Abstract
Anthranilate and indole are alternative degradation products of tryptophan, depending on the bacterial species. While indole enhances the biofilm formation of Pseudomonas aeruginosa, we found that anthranilate, the tryptophan degradation product of P. aeruginosa, had an opposite effect on P. aeruginosa biofilm formation, in which anthranilate deteriorated the mushroom structure of biofilm. The anthranilate effect on biofilm formation was differentially exerted depending on the developmental stage and the presence of shear force. Anthranilate slightly accelerated the initial attachment of P. aeruginosa at the early stage of biofilm development and appeared to build more biofilm without shear force. But anthranilate weakened the biofilm structure in the late stage, deteriorating the mushroom structure of biofilms with shear force to make a flat biofilm. To investigate the interplay of anthranilate with indole in biofilm formation, biofilms were cotreated with anthranilate and indole, and the results showed that anthranilate antagonized the biofilm-enhancing effect of indole. Anthranilate was able to deteriorate the preformed biofilm. The effect of anthranilate and indole on biofilm formation was quorum sensing independent. AntR, a regulator of anthranilate-degrading metabolism was synergistically activated by cotreatment with anthranilate and indole, suggesting that indole might enhance biofilm formation by facilitating the degradation of anthranilate. Anthranilate slightly but significantly affected the cyclic diguaniylate (c-di-GMP) level and transcription of major extracellular polysaccharide (Psl, Pel, and alginate) operons. These results suggest that anthranilate may be a promising antibiofilm agent and antagonize the effect of indole on P. aeruginosa biofilm formation.
INTRODUCTION
Biofilms are a representative example of bacterial group behavior that provides cells with many biological advantages, such as high infectivity, antibiotic resistance, and strong survivability (1, 2). Currently, most persistent bacterial infections are believed to be associated with antibiotic-resistant biofilms of pathogenic bacteria (3, 4). Pseudomonas aeruginosa, a Gram-negative bacterium, is a ubiquitous and major opportunistic human pathogen. The colonization and biofilm formation of P. aeruginosa cause great losses in many industrial facilities and serious infections such as cystic fibrosis, microbial keratitis, and burn wound infections in humans (4–8). Therefore, control of P. aeruginosa biofilms is a very important issue in medicine, public health, and industry.
Anthranilate and indole are both aromatic compounds produced from tryptophan metabolism. In bacteria, tryptophan is metabolized differently depending on the bacterial species, and the key enzyme of this differentiation is tryptophanase, encoded by the tnaA gene (9). Many bacteria, such as Escherichia coli, Haemophilus influenzae, and Vibrio vulnificus, produce indole from tryptophan, since they have tnaA, which converts tryptophan into indole, pyruvate, and ammonia (9, 10). However, some other bacteria, including P. aeruginosa, degrade tryptophan to anthranilate through a kynurenine pathway using the kynBAU genes (9). Therefore, anthranilate and indole are alternative degradation products of tryptophan in the microbial community, and if P. aeruginosa exists in tryptophan-rich environments with other indole-producing bacteria, it will encounter indole from other bacteria as it produces anthranilate.
The effects of tryptophan and indole on the biofilm formation of P. aeruginosa have been recently reported. Tryptophan had an inhibitory effect on biofilm formation of E. coli and P. aeruginosa (11, 12). Indole also inhibited the biofilm formation of E. coli, which metabolizes tryptophan to indole (13). Interestingly, indole enhanced the biofilm formation of P. aeruginosa, which degrades tryptophan to anthranilate but not to indole (13, 14). While P. aeruginosa does not produce indole, P. aeruginosa may encounter indole produced by indole-producing bacteria, such as E. coli, in mixed bacterial communities in nature, and its physiology can be influenced by indole (10, 14). However, the effect of anthranilate on biofilm formation of P. aeruginosa has not been addressed yet, although anthranilate is a real product of the tryptophan metabolism of P. aeruginosa. A recent study showed that P. aeruginosa biofilm cells have enhanced anthranilate-degrading activity (15), implying the possible involvement of anthranilate in biofilm physiology.
Biofilm formation by P. aeruginosa can be controlled in a cell density-dependent manner by a quorum-sensing (QS) system that allows bacteria to communicate with each other via signaling molecules, acyl-homoserine lactones (AHLs) and Pseudomonas quinolone signal (PQS; 2-heptyl-3-hydroxy-4-quinolone) (16, 17). Major AHLs of P. aeruginosa are N-3-oxododecanoyl-l-homoserine lactone (3OC12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL), which are synthesized by LasI and RhlI, respectively, and bind to their cognate receptors, LasR (for 3OC12-HSL), QscR (for 3OC12-HSL), and RhlR (for C4-HSL), to regulate target genes (18–20). PQS, another important QS signal, also plays a significant role in the regulation of virulence genes and biofilm formation (21). In P. aeruginosa, indole inhibits PQS production (13) and anthranilate is a precursor of PQS biosynthesis (22). Moreover, the metabolism of anthranilate is growth phase-differentially regulated by QS system in P. aeruginosa (23). Anthranilate and indole therefore intersect with QS regulation and biofilm formation. Since anthranilate is also a precursor of tryptophan biosynthesis and an intermediate that is metabolized through the tricarboxylic acid (TCA) cycle, it is a key metabolite of P. aeruginosa at the metabolic branch point (22, 23). The complex relationship among QS, biofilm, anthranilate, and indole in P. aeruginosa is schematically described in Fig. S1 in the supplemental material.
In this study, we investigated the effects of anthranilate and indole on biofilm formation of P. aeruginosa and found that anthranilate deteriorated the biofilm, making a flat biofilm. We suggest that anthranilate may be a promising antibiofilm agent and can antagonize the effect of indole on P. aeruginosa biofilm formation.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa strains were grown at 37°C in Luria-Bertani (LB) (yeast, 5 g/liter; Bacto tryptone, 10 g/liter; and NaCl, 5 g/liter) medium with vigorous shaking at 170 rpm. Growth was measured by optical density at 600 nm (OD600). Antibiotics were used at the following concentrations: carbenicillin, 100 μg/ml; ampicillin, 50 μg/ml; and gentamicin, 10 μg/ml (for E. coli) and 50 μg/ml (for P. aeruginosa). Indole and anthranilate were dissolved at 1 M in dimethyl sulfoxide (DMSO) for stock solution and diluted into the media at final concentrations of 0.4 mM and 0.1 mM, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Name | Descriptiona | Reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Wild-type P. aeruginosa | 43 |
| MW1 | lasI rhlI double mutant of PAO1 | 44 |
| E. coli strain DH5α | supE44 ΔlacU169 (ф80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 45 |
| Plasmids | ||
| pAB1 | gfp-mut2 gene in pMF54; Apr | 46 |
| pQF50 | Broad-host-range lacZ fusion plasmid; Apr | 29 |
| pJN105 | araC-pBAD cassette cloned in pBBR1MCS-5; Gmr | 47 |
| pJN105A | Plasmid for AntR overexpression; antR ORF in pJN105; Gmr | 23 |
| pSC11 | lasIp-lacZ reporter in pQF50; Apr | 48 |
| pJL101 | PA1897p-lacZ reporter in pQF50; Apr | 24 |
| pJL201 | antAp-lacZ fusion in pQF50; Apr | 23 |
| pSKcdrA | cdrA-lacZ fusion in pQF50; Apr | This study |
Gmr, gentamicin-resistance; Apr, ampicillin and carbenicillin resistance; ORF, open reading frame.
Measurement of QS regulators and AntR in P. aeruginosa.
To make P. aeruginosa reporter strains for measuring the activity of the QS regulators, the specific promoter-lacZ fusion plasmids, pSC11 (lasIp-lacZ fusion for measuring the LasR activity), pJL101 (PA1897p-lacZ fusion for measuring the QscR activity), and pJL201 (antAp-lacZ fusion for measuring the AntR activity), were transformed into PAO1 (Table 1). The overnight cultures of each reporter strain were inoculated at 1% into fresh LB broth containing carbenicillin and cultivated with and without 0.1 mM anthranilate, 0.4 mM indole, or both. During growth, aliquots were taken every hour and β-galactosidase activities were measured as described below.
Measurement of AntR activity in E. coli (E. coli reconstitution analysis).
To overexpress AntR of P. aeruginosa in E. coli, pJN105A plasmid was used (Table 1). Two compatible plasmids, pJL201 and pJN105A, were transformed into E. coli DH5α and the transformants were grown overnight in LB medium. The cells were then inoculated into fresh LB medium at an initial OD600 of 0.04 and grown to an OD600 of 0.3 without arabinose. After 0.4% arabinose was added for the induction of AntR, 0.1 mM anthranilate, 0.4 mM indole, or both were added for 2 h. The β-galactosidase activity was then measured as described below. As a control, pJN105, the parental plasmid of pJN105A, was cotransformed with pJL201, the transformed cells were grown in the same way, and the β-galactosidase activity was measured.
β-Galactosidase activity assay.
β-Galactosidase activity was assayed with a Tropix-plus kit (Applied Biosystems, USA), with slight modification of the manufacturer's instructions, as described elsewhere (24). First, the OD600 of cultures was measured. One-hundred-microliter quantities of the cultures were taken and 10 μl of chloroform was added. After vigorous vortexing and a 15-min incubation at room temperature, 10 μl from the top was transferred to a new tube and 100 μl of 1:100-diluted substrate solution was added. After a 45-min incubation at room temperature in the dark, 150 μl of light emission solution (Accelerator II) was added and the luminescence was promptly measured with multiwell plate reader (Tristar LB941; Berthold). The luminescence was normalized with the OD600 of the cultures, and the activity was presented as luminescence/OD600.
Biofilm formation and assay.
The static biofilm assay was carried out as described elsewhere (25, 26). P. aeruginosa PAO1 cells were grown to an OD600 of 3 in LB broth with vigorous shaking. These cells were diluted to an OD600 of 0.06 in fresh M63 medium (M63 salt [KH2PO4, 12 g/liter; K2HPO4, 28 g/liter; and NH4SO4, 8 g/liter], 1 mM MgSO4, 0.5% Casamino Acids, 0.2% citrate) on 96-well polystyrene plates and incubated at 37°C for 24 h without shaking. After cell growth was measured by OD600, planktonic cells were poured out and the plate was washed with water and dried for 10 min. Then, 180 μl of crystal violet (0.1%, wt/vol) was added to each well and incubated for 7 min to stain biofilms attached to the well surface. After a brief wash, the biofilm-staining crystal violet was dissolved in 200 μl of absolute ethanol. The absorbance was then measured at 550 nm (A550) to determine the amount of crystal violet, which was normalized with cell growth (OD600).
The drip-flow biofilm formation was carried out as previously described (27), with slight modification. P. aeruginosa cells harboring the green fluorescent protein (GFP)-expressing plasmid pAB1 (Table 1) were cultured overnight and diluted to an OD600 of 1 in fresh LB broth, 1 ml of which was dropped on to slide glass. This inoculation was repeated 3 times, with 10-min intervals. After a 40-min incubation, continuous dropping of 1% Bacto tryptic soy broth (TSB) (tryptone, 5.6 g/liter; soytone, 1.6 g/liter; NaCl, 1.6 g/liter; dextrose, 0.8 g/liter; and K2HPO4, 0.8 g/liter) containing 100 μg/ml of carbenicillin and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) started at a flow rate of 0.5 ml/min.
For flow cell biofilm formation, the same GFP-expressing P. aeruginosa cells were grown overnight and diluted to an OD600 of 0.5 in LB, 200 μl of which was injected into a flow cell chamber (2 mm by 2 mm by 50 mm). Cells were incubated for 1 h without flow for attachment, and then the TSB media containing 100 μg/ml of carbenicillin and 1 mM IPTG flowed at 200 μl/min. Biofilms in the flow cell were grown for 5 days at room temperature and visualized by GFP or staining with 0.1% calcofluor white for 15 min. For the dispersion of preformed biofilm, biofilm was formed in flow cell for 3 days and treated with 0.1 mM anthranilate for 1 day. Biofilms were observed on fluorescence microscopes as described below.
Biofilm imaging and quantification.
Biofilm images were obtained using confocal laser scanning microscopy (CLSM) (Olympus; FV10i) or fluorescence microscopy (Zeiss; Axioskop FL). The excitation wavelength for GFP was 488 nm, and the emission wavelength was 500 nm. For calcofluor white, the excitation and emission wavelengths were 355 nm and 440 nm, respectively. The 3-dimensional images of biofilms were reconstructed from plane images by using Bitplane Imaris 6.3.1 image analysis software. Quantification of biofilms was performed using integrated morphometry analysis in Metamorph version 7.7 (Molecular Devices, USA) and statistical analysis in AutoQuant X (Mediacy, USA).
Measurement of cell surface hydrophobicity.
Bacteria were cultured overnight in LB broth at 37°C with vigorous shaking. An aliquot (1.2 ml) of cell suspension was mixed with 200 μl of hexane with vigorous shaking for 2 min. After separation of the aqueous and organic phases, the aqueous phase was carefully taken and OD600 was measured. The hydrophobicity of cells was calculated based on the cell adhesion to organic phase as described elsewhere (26, 28) from the following equation: percentage of adhesion = 100 × [(OD600 of the initial bacterial suspension − OD600 of the aqueous phase)/OD600 of the initial bacterial suspension].
Quantitative analysis of anthranilate by HPLC.
P. aeruginosa cells were grown overnight in LB medium with or without 0.4 mM indole. Cells were then removed by centrifugation at 5,000 rpm for 20 min at 4°C. The cell-free supernatant was extracted with an equal volume of ethyl acetate, and the ethyl acetate fraction was carefully taken. Ethyl acetate was then evaporated to dryness, and the pellet was dissolved in a small volume of ethyl acetate. This was analyzed for anthranilate by reversed-phase high-performance liquid chromatography (HPLC) (Gilson) using a C18 reverse-phase column and UV detector (wavelength, 220 nm) at a 0.5-ml/min flow rate in linear gradient elution (solvent A, H2O plus 0.1% trifluoroacetic acid [TFA]; solvent B, 100% methanol). Commercial anthranilate (Daejung, South Korea) was analyzed under the same conditions as a standard. For the quantification of peaks, we used the integration mode of the HPLC operating software (Unipoint, Gilson, USA).
Reporter-based measurement of c-di-GMP.
For the construction of reporter plasmid (pSKcdrA in Table 1) to measure the cyclic diguaniylate (c-di-GMP) level, the DNA fragment containing cdrA promoter region was prepared by PCR amplification and cloned into BamHI and HindIII sites of pQF50, a promoter probing plasmid (29). The cdrA promoter cloned in pSKcdrA includes the region from −492 to ∼+1 relative to the start codon of cdrA gene. The PCR primer sequences were GATCGGATCCTTGTTGCTGATCGCGGACCCG (forward) and GACGAAGCTTGAAAATCTCCCTATCTGCGT (reverse). For measurement of the c-di-GMP level, P. aeruginosa cells harboring pSKcdrA were grown with or without 0.1 mM anthranilate for 20 h. For comparison, the reporter cells were grown with various concentrations of sodium nitroprusside (SNP; Sigma) under the same condition. Then, β-galactosidase activity, which reflects the c-di-GMP level, was measured. The level of nontreated cells was set to 100%, and the relative levels of treated cells were calculated from the β-galactosidase activity.
RNA isolation and quantification of transcripts.
The transcription levels of three major extracellular polysaccharide (EPS) gene clusters of P. aeruginosa were measured by quantitative real-time PCR analysis. The specific primer sets for pslA (PA2231, for Psl operon), pelA (PA3064, for Pel operon), and alg44 (PA3542, for alginate operon) were designed as follows: Psl-forward, CGCTCACGGTGATTATGTTC; Psl-reverse, TACATGAACAACAGCAGGCA; Pel-forward, ACAGCCAGGTAATGGACCTC; Pel-reverse, AAGCTGTCCAGGGTATCGAG; alginate-forward, CTACCTTCTCGGCCAACCT; and alginate-reverse, GTCAGGGTCCCTTTCATCTG. P. aeruginosa cells were cultivated with or without 0.1 mM anthranilate. The cultures were directly mixed with RNA Protect Bacteria reagent (Qiagen) to stabilize RNA and then lysed by lysozyme treatment and brief sonication. RNA was purified by RNeasy minicolumns (Qiagen) as instructed by the manufacturer's manual. Contaminated DNA was removed by on-column DNase I (Qiagen) digestion and additional RNeasy column purification. The cDNA synthesis was accomplished with a HelixCrip Thermo reverse transcriptase kit (NanoHelix, South Korea) according to the supplied protocol, in which 10 μg of RNA, 100 pmol of semirandom primer with average G+C content of 75%, and deoxynucleoside triphosphate (dNTP) were used. For quantitative real-time PCR, 10 ng of cDNA and specific primers were mixed with 20 μl of SYBR Premix Ex Taq (TaKaRa) and analyzed in thermal cycler real-time PCR system model TP8000 (TaKaRa, Japan). Genomic DNA was used as a standard for quantification, and nadB (PA0761) was used as an internal control, as described elsewhere (20).
Statistical analysis.
In order to ensure the significance of the results, the data were statistically analyzed using t test (two-sample assuming equal variances) in MS Office Excel (Microsoft, USA). Any P value lower than 0.05 was considered significant.
RESULTS
Biofilm-enhancing effect of indole on P. aeruginosa is QS independent.
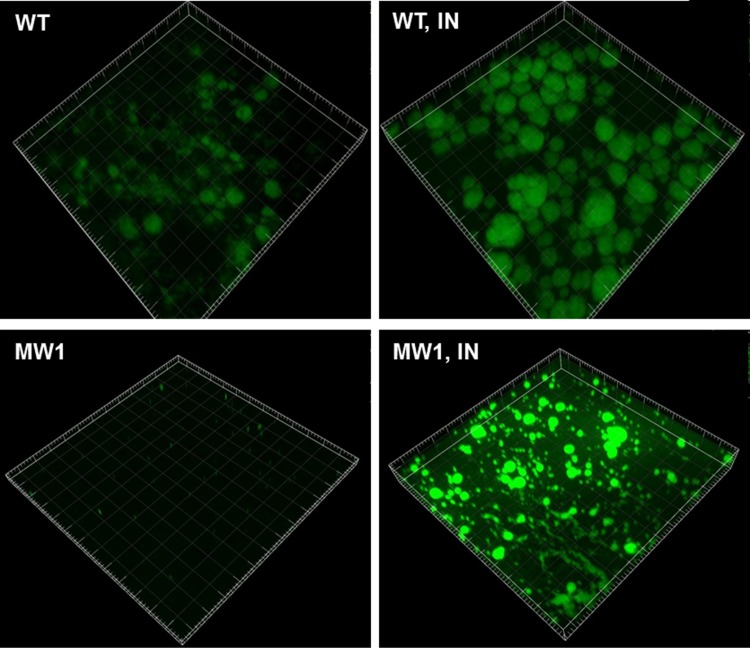
Indole was reported to both inhibit PQS production and enhance biofilm formation in P. aeruginosa (10, 13). Since both PQS production and biofilm formation were positively regulated by QS systems (see Fig. S1 in the supplemental material), we investigated whether the indole effect on the biofilm formation is exerted through QS regulation. When we treated a QS mutant strain, MW1 (lacking lasI and rhlI), which is deficient in the production of QS signals, with 0.4 mM indole, the biofilm formation of MW1 was still enhanced by the indole treatment (Fig. 1). When we investigated the influence of indole on the activities of QS regulators, LasR and QscR, by using reporter fusions, none was found to be significantly affected by 0.4 mM indole treatment (see Fig. S2A and B in the supplemental material). Indole did not affect cell growth at this concentration (data not shown). These results demonstrate that the effect of indole on biofilm formation is exerted independently of the acyl-HSL-based QS systems.
FIG 1.
Biofilm-enhancing effect of indole is QS independent. PAO1 (wild type [WT]) and MW1 (QS mutant lacking lasI and rhlI) cells harboring pAB1 were grown in the drip-flow chamber to form biofilms with indole treatment. WT and MW1 cells in the left column were treated with dimethyl sulfoxide (DMSO) as a buffer control, because indole was dissolved in DMSO before use. Biofilms were imaged by green fluorescence using CLSM at 40 h after inoculation. IN, 0.4 mM indole.
The effect of anthranilate differs depending on the developmental stage and the presence of shear force.
We investigated the effect of anthranilate on the biofilm formation of P. aeruginosa. When we measured the static biofilm formation with anthranilate treatment in shear force-free conditions, anthranilate appeared to enhance the biofilm formation, like indole (Fig. 2). When the biofilm was cotreated with anthranilate and indole together, static biofilm assay showed the additive enhancement of biofilm formation (Fig. 2).
FIG 2.
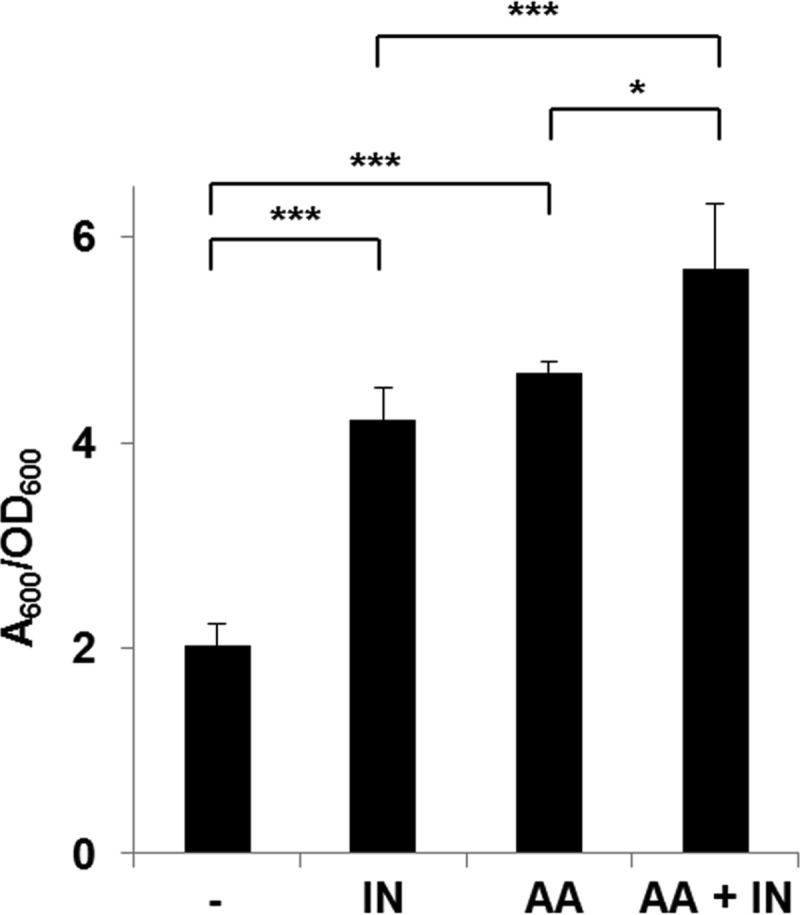
Anthranilate enhances static biofilm formation. Static biofilm assay was carried out as described in Materials and Methods. Wild-type PAO1 cells were grown in 96-well plates and the measurement was taken at 24 h after inoculation. -, DMSO as a buffer control; IN, 0.4 mM indole; AA, 0.1 mM anthranilate; AA + IN, 0.1 mM anthranilate plus 0.4 mM indole. *, P < 0.05; ***, P < 0.005.
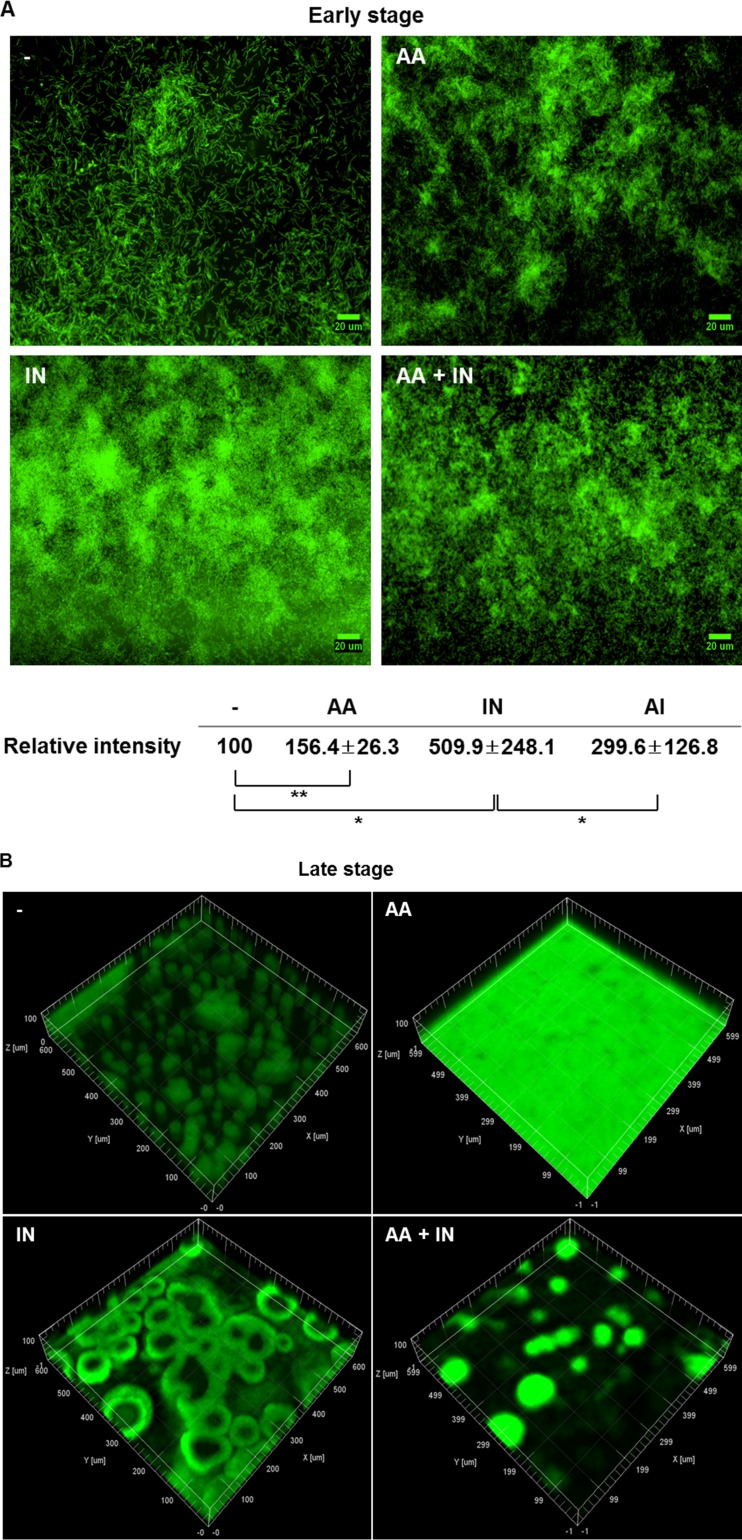
To better investigate this collaborative effect of indole and anthranilate, biofilm formation was measured in the presence of shear force in the flow cell system. We first investigated biofilm formation in the early stage of biofilm development and found that anthranilate and indole were each able to facilitate the attachment of cells. But interestingly, cotreatment with both failed to additively enhance cell attachment (Fig. 3A). Instead, anthranilate appeared to antagonize the biofilm-enhancing effect of indole (Fig. 3A). When we further grew the biofilm and investigated the effect of anthranilate at the late stage of biofilm development, anthranilate hindered the maturation of the biofilm structure, finally making a flat biofilm (Fig. 3B). As previously reported, indole treatment enhanced and advanced the maturation of biofilm and facilitated the dispersion of biofilm in the late stage, leaving a “central void form” at the center of a mushroom structure (Fig. 3B). Anthranilate was also able to dramatically antagonize this indole effect in the late stage of biofilm development (Fig. 3B). Anthranilate did not affect cell growth at the concentration used in this experiment (data not shown).
FIG 3.
Shear force differentiates the anthranilate effect according to developmental stage. (A) Initial attachment of PAO1 cells was observed in the early stage of biofilm development (15 h after inoculation) with indole or anthranilate treatment. The fluorescence of images was quantified and is presented at the bottom as relative intensity. (B) P. aeruginosa biofilm was observed at a late stage of biofilm development (4 days after inoculation). GFP-expressing PAO1 cells that harbor pAB1 were used in these experiments. -, DMSO as a buffer control; IN, 0.4 mM indole; AA, 0.1 mM anthranilate; AA + IN, 0.1 mM anthranilate plus 0.4 mM indole. *, P < 0.05; **, P < 0.01.
Taken together, our results demonstrate that anthranilate has a complex effect on biofilm formation dependent on the presence of shear force and the developmental stage of the biofilm. Without shear force, anthranilate was able to enhance the biofilm formation, and this effect was additive with indole. But with shear force, although anthranilate facilitated the initial colonization in the early stage, it ultimately hindered the maturation of the biofilm and antagonized the effect of indole.
Anthranilate deteriorates the biofilm structure.
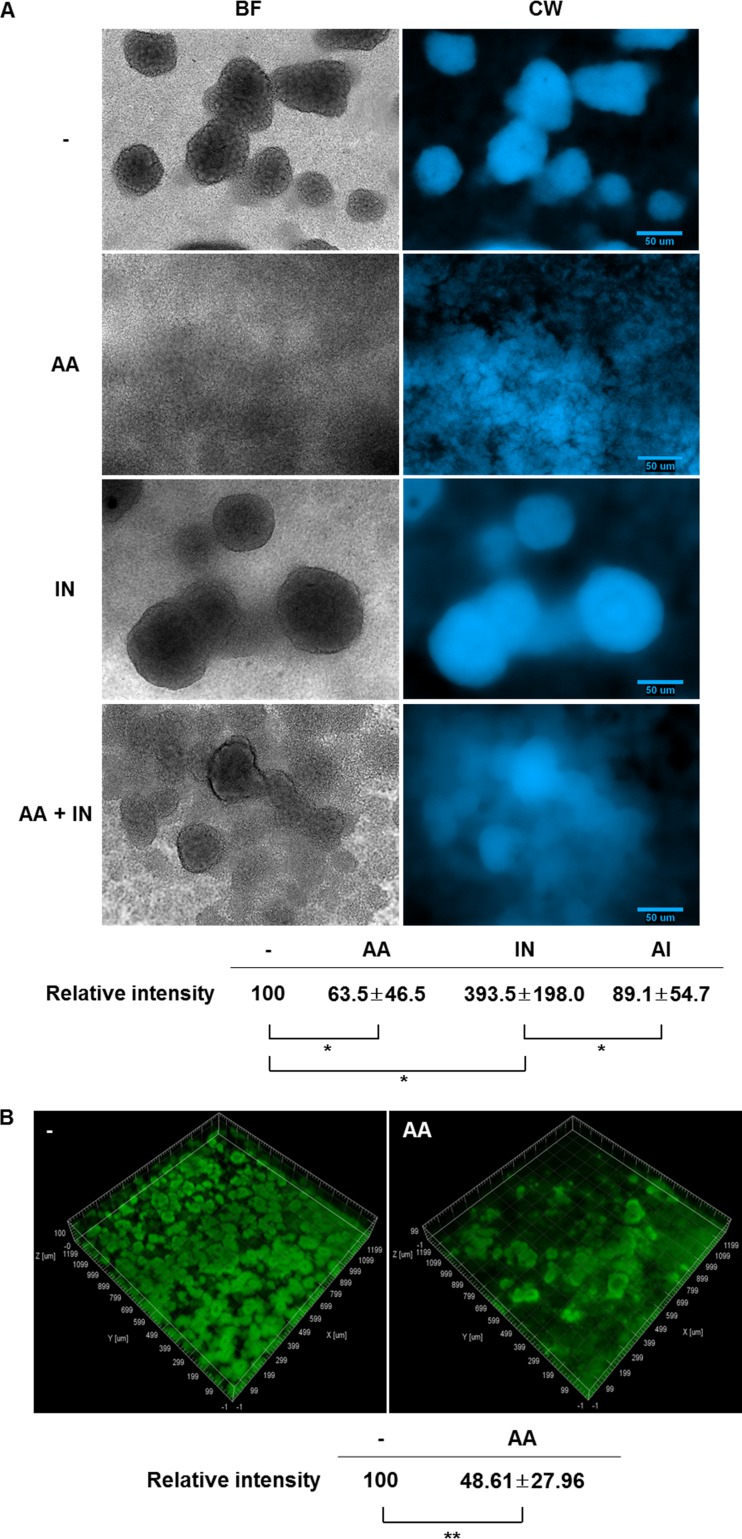
Since the biofilm inhibition by anthranilate occurred in the presence of shear force, we hypothesized that anthranilate weakens the biofilm and destabilizes the structure. To better see the influence of anthranilate and indole on the biofilm, we used calcofluor white staining of extracellular polysaccharides (EPSs) in the biofilms and simple bright-field microscopy. Calcofluor white is a fluorescent dye that strongly binds to extracellular structural polysaccharides, specifically to β-1,4 linkages of glycosidic bonds (30). Both bright-field and EPS staining microscopy showed that the anthranilate-treated biofilm contained a more deteriorated structure, implying that anthranilate likely weakens the biofilm and crumbles the structure in the presence of shear force (Fig. 4A). In contrast, indole enhanced the biofilm structure, forming bigger mushroom bodies. Anthranilate treatment seemed to cause biofilm cells to fall away to the interstitial space and even caused the indole-enhanced biofilm to crumble (Fig. 4A). In order to confirm this biofilm-deteriorating effect of anthranilate, we treated preformed biofilm with anthranilate. As shown in Fig. 4B, the preformed biofilm was destroyed by anthranilate treatment. These results also demonstrate that anthranilate deteriorates the biofilm structure.
FIG 4.
Anthranilate deteriorates the biofilm structure. (A) Biofilm of PAO1 cells formed in a flow cell system with treatment of anthranilate or indole. At 5 days after inoculation, biofilms were directly observed with a bright-field microscope (BF) or stained with calcofluor white and imaged by fluorescence microscopy (CW). -, DMSO as a buffer control; IN, 0.4 mM indole; AA, 0.1 mM anthranilate; AA + IN, 0.1 mM anthranilate plus 0.4 mM indole. The fluorescence intensity was quantified and is presented at the bottom as relative intensity. (B) A biofilm of GFP-expressing PAO1 cells was formed without anthranilate for 3 days in the flow cell system, and this preformed biofilm was then treated with 0.1 mM anthranilate for 24 h (AA). As a control, the preformed biofilm was further incubated without anthranilate under the same conditions (-). The fluorescence was quantified and is presented at the bottom. *, P < 0.05; **, P < 0.01.
The effect of anthranilate is also QS independent.
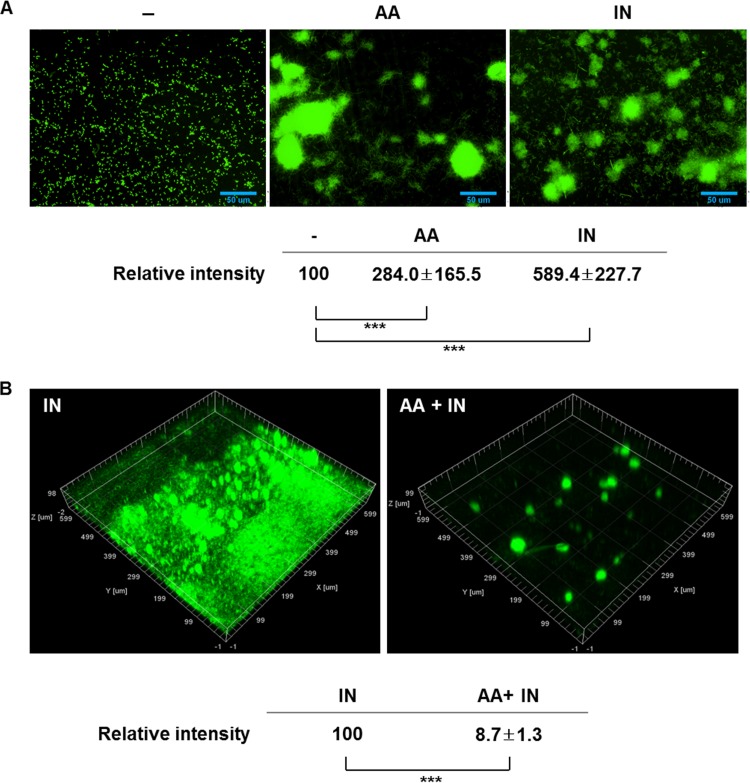
We investigated whether the anthranilate effects occurred in a QS-independent manner like for indole. We treated the QS mutant strain MW1 with anthranilate and observed the biofilm formation using fluorescence microscopy. The facilitation of the initial attachment by anthranilate treatment was still observed in the QS mutant strain as in the wild type (Fig. 5A). Anthranilate also antagonized the biofilm-enhancing effect of indole in the QS mutant (Fig. 5B). These results demonstrate that the anthranilate effects are exerted in a QS-independent manner, like the indole effect. We note that the attachment-facilitating effect of anthranilate in the early stage was more dramatic in the QS mutant than in the wild type.
FIG 5.
The effect of anthranilate is QS independent. (A) MW1 harboring pAB1 was used for biofilm formation. The biofilms of GFP-expressing MW1 cells were grown with 0.1 mM anthranilate (AA) or 0.4 mM indole (IN) in a flow cell system for 24 h. Biofilms were observed on a fluorescence microscope by green fluorescence. The intensity of fluorescence was quantified and is presented at the bottom. −, buffer control. (B) Biofilms of GFP-expressing MW1 cells were grown in a flow cell system for 3 days with 0.4 mM indole (IN) or 0.4 mM indole plus 0.1 mM anthranilate (AA + IN). Fluorescence was quantified and is presented at the bottom. ***, P < 0.005.
Indole boosts the anthranilate-degrading activity.
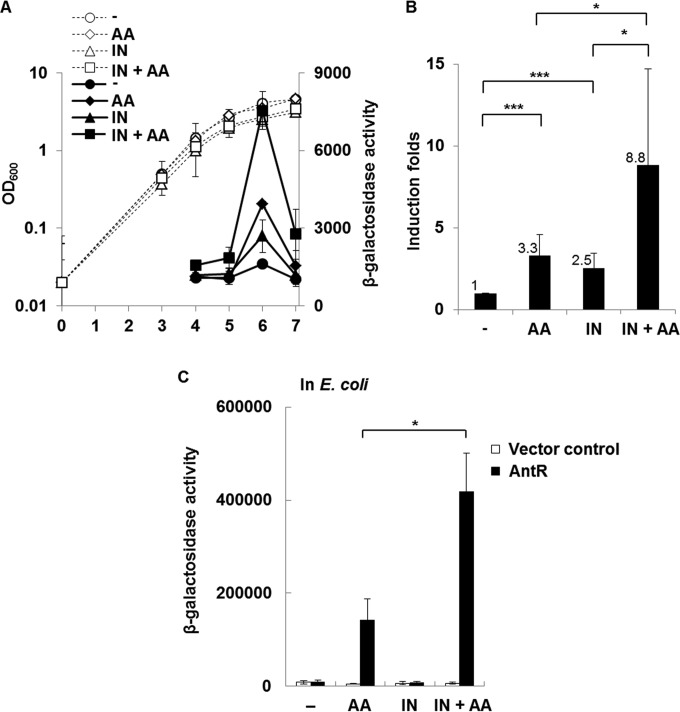
Anthranilate is an activating cofactor of AntR, a transcriptional regulator of the antABC operon encoding the anthranilate dioxygenase complex that functions to degrade anthranilate through the TCA cycle (22, 31). So, anthranilate treatment induces the expression of antABC through direct activation of AntR (22, 31). A recent microarray study showed that indole treatment also increased the transcription of the antABC operon (10). To examine the effects of anthranilate and indole on the expression of the antABC operon, P. aeruginosa cells harboring antAp-lacZ fusion were treated with either anthranilate, indole, or both. The measurement of β-galactosidase activity showed that antA expression was collaboratively augmented by cotreatment of anthranilate and indole in P. aeruginosa (Fig. 6A). This collaborative induction appeared to be significant and synergistic (Fig. 6B). While it is well documented that anthranilate is a cofactor of AntR to induce antA transcription (22, 31), indole was never reported to activate AntR. To investigate whether indole can activate AntR, we carried out E. coli reconstitution analysis using two compatible plasmids, the AntR-expressing plasmid and antAp-lacZ fusion plasmid, as previously described (23, 31). Interestingly, although indole alone did not induce antA expression, it boosted the induction of antA by anthranilate (Fig. 6C). This is consistent with the results of the P. aeruginosa experiment shown in Fig. 6A and B, because indole also synergistically induced antA expression when used with anthranilate. The slight activation of antA by single treatment with indole in P. aeruginosa is likely due to endogenously produced anthranilate.
FIG 6.
Indole activates antABC expression synergistically with anthranilate. (A) AntR activity in P. aeruginosa was measured through growth using the reporter strain (pJL201) with treatment with indole, anthranilate, or both. Solid lines indicate β-galactosidase activity, and dotted lines indicate cell growth (OD600). (B) Induction of AntR activity at stationary phase is presented as fold induction with statistical significance. (C) AntR-expressing plasmid (pJN105A) and pJL201 were cotransformed into E. coli. The transformed E. coli cells were grown to an OD600 of 0.3 and treated with indole or anthranilate for 2 h; β-galactosidase activity was then measured. -, buffer control; IN, 0.4 mM indole; AA, 0.1 mM anthranilate; AA + IN, 0.1 mM anthranilate plus 0.4 mM indole. *, P < 0.05; ***, P < 0.005.
We hypothesized that the extra-enhanced expression of antABC by indole might accelerate the degradation of anthranilate and reduce the local concentration of anthranilate within the biofilm, which might cause the biofilm enhancement by indole treatment. To prove this hypothesis, we measured the anthranilate levels in P. aeruginosa culture supernatants with and without indole treatment. The results showed that the anthranilate level was reduced to half by the indole treatment (Fig. 7), supporting our hypothesis.
FIG 7.
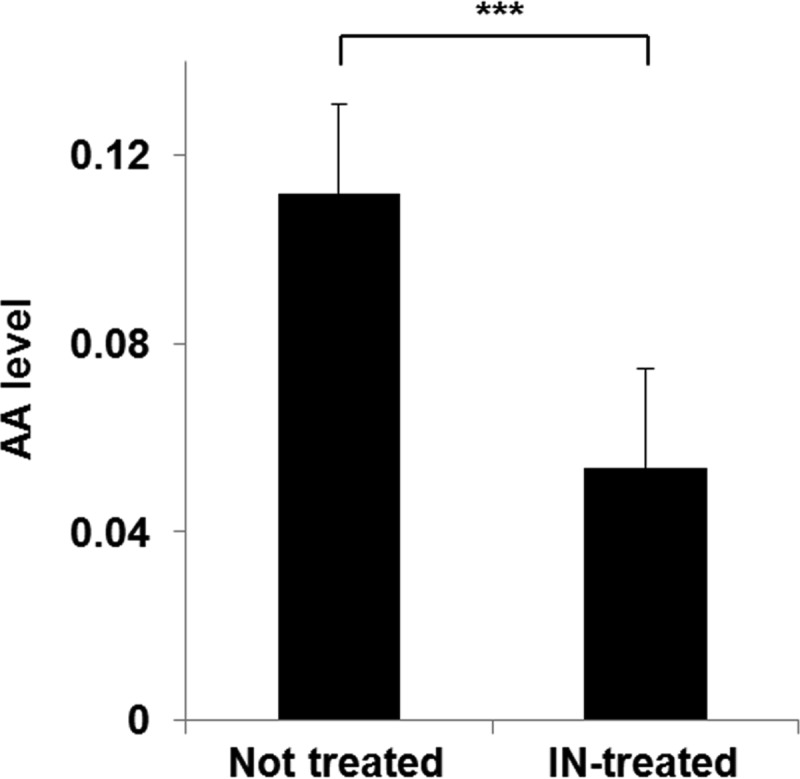
The anthranilate level in a culture supernatant of P. aeruginosa was reduced by indole treatment. P. aeruginosa cells were cultivated to an OD600 of 3 with or without 0.4 mM indole, and the anthranilate level in the culture supernatant was measured by HPLC analysis. ***, P < 0.005.
Anthranilate affects the c-di-GMP level and transcription of EPS operons.
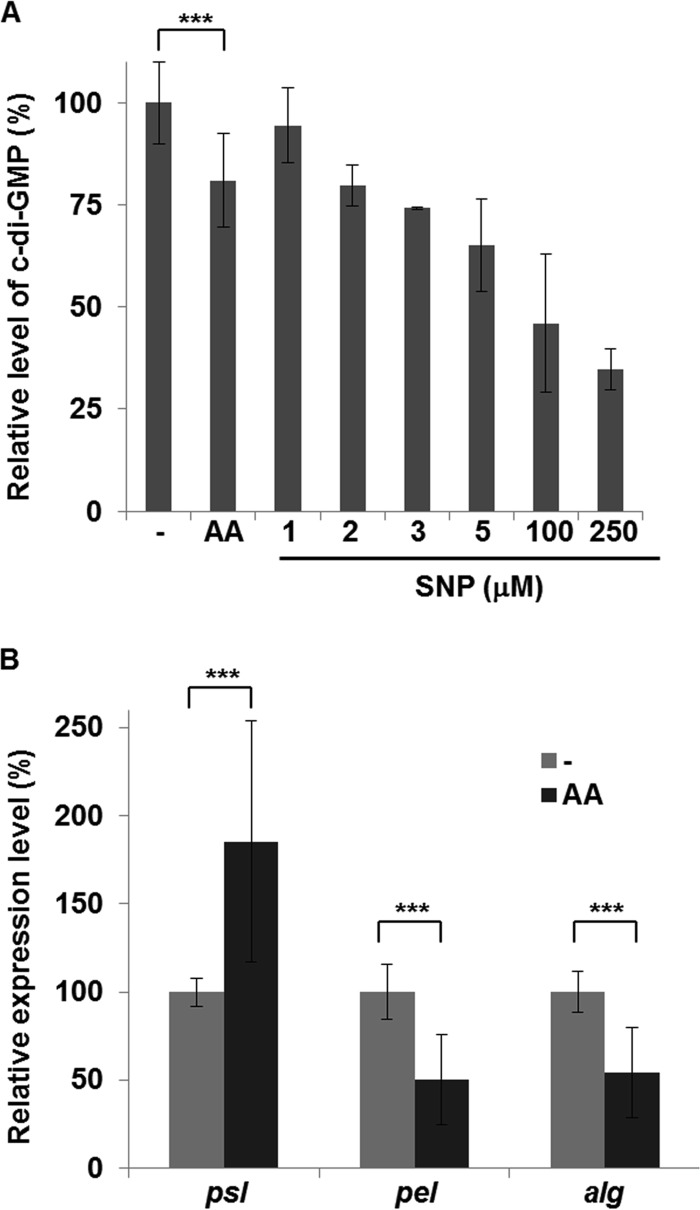
We tried to address how anthranilate deteriorates the biofilm structure. We first tested whether the treatment of anthranilate could change the hydrophobicity of cell surface, but there was no significant change, indicating that it is not the cause (see Fig. S3 in the supplemental material). Next, we investigated the level of an intracellular signaling molecule, c-di-GMP, which plays an important role in controlling biofilm formation in many Gram-negative bacteria (32). We constructed a cdrA-lacZ fusion for gauging c-di-GMP level, since the cdrA gene (PA4625), encoding a large adhesin, has been used as a c-di-GMP-responsive reporter (33). cdrA is highly upregulated in response to increased levels of c-di-GMP and downregulated by decreased levels of c-di-GMP (34). When we measured the β-galactosidase activity, which reflects the intracellular c-di-GMP level, the anthranilate-treated cells showed a level decreased by 20% (Fig. 8A). Sodium nitroprusside (SNP), a nitrogen monoxide (NO)-releasing agent, has been reported to decrease the intracellular c-di-GMP level (35). A previous study using liquid chromatography-tandem mass spectrometry (LC-MS-MS) analysis reported about a 44% reduction of intracellular c-di-GMP by 5 μM SNP treatment (35), and our results showed a 35% reduction by same concentration of SNP (Fig. 8A). When the reporter cells were treated with various concentrations of SNP for comparison, it demonstrated that the 0.1 mM anthranilate treatment had an effect comparable to that of 2 μM SNP treatment (Fig. 8A).
FIG 8.
Anthranilate effects on the levels of c-di-GMP and expression of EPS biosynthetic genes. (A) P. aeruginosa cells harboring pSKcdrA (cdrA-lacZ fusion) were grown without (−) or with 0.1 mM anthranilate for 20 h, and β-galactosidase activity, which reflects the c-di-GMP level, was measured. For comparison, the reporter cells were grown with various concentrations of SNP under the same condition. The c-di-GMP level of nontreated cells was set to 100%, and the relative c-di-GMP levels of treated cells are presented from β-galactosidase activity. (B) P. aeruginosa cells were cultivated with or without 0.1 mM anthranilate, and the transcripts of Psl, Pel, and alginate biosynthetic operons were measured by quantitative real-time PCR analysis. The transcription levels are presented relative to the transcription level of nontreated cells. ***, P < 0.005.
The c-di-GMP level regulates the production of EPSs in P. aeruginosa. In order to find a clue about what happens to the biofilm matrix with anthranilate treatment, we measured the transcription levels of the EPS biosynthetic operons. P. aeruginosa has three major EPSs in the biofilm matrix: Psl, Pel, and alginate (36). Our results showed that the anthranilate treatment increased the transcription of Psl operon by 85% but decreased the transcriptions of Pel and alginate operons by 50% and 54%, respectively (Fig. 8B).
DISCUSSION
In this study, we investigated the effects of anthranilate on P. aeruginosa biofilm formation. Our results demonstrated the following: (i) anthranilate causes the destabilization of biofilm, deteriorating the mushroom structure; (ii) anthranilate affects biofilm formation differently depending on the developmental stage and the presence of shear force; (iii) the effects of anthranilate and indole on biofilm formation do not involve QS regulation; and (iv) indole boosts anthranilate-degrading activity.
Anthranilate facilitated the attachment of cells to the surface in the early stage of biofilm development, but it seemed to deteriorate the mushroom structure in the late stage. Shear force played an important role in this effect: without shear force, anthranilate treatment increased the total amount of biofilm even with long cultivation (Fig. 2), but with shear force, anthranilate treatment caused the biofilm to crumble (Fig. 3B and 4A and B). Apparently, the shear force holds the key to determining the final form of the biofilm structure between the two anthranilate effects: increasing cell attachment to the surface or weakening the mushroom structure. Strong shear force will crumble and wash away the weakened biofilm. Still, the cells falling from the weakened biofilm may be retained in the interstitial space around the biofilm stalks if the shear force is not strong enough to wash the cells off, because anthranilate facilitates surface attachment while weakening the biofilm structure.
Then, how does anthranilate deteriorate and weaken the biofilm structure? Multiple studies have suggested that Psl is important in initial attachment to abiotic and biotic surfaces (32, 37), Pel primarily plays a role after surface attachment (38), and alginate is associated with chronic stages of biofilm-mediated infection (39). Therefore, our results regarding the expression of EPS operons imply that anthranilate treatment may facilitate initial attachment by increasing Psl production but weaken the biofilm structure at late stages by decreasing Pel and alginate production. This is consistent with our observations regarding the effects of anthranilate on biofilm formation. Recently, it was reported that the mucoid strains recovered from chronic pulmonary infections in cystic fibrosis patients express elevated levels of alginate and reduced levels of Psl, and the transcription factor responsible for this inverse regulation of alginate and Psl operons is AmrZ (40). Since AmrZ positively regulates the expression of alginate operon but represses the transcription of Psl operon, it was suggested that AmrZ may mediate transition of P. aeruginosa biofilm infection from colonizing to chronic stages. Interestingly, the effect of anthranilate on the transcription of Psl and alginate operons is opposite to that on AmrZ function.
Among three major EPSs of P. aeruginosa, the production of Pel is positively regulated by c-di-GMP at the transcriptional and allosteric levels in P. aeruginosa. FleQ, a transcriptional repressor, derepresses the transcription of pel operon when intracellular c-di-GMP levels are high (41). c-di-GMP also binds to PelD, an inner membrane protein, and enhances Pel production (36). The production of alginate is positively regulated by c-di-GMP at allosteric levels. Like for PelD, c-di-GMP binding to Alg44, an inner membrane protein, is required for alginate production (36). Since anthranilate treatment decreased both c-di-GMP and the transcription of Pel and alginate operons (Fig. 8A and B), final production of Pel and alginate should be decreased by anthranilate treatment at both transcriptional and allosteric levels.
Anthranilate is naturally produced by P. aeruginosa. It is produced at very low levels in P. aeruginosa culture medium during exponential growth but rapidly accumulates at late stationary phase up to 0.05 mM (23). The accumulation of anthranilate activates AntR, which activates the expression of the antABC operon to degrade anthranilate (23, 31). Our results imply that anthranilate should be degraded for P. aeruginosa to form mature biofilms. Actually, a recent study showed that P. aeruginosa cells in biofilms enhanced anthranilate-degrading activity (15). Another study showed that tryptophan had an inhibitory effect on biofilm formation by P. aeruginosa (11). This also supports our results in that P. aeruginosa degrades tryptophan to anthranilate. The anthranilate concentration goes up with supplementation of tryptophan in culture medium, which can deteriorate biofilm. A study actually showed that the anthranilate concentration increased with tryptophan supplementation (42).
Indole has recently received much attention due to its diverse biological roles in bacterial physiology and its potential to modulate biofilm formation (14). In P. aeruginosa, indole enhances biofilm formation, but it has the opposite effect in E. coli, repressing biofilm formation (13). Since there are generally many indole-producing bacteria in environmental habitats where P. aeruginosa lives (e.g., in the gut, where E. coli is abundant), occasional input of tryptophan-rich nutrients may increase both indole and anthranilate around P. aeruginosa. Our study suggests that anthranilate produced by P. aeruginosa can antagonize the effect of indole on biofilm. So, the effect of indole on P. aeruginosa may be finely tuned in the balance between endogenously produced anthranilate and exogenous indole.
In addition, our results suggest that the biofilm-enhancing effect of indole might be exerted through the reduction of anthranilate by indole treatment, as shown in Fig. 7. The extrainduction of antABC by indole may accelerate the degradation of anthranilate, which can remove the biofilm-deteriorating effect of anthranilate by reducing the local concentration of anthranilate within the biofilm. This may result in the enhancement of biofilm formation. This postulation is also supported by a study showing that P. aeruginosa cells in biofilm have enhanced anthranilate-degrading activity (15).
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number 2013R1A1A2012220). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number 2010-0006622).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03551-14.
REFERENCES
- 1.Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Parsek MR, Greenberg EP. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Ymele-Leki P, Ross JM. 2007. Erosion from Staphylococcus aureus biofilms grown under physiologically relevant fluid shear forces yields bacterial cells with reduced avidity to collagen. Appl Environ Microbiol 73:1834–1841. doi: 10.1128/AEM.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Hancock RE, Speert DP. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat 3:247–255. doi: 10.1054/drup.2000.0152. [DOI] [PubMed] [Google Scholar]
- 6.Willcox MD, Zhu H, Conibear TC, Hume EB, Givskov M, Kjelleberg S, Rice SA. 2008. Role of quorum sensing by Pseudomonas aeruginosa in microbial keratitis and cystic fibrosis. Microbiology 154:2184–2194. doi: 10.1099/mic.0.2008/019281-0. [DOI] [PubMed] [Google Scholar]
- 7.Huq A, Whitehouse CA, Grim CJ, Alam M, Colwell RR. 2008. Biofilms in water, its role and impact in human disease transmission. Curr Opin Biotechnol 19:244–247. doi: 10.1016/j.copbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Kumar CG, Anand SK. 1998. Significance of microbial biofilms in food industry: a review. Int J Food Microbiol 42:9–27. doi: 10.1016/S0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 9.Kurnasov O, Jablonski L, Polanuyer B, Dorrestein P, Begley T, Osterman A. 2003. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiol Lett 227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Attila C, Cirillo SL, Cirillo JD, Wood TK. 2009. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol 2:75–90. doi: 10.1111/j.1751-7915.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandenburg KS, Rodriguez KJ, McAnulty JF, Murphy CJ, Abbott NL, Schurr MJ, Czuprynski CJ. 2013. Tryptophan inhibits biofilm formation by Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:1921–1925. doi: 10.1128/AAC.00007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimazaki J, Furukawa S, Ogihara H, Morinaga Y. 2012. l-Tryptophan prevents Escherichia coli biofilm formation and triggers biofilm degradation. Biochem Biophys Res Commun 419:715–718. doi: 10.1016/j.bbrc.2012.02.085. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Jayaraman A, Wood TK. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Lee J. 2010. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. [DOI] [PubMed] [Google Scholar]
- 15.Costaglioli P, Barthe C, Claverol S, Brozel VS, Perrot M, Crouzet M, Bonneu M, Garbay B, Vilain S. 2012. Evidence for the involvement of the anthranilate degradation pathway in Pseudomonas aeruginosa biofilm formation. Microbiologyopen 1:326–339. doi: 10.1002/mbo3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 17.Bassler BL. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109:421–424. doi: 10.1016/S0092-8674(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 18.Fuqua C, Greenberg EP. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol 3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 19.Park SJ, Liu HB, Park S, Lee JH. 2013. Modulation of QscR, a quorum sensing receptor of Pseudomonas aeruginosa, by truncation of a signal binding domain. Res Microbiol 164:375–381. doi: 10.1016/j.resmic.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Ha C, Park SJ, Im SJ, Lee JH. 2012. Interspecies signaling through QscR, a quorum receptor of Pseudomonas aeruginosa. Mol Cells 33:53–59. doi: 10.1007/s10059-012-2208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Camara M, Williams P. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Oglesby AG, Farrow JM III, Lee JH, Tomaras AP, Greenberg EP, Pesci EC, Vasil ML. 2008. The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J Biol Chem 283:15558–15567. doi: 10.1074/jbc.M707840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi Y, Park HY, Park SJ, Kim SK, Ha C, Im SJ, Lee JH. 2011. Growth phase-differential quorum sensing regulation of anthranilate metabolism in Pseudomonas aeruginosa. Mol Cells 32:57–65. doi: 10.1007/s10059-011-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Lequette Y, Greenberg EP. 2006. Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Mol Microbiol 59:602–609. doi: 10.1111/j.1365-2958.2005.04960.x. [DOI] [PubMed] [Google Scholar]
- 25.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee KJ, Kim JA, Hwang W, Park SJ, Lee KH. 2013. Role of capsular polysaccharide (CPS) in biofilm formation and regulation of CPS production by quorum-sensing in Vibrio vulnificus. Mol Microbiol 90:841–857. doi: 10.1111/mmi.12401. [DOI] [PubMed] [Google Scholar]
- 27.Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. 1998. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol 64:4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balebona MC, Morinigo MA, Borrego JJ. 2001. Hydrophobicity and adhesion to fish cells and mucus of Vibrio strains isolated from infected fish. Int Microbiol 4:21–26. doi: 10.1007/s101230100004. [DOI] [PubMed] [Google Scholar]
- 29.Farinha MA, Kropinski AM. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol 172:3496–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herth W, Schnepf E. 1980. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 105:129–133. doi: 10.1007/BF01279855. [DOI] [Google Scholar]
- 31.Kim SK, Im SJ, Yeom DH, Lee JH. 2012. AntR-mediated bidirectional activation of antA and antR, anthranilate degradative genes in Pseudomonas aeruginosa. Gene 505:146–152. doi: 10.1016/j.gene.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Wei Q, Ma LZ. 2013. Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int J Mol Sci 14:20983–21005. doi: 10.3390/ijms141020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. 2012. Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa. Appl Environ Microbiol 78:5060–5069. doi: 10.1128/AEM.00414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borlee BR, Goldman AD, Murakami K, Samudrala R, Wozniak DJ, Parsek MR. 2010. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol Microbiol 75:827–842. doi: 10.1111/j.1365-2958.2009.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. 2009. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol 191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin MJ, Nivens DE, Weadge JT, Howell PL. 2011. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol 2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. 2006. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188:8213–8221. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasseur P, Vallet-Gely I, Soscia C, Genin S, Filloux A. 2005. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 151:985–997. doi: 10.1099/mic.0.27410-0. [DOI] [PubMed] [Google Scholar]
- 39.Schurr MJ. 2013. Which bacterial biofilm exopolysaccharide is preferred, Psl or alginate? J Bacteriol 195:1623–1626. doi: 10.1128/JB.00173-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones CJ, Ryder CR, Mann EE, Wozniak DJ. 2013. AmrZ modulates Pseudomonas aeruginosa biofilm architecture by directly repressing transcription of the psl operon. J Bacteriol 195:1637–1644. doi: 10.1128/JB.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrow JM III, Pesci EC. 2007. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J Bacteriol 189:3425–3433. doi: 10.1128/JB.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson JP, Pesci EC, Iglewski BH. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179:5756–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiteley M, Lee KM, Greenberg EP. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 46.Walters MC III, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman JR, Fuqua C. 1999. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197–203. doi: 10.1016/S0378-1119(98)00601-5. [DOI] [PubMed] [Google Scholar]
- 48.Chugani SA, Whiteley M, Lee KM, D'Argenio D, Manoil C, Greenberg EP. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.