Abstract
Acetoin in vinegar is an attractant to fruit flies when combined with acetic acid. To make vinegar more effective in attracting fruit flies with increased acetoin production, Komagataeibacter europaeus KGMA0119 was modified by specific gene disruption of the acetohydroxyacid isomeroreductase gene (ilvC). A previously constructed mutant lacking the putative ligand-sensing region in the leucine-responsive regulatory protein (KeLrp, encoded by Kelrp) was also used. The ilvC and Kelrp disruptants (KGMA5511 and KGMA7203, respectively) produced greater amounts of acetoin (KGMA5511, 0.11%; KGMA7203, 0.13%) than the wild-type strain KGMA0119 (0.069%). KGMA7203 produced a trace amount of isobutyric acid (0.007%), but the other strains did not. These strains produced approximately equal amounts of acetic acid (0.7%). The efficiency of fruit fly attraction was investigated with cultured Drosophila melanogaster. D. melanogaster flies (approximately 1,500) were released inside a cage (2.5 m by 2.5 m by 1.5 m) and were trapped with a device containing vinegar and a sticky sheet. The flies trapped on the sticky sheet were counted. The cell-free supernatant from KGMA7203 culture captured significantly more flies (19.36 to 36.96% of released flies) than did KGMA0119 (3.25 to 11.40%) and KGMA5511 (6.87 to 21.50%) cultures. Contrastingly, a 0.7% acetic acid solution containing acetoin (0.13%) and isobutyric acid (0.007%), which mimicked the KGMA7203 supernatant, captured significantly fewer flies (0.88 to 4.57%). Furthermore, the KGMA0119 supernatant with additional acetoin (0.13%) and isobutyric acid (0.007%) captured slightly more flies than the original KGMA0119 supernatant but fewer than the KGMA7203 supernatant, suggesting that the synergistic effects of acetic acid, acetoin, isobutyric acid, and unidentified metabolites achieved the efficient fly trapping of the KGMA7203 supernatant.
INTRODUCTION
Bacterial cultures can function as attractants for insects (1). Fruit flies, such as Drosophila melanogaster, are recognized as an index for unsanitary conditions in urban areas because the flies infest damaged and overripe fruits and rotten foods. One of the important challenges is to keep the numbers of flies low, particularly in the food industries. A closely related species, the spotted-wing drosophila (SWD) (Drosophila suzukii), which is native to southeastern Asia, is a newly emerging invasive pest for soft-skinned fruits such as blueberries, strawberries, and peaches in North America and Europe (2–4). Because the SWD breaks the skin of maturing healthy fruits using a serrated ovipositor to oviposit, unlike other Drosophila species, the fly causes significant damage to soft-skinned fruits (2). The damage caused by the SWD promotes microbial decay in the fruits, which results in a secondary infestation of other Drosophila species. The damaged fruits are likely to be rejected at the processing plant or export terminal (5). Although fruits are protected with sprays of chemical insecticides once the SWD is detected, growers risk the rejection of harvested fruits when the levels of residual insecticides exceed the maximum limits (5). Serious economic losses are estimated to be caused by the SWD, including increased management costs and rejection of crops, in the fruit-growing industries of the United States and Europe (6, 7). Therefore, effective flytraps are important to establish a systematic pest management program to prevent economic damage by protecting fruits from the attack of the SWD and other Drosophila species. Such traps could also help growers reduce pesticide applications, which is preferable for the safety of the consumer.
Recently, flytraps that used fermented foods instead of chemicals were developed for general household use in response to increasing consumer demands. Vinegar and wine strongly attract fruit flies (1, 8). Several physiologically active compounds in vinegar and wine were identified by gas chromatography coupled with electroantennographic detection (GC-EAD) (7, 9, 10). Acetic acid is a primary volatile compound in vinegar and plays a key role in fruit fly attraction (7, 8, 11). Combined with acetic acid, acetoin, which is a fermentation compound primarily found in dairy foods (12) that has a heavy cheese-like odor, exerts a strong synergistic effect on fruit fly attraction (7, 11). These volatile compounds are important signals for fruit flies to find feeding and oviposition sites (10). Therefore, vinegar with increased amounts of acetoin is expected to be a powerful attractant for effective trapping of flies.
Acetic acid bacteria (AAB) are Gram-negative obligately aerobic microorganisms. Recently, it has been reported that some unique AAB are applicable not only for vinegar production but also for other beneficial purposes, such as cellulose production for transplantation therapy (13), production of shikimate (precursor of oseltamivir) (14), and nitrogen fixation in agriculture (15). Komagataeibacter europaeus (previously classified as Gluconacetobacter europaeus) (16) is widely used in the industrial production of vinegar because of its ability to oxidize ethanol and high tolerance to acetic acid (17). In our previous study, a targeted gene disruption system using the endogenous pyrE gene as a selectable marker was developed for use in K. europaeus, and the pathway for acetoin biosynthesis in K. europaeus was determined (18). The pathway shares intermediary metabolites (pyruvate and 2-acetolactate) with the pathway for branched-chain amino acid (BCAA; valine, leucine, or isoleucine) biosynthesis (Fig. 1). The disruption of ilvC, which is one of the genes for BCAA biosynthesis and encodes acetohydroxyacid isomeroreductase, was expected to promote acetoin production by shifting the carbon flux from valine to acetoin (Fig. 1). Our previous study also revealed that the K. europaeus leucine-responsive regulatory protein (KeLrp [previously designated GeLrp]; encoded by Kelrp) repressed the transcription of ilvIH, encoding a rate-limiting enzyme in BCAA biosynthesis (acetohydroxyacid synthase). Increased expression of ilvIH occurred when the repression was released by the truncation of the C-terminal ligand-sensing region in KeLrp (Fig. 1) (19). Thus, the Kelrp disruptant (designated KGMA7203) was predicted to accumulate increased amounts of acetoin as a by-product (Fig. 1).
FIG 1.
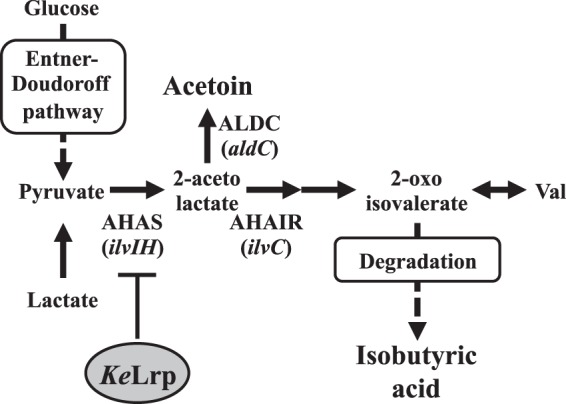
Biosynthetic pathway for the production of valine, acetoin, and isobutyric acid. AHAS, acetohydroxyacid synthase; AHAIR, acetohydroxyacid isomeroreductase; ALDC, α-acetolactate decarboxylase; KeLrp, leucine-responsive regulatory protein in K. europaeus.
To enhance attractiveness to fruit flies, changes in raw materials and their blending ratios are effective techniques. Currently, a variety of flytraps that use fermented foods (e.g., apple cider vinegar, rice vinegar, and balsamic vinegar) as attractants are available on the market. In this study, we first constructed an ilvC disruptant (designated KGMA5511). Fly-trapping experiments were then conducted with traps baited with cell-free supernatants from cultures of the ilvC disruptant KGMA5511 and the previously constructed Kelrp disruptant KGMA7203 (19), and the attractiveness of the supernatants to fruit flies was investigated. Fruit fly trapping was effectively enhanced by the culture supernatants of these metabolically modified AAB.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used in the study are presented in Table 1. Strain KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE {where “Δ(397-511)” indicates deletion of nucleotides 397 to 511}] was a valine- and leucine-overproducing strain with a specific deletion at the C-terminal ligand-binding domain in KeLrp (19). Strain KGMA0119 (wild type, isolated from rice vinegar) (18) and its derivatives were cultured in yeast peptone dextrose (YPD) broth (18) at 30°C with reciprocal shaking at 150 rpm. To isolate the ilvC disruptant strain KGMA5511 (see below), a minimal medium (18) containing 12 mM BCAAs (12 mM valine, 12 mM leucine, and 12 mM isoleucine) was used. As well, 12 mM BCAAs were added to YPD broth when the strain KGMA5511 was cultured. Unless otherwise indicated, 0.4% (wt/vol) ethanol and 0.5% (wt/vol) acetic acid were added to both media. For plate cultures, 0.9% (wt/vol) agar was added to both media.
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristics or sequence (5′ to 3′)a | Source or purpose |
|---|---|---|
| Strains | ||
| E. coli DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | 20 |
| K. europaeus | ||
| KGMA0119 | Wild type isolated from rice vinegar | 18 |
| KGMA0704 | KGMA0119 derivative; ΔpyrE | 18 |
| KGMA5511 | KGMA0704 derivative; ΔpyrE ΔilvC::pyrE | This work |
| KGMA7203 | KGMA0704 derivative; ΔpyrE KelrpΔ(397-511)::pyrE | 19 |
| Plasmids | ||
| pBR322 | Ampr | 21 |
| pBR322-ΔilvC::pyrE | pBR322 derivative; ΔilvC::pyrE cassette composed of 5′ upstream and 3′ downstream regions of ilvC and a pyrE cassette (3,374 bp) | This work |
| Primers | ||
| ilvC-F | AAGAATTCTCACGGTATTCCGAAAGCTACAGT | ilvC disruption |
| ilvC-R | AAGGATCCGTATTCAGGCGGTATAGGTGACGTT | ilvC disruption |
| ilvC-i1 | ATGATGTCATGGATCGGCAAGAACAAGCTG | ilvC disruption |
| ilvC-i2 | ATCGCGATCGTAATAGACGCGCATGGTCTT | ilvC disruption |
| EP-F2 | CTGCCATATCCCGTGTTCGT | ilvC disruption |
| E-R4 | TCGCCATAGGGAAAGACTGC | ilvC disruption |
| ilvC-F2 | GTGGATACAATATCGAAAGCCTGAC | Genotyping |
| ilvC-R2 | CTGGCTTGGAAAAGAAGAAAGGTTC | Genotyping |
Restriction enzyme sites are underlined. Amp, ampicillin.
Escherichia coli DH5α (20) (TaKaRa Bio, Ohtsu, Shiga, Japan) and pBR322 (21) (TaKaRa Bio) were used to construct the ilvC disruption vector (see Fig. S1A in the supplemental material). E. coli DH5α was cultured in lysogeny broth (22) containing 50 μg/ml of ampicillin at 37°C.
DNA manipulation and sequencing.
General DNA manipulations were performed as described by Green and Sambrook (22). PCR was conducted using KOD plus (Toyobo, Osaka, Japan) as a DNA polymerase. Restriction and modifying enzymes were purchased from TaKaRa Bio or Nippon Gene (Tokyo, Japan). PCR products and restriction fragments were separated by agarose gel electrophoresis, and the DNA fragments were recovered using the NucleoSpin Gel and PCR Cleanup kit (TaKaRa Bio). DNA sequencing was performed with a BigDye Terminator Cycle Sequencing kit, ver. 3.1, and model 3130 capillary sequencer (Applied Biosystems, Foster City, CA, USA).
Construction of ilvC disruption vector and ilvC disruptant.
The theoretical background for the specific gene disruption was described previously (18, 23–25). The pyrE gene, encoding orotate phosphoribosyltransferase, and strain KGMA0704, lacking pyrE, were used as a selectable marker and a host strain, respectively. The primers used for the construction of the ilvC disruption vector are listed in Table 1 and are shown in Fig. S1A in the supplemental material. A 1.5-kb pyrE marker cassette containing its promoter region was amplified from KGMA0119 chromosomal DNA using 5′-phosphorylated primers EP-F2 and E-R4. A 2.8-kb fragment containing the ilvC open reading frame (ORF) together with its 5′ (1.0 kb)- and 3′ (0.9 kb)-flanking regions was amplified using primers ilvC-F and ilvC-R (see Fig. S1A in the supplemental material). The amplified fragment was subcloned into pBR322 between the EcoRI and BamHI sites. The 5′- and 3′-flanking regions of ilvC and the plasmid backbone, excluding the 945 bp of the ilvC ORF, were amplified from the intermediary plasmid using primers ilvC-i1 and ilvC-i2 (see Fig. S1A in the supplemental material). This PCR fragment was then ligated with a 5′-phosphorylated pyrE cassette, and the resultant plasmid was designated pBR322-ΔilvC::pyrE (see Fig. S1A in the supplemental material). The pBR322-ΔilvC::pyrE plasmid was introduced into KGMA0704 competent cells by electroporation, and the cells were allowed to recover in 1 ml of YPD broth for 3 h at 30°C. The cells were then harvested by centrifugation, washed with saline (0.85% [wt/vol] NaCl), and transferred to 5 ml of the minimal medium lacking uracil and containing 12 mM BCAAs. The cells were cultured for at least 48 h to concentrate uracil prototrophic and BCAA auxotrophic cells. An aliquot of the culture was diluted with saline, spread onto the minimal agar plate lacking uracil and containing BCAAs, and incubated at 30°C for 4 to 5 days. The genotypes of positive clones were investigated with PCR using primers ilvC-F2 and ilvC-R2 (see Fig. S1A in the supplemental material) and by sequencing the obtained PCR fragments. The resultant ilvC disruptant was designated KGMA5511 (ΔpyrE ΔilvC::pyrE) (see Fig. S1 in the supplemental material).
Measurements of volatile compounds and organic acids and preparation of culture supernatants for fly trapping.
The strains KGMA0119, KGMA5511, and KGMA7203 (19) were cultured in 30 ml of YPD broth supplemented with 1.0% (wt/vol) sodium l-lactate (and 12 mM BCAAs for KGMA5511). Aliquots of cultures were sampled sequentially, and cell-free supernatants were prepared by centrifugation (4°C, 10,000 × g, 5 min) and filtration (0.45-μm-pore-size Millex LH filter; Millipore, Bedford, MA, USA). The concentrations of ethanol, acetic acid, and acetoin in the supernatants were measured with a GC-2014 gas chromatograph (GC; Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a packed column (PEG20M 10%, Shincarbon A 60/80, 2.1 m by 3.2 mm; Shinwa Chemical, Kyoto, Japan). The column oven temperature was initially held at 60°C for 3 min, which was followed by an increase of 10°C per min up to 200°C.
The level of isobutyric acid in the supernatants (filtered with a 0.45-μm filter) was quantified by high-performance liquid chromatography (HPLC; Organic Acid Analysis System Prominence; Shimadzu) on an ion-exclusion column (Shim-pack SCR-102H; Shimadzu). The column was equilibrated with a mobile phase (5 mM p-toluenesulfonate; Wako Pure Chemicals, Osaka, Japan) at a flow rate of 0.8 ml/min at 40°C. The eluted organic acids were automatically mixed with a reaction buffer composed of 5 mM p-toluenesulfonate, 100 μM EDTA (Dojindo Molecular Technologies, Kumamoto, Japan), and 20 mM Bis-Tris [bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane; Dojindo Molecular Technologies] at a flow rate of 0.8 ml/min at 40°C and were monitored with an electrical conductivity detector (CDD-10AVP; Shimadzu).
After cultivation for 34 h, cell-free supernatants of the three strains were prepared as described above using the entire cultures, which were then used for the fly-trapping experiments (see below).
To evaluate concentrations of acetic acid and acetoin in supernatants and the final cell yields (optical density at 600 nm [OD660]) of the three strains after 34 h of cultivation, mean values (n = 3) were statistically analyzed by Tukey's test at P values of 0.05 following an analysis of variance (ANOVA).
Breeding of fruit flies.
D. melanogaster was kindly provided by the Department of Biological Sciences, Tokyo Metropolitan University, Japan. A banana medium (10 g of pasted banana supplemented with 0.5 g of dry yeast [Oriental Yeast, Tokyo, Japan] and 3 ml of beer [Kirin, Tokyo, Japan]) was used routinely as a feed for the fruit flies. The flies were transferred to a 30-ml glass vial containing 6 ml of the banana medium and capped with a cotton plug, and the flies were reared at room temperature (22 to 25°C) for 10 to 14 days. To maintain the fly population, newly eclosed flies were transferred to glass vials containing fresh medium, and this was repeated every 10 days. For the trapping experiments, the adult flies, larvae, and pupae in the glass vials were transferred to a 5-liter glass bottle baited with fresh banana medium and further reared for 5 to 10 days until the population reached approximately 1,500 individuals. The cultured flies were used in the subsequent trapping experiments.
Fly-trapping experiments.
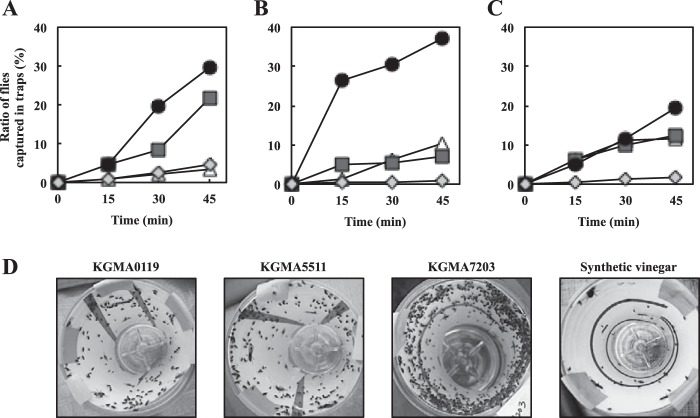
As a flytrap device, a plastic bottle of the Victor Fly Magnet system (Woodstream Co., Lititz, PA, USA) was used with modifications as follows: a sticky sheet (11 cm by 21 cm; Sankyo-Shodoku Co., Tokyo, Japan) was formed into a cylinder and attached to a clear plastic bottle (Fig. 2A). Thirty milliliters of the cell-free supernatant was added to the bottle as an attractant, and the bottle was capped by a lid with entry holes for the attracted flies and a shade that promoted the spread of attractant (Fig. 2A). Synthetic vinegar composed of 0.7% (wt/vol) acetic acid, 0.13% (wt/vol) acetoin, and 0.007% (wt/vol) isobutyric acid was used as a reference solution. The traps were hung in the corners of a cage (2.5 m by 2.5 m by 1.5 m) placed outside and fixed with a mosquito net. The height at which the traps were hung was 1.0 m from the ground (Fig. 2B). To evaluate the attractiveness of the supernatants, the cultured flies (approximately 1,500) were released inside the cage, and the flies captured on the sticky sheets were monitored every 15 min for 45 min. At the conclusion of the trapping experiments, the flies not captured in the traps were killed with an insecticide and enumerated. The number of flies killed was added to that of captured flies to determine the total number of released flies. The attractiveness of supernatants was indexed as the ratio of the number of flies captured in traps to the fly population released (number of captured flies/total number of released flies [%]). The trapping experiments were conducted in Sanda City, Hyogo, Japan. The first trapping experiments were conducted in triplicate (n = 3) on 1 and 9 July 2014 (see Fig. 6). The first, second, and third trials were performed from 13:30 to 14:15 on July 1 under sunny conditions (30°C) (see Fig. 6A), from 11:00 to 11:45 on July 9 under cloudy conditions (28°C) (see Fig. 6B), and from 14:00 to 14:45 on July 9 under cloudy conditions (28°C) (see Fig. 6C), respectively. The second trapping experiments to examine an effect of additional acetoin and isobutyric acid on fly-trapping efficiency were also conducted in triplicate (n = 3) on 9 and 20 October 2014 (see Fig. 7). The first, second, and third trials were performed from 13:40 to 14:25 on 9 October under cloudy conditions (26°C) (see Fig. 7A), from 15:05 to 15:50 on 9 October under cloudy conditions (25°C) (see Fig. 7B), and from 13:40 to 14:25 on 20 October under cloudy conditions (25°C) (see Fig. 7C), respectively.
FIG 2.
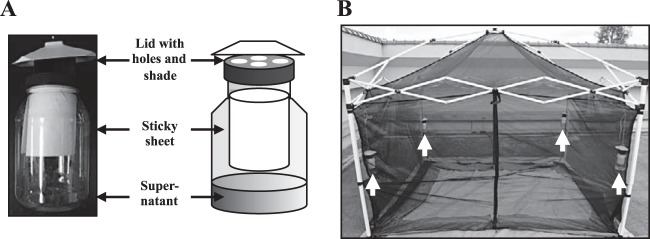
Design of fly-trapping experiment. (A) Trap design. Victor Fly Magnet (Woodstream Co.) with sticky sheet. The cell-free supernatants of cultures from strains KGMA0119 (wild type), KGMA5511 (ΔpyrE ΔilvC::pyrE), and KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE] were used as attractants. (B) Cage with a mosquito net used for trapping experiments. Traps were hung in the corners of the cage (white arrows). The cultured flies were released inside the cage, and the flies captured in traps were monitored (Fig. 6 and 7).
FIG 6.
The fly-trapping experiments with culture supernatants from various strains. Strains KGMA0119 (wild type), KGMA5511 (ΔpyrE ΔilvC::pyrE), and KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE] were cultured in YPD broth supplemented with 1.0% sodium l-lactate (and 12 mM BCAAs for KGMA5511). After culture for 34 h, cell-free supernatants were prepared and used for fly-trapping experiments. (A to C) Attractiveness of the supernatants to fruit flies. The attractiveness of the supernatants was indicated as a ratio of flies captured in traps to the total fly population released (%). The synthetic vinegar was composed of 0.7% acetic acid, 0.13% acetoin, and 0.007% isobutyric acid. The trapping experiments were conducted in triplicate. The first (A), second (B), and third (C) trials were conducted in the sunny afternoon (30°C), in the cloudy morning (28°C), and in the cloudy afternoon (28°C), respectively. See Materials and Methods for more-detailed conditions during the experiments (date and time). White triangles, KGMA0119; dark gray squares, KGMA5511; black circles, KGMA7203; light gray diamonds, synthetic vinegar. (D) Flies captured in traps. The results are consistent with the data of 45 min in panel B.
FIG 7.
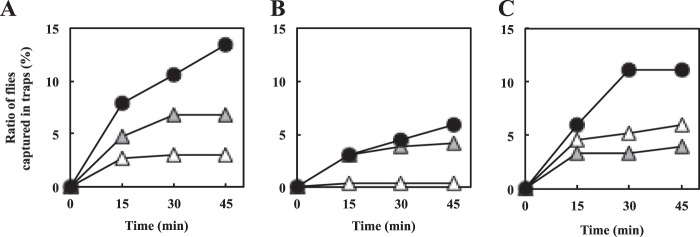
Effects of additional acetoin and isobutyric acid on the fly-trapping efficiency of KGMA0119 supernatant. Strains KGMA0119 (wild type) and KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE] were cultured in YPD broth supplemented with 1.0% sodium l-lactate. After 34 h of cultivation, cell-free supernatants were prepared and used for fly-trapping experiments. To investigate the effects of increased amounts of attractive compounds, the KGMA0119 supernatant, whose final concentrations of acetoin (0.13%) and isobutyric acid (0.007%) were adjusted to those in KGMA7203 supernatant, was used. The attractiveness of the supernatants was indicated as the ratio of the number of flies captured in traps to the total fly population released (%). The trapping experiments were conducted in triplicate. The first (A), second (B), and third (C) trials were conducted in the cloudy afternoon (26°C), in the cloudy late afternoon (25°C), and in the cloudy afternoon (25°C), respectively. White triangles, KGMA0119; light gray triangles, KGMA0119 with additional acetoin (0.13%) and isobutyric acid (0.007%); black circles, KGMA7203. See Materials and Methods for more-detailed conditions during the experiments (date and time).
Nucleotide sequence accession numbers.
The nucleotide sequences of ilvC and Kelrp were deposited in the DDBJ, EMBL, and GenBank databases under accession numbers AB899162 and AB899159, respectively.
RESULTS
Construction of the ilvC disruptant.
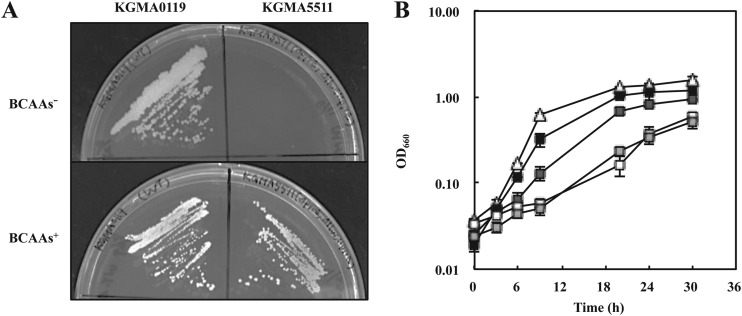
The biosynthesis pathway for acetoin shares intermediary metabolites with the pathway for BCAAs in K. europaeus (Fig. 1). The disruption of the ilvC gene was expected to change the carbon flux toward acetoin and result in increased production of acetoin. The ilvC disruption vector pBR322-ΔilvC::pyrE was introduced into the pyrE-lacking strain KGMA0704 (see Fig. S1A in the supplemental material). The resultant ilvC disruptant was designated KGMA5511 (ΔpyrE ΔilvC::pyrE) (see Fig. S1A in the supplemental material). The PCR analysis confirmed that the ilvC locus of KGMA5511 was longer than that of each of the two parental strains, KGMA0704 and the wild-type strain KGMA0119 (see Fig. S1B in the supplemental material). Sequence analysis of the targeted region also confirmed that the ilvC locus in KGMA5511 was replaced with the pyrE marker as expected. The KGMA5511 strain strictly required three types of BCAA (valine, leucine, and isoleucine) for its growth (Fig. 3A) and showed impaired growth when it was cultured in a nutrient-rich YPD broth in the absence of BCAAs (Fig. 3B). To restore this growth defect, 12 mM BCAAs (12 mM valine, 12 mM leucine, and 12 mM isoleucine) was needed. The impaired growth of KGMA5511 was not fully restored by the addition of lower concentrations (0.5 or 4 mM each) of BCAAs to the medium (Fig. 3B).
FIG 3.
The BCAA auxotrophy of KGMA5511 (ΔpyrE ΔilvC::pyrE). (A) BCAA auxotrophy of strain KGMA5511. Strains KGMA0119 (wild type) and KGMA5511 were streaked onto a minimal agar plate lacking (upper panel) or containing (lower panel) 12 mM BCAAs. (B) Growth profiles of KGMA0119 and KGMA5511 strains. KGMA0119 was cultured in YPD broth lacking BCAAs, and KGMA5511 was cultured in YPD broth lacking or containing various concentrations of BCAAs. White triangles, KGMA0119 without BCAAs; white squares, KGMA5511 without BCAAs; light gray squares, KGMA5511 with 0.5 mM BCAAs; dark gray squares, KGMA5511 with 4 mM BCAAs; black squares, KGMA5511 with 12 mM BCAAs. Experiments were conducted in triplicate, and error bars indicate standard deviations.
Volatile compounds and organic acid profiles of gene disruptants.
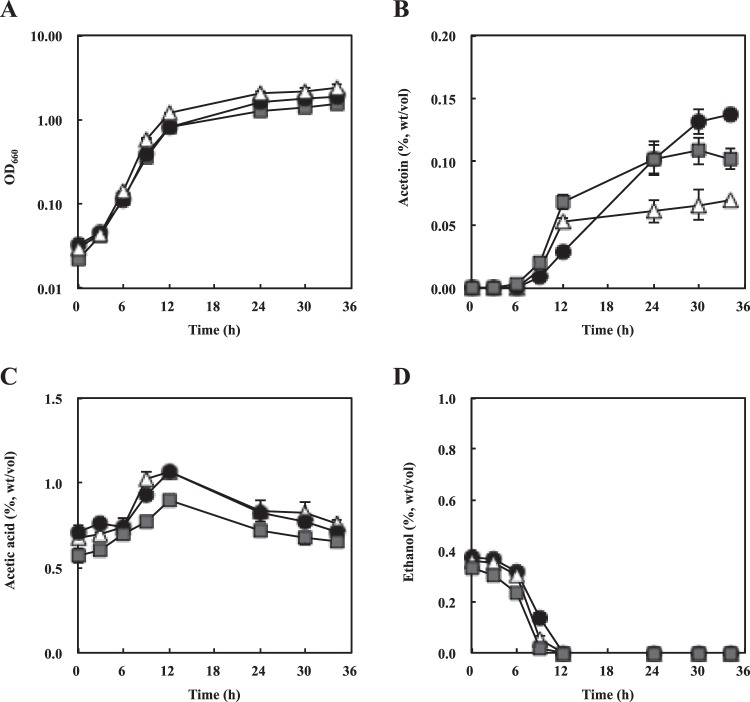
Our previous study revealed that the genes for BCAAs biosynthesis (ilvI and ilvH) were highly expressed in the Kelrp disruptant KGMA7203 (19). Thus, it was expected that the strain accumulated increased amounts of acetoin as a by-product (Fig. 1). To estimate acetoin productivity, the strains KGMA0119, KGMA5511, and KGMA7203 were cultured in YPD broth supplemented with 1.0% sodium l-lactate (and 12 mM BCAAs for KGMA5511), and the concentrations of acetoin, acetic acid, and ethanol in the cultures were measured at various growth phases by GC (Fig. 4). The growth profiles were also monitored. The final cell yields of KGMA5511 and KGMA7203 were slightly lower than those of KGMA0119 (Fig. 4A); however, there were no statistical differences among the cell yields of the three strains (Tukey's test following ANOVA, F = 5.14, df = 8, P = 0.017) (Table 2). Whereas all the strains produced acetoin from the late logarithmic phase of growth, strains KGMA5511 (0.11%) and KGMA7203 (0.13%) accumulated significantly greater amounts of acetoin than the wild-type KGMA0119 (0.069%) (Tukey's test following ANOVA, F = 5.14, df = 8, P = 0.00011) (Fig. 4B and Table 2). The profiles of ethanol oxidization and acetic acid production were not significantly different in the three strains (Fig. 4C and D), and the concentrations of acetic acid after 34 h of culture were approximately 0.7% (Tukey's test following ANOVA, F = 5.14, df = 8, P = 0.15) (Fig. 4C and Table 2). Ethanol was not detected in cultures of the three strains after 34 h of growth (Fig. 4D).
FIG 4.
Growth and volatile compound profiles of strains KGMA0119 (wild type), KGMA5511 (ΔpyrE ΔilvC::pyrE), and KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE]. The three strains were cultured in YPD broth supplemented with 1.0% sodium l-lactate (and 12 mM BCAAs for KGMA5511). The amounts of acetoin, acetic acid, and ethanol in cultures were measured by GC. Shown are profiles of growth (OD660) (A), acetoin (% [wt/vol]) (B), acetic acid (% [wt/vol]) (C), and ethanol (% [wt/vol]) (D). White triangles, KGMA0119; dark gray squares, KGMA5511; black circles, KGMA7203. Experiments were conducted in triplicate, and error bars indicate standard deviations.
TABLE 2.
Volatile compounds in cultures and cell yields of KGMA0119 (wild type) and its derivatives after 34 h of cultivationa
| Strain (relevant characteristics) | Mean amt ± SD of volatile compound (%, wt/vol) |
Final cell yield (OD660 at 34 h) | |
|---|---|---|---|
| Acetic acid | Acetoin | ||
| KGMA0119 (wild type) | 0.76 ± 0.022 A | 0.069 ± 0.00082 A | 2.33 ± 0.28 A |
| KGMA5511 (ΔpyrE ΔilvC::pyrE) | 0.69 ± 0.046 A | 0.11 ± 0.0059 B | 1.56 ± 0.12 A |
| KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE] | 0.71 ± 0.025 A | 0.13 ± 0.0081 B | 1.87 ± 0.11 A |
Values shown are consistent with the date of 34 h of cultivation in Fig. 4. Values followed by different capital letters (A or B) are significantly different by Tukey's test at P values of 0.05. For acetic acid, ANOVA F = 5.14, df = 8, P = 0.15. For acetoin, ANOVA F = 5.14, df = 8, P = 0.00011. For cell yield, ANOVA F = 5.14, df = 8, P = 0.017.
To investigate the composition in more detail, the organic acids that accumulated in the cultures after 34 h growth were analyzed with HPLC. Strain KGMA7203 accumulated a trace amount of isobutyric acid (0.007%), whereas the other two strains did not (Fig. 5). Cell-free supernatants were then prepared and used for the fly-trapping experiments.
FIG 5.
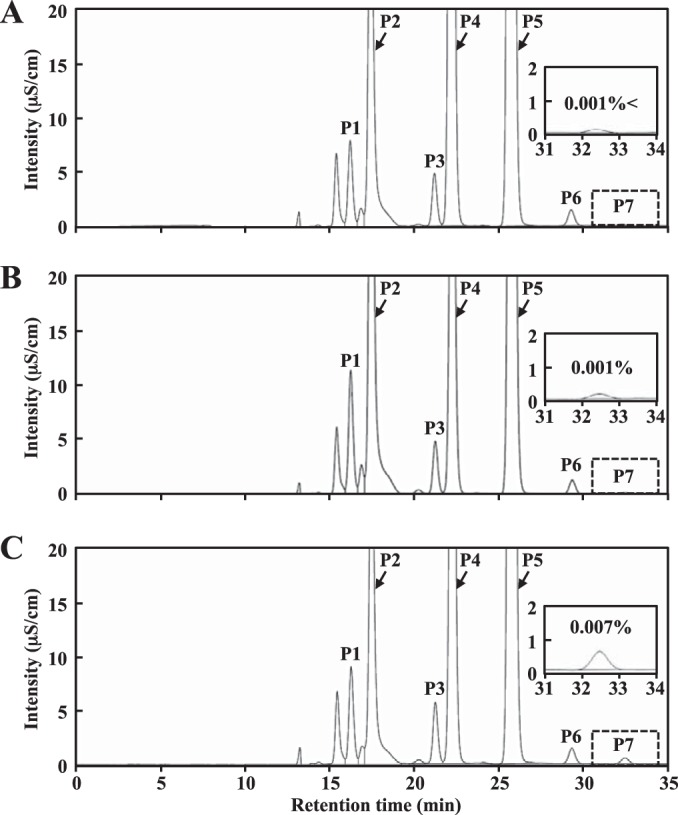
HPLC profiles of organic acids that accumulated in cultures after 34 h of growth. The chromatograms are consistent with the data of 34 h of cultivation in Fig. 4. The data of chromatograms (31 to 34 min) denoted by dotted-line boxes are highlighted. Peaks: P1, citric acid (16.3 min); P2, gluconic acid (17.5 min); P3, succinic acid (21.2 min); P4, lactic acid (22.2 min); P5, acetic acid (25.8 min); P6, propionic acid (29.3 min); P7, isobutyric acid (32.5 min). (A) KGMA0119 (wild type); (B) KGMA5511 (ΔpyrE ΔilvC::pyrE); (C) KGMA7203 [ΔpyrE KelrpΔ(397-511)::pyrE].
Fly-trapping experiments.
The attractiveness to fruit flies was evaluated using traps baited with the supernatants of the three strains and a synthetic vinegar with concentrations of acetic acid (0.7%), acetoin (0.13%), and isobutyric acid (0.007%) that were adjusted to those of the KGMA7203 supernatant obtained after 34 h of growth. The synthetic vinegar captured fewer than 5% of released flies (0.88 to 4.57%) (Fig. 6), which indicated that it was not attractive to flies. In contrast, the culture supernatants of the two gene disruptants were more attractive to flies than that of the KGMA0119 strain (Fig. 6). The supernatant of KGMA7203 had the highest attractiveness to flies and captured significantly more flies (19.36 to 36.96%) than those of KGMA0119 (3.25 to 11.40%) and KGMA5511 (6.87 to 21.50%) during a 45-min period (Fig. 6).
Effects of additional acetoin and isobutyric acid on the fly-trapping efficiency of KGMA0119 supernatant.
The data described above indicated that acetic acid, acetoin, and isobutyric acid were important to attract fruit flies. Therefore, acetoin and isobutyric acid were added to the KGMA0119 supernatant for final concentrations of 0.13% and 0.007%, respectively, and then used in a fruit fly-trapping experiment. The KGMA0119 supernatant that contained additional acetoin (0.13%) and isobutyric acid (0.007%) captured slightly more flies (4.11 to 6.85%) than the original KGMA0119 supernatant (0.34 to 3.08%) (Fig. 7A and B), although the opposite tendency was observed in the third trial (KGMA0119, 5.92%; KGMA0119 with additional acetoin and isobutyric acid, 3.95%) (Fig. 7C). The KGMA7203 supernatant showed the highest attractiveness to fruit flies in all the trials conducted (5.82 to 13.36%) (Fig. 7).
DISCUSSION
To enhance the attractiveness of vinegar to fruit flies, we focused on acetoin and constructed two gene disruptants that were expected to produce increased amounts of acetoin (Fig. 4B and Table 2). The KGMA7203 supernatant attracted significantly more flies than the wild-type supernatant or synthetic vinegar (Fig. 6). Although acetoin levels in the cultures of the KGMA5511 and KGMA7203 strains were approximately equal (Fig. 4B and Table 2), the supernatant of KGMA7203 was more attractive to flies than that of KGMA5511, which suggested that KGMA7203 produced other attractants in addition to acetoin. The HPLC analyses revealed that KGMA7203 accumulated a trace amount of isobutyric acid in the culture, whereas the other strains did not (Fig. 5). Our previous study showed that the genes for BCAAs biosynthesis were highly expressed in KGMA7203 and resulted in the overproduction of valine (19). The genome analysis predicted that K. europaeus possessed the degradation pathways of BCAAs and their corresponding precursors, which were similar to those in other bacteria (26, 27). The isobutyric acid in the KGMA7203 supernatant would be derived from the degradation of the overproduced valine and its precursor (2-oxoisovalerate) (Fig. 1). Kleiber et al. reported that some short-chain acids, such as formic acid and valeric acid, attracted fruit flies in greenhouse assays (28). The high attractiveness of the KGMA7203 supernatant might be attributed to a synergistic effect of acetic acid, acetoin, and isobutyric acid. In contrast, synthetic vinegar (0.7% acetic acid, 0.13% acetoin, and 0.007% isobutyric acid), which mimicked the KGMA7203 supernatant, captured significantly fewer flies (Fig. 6). Furthermore, the KGMA0119 supernatant that contained additional acetoin (0.13%) and isobutyric acid (0.007%) captured slightly more flies than the original KGMA0119 supernatant but fewer than the KGMA7203 supernatant (Fig. 7). These results suggested that the efficient fly trapping of the KGMA7203 culture was achieved by the synergistic effect of acetic acid, acetoin, isobutyric acid, and unidentified metabolites.
The disruption of ilvC was effective in increasing the production of acetoin (Fig. 4B and Table 2). However, the ilvC disruptant KGMA5511 required abnormally large amounts of BCAAs (12 mM) for normal growth (Fig. 3B), and the impaired growth of KGMA5511 was not fully restored by the addition of lower concentrations (0.5 or 4 mM) of BCAAs to the growth medium (Fig. 3B). E. coli possesses effective BCAA transport systems encoded by the livKHMGF gene cluster and livJ gene (29). The systems are composed of periplasmic BCAA-binding proteins (LivJ and LivK), hydrophobic transmembrane proteins (LivH and LivM), and cytoplasmic ATP-binding components (LivG and LivF). The active transport of BCAAs is driven by the energy from hydrolysis of ATP (29). Genome analysis predicted that K. europaeus lacked some orthologs (LivJ, LivK, LivH, and LivM) of the proteins for BCAA transport reported in E. coli (26). Abnormal BCAA auxotrophy of the strain KGMA5511 might be attributed to the lack of essential components for BCAA transport systems. Such a characteristic of this strain would be a disadvantage for the production of attractants, particularly at a larger scale. Instead of ilvC disruption, however, the enhanced expression of the aldC gene (which encodes α-acetolactate decarboxylase) (Fig. 1) would be more practical for the efficient production of acetoin. Shuttle plasmid vectors between E. coli and K. europaeus were developed recently (30), and trials to overexpress aldC are in progress.
Lrp is a global transcriptional regulator that controls the expression of approximately 10% of all genes in E. coli, and the Lrp regulon coordinates various cellular processes such as the metabolism and transport of amino acids (31, 32). The binding of leucine to its C-terminal region modulates the activity of Lrp on target genes (31, 32). KeLrp is an Lrp ortholog in K. europaeus and was predicted to regulate the expression of numerous genes and control BCAA biosynthesis and export (19). Strain KGMA7203 expressed a mutated KeLrp with the C-terminal ligand-binding region deleted (19). Thus, it was anticipated that the KeLrp variant would be insensitive to a signal molecule and that a number of downstream metabolisms would be significantly affected in KGMA7203. As a result, strain KGMA7203 might accumulate unidentified metabolites attractive to fruit flies as well as acetoin. Cha et al. reported that methionol increased fly response to a mixture of acetic acid and ethanol (7). Further analyses are needed to identify the other attractants in the KGMA7203 culture.
The numbers of captured flies in the experiments conducted in the afternoon (Fig. 6A and C) appeared to be lower than those in the experiment conducted in the morning (Fig. 6B). It has been well known that endogenous circadian clocks control rhythmic phenomena, such as locomotor activity, physiology, and metabolisms in D. melanogaster (33). The clocks can synchronize these phenomena to the environmental cycles through Zeitgebers (“time giver”) such as light intensity and temperature (34). D. melanogaster generally shows peaks of locomotor activity at dawn and dusk, and the activity falls down to its minimum at several hours before dusk (35). The fluctuation and decrease in captured flies in the afternoon experiments might be due to the lower locomotor activity of flies caused by the circadian clocks. Similarly, the numbers of captured flies in the second trapping experiments (Fig. 7) were smaller than those in the first trapping experiments (Fig. 6). Since D. melanogaster is an ectotherm, its locomotor activity would be significantly affected by the slight change in temperature (as little as 3°C) (34, 36, 37). The lower efficiency in fly attraction in the second experiments could be attributed to the temperature being lower (25 to 26°C) (Fig. 7) than that of the first experiments (28 to 30°C) (Fig. 6). Temperature also influences the release rate of attractants. Overall, however, the supernatant of KGMA7203 exerted the highest attractiveness to fruit flies in all the experiments that we conducted, emphasizing the advantage of strain KGMA7203 in fruit fly attraction.
For effective fly trapping, several factors such as the concentration, blending ratio, release rate of attractants, and trap device shape (5, 38) should be optimized. Additionally, the raw materials and bacterial strains used for vinegar fermentation are important to enhance attractiveness. From our observations, the odor of vinegar varies significantly depending on the raw materials and bacterial strains used in its production. In the United States, apple cider vinegar has been recommended as a desirable attractant to fruit flies (1). According to a report of Landolt et al. (8), rice vinegar is also preferable for the effective trapping of flies. Our results indicate that KGMA7203 is a suitable strain for developing effective flytraps with boosted attractiveness, and we hypothesize that the vinegars fermented by the strain are expected to be useful to attract fruit flies, including the SWD. We are now investigating the trapping efficiency of the KGMA7203 supernatant to various fruit flies besides D. melanogaster.
Supplementary Material
ACKNOWLEDGMENTS
We are deeply grateful for the technical assistance with the breeding of fruit fly that was provided by T. Ide (Department of Biological Sciences, Tokyo Metropolitan University).
This study was supported in part by grant of The Senshu Ikeda Bank Ltd.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03678-14.
REFERENCES
- 1.Landolt PJ, Adams T, Rogg H. 2012. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J Appl Entomol 136:148–154. doi: 10.1111/j.1439-0418.2011.01646.x. [DOI] [Google Scholar]
- 2.Rota-Stabelli O, Blaxter M, Anfora G. 2013. Drosophila suzukii. Curr Biol 23:R8–R9. doi: 10.1016/j.cub.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Hauser M. 2011. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67:1352–1357. doi: 10.1002/ps.2265. [DOI] [PubMed] [Google Scholar]
- 4.Lee JC, Bruck DJ, Curry H, Edwards D, Haviland DR, Van Steenwyk RA, Yorgey BM. 2011. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag Sci 67:1358–1367. doi: 10.1002/ps.2225. [DOI] [PubMed] [Google Scholar]
- 5.Lee JC, Shearer PW, Barrantes LD, Beers EH, Burrack HJ, Dalton DT, Dreves AJ, Gut LJ, Hamby KA, Haviland DR, Isaacs R, Nielsen AL, Richardson T, Rodriguez-Saona CR, Stanley CA, Walsh DB, Walton VM, Yee WL, Zalom FG, Bruck DJ. 2013. Trap designs for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ Entomol 42:1348–1355. doi: 10.1603/EN13148. [DOI] [PubMed] [Google Scholar]
- 6.Beers EH, Van Steenwyk RA, Shearer PW, Coates WW, Grant JA. 2011. Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag Sci 67:1386–1395. doi: 10.1002/ps.2279. [DOI] [PubMed] [Google Scholar]
- 7.Cha DH, Adams T, Werle CT, Sampson BJ, Adamczyk JJ Jr, Rogg H, Landolt PJ. 2014. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag Sci 70:324–331. doi: 10.1002/ps.3568. [DOI] [PubMed] [Google Scholar]
- 8.Landolt PJ, Adams T, Davis TS, Rogg H. 2012. Spotted wing drosophila, Drosophila suzukii (Diptera: Drosophilidae), trapped with combinations of wines and vinegars. Fla Entomol 95:326–332. doi: 10.1653/024.095.0213. [DOI] [Google Scholar]
- 9.Zhu J, Park KC, Baker TC. 2003. Identification of odors from overripe mango that attract vinegar flies, Drosophila melanogaster. J Chem Ecol 29:899–909. doi: 10.1023/A:1022931816351. [DOI] [PubMed] [Google Scholar]
- 10.Cha DH, Adams T, Rogg H, Landolt PJ. 2012. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J Chem Ecol 38:1419–1431. doi: 10.1007/s10886-012-0196-5. [DOI] [PubMed] [Google Scholar]
- 11.Becher PG, Bengtsson M, Hansson BS, Witzgall P. 2010. Flying the fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J Chem Ecol 36:599–607. doi: 10.1007/s10886-010-9794-2. [DOI] [PubMed] [Google Scholar]
- 12.García-Quintáns N, Repizo G, Martín M, Magni C, López P. 2008. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp. lactis bv. diacetylactis CRL264 by acidic growth. Appl Environ Microbiol 74:1988–1996. doi: 10.1128/AEM.01851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav V, Paniliatis BJ, Shi H, Lee K, Cebe P, Kaplan DL. 2010. Novel in vivo-degradable cellulose-chitin copolymer from metabolically engineered Gluconacetobacter xylinus. Appl Environ Microbiol 76:6257–6265. doi: 10.1128/AEM.00698-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adachi O, Ano Y, Toyama H, Matsushita K. 2006. High shikimate production from quinate with two enzymatic systems of acetic acid bacteria. Biosci Biotechnol Biochem 70:2579–2582. doi: 10.1271/bbb.60259. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Flores-Encarnación M, Contreras-Zentella M, Garcia-Flores L, Escamilla JE, Kennedy C. 2004. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome c biogenesis genes. J Bacteriol 186:5384–5391. doi: 10.1128/JB.186.16.5384-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada Y. 2014. Transfer of Gluconacetobacter kakiaceti, Gluconacetobacter medellinensis and Gluconacetobacter maltaceti to the genus Komagataeibacter as Komagataeibacter kakiaceti comb. nov., Komagataeibacter medellinensis comb. nov. and Komagataeibacter maltaceti comb. nov. Int J Syst Evol Microbiol 64(Part 5):1670–1672. doi: 10.1099/ijs.0.054494-0. [DOI] [PubMed] [Google Scholar]
- 17.Trček J, Jernejc K, Matsushita K. 2007. The highly tolerant acetic acid bacterium Gluconacetobacter europaeus adapts to the presence of acetic acid by changes in lipid composition, morphological properties and PQQ-dependent ADH expression. Extremophiles 11:627–635. doi: 10.1007/s00792-007-0077-y. [DOI] [PubMed] [Google Scholar]
- 18.Akasaka N, Sakoda H, Hidese R, Ishii Y, Fujiwara S. 2013. An efficient method using Gluconacetobacter europaeus to reduce an unfavorable flavor compound, acetoin, in rice vinegar production. Appl Environ Microbiol 79:7334–7342. doi: 10.1128/AEM.02397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akasaka N, Ishii Y, Hidese R, Sakoda H, Fujiwara S. 2014. Enhanced production of branched-chain amino acids by Gluconacetobacter europaeus with a specific regional deletion in a leucine responsive regulator. J Biosci Bioeng 118:607–615. doi: 10.1016/j.jbiosc.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Bolívar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113. [PubMed] [Google Scholar]
- 22.Green MR, Sambrook JF. 2012. Molecular cloning: a laboratory manual, 4th ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 23.Sato T, Fukui T, Atomi H, Imanaka T. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol 185:210–220. doi: 10.1128/JB.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada K, Hidese R, Fukuda W, Niitsu M, Takao K, Horai Y, Umezawa N, Higuchi T, Oshima T, Yoshikawa Y, Imanaka T, Fujiwara S. 2014. Identification of a novel aminopropyltransferase involved in the synthesis of branched-chain polyamines in hyperthermophiles. J Bacteriol 196:1866–1876. doi: 10.1128/JB.01515-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidese R, Inoue T, Imanaka T, Fujiwara S. 2014. Cysteine desulphurase plays an important role in environmental adaptation of the hyperthermophilic archaeon Thermococcus kodakarensis. Mol Microbiol 93:331–345. doi: 10.1111/mmi.12662. [DOI] [PubMed] [Google Scholar]
- 26.Andrés-Barrao C, Falquet L, Calderon-Copete SP, Descombes P, Ortega Pérez R, Barja F. 2011. Genome sequences of the high-acetic acid-resistant bacteria Gluconacetobacter europaeus LMG 18890T and G. europaeus LMG 18494 (reference strains), G. europaeus 5P3, and Gluconacetobacter oboediens 174Bp2 (isolated from vinegar). J Bacteriol 193:2670–2671. doi: 10.1128/JB.00229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harwood CS, Canale-Parola E. 1981. Adenosine 5′-triphosphate-yielding pathways of branched-chain amino acid fermentation by a marine spirochete. J Bacteriol 148:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiber JR, Unelius CR, Lee JC, Suckling DM, Qian MC, Bruck DJ. 2014. Attractiveness of fermentation and related products to spotted wing drosophila (Diptera: Drosophilidae). Environ Entomol 43:439–447. doi: 10.1603/EN13224. [DOI] [PubMed] [Google Scholar]
- 29.Adams MD, Wagner LM, Graddis TJ, Landick R, Antonucci TK, Gibson AL, Oxender DL. 1990. Nucleotide sequence and genetic characterization reveal six essential genes for the LIV-I and LS transport systems of Escherichia coli. J Biol Chem 265:11436–43. [PubMed] [Google Scholar]
- 30.Akasaka N, Astuti W, Ishii Y, Hidese R, Sakoda H, Fujiwara S. 2015. Change in the plasmid copy number in acetic acid bacteria in response to growth phase and acetic acid concentration. J Biosci Bioeng pii:S1389-1723(14)00442-3. doi: 10.1016/j.jbiosc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Newman EB, Lin R. 1995. Leucine-responsive regulatory protein: a global regulator of gene expression in E. coli. Annu Rev Microbiol 49:747–775. doi: 10.1146/annurev.mi.49.100195.003531. [DOI] [PubMed] [Google Scholar]
- 32.Cho BK, Barrett CL, Knight EM, Park YS, Palsson BØ. 2008. Genome-scale reconstruction of the Lrp regulatory network in Escherichia coli. Proc Natl Acad Sci U S A 105:19462–19467. doi: 10.1073/pnas.0807227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardin PE. 2005. The circadian timekeeping system of Drosophila. Curr Biol 15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Boothroyd CE, Wijnen H, Naef F, Saez L, Young MW. 2007. Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genet 3(4):e54. doi: 10.1371/journal.pgen.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allada R, Chung BY. 2010. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol 72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. 1993. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms 8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 37.Dillon ME, Wang G, Garrity PA, Huey RB. 2009. Review: thermal preference in Drosophila. J Therm Biol 34:109–119. doi: 10.1016/j.jtherbio.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birmingham AL, Kovacs E, Lafontaine JP, Avelino N, Borden JH, Andreller IS, Gries G. 2011. A new trap and lure for Drosophila melanogaster (Diptera: Drosophilidae). J Econ Entomol 104:1018–1023. doi: 10.1603/EC10432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.