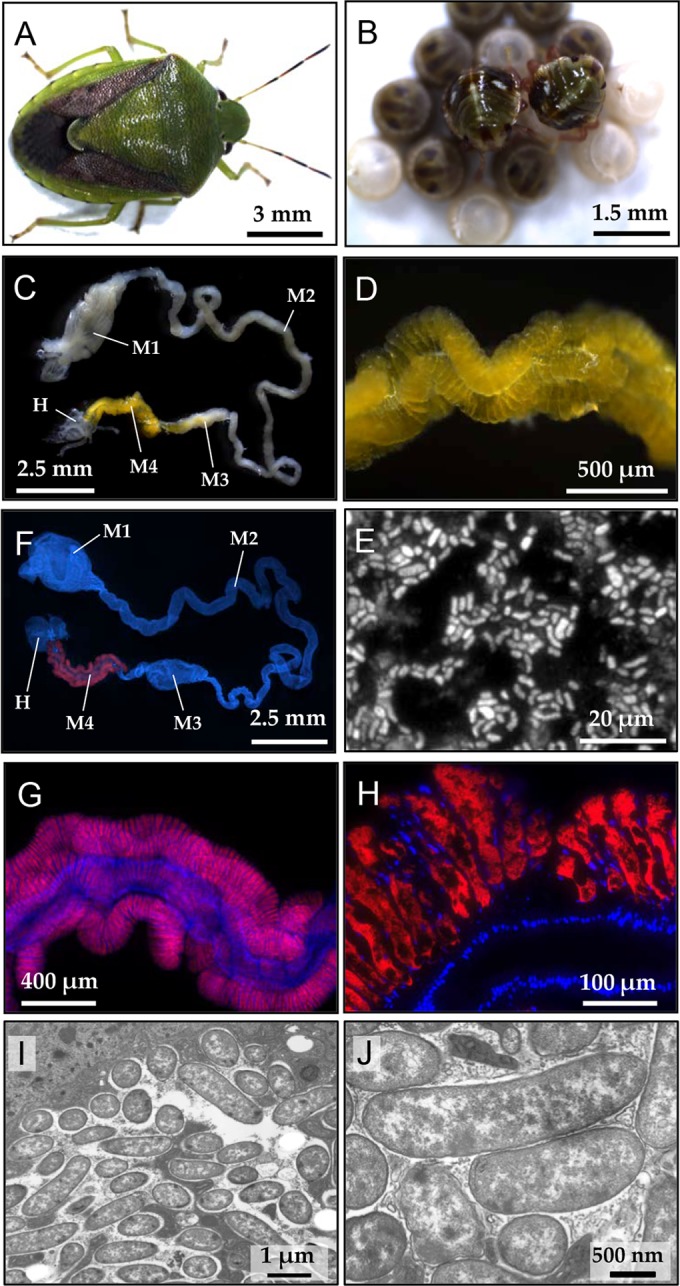
Plautia splendens and its midgut symbiotic system. (A) An adult female. (B) Newborn nymphs probing the surface of eggshell. (C) A dissected alimentary tract. Abbreviations: M1, midgut first section; M2, midgut second section; M3, midgut third section; M4, midgut fourth section with crypts (symbiotic organ); H, hindgut. (D) An enlarged image of the symbiotic organ with numerous crypts. (E) Symbiont cells released from the dissected symbiotic organ. The cells were stained with SYTOX green. (F to H) Visualization of the symbiotic bacteria by in situ hybridization in the whole dissected alimentary tract (F), in the symbiotic organ (G), and within the crypts (H). Red and blue signals indicate the symbiotic bacteria and host's nuclear DNA, respectively. (I, J) Transmission electron microscopic images of the symbiotic bacteria within the crypt cavity.
Characterization of the symbiont.
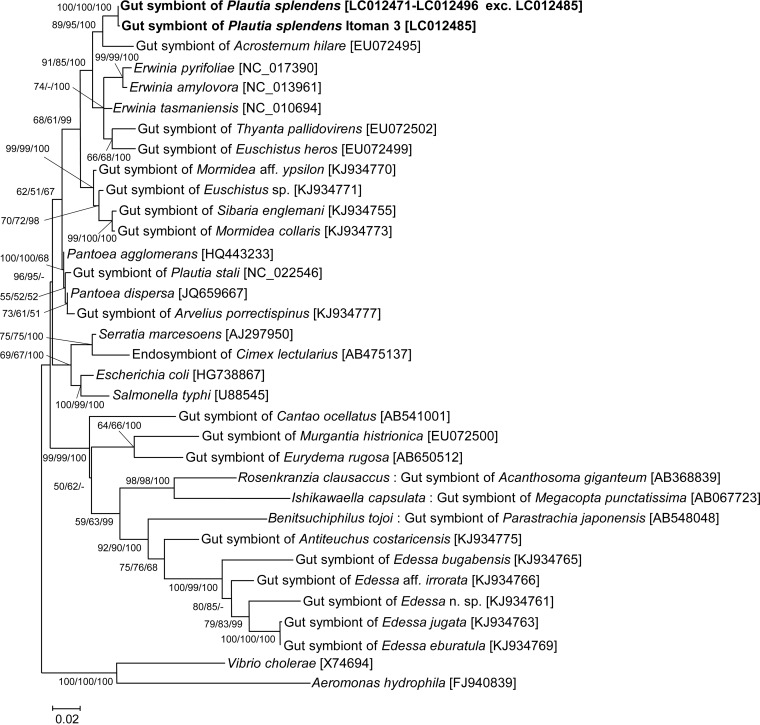
We dissected 26 adult insects collected from six natural populations of P. splendens (Table 1). The dissected midgut fourth sections were individually subjected to DNA extraction and PCR amplification of a bacterial 16S rRNA gene. Direct sequencing of the PCR products consistently yielded clear fluorescence profiles, indicating that a single bacterial species was dominant in each symbiotic organ. Almost all the sequences were identical to each other, except for a sequence from the insect sample Itoman3, containing a single nucleotide substitution. Moreover, 4 of the 26 samples (Ishigaki1, Ishigaki2, Nago4, and Itoman5) were subjected to cloning of the PCR products and sequencing of multiple clones. For 8 clones from each sample, all the sequences were identical to each other. Molecular phylogenetic analysis based on the 16S rRNA gene sequences showed that the gut symbionts of P. splendens formed a distinct lineage in the Enterobacteriaceae of the Gammaproteobacteria, clustering with the gut symbionts of stinkbugs Acrosternum hilare, Euschistus heros, and Thyanta pallidovirens, and also with several Erwinia species (Fig. 2). Notably, the gut symbionts of P. splendens were not allied to the gut symbiont of a closely related congenic stinkbug P. stali (Fig. 2). On the other hand, when the dissected ovaries and testes were individually subjected to DNA extraction and PCR of the bacterial 16S rRNA gene, no amplification was observed, indicating the absence of the gut symbiont and other symbiotic bacteria like Wolbachia in the reproductive organs of P. splendens.
FIG 2.
Molecular phylogenetic analysis of the gut symbiont of Plautia splendens. A maximum likelihood phylogeny inferred from 1,151 aligned nucleotide sites of 16S rRNA gene sequences is displayed with statistical support values no less than 50% at each node in the order of maximum likelihood/neighbor-joining/Bayesian. Sequence accession numbers are shown in brackets.
Localization of the symbiont.
Whole-mount in situ hybridization targeting bacterial 16S rRNA confirmed the symbiont localization within the midgut fourth section (Fig. 1F and G). In situ hybridization of tissue sections of the symbiotic organ visualized the symbiont cells densely populating the inner cavity of the crypts, whereas few symbiont signals were found in the main tract of the midgut fourth section (Fig. 1H). Transmission electron microscopy detected numerous rod-shaped bacterial cells within the crypt cavities (Fig. 1I and J).
Vertical transmission of the symbiont.
We collected 29 newly laid egg masses of P. splendens and randomly allocated them to two experimental groups: 15 control egg masses without treatment and 14 surface-sterilized egg masses treated with formalin. Nymphs from these egg masses were harvested after the second-instar molt and subjected to diagnostic PCR detection of the symbiont. In the control group, most of the nymphs (44/48 = 91.7%) were symbiont positive, which was in sharp contrast with the sterilized group, wherein all the nymphs (0/48 = 0%) were symbiont negative. These results indicate that the symbiont is vertically transmitted to the next generation of P. splendens via egg surface contamination, as known for many other stinkbugs (1, 12–21).
Population dynamics of the symbiont.
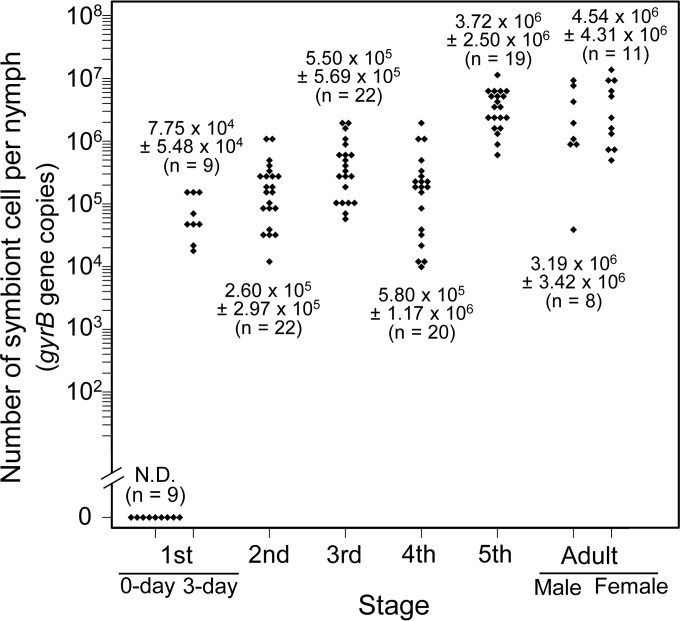
We examined the infection dynamics of the symbiont through the developmental course of P. splendens by quantitative PCR targeting a gyrB gene of the symbiont (Fig. 3). While no symbiont was detected in first-instar nymphs just after hatching, 3-day-old first instar nymphs exhibited the symbiont titers around 7.8 × 104 per insect. As the nymphs grew, the symbiont titers steadily increased, finally attaining 4.5 × 106 per insect in adult females (Fig. 3).
FIG 3.
Population dynamics of the gut symbiont during the developmental course of Plautia splendens. Infection titers were evaluated by quantitative PCR in terms of symbiont gyrB gene copies per insect.
Fitness effects of the symbiont.
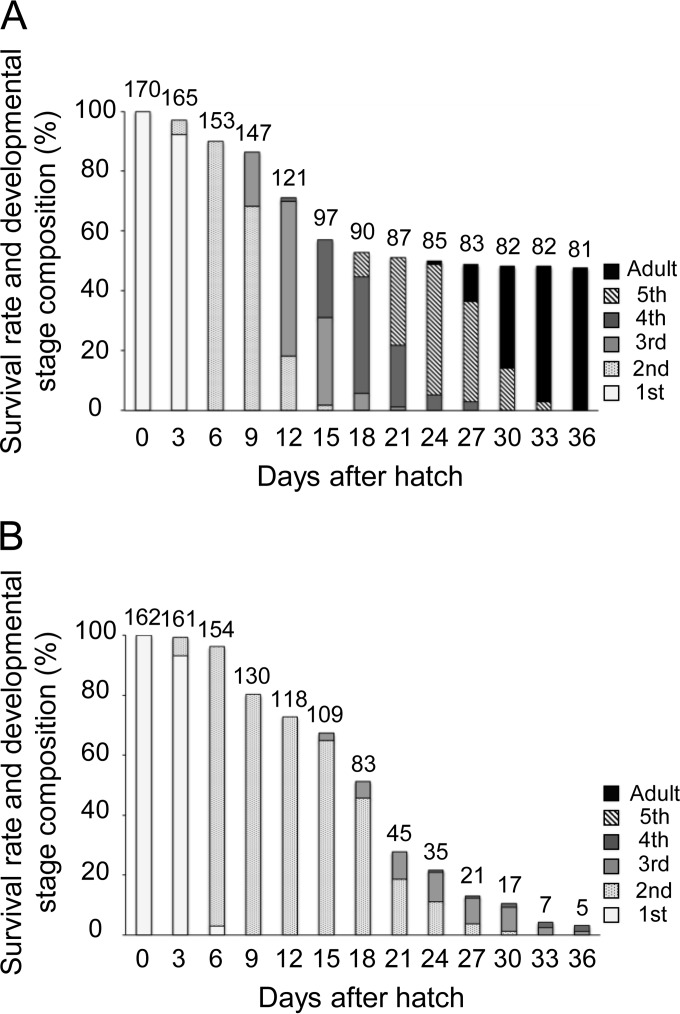
We collected 25 newly laid egg masses of P. splendens and randomly allocated them to two experimental groups: 13 control egg masses (in total 177 eggs) without treatment and 12 surface-sterilized egg masses (in total 172 eggs) treated with formalin. These egg masses were separately reared for 36 days in sterilized plastic petri dishes with sterilized raw peanuts and sterilized distilled water containing 0.05% ascorbic acid. The hatching rates were 96.0% (170/177) in the control group and 94.2% (162/172) in the sterilized group, indicating no detrimental effects of the sterilizing treatment. In the control group, 47.6% (81/170) of the newborn nymphs survived for 36 days, and all of them became adult (Fig. 4A). In the sterilized group, by contrast, only 3.1% (5/162) of the newborn nymphs survived for 36 days, and none of them attained adulthood (Fig. 4B). These results indicate that the symbiont is important for normal growth and survival of P. splendens.
FIG 4.
Survival and growth of Plautia splendens with and without symbiont-sterilizing treatment. (A) Insects from the control egg masses without treatment; (B) insects from the sterilized egg masses whose surface was treated with formalin.
Female-specific enlarged crypts at the posterior end of the midgut symbiotic organ.
Through close inspection of the midgut symbiotic organs of P. splendens, we found a notable sex-related structural difference. In adult females, several crypts at the posterior end of the midgut fourth section were swollen and looked translucent (Fig. 5A and B), while such structure was not found in adult males (Fig. 5C and D). Numbers of the swollen end crypts were 3 to 6 end crypts in 4 rows (17.1 ± 2.1; n = 13). In females at the fifth instar, the end crypts were not swollen but were structurally distinct from the adjacent normal crypts: smaller in size and without yellowish coloration (Fig. 5E). In males at the fifth instar, in contrast, the end crypts looked similar to the adjacent normal crypts (Fig. 5F). In situ hybridization of tissues sections revealed that (i) unlike the normal crypts, the inner cavity of the swollen crypts were often not full of the symbiont cells, which were associated mainly with the crypt wall (Fig. 5G), and (ii) it was frequently observed that, from the swollen crypts, the symbiont cells were released to the midgut main tract (Fig. 5H), which was seldom observed with the normal crypts throughout the stretch of the midgut symbiotic organ. We also inspected the midgut symbiotic organs of other pentatomid stinkbugs, Plautia stali (Fig. 6A) and Carbula crassiventris (Fig. 6B). In both species, the female-specific swollen end crypts were identified (Fig. 6C to F), which were 4 to 7 end crypts in 4 rows (20.5 ± 1.6; n = 10) in P. stali and 3 to 5 end crypts in 4 rows (15.6 ± 1.7; n = 10) in C. crassiventris.
FIG 5.
Female-specific enlarged crypts at the posterior midgut symbiotic organ of Plautia splendens. (A, B) The posterior end of the symbiotic organ of adult females, in which the enlarged end crypts are highlighted by brackets; (C, D) the posterior end of the symbiotic organ of adult males, in which no enlarged crypts are seen; (E) the posterior symbiotic organ of a fifth-instar female, in which the end crypts are not enlarged but structurally distinct from the adjacent normal crypts; (F) the posterior symbiotic organ of a fifth-instar male; (G) in situ hybridization of the symbiotic bacteria in the enlarged end crypts of an adult female; (H) an enlarged image of the junction between the crypt and the main tract of the midgut. Arrows indicate the symbiont cells discharged from the enlarged crypt to the main tract. Panels A, C, E, and F are bright-field images, whereas panels B, D, G, and H are fluorescence images.
FIG 6.
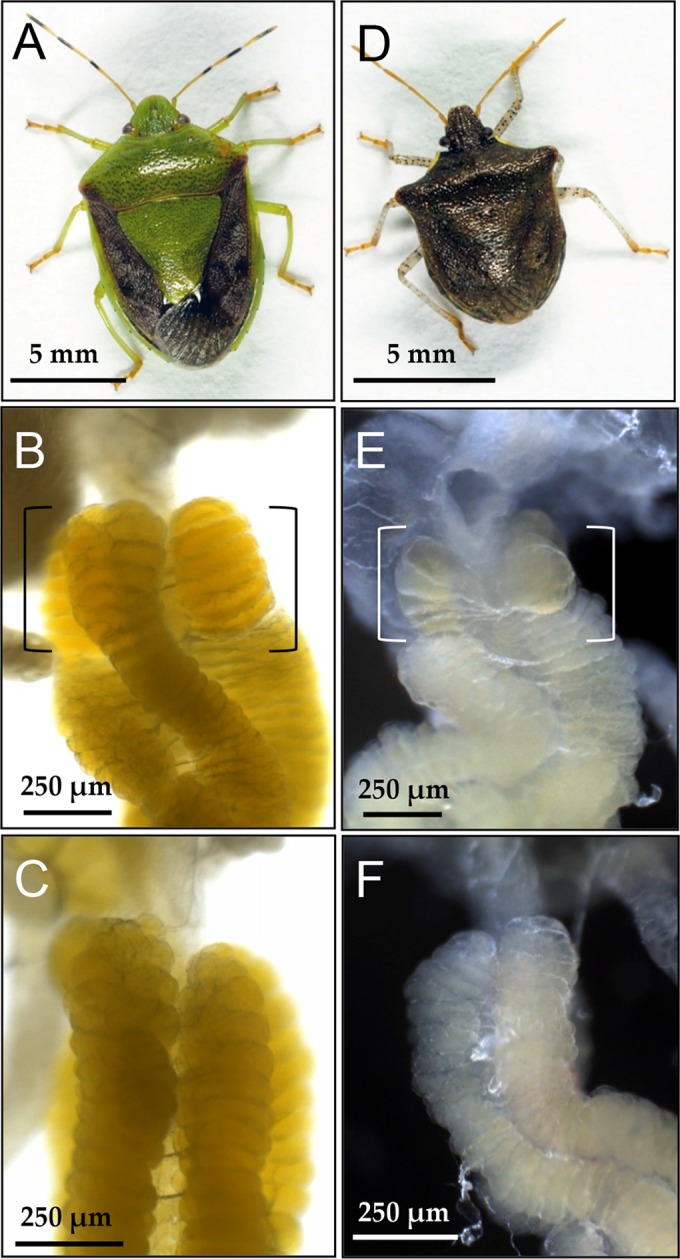
Female-specific enlarged crypts at the posterior midgut symbiotic organ of other stinkbugs of the family Pentatomidae. (A to C) Plautia stali; (D to F) Carbula crassiventris; (A, D) adult females; (B, E) the posterior end of the symbiotic organ of adult females, in which the enlarged end crypts are highlighted by brackets; (C, F) the posterior end of the symbiotic organ of adult males, in which no enlarged crypts are seen.
DISCUSSION
Here, we demonstrated that the bacterial symbiont of P. splendens is localized within the crypt cavity of the midgut fourth section extracellularly (Fig. 1F to H), smeared on the egg surface upon oviposition by adult females, orally acquired by newborn nymphs (Fig. 1B), and thereby transmitted vertically to the next generation (Fig. 3) and important for growth and survival of the host insects (Fig. 4). Such obligate symbiotic bacteria associated with the midgut symbiotic organs have been described from a variety of stinkbugs of the families Pentatomidae (15, 17, 18, 20, 21, 34, 44), Scutelleridae (16, 45, 46), Cydnidae (19), Plataspidae (24, 25, 47, 48), Acanthosomatidae (14), Parastrachiidae (22), Urostylididae (26), and others. As are the cases of the gut symbiotic bacteria associated with the stinkbugs representing the superfamily Pentatomoidea (21, 49, 50), the gut symbiont of P. splendens was placed within the Enterobacteriaceae of the Gammaproteobacteria, in contrast to the betaproteobacterial gut symbionts widely found across the superfamilies Lygaeoidea and Coreoidea (5). Although P. splendens is closely related and congenic to the brown-winged green stinkbug P. stali, the gut symbiont of P. splendens was phylogenetically distinct from the gut symbiont of P. stali (Fig. 2) (49, 51), reflecting the polyphyletic and promiscuous nature of the host-symbiont associations in the Pentatomidae (21, 49).
In this study, the gut symbiont was shown to be important for normal growth and survival of P. splendens (Fig. 4), but exact biological roles of the symbiont deserve future studies. On the grounds that nutritional bacterial symbionts that supply essential amino acids, vitamins, or other nutrients deficient in their host's diet are widespread among plant-sucking members of the Hemiptera (52, 53), the symbiont of P. splendens may also play a nutritional role. Since P. splendens was experimentally fed with protein-rich peanuts and soybeans in this study, amino acid provisioning by the symbiont seems not relevant, raising the possibility of vitamin provisioning by the symbiont, as demonstrated in the seed-sucking cotton stainer bug Dysdercus fasciatus (54). On the other hand, it should be noted that in the field, adults and nymphs of P. splendens are frequently found sucking flower buds and receptacles of wild thistles, which are plausibly less nutritious than peanuts and soybeans. Under the natural condition, therefore, the symbiont's provisioning of other nutrients should also be taken into account. Considering that a variety of biological roles other than nutritional ones are attributed to facultative bacterial symbionts of diverse insects (55) and that many stinkbugs harbor not only the obligate gut symbionts but also facultative bacterial symbionts like Wolbachia, Sodalis, Rickettsia, Spiroplasma, Lariskella, etc. (44, 56–58), the possibility of nonnutritional roles of the symbiont should also be considered.
In previous studies, elaborate, and often spectacular, female-specific cellular, morphological, and developmental traits specialized for vertical symbiont transmission have been reported from stinkbugs of the families Plataspidae, Urostylididae, Parastrachiidae, etc. (14, 22, 26, 27). In this study, although less conspicuous, we found that several rows of crypts at the posterior end region of the symbiotic midgut are morphologically differentiated and conspicuously enlarged specifically in adult females of P. splendens (Fig. 5). While the symbiont cells are confined within the crypts and seldom found in the main tract throughout the stretch of the midgut symbiotic organ (Fig. 1H), we observed that the female-specific enlarged end crypts often released the symbiont cells to the main tract cavity (Fig. 5G and H). The enlarged end crypts were also found in adult females of other pentatomid species P. stali and C. crassiventris (Fig. 6). Early morphological studies on the alimentary tract of stinkbugs documented that the midgut crypts closest to the hindgut are substantially enlarged in adult females of such pentatomid species as Graphosoma italicum, Palomena prasina, Pentatoma rufipes, and Peribalus vernalis (1, 30, 31). These results suggest that the enlarged end crypts represent a female-specific morphological trait specialized for vertical symbiont transmission commonly found among stinkbugs of the family Pentatomidae. The hypothesis that the symbiont transmission organ presumably evolved in the common ancestor of extant pentatomid stinkbugs is, although further experimental works are needed to verify this idea, in impressive contrast with the polyphyletic evolutionary origins of the gut symbiotic bacteria in the Pentatomidae (21, 49).
In the southern green stinkbug Nezara viridula, it has been reported that a basal region of the ovarioles, called the ovarial pedicel, excretes a glue-like substance for attaching eggs onto substrata upon oviposition, and the gut symbiotic bacteria are excreted from the anus and smeared on the egg surface together with the glue-like substance (13, 17, 59). Considering that all pentatomid species attach their eggs onto substrata (32), the glue-like substance is likely also produced by the ovarial pedicel of P. splendens and other pentatomid stinkbugs. If so, the symbiont-containing material smeared on the egg surface upon oviposition may consist of at least two types of secretions derived from distinct organs: the symbiont-containing secretion from the enlarged end crypts and the glue-like secretion from the ovarial pedicel. Conceivably, though speculative, the glue-like secretion may attach and stabilize the symbiont cells on the egg surface, whereas the crypt secretion may play a role in ex-host survival of the symbiotic bacteria. In this context, transcriptomic analyses of the enlarged end crypts and the ovarial pedicel of the pentatomid stinkbugs will provide further insights into molecular, biochemical, and functional aspects of the symbiont-containing materials involved in vertical symbiont transmission.
Through continuous host-symbiont interactions over evolutionary time, not only the symbiotic bacteria but also the host organisms often develop specialized cells, tissues, and organs, such as root nodules in the legume-Rhizobium nitrogen-fixing symbiosis (60), symbiotic light organs in the squid-Vibrio luminescent symbiosis (61), and bacteriocytes, bacteriomes, and gut symbiotic organs in diverse insect-microbe symbioses (1). The elaborate female-specific tissues and organs found in a variety of stinkbugs for ensuring vertical symbiont transmission, which have presumably evolved in the different lineages independently, will provide unique model systems for molecular, developmental, and evolutionary understanding of the symbiosis-driven coevolution. In this context, it is notable that P. splendens and allied stinkbugs are easily maintainable in the laboratory and experimentally tractable and that the female-specific tissues and organs are suitable for comparative transcriptomics between female insects and male insects and also between symbiotic insects and aposymbiotic insects.
ACKNOWLEDGMENTS
We thank J. Makino, N. Tanifuji, U. Asaga, and W. Kikuchi for technical and secretarial assistance and M. Moriyama for providing the laboratory strain of P. stali.
This study was supported by JSPS KAKENHI grant 25221107 to T.F.
REFERENCES
- 1.Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY. [Google Scholar]
- 2.Bourtzis K, Miller TA. 2003. Insect symbiosis. CRC Press, Boca Raton, FL. [Google Scholar]
- 3.Bright M, Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol 8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs JL, Essenberg CJ, Turcotte MM. 2011. New paradigms for the evolution of beneficial infections. Trends Ecol Evol 26:202–209. doi: 10.1016/j.tree.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi Y, Hosokawa T, Fukatsu T. 2011. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J 5:446–460. doi: 10.1038/ismej.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura T, Braendle C, Shingleton A, Sisk G, Kambhampati S, Stern D. 2003. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J Exp Zool B Mol Dev Evol 295:59–81. doi: 10.1002/jez.b.3. [DOI] [PubMed] [Google Scholar]
- 7.Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. 2008. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J Insect Physiol 54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koga R, Meng X-Y, Tsuchida T, Fukatsu T. 2012. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface. Proc Natl Acad Sci U S A 109:E1230–E1237. doi: 10.1073/pnas.1119212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalepa CA, Bignell DE, Bandi C. 2001. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insect Soc 48:194–201. doi: 10.1007/PL00001767. [DOI] [Google Scholar]
- 10.Kikuchi Y, Hosokawa T, Fukatsu T. 2008. Diversity of bacterial symbiosis in stinkbugs, p 39–63. In Dijk TV. (ed), Microbal ecology research trends. Nova Science Publishers, New York, NY. [Google Scholar]
- 11.Glasgow H. 1914. The gastric caeca and the caecal bacteria of the Heteroptera. Biol Bull 3:101–171. [Google Scholar]
- 12.Abe Y, Mishiro K, Takanashi M. 1995. Symbiont of brown-winged green bug, Plautia stali SCOTT. Jpn J Appl Entomol Zool 39:109–115. doi: 10.1303/jjaez.39.109. [DOI] [Google Scholar]
- 13.Prado SS, Rubinoff D, Almeida RPP. 2006. Vertical transmission of a pentatomid caeca-associated symbiont. Ann Entomol Soc Am 99:577–585. doi: 10.1603/0013-8746(2006)99[577:VTOAPC]2.0.CO;2. [DOI] [Google Scholar]
- 14.Kikuchi Y, Hosokawa T, Nikoh N, Meng X-Y, Kamagata Y, Fukatsu T. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol 7:2. doi: 10.1186/1741-7007-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prado SS, Almeida RPP. 2009. Role of symbiotic gut bacteria in the development of Acrosternum hilare and Murgantia histrionica. Entomol Exp Appl 132:21–29. doi: 10.1111/j.1570-7458.2009.00863.x. [DOI] [Google Scholar]
- 16.Kaiwa N, Hosokawa T, Kikuchi Y, Nikoh N, Meng XY, Kimura N, Ito M, Fukatsu T. 2010. Primary gut symbiont and secondary, Sodalis-allied symbiont of the scutellerid stinkbug Cantao ocellatus. Appl Environ Microbiol 76:3486–3494. doi: 10.1128/AEM.00421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tada A, Kikuchi Y, Hosokawa T, Musolin DL, Fujisaki K, Fukatsu T. 2011. Obligate association with gut bacterial symbiont in Japanese populations of the southern green stinkbug Nezara viridula (Heteroptera: Pentatomidae). Appl Entomol Zool 46:483–488. doi: 10.1007/s13355-011-0066-6. [DOI] [Google Scholar]
- 18.Kikuchi Y, Hosokawa T, Nikoh N, Fukatsu T. 2012. Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae). Appl Entomol Zool 47:1–8. doi: 10.1007/s13355-011-0081-7. [DOI] [Google Scholar]
- 19.Hosokawa T, Hironaka M, Inadomi K, Mukai H, Nikoh N, Fukatsu T. 2013. Diverse strategies for vertical symbiont transmission among subsocial stinkbugs. PLoS One 8:e65081. doi: 10.1371/journal.pone.0065081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor CM, Coffey PL, DeLay BD, Dively GP. 2014. The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål). PLoS One 9:e90312. doi: 10.1371/journal.pone.0090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bistolas KS, Sakamoto RI, Fernandes JA, Goffredi SK. 2014. Symbiont polyphyly, co-evolution, and necessity in pentatomid stinkbugs from Costa Rica. Front Microbiol 5:349. doi: 10.3389/fmicb.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosokawa T, Hironaka M, Mukai H, Inadomi K, Suzuki N, Fukatsu T. 2012. Mothers never miss the moment: a fine-tuned mechanism for vertical symbiont transmission in a subsocial insect. Anim Behav 83:293–300. doi: 10.1016/j.anbehav.2011.11.006. [DOI] [Google Scholar]
- 23.Schorr H. 1957. Zur Verhaltensbiologie und Symbiose von Brachypelta aterrima Förest (Cydnidae, Heteroptera). Z Morphol Ökol Tiere 45:561–602. [Google Scholar]
- 24.Fukatsu T, Hosokawa T. 2002. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl Environ Microbiol 68:389–396. doi: 10.1128/AEM.68.1.389-396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol 4:1841–1851. doi: 10.1371/journal.pbio.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiwa N, Hosokawa T, Nikoh N, Tanahashi M, Moriyama M, Meng X-Y, Maeda T, Yamaguchi K, Shigenobu S, Ito M, Fukatsu T. 2014. Symbiont-supplemented maternal investment underpinning host's ecological adaptation. Curr Biol 24:2465–2470. doi: 10.1016/j.cub.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 27.Hosokawa T, Kikuchi Y, Meng XY, Fukatsu T. 2005. The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol Ecol 54:471–477. doi: 10.1016/j.femsec.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Hosokawa T, Kikuchi Y, Fukatsu T. 2007. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect-bacterium mutualism? Mol Ecol 16:5316–5325. doi: 10.1111/j.1365-294X.2007.03592.x. [DOI] [PubMed] [Google Scholar]
- 29.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2008. Symbiont acquisition alters behaviour of stinkbug nymphs. Biol Lett 4:45–48. doi: 10.1098/rsbl.2007.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuskop M. 1924. Bakteriensymbiosen bei Wanzen: (Hemiptera Heteroptera). Arch Protistenkde 47:350–385. [Google Scholar]
- 31.Rosenkranz W. 1939. Die symbiose der pentatomiden. Z Morphol Ökol Tiere 36:279–309. [Google Scholar]
- 32.Schuh RT, Slater JA. 1995. True bugs of the world (Hemiptera: Heteroptera). Cornell University Press, New York, NY. [Google Scholar]
- 33.Weirauch C, Schuh RT. 2011. Systematics and evolution of Heteroptera: 25 years of progress. Annu Rev Entomol 56:487–510. doi: 10.1146/annurev-ento-120709-144833. [DOI] [PubMed] [Google Scholar]
- 34.Bansal R, Michel AP, Sabree ZL. 2014. The crypt-dwelling primary bacterial symbiont of the polyphagous pentatomid pest Halyomorpha halys (Hemiptera: Pentatomidae). Environ Entomol 43:617–625. doi: 10.1603/EN13341. [DOI] [PubMed] [Google Scholar]
- 35.Fukatsu T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol Ecol 8:1935–1945. doi: 10.1046/j.1365-294x.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 36.Sandström JP, Russell JA, White JP, Moran NA. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol 10:217–228. doi: 10.1046/j.1365-294X.2001.01189.x. [DOI] [PubMed] [Google Scholar]
- 37.Fukatsu T, Nikoh N. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl Environ Microbiol 64:3599–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 41.Tanabe AS. 2011. Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol Ecol Resour 11:914–921. doi: 10.1111/j.1755-0998.2011.03021.x. [DOI] [PubMed] [Google Scholar]
- 42.Koga R, Tsuchida T, Fukatsu T. 2009. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl Entomol Zool 44:281–291. doi: 10.1303/aez.2009.281. [DOI] [Google Scholar]
- 43.Moriyama M, Koga R, Hosokawa T, Nikoh N, Futahashi R, Fukatsu T. 2012. Comparative transcriptomics of the bacteriome and the spermalege of the bedbug Cimex lectularius (Hemiptera: Cimicidae). Appl Entomol Zool 47:233–243. doi: 10.1007/s13355-012-0112-z. [DOI] [Google Scholar]
- 44.Matsuura Y, Hosokawa T, Serracin M, Tulgetske GM, Miller TA, Fukatsu T. 2014. Bacterial symbionts of a devastating coffee plant pest, the stinkbug Antestiopsis thunbergii (Hemiptera: Pentatomidae). Appl Environ Microbiol 80:3769–3775. doi: 10.1128/AEM.00554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiwa N, Hosokawa T, Kikuchi Y, Nikoh N, Meng XY, Kimura N, Ito M, Fukatsu T. 2011. Bacterial symbionts of the giant jewel stinkbug Eucorysses grandis (Hemiptera: Scutelleridae). Zool Sci 28:169–174. doi: 10.2108/zsj.28.169. [DOI] [PubMed] [Google Scholar]
- 46.Kafil M, Bandani AR, Kaltenpoth M, Goldansaz SH, Alavi SM. 2013. Role of symbiotic bacteria in the growth and development of the Sunn pest, Eurygaster integriceps. J Insect Sci 13:99. doi: 10.1673/031.013.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc R Soc B 274:1979–1984. doi: 10.1098/rspb.2007.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikoh N, Hosokawa T, Oshima K, Hattori M, Fukatsu T. 2011. Reductive evolution of bacterial genome in insect gut environment. Genome Biol Evol 3:702–714. doi: 10.1093/gbe/evr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prado SS, Almeida RPP. 2009. Phylogenetic placement of pentatomid stink bug gut symbionts. Curr Microbiol 58:64–69. doi: 10.1007/s00284-008-9267-9. [DOI] [PubMed] [Google Scholar]
- 50.Hosokawa T, Kikuchi Y, Nikoh N, Fukatsu T. 2012. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol 78:4758–4761. doi: 10.1128/AEM.00867-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi H, Kawasaki K, Takeishi K, Noda H. 2011. Symbiont of the stink bug Plautia stali synthesizes rough-type lipopolysaccharide. Microbiol Res 167:48–54. doi: 10.1016/j.micres.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 53.Douglas AE. 2009. The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47. doi: 10.1111/j.1365-2435.2008.01442.x. [DOI] [Google Scholar]
- 54.Salem H, Bauer E, Strauss AS, Vogel H, Marz M, Kaltenpoth M. 2014. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc R Soc B 281:20141838. doi: 10.1098/rspb.2014.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 56.Kikuchi Y, Fukatsu T. 2003. Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl Environ Microbiol 69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuura Y, Kikuchi Y, Meng XY, Koga R, Fukatsu T. 2012. Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl Environ Microbiol 78:4149–4156. doi: 10.1128/AEM.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosokawa T, Kaiwa N, Matsuura Y, Kikuchi Y, Fukatsu T. 2015. Infection prevalence of Sodalis symbionts among stinkbugs. Zool Lett 1:5. doi: 10.1186/s40851-014-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bin F, Vinson SB, Strand MR, Colazza S, Jones WAJ. 1993. Source of an egg kairomone for Trissolcus basalis, a parasitoid of Nezara viridula. Physiol Entomol 18:7–15. doi: 10.1111/j.1365-3032.1993.tb00443.x. [DOI] [Google Scholar]
- 60.Kondorosi E, Mergaert P, Kereszt A. 2013. A paradigm for endosymbiotic life: cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu Rev Microbiol 67:611–628. doi: 10.1146/annurev-micro-092412-155630. [DOI] [PubMed] [Google Scholar]
- 61.Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid-vibrio symbiosis. Nat Rev Microbiol 2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]