Abstract
Bacillus cereus is an opportunistic human pathogen responsible for food poisoning and other, nongastrointestinal infections. Due to the emergence of multidrug-resistant B. cereus strains, the demand for alternative therapeutic options is increasing. To address these problems, we isolated and characterized a Siphoviridae virulent phage, PBC1, and its lytic enzymes. PBC1 showed a very narrow host range, infecting only 1 of 22 B. cereus strains. Phylogenetic analysis based on the major capsid protein revealed that PBC1 is more closely related to the Bacillus clarkii phage BCJA1c and phages of lactic acid bacteria than to the phages infecting B. cereus. Whole-genome comparison showed that the late-gene region, including the terminase gene, structural genes, and holin gene of PBC1, is similar to that from B. cereus temperate phage 250, whereas their endolysins are different. Compared to the extreme host specificity of PBC1, its endolysin, LysPBC1, showed a much broader lytic spectrum, albeit limited to the genus Bacillus. The catalytic domain of LysPBC1 when expressed alone also showed Bacillus-specific lytic activity, which was lower against the B. cereus group but higher against the Bacillus subtilis group than the full-length protein. Taken together, these results suggest that the virulent phage PBC1 is a useful component of a phage cocktail to control B. cereus, even with its exceptionally narrow host range, as it can kill a strain of B. cereus that is not killed by other phages, and that LysPBC1 is an alternative biocontrol agent against B. cereus.
INTRODUCTION
Bacillus cereus is a spore-forming, opportunistic Gram-positive bacterium that is widely distributed in the environment. Because B. cereus produces emetic toxin and enterotoxins, it causes food poisoning, including vomiting and diarrhea, particularly after the consumption of rice-based dishes (1). The Centers for Disease and Control and Prevention (CDC) reported that B. cereus outbreaks account for 2 to 5% of food-borne diseases in the United States (2). In addition to food poisoning, B. cereus is also associated with potentially fatal non-gastrointestinal tract infections due to the various types of toxins, including phospholipases, proteases, and hemolysins, produced during growth (3). For these reasons, there is an urgent need to control B. cereus. Since B. cereus is generally resistant to beta-lactam antibiotics and several strains of B. cereus are also resistant to erythromycin, tetracycline, and fluoroquinolones (1, 4), the demand for alternative methods to control B. cereus has grown (3).
Bacteriophages, natural killers of bacteria, have gained increasing attention in the past few decades, particularly in light of emerging antibiotic resistance (5, 6). Bacteriophages are much more specific than antibiotics, so they have minimal impact on commensal bacteria other than the host. Although bacteria can develop resistance to their viral predators, finding a new phage that can kill resistant bacteria is not difficult, because the phage itself continually evolves against the mutated bacteria. Furthermore, phage cocktails, containing different kinds of phages, can efficiently prevent bacteria from developing phage resistance (7, 8). To date, however, only a few B. cereus phages have been characterized in detail (9–16). Thus, isolating and characterizing new B. cereus phages is essential for developing efficient biocontrol agents against B. cereus.
Previously, we isolated the B. cereus-infecting Siphoviridae phage PBC1 and announced its genome sequence (17). PBC1 contains 41,164 bp of linear double-stranded DNA, including 50 predicted open reading frames (ORFs). Because PBC1 forms clear plaques and does not contain any lysogeny-related genes in its genome, PBC1 is considered to be a virulent phage. Although several virulent variant Bacillus anthracis Siphoviridae phages have been reported so far (18–20), they are derivatives of B. anthracis prophage W, and their genomes contain genes for several lysogeny-related proteins, including an integrase-like protein (21). Therefore, to our knowledge, PBC1 is the only naturally isolated virulent Siphoviridae phage that infects B. cereus.
In this paper, we present the results of microbiological and genomic characterization of the novel virulent B. cereus phage PBC1. In addition, the predicted endolysin of PBC1 (LysPBC1) and its catalytic domain (LysPBC1_EAD) were produced recombinantly in Escherichia coli and characterized. The results described here will not only broaden our knowledge of B. cereus phages and their endolysins, but will also be useful for developing efficient biocontrol agents against the notorious human pathogen B. cereus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. B. cereus strain ATCC 21768 was used for the isolation and propagation of the PBC1 phage. All of the Bacillus strains and Gram-negative bacteria were grown in Luria-Bertani (LB) broth at 37°C. Listeria, Staphylococcus, and Enterococcus strains were grown in brain heart infusion broth at 37°C. Clostridium strains were grown in reinforced clostridial medium at 37°C under anaerobic conditions. To create agar medium, the broth medium was supplemented with 1.5% agar. All of the media used in this study were purchased from Difco and used according to the manufacturer's instructions.
TABLE 1.
Host range of phage PBC1 and lytic activity of LysPBC1 and its enzymatic active domain LysPBC1_EAD
| Species | Strain no.a | Plaque (PBC1) | Relative lytic activityb |
|
|---|---|---|---|---|
| LysPBC1 | LysPBC1_EAD | |||
| B. cereus group strains | ||||
| B. cereus | ATCC 27348 | − | +++ | + |
| ATCC 21768 | + | +++ | + | |
| ATCC 13061 | − | +++ | + | |
| ATCC 14579 | − | ++ | + | |
| ATCC 21772 | − | +++ | ++ | |
| ATCC 10876 | − | ++ | + | |
| KCTC 3674 | − | ++ | + | |
| ATCC 10987 | − | +++ | + | |
| KCTC 1094 | − | ++ | − | |
| NCCP 10623 | − | ++ | + | |
| NCCP 10624 | − | +++ | + | |
| NCCP 10634 | − | + | + | |
| NCCP 10715 | − | ++ | − | |
| NCCP 10841 | − | ++ | + | |
| NCCP 10856 | − | + | − | |
| NCCP 11306 | − | ++ | + | |
| NCCP 11308 | − | +++ | ++ | |
| NCCP 11309 | − | ++ | + | |
| NCCP 11311 | − | ++ | + | |
| NCCP 12448 | − | +++ | + | |
| NCCP 14043 | − | ++ | ++ | |
| NCCP 14796 | − | ++ | + | |
| B. thuringiensis | ATCC 10792 | − | ++ | + |
| B. mycoides | ATCC 6462 | − | ++ | + |
| B. weihenstephanensis | KCTC 3975 | − | + | − |
| Other Gram-positive strains | ||||
| B. megaterium | JCM 2506 | − | + | + |
| B. subtilis | ATCC 6051 | − | + | ++ |
| ATCC 6633 | − | + | ++ | |
| ATCC 23857 | − | + | ++ | |
| JCM 2508 | − | − | + | |
| B. licheniformis | JCM 2505 | − | + | ++ |
| Bacillus sphaericus | JCM 2502 | − | + | + |
| Bacillus circulans | JCM 2504 | − | + | + |
| L. monocytogenes | Scott A | − | − | − |
| Listeria innocua | ATCC 33090 | − | − | − |
| Clostridium perfringens | ATCC 3624 | − | − | − |
| Clostridium indolis | ATCC 25771 | − | − | − |
| Staphylococcus aureus | ATCC 29213 | − | − | − |
| Enterococcus faecalis | ATCC 29212 | − | − | − |
| Gram-negative strains | ||||
| E. coli | MG1655 | − | ++ | ++ |
| Shigella flexneri | 2a strain 2457T | − | ++ | +++ |
| Cronobacter sakazakii | ATCC 29544 | − | ++ | ++ |
ATCC, American Type Culture Collection; KCTC, Korean Collection for Type Cultures; NCCP, National Culture Collection for Pathogens; JCM, Japan Collection of Microorganisms.
Gram-negative bacteria were pretreated with EDTA. The relative lytic activity of endolysin was obtained by measuring the percent drop in OD600 in 8 min. −, no lysis; +, limited lysis; ++, medium lysis; +++, rapid lysis.
Isolation of PBC1 phage.
A sewage sample was collected from the Seonam Water Reclamation Center at Seoul, South Korea. Briefly, a 25-ml sample was added to equal volumes of 2× LB broth and incubated with shaking at 37°C for 24 h. After centrifugation (15,000 × g for 10 min), the supernatant was filtered using a 0.22-μm-pore-size filter (Millipore). Then, 10 ml of the filtrate was mixed with 50 ml of LB broth and 500 μl of B. cereus ATCC 21768 overnight cultures, and the mixture was incubated at 37°C for 12 h with shaking. The culture was centrifuged, and the supernatant was filter sterilized as described above. The presence of phages was confirmed using a plaque-forming assay with molten 0.4% LB soft agar inoculated with B. cereus ATCC 21768 overnight cultures. After incubation at 37°C for 12 h, a single phage plaque was picked with a sterile pipette tip and eluted in 1 ml of SM buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, and 10 mM MgCl2). These plaque isolation and elution steps were repeated at least three times to purify single phage.
Host range.
Host range studies were performed by plaque-forming assays. Isolated PBC1 phage (1 × 107 PFU/ml) were serially diluted 10-fold, and 10-μl aliquots were spotted onto the 0.4% LB soft-agar overlay containing the test bacteria grown overnight. The plate was incubated at 37°C overnight, and single-plaque formation was monitored.
Purification and high-titer stock preparation of PBC1.
Phage particles were precipitated with 10% polyethylene glycol 6000 (Sigma) in the presence of 1 M NaCl at 4°C overnight. After centrifugation (10,000 × g; 20 min; 4°C), the precipitated phage were resuspended in SM buffer and purified by CsCl density gradient ultracentrifugation (78,500 × g; 2 h; 4°C). Separated phage were dialyzed against 1,000 volumes of SM buffer using a Spectra/Por 4 dialysis membrane tube (Spectrum) for 2 h with one buffer change.
Morphological analysis by TEM.
Purified PBC1 (1 × 1010 PFU/ml) was placed on carbon-coated copper grids and negatively stained with 2% aqueous uranyl acetate (pH 4.0) for 20 s. PBC1 was visualized by transmission electron microscopy (TEM) (Leo 912AB transmission electron microscope; Carl Zeiss) at 80 kV. Images were scanned with a Proscan 1,024- by 1,024-pixel charge-coupled-device camera at the National Academy of Agricultural Science (Suwon, South Korea). PBC1 was classified according to the guidelines of the International Committee on Taxonomy of Viruses.
Bacterial-challenge test in liquid culture.
Exponentially growing B. cereus ATCC 21786 cells (2 × 107 CFU/ml) in 50 ml LB broth were infected with 1 ml of PBC1 (1010, 109, and 108 PFU/ml) and incubated for another 10 h at 37°C with vigorous shaking. Bacterial growth was monitored by measuring the optical density at 600 nm (OD600) at various time points. As a negative control, one bacterial culture was inoculated with 100 μl SM buffer instead of PBC1.
Inhibition of B. cereus growth in boiled rice.
To evaluate the effect of phage PBC1 on the growth of B. cereus cells in foods, we chose boiled rice as a food sample, because rice-based dishes are frequently involved in B. cereus poisoning (22). Round-grain white rice was purchased at a local market, and boiled-rice samples were prepared as described previously with slight modification (23). Briefly, rice (200 g) was boiled in water (250 ml) for 30 min, and the resulting cooked rice (10 g) was diluted in 40 ml of distilled water under vigorous shaking for 10 min. Then, the homogeneous slurry samples (10 ml) were incubated at the desired temperatures (25°C or 37°C) for 1 h, and the samples were artificially contaminated with B. cereus ATCC 21768 (103 CFU/ml). Next, aliquots of PBC1 (0.01 ml) were added to the samples to achieve a final concentration of 108 PFU/ml (10, 24). Following phage addition, the samples were thoroughly mixed and incubated at the desired temperatures (25°C or 37°C). After the desired intervals of incubation, the food samples were serially diluted in PBS and plated in duplicate on LB agar. The plates were incubated at 37°C for 12 h, and the average number of colonies was determined. The experiments were carried out independently in triplicate.
In vitro phage adsorption assays.
Bacterial cells (20 ml; OD600 = 1.0 to 1.5) were harvested by centrifugation (6,000 × g; 10 min; 4°C) and resuspended in 20 ml fresh LB broth. The bacterial suspension was adjusted to produce an OD600 of 0.1 (approximately 107 CFU/ml), and the sample was aliquoted into 15-ml Falcon tubes (10-ml suspension per tube). The cells were supplemented with 25 μg/ml of chloramphenicol to prevent cell growth and phage multiplication (25). Then, PBC1 was added to each tube with 10 mM CaCl2 andMgCl2 to achieve the final concentration of 104 PFU/ml and incubated at 37°C. Samples were taken at 5-min intervals, and the cells were immediately removed by centrifugation (16,000 × g; 1 min; 4°C) and filtration (0.22-μm-pore-size filter). The number of unbound free phage particles was determined by overlay assay. Because phages can exploit both carbohydrates and surface proteins as receptors, we treated bacteria with periodate or proteinase K and tested how these treatments affected PBC1 adsorption. For the periodate and proteinase K treatment assay, we followed the method described previously (26, 27). Briefly, exponentially growing B. cereus ATCC 21768 cells were harvested and washed once with fresh LB broth. The bacterial cells were then treated with proteinase K (0.2 mg/ml; Qiagen) at 45°C for 2 h. For the periodate treatment group, the cells were centrifuged, and the bacterial pellet was suspended in sodium acetate (50 mM; pH 5.2) or sodium acetate containing either 10 or 100 mM periodate (Sigma) before being incubated for 2 h in a dark area. Following incubation, the samples were washed three times with fresh LB broth. Adsorption assays were performed for the various treated cells as described above.
One-step growth curve.
When the OD600 of the B. cereus ATCC 21768 culture reached 1.5 (108 CFU/ml), 50 ml of the culture was harvested. The PBC1 phage (1 ml; 5 × 106 PFU/ml) was added with 10 mM CaCl2 and MgCl2 and allowed to adsorb for 5 min. The mixture was centrifuged at 6,000 × g for 10 min, and the supernatant was discarded to remove the residual phage. The cell pellet was then resuspended with the same volume of fresh LB broth, and the culture was further incubated at 37°C with shaking. Two sets of samples were collected every 5 min before dilution and overlay onto the bacterial lawn containing 10 mM CaCl2 and MgCl2 for phage titration. To determine the eclipse period, the second set of samples was treated with 1% chloroform to release the intracellular phage before the overlay assay.
Phylogenetic analysis.
To determine the phylogenetic position of the PBC1 phage, we made phylogenetic trees based on the alignment of the amino acid sequences from the large subunit of terminase (TerL) and the major capsid proteins (MCPs) from various bacteriophages. The amino acid sequences used for phylogenetic trees are available online in the NCBI nucleotide databases (http://www.ncbi.nlm.nih.gov/nuccore). An alignment of amino acid sequences was created using the Clustal X2 program (28). The phylogenetic tree was constructed with MEGA5 by the neighbor-joining method and bootstrap analysis (1,000 replicates) with P distance values (29).
Endolysin production.
The endolysin gene (gp25) from the PBC1 genome was PCR amplified with the following primers: gcgCATATGGATAAATTTACAGTACATGCAGGGC (forward) and gcgGGATCCTTAATACTCAATCACTTCTTCTTGATACCACCA (reverse) (where underlined sequences represent restriction enzyme recognition sites and lowercase letters represent extra bases at the terminal restriction site for efficient digestion by restriction enzymes). The PCR product was cloned into pET15b (Novagen), which has an N-terminal hexahistidine (His) tag sequence. The correctly cloned plasmid was transformed into E. coli BL21(DE3). To express the endolysin (LysPBC1), the clone was grown in LB broth containing 50 μg/ml ampicillin to an OD600 of 0.7 and induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Then, the induced culture was shaken for 20 h at 18°C. The harvested cells were resuspended in lysis buffer (50 mM Tris-Cl, 200 mM NaCl, pH 8.0) and disrupted by sonication (Branson Ultrasonics). After centrifugation at 20,000 × g for 40 min, the supernatant was filtered (0.22-μm pore size; Millipore) and purified using a Ni-nitrilotriacetic acid (NTA) Superflow column (Qiagen) according to the manufacturer's instructions. The identity and purity of the protein were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (30). The purified LysPBC1 was stored at −80°C after the buffer was changed to the storage buffer (40 mM Tris-Cl, 200 mM NaCl, 30% glycerol, pH 8.0) using a PD Miditrap G-25 (GE Healthcare). The catalytic domain of the endolysin (LysPBC1_EAD) sequence was PCR amplified from LysPBC1-pET15b using the primers gcgCATATGGATAAATTTACAGTACATGCAGGGC (forward) and gcgGGATCCTTAATTTACTGCTTTGCCTGTAACGGCTT (reverse) to insert a stop codon after Asn174. Protein expression and purification of LysPBC1_EAD was conducted in the same way as for LysPBC1.
Lytic activity of endolysin.
The lytic activity of the endolysin was assessed by turbidity reduction assay as described previously (31). Bacterial cells were grown to exponential phase and resuspended with the reaction buffer (20 mM Tris-Cl, pH 8.0) to adjust the OD600 to approximately 1.0. Then, the purified endolysin was added to a final concentration of 0.4 μM, and the OD600 values were monitored over time. For the lytic activity of LysPBC1_EAD, an equimolar concentration (0.4 μM) was used to take into account the difference in protein molecular weight. For Gram-negative bacteria, the exponentially growing bacterial cells were pretreated with a buffer containing 20 mM Tris-Cl (pH 8.0) and 0.1 M EDTA for 5 min at room temperature (RT). Then, the cells were washed three times with reaction buffer to remove residual EDTA, and the endolysin was added.
GenBank accession numbers.
The GenBank accession numbers of PBC1 and LysPBC1 are JQ619704 and AFE86261.1, respectively.
RESULTS AND DISCUSSION
Host range of phage PBC1.
A bacteriophage, PBC1, was isolated from a sewage sample using B. cereus ATCC 21768 as a host strain (17). Since the host range of a phage is an important factor for developing biocontrol agents, we performed a plaque assay with a variety of bacterial species. As shown in Table 1, the PBC1 phage has extreme host specificity, producing clear plaques only against B. cereus strain ATCC 21768 out of the 22 B. cereus strains tested. Other B. cereus group species (Bacillus thuringiensis, Bacillus mycoides, and Bacillus weihenstephanensis) were also resistant to the PBC1 phage (32). Other Gram-positive or Gram-negative bacterial species were resistant to PBC1. These results indicate that the phage PBC1 has very narrow host specificity, and consequently, PBC1 alone would not be an efficient biocontrol agent against diverse strains of B. cereus. However, since the host strain (ATCC 21768) was resistant to all other phages isolated by our group (10, 17), we suspected that PBC1 could be a potential candidate for an effective phage cocktail lytic for B. cereus. Therefore, we further characterized PBC1.
Morphology and one-step growth curve of the phage PBC1.
To observe the morphology of the PBC1 phage, we first needed to obtain pure phage with high titers. Supplementation with 10 mM calcium chloride led to clearer plaque formation and increased the efficiency of plating (EOP), suggesting that calcium supplementation promoted productive PBC1 infection and could therefore be used for high-titer phage preparation (data not shown). TEM analysis revealed that PBC1 belongs to the family Siphoviridae, featuring an isometric head with a diameter of 70 nm and a noncontractile tail with a length of approximately 200 nm (Fig. 1A). The one-step growth kinetics of PBC1 indicated that PBC1 had an eclipse period of 10 min, a latent period of 20 min, and a burst size of 45 PFU/infected cell when infecting its host bacterium, B. cereus ATCC 21768 (Fig. 1B).
FIG 1.
(A) Transmission electron microscopy image of PBC1. PBC1 was negatively stained with 2% uranyl acetate and observed by TEM. (B) One-step growth curve of PBC1. E, eclipse period; L, latent period; B, burst size. Means and standard deviations (SD) of three independent assays are shown.
Inhibition of B. cereus growth by the phage PBC1 in liquid culture and boiled rice.
To evaluate the ability of PBC1 to lyse host bacteria in broth, we performed a host growth inhibition assay in the presence of PBC1. PBC1 inhibited bacterial growth, and the inhibition patterns were dependent on the phage concentrations added (Fig. 2A). After infection of B. cereus ATCC 21768 with 1010 PFU/ml of PBC1 (multiplicity of infection [MOI], 10), the host bacteria were lysed rapidly, and clear lysate containing cell debris was formed only after 30 min. With 108 PFU/ml of PBC1 (MOI, 0.1), host growth inhibition was observed 1.5 h after phage infection, and the broth culture became clear approximately 2 h after infection. However, the growth inhibition lasted only approximately 5 h, and regrowth occurred regardless of the MOI, even though growth inhibition started faster at a high MOI (Fig. 2A). Several isolates from the regrowth culture showed PBC1 insensitivity, indicating that PBC1-resistant mutants were generated. These results showed that phage infection at an MOI of 10 efficiently lysed host bacteria, whereas phage-resistant mutants appeared more rapidly than during infection at an MOI of 0.1. It has been stated that increasing the phage inoculum density could lead to increased emergence of mutant bacteria by limiting the opportunity for reproduction of mutant phage variants (33). These results led to the conclusion that the appropriate MOI should be considered for the successful use of PBC1 for biocontrol purposes. To confirm the lytic activity of PBC1 in foods, B. cereus and PBC1 were added to boiled rice, and bacterial growth was monitored. Since several studies showed the efficacy of a high concentration of phage for food control purposes (24, 34), we used a sufficiently high concentration (108 PFU/ml) of PBC1 in the assay. As shown in Fig. 2B, no viable cells were detected from phage-treated samples, indicating that phage PBC1 was very effective in preventing the growth of B. cereus ATCC 21768 in boiled rice. In addition, prolonged incubation time did not neutralize the lytic activity of phage, suggesting that the virulent phage PBC1 could be a useful component for the development of a phage cocktail against B. cereus.
FIG 2.
Bacterial-challenge test of PBC1 with B. cereus ATCC 21768. (A) PBC1 inhibited the growth of B. cereus in liquid medium. The growth inhibition pattern was dependent on the number of phage added. Non-PBC1-infected cells (circles) are shown as a control. (B) Boiled-rice samples were spiked with bacteria (1 × 103 CFU/ml), and PBC1 (1 × 108 PFU/ml) was applied to the samples. The treated samples were stored at different incubation temperatures, and the concentration of viable cells was determined at the time points indicated.
The PBC1 phage binds to a carbohydrate moiety of B. cereus ATCC 21768.
With 10 mM calcium and magnesium chloride, the majority (∼90%) of PBC1 particles were found to be attached to the bacteria after 15 min of incubation (Fig. 3A). To explain the narrow host specificity of PBC1, five different B. cereus strains were used for the PBC1 adsorption assay (Fig. 3B). Among the five B. cereus strains, only B. cereus strain ATCC 21768 significantly adsorbed the PBC1 phage (P < 0.05). Treatments of bacteria with periodate or proteinase K before the phage adsorption assay revealed that PBC1 may use carbohydrate structure as a phage receptor. As seen in Fig. 3C, treatment with periodate, which cleaves saccharide rings with vicinal diols (26), reduced phage binding, whereas treatment with proteinase K did not affect the binding of PBC1 to the cell surface. Although further studies are needed to identify the exact PBC1 receptor of the host, these results suggest that the host strain, B. cereus ATCC 21768, has a distinct carbohydrate structure on its cell surface that is used by PBC1 as a phage receptor.
FIG 3.
PBC1 adsorption assay. (A) The majority (∼90%) of PBC1 particles were adsorbed onto B. cereus ATCC 21768 within 15 min. (B) PBC1 adsorption onto different B. cereus strains showed that PBC1 specifically bound to the host strain, B. cereus ATCC 21768. LB medium without bacterial cells was used as a control (LB), and the treatments are indicated below the bars. (C) PBC1 binding was inhibited by periodate treatment of the host cell, whereas proteinase K treatment did not affect phage adsorption. The means and SD of three independent assays are given.
Comparison with other related phage genomes.
Previously, we announced the whole genome sequence of phage PBC1 (17). To compare its genome with those of other phages, we first conducted phylogenetic analysis based on the amino acid sequence of a terminase large subunit (TerL) and an MCP. Phylogenetic analysis of the TerL showed that the TerL of PBC1 shared maximum identity (69%) with that of B. cereus phage 250 (GenBank accession no. GU229986.1) (Fig. 4A) (66). Although both phages belong to the family Siphoviridae and show significant amino acid sequence similarities in the late-gene regions, the genome size of PBC1 (41.2 kb) is smaller than that of phage 250 (56.5 kb) (Fig. 5). Phage 250 is a mitomycin C-induced temperate phage whose genome contains two transposase genes; on the other hand, PBC1 is a virulent phage lacking genes associated with lysogeny control, potential virulence, and antibiotic resistance. These results suggest that PBC1 is more appropriate for biocontrol or as a therapeutic agent than phage 250. The TerL in PBC1 showed 54% amino acid sequence identity with that of Listeria monocytogenes phage A006 (GenBank accession no. DQ003642) (35). Considering that TerL is responsible for packaging the phage genome and determining the DNA-packaging strategy of the phage (36), the terminally redundant and circularly permuted genome of phage A006 suggest that PBC1 engages in a headful packaging mechanism (37).
FIG 4.
Comparative phylogenetic analysis of the terminase large subunits (A) and the major capsid proteins (B). The host species is shown after the name of each phage (BC, B. cereus; BA, B. anthracis; BT, B. thuringiensis). The scale bars indicate the number of nucleotide substitutions per site. The numbers at the nodes represent the bootstrap probabilities.
FIG 5.
Alignment of the proteomes among the following PBC1-related phages: B. clarkii temperate phage BCJA1c, B. cereus virulent phage PBC1, B. cereus temperate phage vB_BceS-IEBH, and B. cereus temperate phage 250. The shading indicates the degrees of amino acid sequence identity of the gene products with an identity of >26% (scale at lower right). To facilitate the genome comparison, the genome of IEBH was rearranged commencing with the small terminase gene.
Phylogenetic analysis of the MCP revealed that the MCP of PBC1 (gp8) showed low homology (less than 15%) with other MCPs of the B. cereus group phages (Fig. 4B). Instead, the MCP of PBC1 displayed the highest homology (67% identity) with the MCP of the Bacillus clarkii temperate phage BCJA1c (GenBank accession no. AY616446) (38). The alkaliphilic Siphoviridae phage BCJA1c shares similar morphology, genome length (∼ 1 kb), and G+C content (41.7 mol%) with PBC1. As shown in Fig. 5, a portal protein and other structural proteins of PBC1 also showed significant homology with those of BCJA1c. Interestingly, the MCP of PBC1 exhibited considerable homology with the MCPs from phages of lactic acid bacteria, such as Lactobacillus phage ATCC 8014-B1 (50% identity), Pediococcus phage cIP1 (50% identity), Lactobacillus phage phiJL-1 (48% identity), and Lactococcus phage BM13 (44% identity). These results demonstrate that phage PBC1 has characteristics distinct from those of other phages of the B. cereus group.
Phylogenetic analysis of TerL and the MCP of PBC1 prompted us to make whole-genome comparisons among PBC1-related phages at the protein level (Fig. 5). Note that genome comparison at the DNA level did not identify any genes with significant similarity (data not shown). The structural proteins, including head and tail morphogenesis proteins, of PBC1 showed significant similarity to those of phage 250 and the B. cereus temperate phage vB_BceS-IEBH (GenBank accession no. NC_011167), which shares more than 98% DNA sequence identity with phage 250 (11). Interestingly, while the holins of PBC1 and phage 250 share significant homology (55% identity), their endolysins showed only limited amino acid sequence similarity (14% identity).
Broad lytic activity of the PBC1 endolysin.
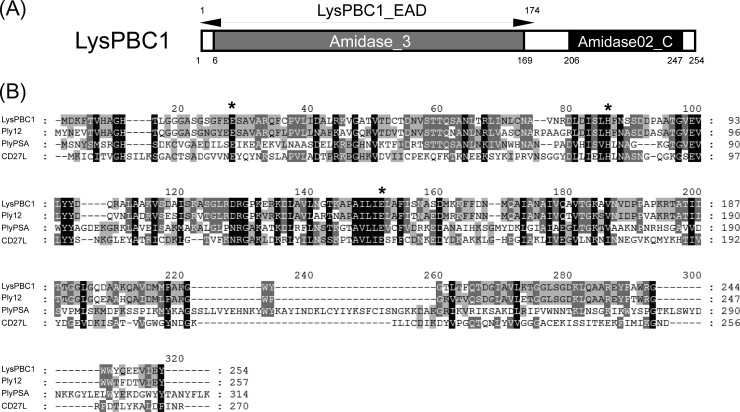
Although PBC1 has a virulent lifestyle and rapidly lyses the host B. cereus strain, its extremely narrow host range may limit its efficiency as a biocontrol agent unless it is used in a phage cocktail. It has been reported that the endolysin, a phage-encoded peptidoglycan hydrolase, generally shows a broader lytic spectrum than the phage (39–43). Moreover, the development of resistance against endolysins has not been reported (44–47). Therefore, we chose the endolysin of PBC1 (LysPBC1) as an alternative or complementary antimicrobial against B. cereus and tested its lytic activity. Pfam and Conserved Domain Database analyses showed that LysPBC1 is a putative N-acetylmuramoyl-l-alanine amidase consisting of an N-terminal type 3 amidase domain (PF01520) and a C-terminal amidase02_C domain (PF12123) (Fig. 6A). Amino acid sequence alignment revealed that LysPBC1 presents 73% overall identity with an endolysin of B. cereus phage 12826 (48) (Fig. 6B). In addition, LysPBC1 was aligned with non-Bacillus lysins, such as the L. monocytogenes phage endolysin PlyPSA (49) and Clostridium difficile phage endolysin CD27L (50), due to the conserved catalytic amidase_3 domains. LysPBC1 has three Zn2+-coordinating residues (Glu23, His79, and Glu140) in the catalytic domain, and these residues are conserved in all four endolysins. Considering that the C-terminal amidase02_C domain of LysPBC1 did not show any homology to PlyPSA and CD27L and that several endolysins have an amidase02_C domain (PF12123) as a cell wall binding domain (42, 51), the C-terminal domain is presumed to function in binding the B. cereus cell wall.
FIG 6.
Modular structure of B. cereus phage endolysin LysPBC1. (A) Schematic representation of LysPBC1, which has a conserved N-terminal amidase_3 domain and a C-terminal amidase02_C domain. (B) Sequence alignment of LysPBC1-related endolysins. Ply12, B. cereus phage 12826 endolysin; PlyPSA, L. monocytogenes phage PSA endolysin; CD27L, Clostridium difficile phage ΦCD27 endolysin. Conserved and identical residues are shaded in gray (dark gray, >70% conserved; light gray, >40% conserved) and black, respectively. The asterisks represent the conserved Zn2+-coordinating catalytic residues in N-acetylmuramoyl-l-alanine amidase.
LysPBC1 was highly expressed in soluble form in E. coli, and the protein was purified by Ni-NTA affinity chromatography, resulting in a homogeneous preparation (Fig. 7A). The purified LysPBC1 was able to lyse all strains of B. cereus group bacteria, including B. cereus, B. thuringiensis, B. mycoides, and B. weihenstephanensis, when it was added exogenously (Table 1). This broad lytic activity was in sharp contrast to the very narrow host specificity of phage PBC1. In addition, LysPBC1 showed modest lytic activities toward members of the genus Bacillus, such as Bacillus subtilis, Bacillus megaterium, and Bacillus licheniformis. It is interesting that the exogenously added LysPBC1 alone cannot lyse Gram-negative bacteria, whereas the EDTA-treated Gram-negative cells were susceptible to the lytic action of LysPBC1. These results might be attributed to Bacillus and the Gram-negative bacteria tested having the same peptidoglycan type (A1γ) (51). We observed that LysPBC1 did not lyse Listeria and Clostridium species, both of which have the same peptidoglycan type, A1γ (52). These results suggest that the genus- or species-specific moieties of the cell wall are required for the enzymatic action of LysPBC1.
FIG 7.
(A and B) Ni-NTA-purified LysPBC1 (A) and LysPBC1_EAD (B) were loaded on SDS-PAGE gels. Lane M, protein size marker. (C and D) Equimolar concentrations (0.4 μM) of LysPBC1 and LysPBC1_EAD were added to the suspensions of B. cereus (C) and B. subtilis (D), and the decrease in turbidity was monitored. The values are the means and SDs from triplicate assays.
Lytic activity of LysPBC1_EAD.
A number of studies have shown that the catalytic domains of endolysins can also be utilized as efficient antimicrobial agents (53–56). These truncated endolysins often show increased protein solubility and lytic activity compared to the parental endolysins (57–59). Therefore, we designed the N-terminal amidase_3 enzymatic active domain of LysPBC1 (here designated LysPBC1_EAD) by adding a stop codon after Asn174 and expressed the truncated protein in E. coli. Both the full-length endolysin, LysPBC1, and the truncated endolysin, LysPBC1_EAD, were overexpressed in E. coli and purified to homogeneity (Fig. 7A and B). The turbidity reduction assays with the same molar concentrations of the proteins revealed that LysPBC1 showed stronger lytic activity against B. cereus cells than LysPBC1_EAD (Fig. 7C and Table 1). However, LysPBC1_EAD showed higher lytic activity than LysPBC1 against nonnative targets, such as B. subtilis (Fig. 7D). A similar pattern was observed for Bacillus pumilus and B. licheniformis, both of which belong to the B. subtilis group (60). These results indicate that the C-terminal cell wall binding domain of LysPBC1 is essential for full activity of the endolysin only for its natural target.
The purified LysPBC1_EAD showed broad lytic activities toward Bacillus species, as well as Gram-negative bacteria treated with EDTA (Table 1). As with full-length LysPBC1, LysPBC1_EAD did not lyse Listeria and Clostridium species despite the fact that the EAD of LysPBC1 exhibited significant sequence homology with that of PlyPSA (34% identity) and CD27L (29% identity). These results indicate that the catalytic domain alone maintains the host specificity, and the removal of the cell wall binding domain rarely affects the lytic spectrum of LysPBC1. Other studies have also reported the retained host specificity of the catalytic domain of endolysins (57, 61), suggesting that species-specific structures surrounding catalytic sites determine the lytic activity of the endolysin. Alternatively, it could be that the nonconserved region of LysPBC1_EAD is necessary for proper function of the protein on its natural substrate.
It has been proposed that the cell wall binding domain of endolysin has an inhibitory role when it is not bound to the target (58, 62). However, this is not the case for LysPBC1, because the cell wall binding domain of LysPBC1 did not bind several B. cereus group strains (our unpublished data) despite the fact that they were more sensitive to the full-length endolysin. These results suggest that, in addition to cell wall binding, the C-terminal domain of LysPBC1 may play an additional role in full lytic activity of the endolysin against the B. cereus group strains. In the case of B. subtilis group strains, on the other hand, we propose that the differences in cell wall components between the B. cereus group and the B. subtilis group are responsible for the variance in lytic activity. B. subtilis group bacteria display wall teichoic acid (WTA), containing a negatively charged phosphate group, through the peptidoglycan layer, whereas the B. cereus group bacteria lack the WTA (63, 64). Instead, the B. cereus group contains an uncharged branched polysaccharide as a major cell wall component (65). Consequently, the cell wall of B. subtilis is more likely to have a negative charge, and this net charge difference may influence the lytic activity of the endolysin. The theoretical isoelectric point of LysPBC1_EAD (7.15) is higher than that of LysPBC1 (6.79), supporting this explanation. It is also possible that the small size of LysPBC1_EAD (approximately 20.5 kDa) allows it to penetrate the peptidoglycan layer of B. subtilis group bacteria more efficiently than the full-length LysPBC1 (approximately 29.4 kDa), resulting in more rapid lysis of the cell.
In summary, we described potential biocontrol agents derived from B. cereus phage PBC1, the only naturally isolated virulent Siphoviridae phage among the reported B. cereus phages. Comparative genome analysis demonstrated the distinct features of PBC1 in that the MCP of PBC1 did not show close homology to MCPs from other B. cereus phages. Although PBC1 rapidly kills the B. cereus host bacteria, its host specificity is quite narrow, as PBC1 infected only 1 of 22 B. cereus strains. On the other hand, the PBC1-encoded endolysin, LysPBC1, lysed all B. cereus group strains tested, as well as other members of the genus Bacillus. The catalytic amidase domain of LysPBC1, LysPBC1_EAD, also showed lytic activity and maintained host specificity. These results demonstrate that not only the phage PBC1 itself but also its endolysin could be useful as novel biocontrol agents.
ACKNOWLEDGMENT
This research was supported by the R&D Convergence Center Support Program, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
REFERENCES
- 1.Bottone E. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2011. Surveillance for foodborne disease outbreaks—United States, 2008. MMWR Morb Mortal Wkly Rep 60:1197–1202. [PubMed] [Google Scholar]
- 3.Drobniewski FA. 1993. Bacillus cereus and related species. Clin Microbiol Rev 6:324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simm R, Voros A, Ekman JV, Sodring M, Nes I, Kroeger JK, Saidijam M, Bettaney KE, Henderson PJF, Salkinoja-Salonen M, Kolsto AB. 2012. BC4707 is a major facilitator superfamily multidrug resistance transport protein from Bacillus cereus implicated in fluoroquinolone tolerance. PLoS One 7:e36720. doi: 10.1371/journal.pone.0036720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia P, Martinez B, Obeso JM, Rodriguez A. 2008. Bacteriophages and their application in food safety. Lett Appl Microbiol 47:479–485. doi: 10.1111/j.1472-765X.2008.02458.x. [DOI] [PubMed] [Google Scholar]
- 6.Lu TK, Koeris MS. 2011. The next generation of bacteriophage therapy. Curr Opin Microbiol 14:524–531. doi: 10.1016/j.mib.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Gu JM, Liu XH, Li Y, Han WY, Lei LC, Yang YJ, Zhao HL, Gao Y, Song J, Lu R, Sun CJ, Feng X. 2012. A method for generation phage cocktail with great therapeutic potential. PLoS One 7:e31698. doi: 10.1371/journal.pone.0031698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan BK, Abedon ST, Loc-Carrillo C. 2013. Phage cocktails and the future of phage therapy. Future Microbiol 8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- 9.Shin H, Bandara N, Shin E, Ryu S, Kim K. 2011. Prevalence of Bacillus cereus bacteriophages in fermented foods and characterization of phage JBP901. Res Microbiol 162:791–797. doi: 10.1016/j.resmic.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Bandara N, Jo J, Ryu S, Kim K. 2012. Bacteriophages BCP1-1 and BCP8-2 require divalent cations for efficient control of Bacillus cereus in fermented foods. Food Microbiol 31:9–16. doi: 10.1016/j.fm.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Smeesters PR, Dreze PA, Bousbata S, Parikka KJ, Timmery S, Hu X, Perez-Morga D, Deghorain M, Toussaint A, Mahillon J, Van Melderen L. 2011. Characterization of a novel temperate phage originating from a cereulide-producing Bacillus cereus strain. Res Microbiol 162:446–459. doi: 10.1016/j.resmic.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 12.El-Arabi T, Griffiths M, She Y-M, Villegas A, Lingohr E, Kropinski A. 2013. Genome sequence and analysis of a broad-host range lytic bacteriophage that infects the Bacillus cereus group. Virol J 10:48. doi: 10.1186/1743-422X-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J-H, Shin H, Son B, Heu S, Ryu S. 2013. Characterization and complete genome sequence of a virulent bacteriophage B4 infecting food-borne pathogenic Bacillus cereus. Arch Virol 158:2101–2108. doi: 10.1007/s00705-013-1719-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee W, Billington C, Hudson J, Heinemann J. 2011. Isolation and characterization of phages infecting Bacillus cereus. Lett Appl Microbiol 52:456–464. doi: 10.1111/j.1472-765X.2011.03023.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin H, Lee JH, Park J, Heu S, Ryu S. 2014. Characterization and genome analysis of the Bacillus cereus-infecting bacteriophages BPS10C and BPS13. Arch Virol 159:2171–2175. doi: 10.1007/s00705-014-2030-6. [DOI] [PubMed] [Google Scholar]
- 16.Klumpp J, Schmuki M, Sozhamannan S, Beyer W, Fouts DE, Bernbach V, Calendar R, Loessner MJ. 2014. The odd one out: Bacillus ACT bacteriophage CP-51 exhibits unusual properties compared to related Spounavirinae W Ph and Bastille. Virology 462:299–308. doi: 10.1016/j.virol.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Kong M, Kim M, Ryu S. 2012. Complete genome sequence of Bacillus cereus bacteriophage PBC1. J Virol 86:6379–6380. doi: 10.1128/JVI.00706-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minakhin L, Semenova E, Liu J, Vasilov A, Severinova E, Gabisonia T, Inman R, Mushegian A, Severinov K. 2005. Genome sequence and gene expression of Bacillus anthracis bacteriophage Fah. J Mol Biol 354:1–15. doi: 10.1016/j.jmb.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Fouts DE, Rasko DA, Cer RZ, Jiang LX, Fedorova NB, Shvartsbeyn A, Vamathevan JJ, Tallon L, Althoff R, Arbogast TS, Fadrosh DW, Read TD, Gill SR. 2006. Sequencing Bacillus anthracis typing phages Gramma and Cherry reveals a common ancestry. J Bacteriol 188:3402–3408. doi: 10.1128/JB.188.9.3402-3408.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuch R, Fischetti VA. 2006. Detailed genomic analysis of the W beta and gamma phages infecting Bacillus anthracis: implications for evolution of environmental fitness and antibiotic resistance. J Bacteriol 188:3037–3051. doi: 10.1128/JB.188.8.3037-3051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillis A, Mahillon J. 2014. Phages preying on Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: past, present and future. Viruses 6:2623–2672. doi: 10.3390/v6072623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer JM, Gilbert RJ. 1989. Bacillus cereus and other Bacillus species, p 21–70. In Doyle MP. (ed), Foodborne bacterial pathogens. Marcel Dekker Inc., New York, NY. [Google Scholar]
- 23.Grande MJ, Lucas R, Abriouel H, Valdivia E, Omar NB, Maqueda M, Martinez-Bueno M, Martinez-Canamero M, Galvez A. 2006. Inhibition of toxicogenic Bacillus cereus in rice-based foods by enterocin AS-48. Int J Food Microbiol 106:185–194. doi: 10.1016/j.ijfoodmicro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Guenther S, Huwyler D, Richard S, Loessner MJ. 2009. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl Environ Microbiol 75:93–100. doi: 10.1128/AEM.01711-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baptista C, Santos MA, Sao-Jose C. 2008. Phage SPP1 reversible adsorption to Bacillus subtilis cell wall teichoic acids accelerates virus recognition of membrane receptor YueB. J Bacteriol 190:4989–4996. doi: 10.1128/JB.00349-08 (Erratum, J Bacteriol 191:1726, 2009.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen MC, van Alphen LB, Harboe A, Li J, Christensen BB, Szymanski CM, Brondsted L. 2011. Bacteriophage F336 recognizes the capsular phosphoramidate modification of Campylobacter jejuni NCTC11168. J Bacteriol 193:6742–6749. doi: 10.1128/JB.05276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiljunen S, Datta N, Dentovskaya SV, Anisimov AP, Knirel YA, Bengoechea JA, Holst O, Skurnik M. 2011. Identification of the lipopolysaccharide core of Yersinia pestis and Yersinia pseudotuberculosis as the receptor for bacteriophage phiA1122. J Bacteriol 193:4963–4972. doi: 10.1128/JB.00339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Nei M, Dudley J, Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunne M, Mertens HD, Garefalaki V, Jeffries CM, Thompson A, Lemke EA, Svergun DI, Mayer MJ, Narbad A, Meijers R. 2014. The CD27L and CTP1L endolysins targeting Clostridia contain a built-in trigger and release factor. PLoS Pathog 10:e1004228. doi: 10.1371/journal.ppat.1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son B, Yun J, Lim JA, Shin H, Heu S, Ryu S. 2012. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol 12:33. doi: 10.1186/1471-2180-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priest FG, Barker M, Baillie LWJ, Holmes EC, Maiden MCJ. 2004. Population structure and evolution of the Bacillus cereus group. J Bacteriol 186:7959–7970. doi: 10.1128/JB.186.23.7959-7970.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kysela DT, Turner PE. 2007. Optimal bacteriophage mutation rates for phage therapy. J Theor Biol 249:411–421. doi: 10.1016/j.jtbi.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Bigwood T, Hudson JA, Billington C. 2009. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol Lett 291:59–64. doi: 10.1111/j.1574-6968.2008.01435.x. [DOI] [PubMed] [Google Scholar]
- 35.Dorscht J, Klumpp J, Bielmann R, Schmelcher M, Born Y, Zimmer M, Calendar R, Loessner MJ. 2009. Comparative genome analysis of Listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J Bacteriol 191:7206–7215. doi: 10.1128/JB.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casjens SR, Gilcrease EB. 2009. Determining DNA packaging strategy by analysis of the termini of the chromosomes in tailed-bacteriophage virions. Methods Mol Biol 502:91–111. doi: 10.1007/978-1-60327-565-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casjens SR, Gilcrease EB, Winn-Stapley DA, Schicklmaier P, Schmieger H, Pedulla ML, Ford ME, Houtz JM, Hatfull GF, Hendrix RW. 2005. The generalized transducing Salmonella bacteriophage ES18: complete genome sequence and DNA packaging strategy. J Bacteriol 187:1091–1104. doi: 10.1128/JB.187.3.1091-1104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kropinski AM, Hayward M, Agnew MD, Jarrell KF. 2005. The genome of BCJA1c: a bacteriophage active against the alkaliphilic bacterium, Bacillus clarkii. Extremophiles 9:99–109. doi: 10.1007/s00792-004-0425-0. [DOI] [PubMed] [Google Scholar]
- 39.Borysowski J, Weber-Dabrowska B, Gorski A. 2006. Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med 231:366–377. [DOI] [PubMed] [Google Scholar]
- 40.Ganz HH, Law C, Schmuki M, Eichenseher F, Calendar R, Loessner MJ, Getz WM, Korlach J, Beyer W, Klumpp J. 2014. Novel giant siphovirus from Bacillus anthracis features unusual genome characteristics. PLoS One 9:e85972. doi: 10.1371/journal.pone.0085972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang WH, Mi ZQ, Yin XY, Fan H, An XP, Zhang ZY, Chen JK, Tong YG. 2013. Characterization of Enterococcus faecalis phage IME-EF1 and its endolysin. PLoS One 8:e80435. doi: 10.1371/journal.pone.0080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan YH, Peng Q, Gao MY. 2012. Characteristics of a broad lytic spectrum endolysin from phage BtCS33 of Bacillus thuringiensis. BMC Microbiol 12:297. doi: 10.1186/1471-2180-12-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Son JS, Jun SY, Kim EB, Park JE, Paik HR, Yoon SJ, Kang SH, Choi YJ. 2010. Complete genome sequence of a newly isolated lytic bacteriophage, EFAP-1 of Enterococcus faecalis, and antibacterial activity of its endolysin EFAL-1. J Appl Microbiol 108:1769–1779. doi: 10.1111/j.1365-2672.2009.04576.x. [DOI] [PubMed] [Google Scholar]
- 44.Callewaert L, Walmagh M, Michiels CW, Lavigne R. 2011. Food applications of bacterial cell wall hydrolases. Curr Opin Biotechnol 22:164–171. doi: 10.1016/j.copbio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Hermoso JA, Garcia JL, Garcia P. 2007. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol 10:461–472. doi: 10.1016/j.mib.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Rubio L, Martinez B, Rodriguez A, Donovan DM, Gotz F, Garcia P. 2013. The phage lytic proteins from the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 display multiple active catalytic domains and do not trigger staphylococcal resistance. PLoS One 8:e64671. doi: 10.1371/journal.pone.0064671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira H, Azeredo J, Lavigne R, Kluskens LD. 2012. Bacteriophage endolysins as a response to emerging foodborne pathogens. Trends Food Sci Technol 28:103–115. doi: 10.1016/j.tifs.2012.06.016. [DOI] [Google Scholar]
- 48.Loessner MJ, Maier SK, DaubekPuza H, Wendlinger G, Scherer S. 1997. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J Bacteriol 179:2845–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korndorfer IP, Danzer J, Schmelcher M, Zimmer M, Skerra A, Loessner MJ. 2006. The crystal structure of the bacteriophage PSA endolysin reveals a unique fold responsible for specific recognition of Listeria cell walls. J Mol Biol 364:678–689. doi: 10.1016/j.jmb.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 50.Mayer MJ, Narbad A, Gasson MJ. 2008. Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J Bacteriol 190:6734–6740. doi: 10.1128/JB.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mo KF, Li XR, Li HQ, Low LY, Quinn CP, Boons GJ. 2012. Endolysins of Bacillus anthracis bacteriophages recognize unique carbohydrate epitopes of vegetative cell wall polysaccharides with high affinity and selectivity. J Am Chem Soc 134:15556–15562. doi: 10.1021/ja3069962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schleifer K, Kandler O. 1972. Peptidoglycan types of Bacterial cell-walls and their taxonomic implications. Bacteriol Rev 36:407–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sass P, Bierbaum G. 2007. Lytic activity of recombinant bacteriophage phi 11 and phi 12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol 73:347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmelcher M, Powell AM, Becker SC, Camp MJ, Donovan DM. 2012. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl Environ Microbiol 78:2297–2305. doi: 10.1128/AEM.07050-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Zhang Y, Yu JP, Huang YL, Zhang XE, Wei HP. 2014. Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 58:536–542. doi: 10.1128/AAC.01793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer MJ, Garefalaki V, Spoerl R, Narbad A, Meijers R. 2011. Structure-based modification of a Clostridium difficile-targeting endolysin affects activity and host range. J Bacteriol 193:5477–5486. doi: 10.1128/JB.00439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Low LY, Yang C, Perego M, Osterman A, Liddington RC. 2005. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J Biol Chem 280:35433–35439. doi: 10.1074/jbc.M502723200. [DOI] [PubMed] [Google Scholar]
- 59.Oliveira H, Melo LDR, Santos SB, Nobrega FL, Ferreira EC, Cerca N, Azeredo J, Kluskens LD. 2013. Molecular aspects and comparative genomics of bacteriophage endolysins. J Virol 87:4558–4570. doi: 10.1128/JVI.03277-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matarante A, Baruzzi F, Cocconcelli PS, Morea M. 2004. Genotyping and toxigenic potential of Bacillus subtilis and Bacillus pumilus strains occurring in industrial and artisanal cured sausages. Appl Environ Microbiol 70:5168–5176. doi: 10.1128/AEM.70.9.5168-5176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer MJ, Gasson MJ, Narbad A. 2012. Genomic sequence of bacteriophage ATCC 8074-B1 and activity of its endolysin and engineered variants against Clostridium sporogenes. Appl Environ Microbiol 78:3685–3692. doi: 10.1128/AEM.07884-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Low LY, Yang C, Perego M, Osterman A, Liddington R. 2011. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J Biol Chem 286:34391–34403. doi: 10.1074/jbc.M111.244160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson I, Sorokin A, Kapatral V, Reznik G, Bhattacharya A, Mikhailova N, Burd H, Joukov V, Kaznadzey D, Walunas T, D'Souza M, Larsen N, Pusch G, Liolios K, Grechkin Y, Lapidus A, Goltsman E, Chu L, Fonstein M, Ehrlich SD, Overbeek R, Kyrpides N, Ivanova N. 2005. Comparative genome analysis of Bacillus cereus group genomes with Bacillus subtilis. FEMS Microbiol Lett 250:175–184. doi: 10.1016/j.femsle.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Weidenmaier C, Peschel A. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 65.Leoff C, Choudhury B, Saile E, Quinn CP, Carlson RW, Kannenberg EL. 2008. Structural elucidation of the nonclassical secondary cell wall polysaccharide from Bacillus cereus ATCC 10987. Comparison with the polysaccharides from Bacillus anthracis and B. cereus type strain ATCC 14579 reveals both unique and common structural features. J Biol Chem 283:29812–29821. doi: 10.1074/jbc.M803234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YD, Park JH. 2010. Genomic sequence of temperate phage 250 isolated from emetic B. cereus and cloning of putative endolysin. Food Sci Biotechnol 19:1643–1648. doi: 10.1007/s10068-010-0232-6. [DOI] [Google Scholar]