Abstract
CodY is a transcriptional regulator conserved in the low-GC group of Gram-positive bacteria. In this work, we demonstrated the presence in Streptococcus thermophilus ST2017 of a functional member of the CodY family of global regulatory proteins, S. thermophilus CodY (CodYSt). The CodYSt regulon was identified by transcriptome analysis; it consisted predominantly of genes involved in amino acid metabolism but also included genes involved in several other cellular processes, including carbon metabolism, nutrient transport, and stress response. It was revealed that CodYSt repressed the transformation of the central metabolic pathway to amino acid metabolism and improved lactose utilization. Furthermore, the glutamate dehydrogenase gene (gdhA), repressed by CodYSt, was suggested to coordinate the interconversion between carbon metabolism and amino acid metabolism and to play an important role on the optimal growth of S. thermophilus ST2017 in milk. A conserved CodYSt box [AA(T/A)(A/T)TTCTGA(A/C)AATT] was indeed required for in vitro binding of CodYSt to the target regions of DNA. These results provided evidence for the function of CodYSt, by which this strain coordinately regulates its various metabolic pathways so as to adapt to the milk environment.
INTRODUCTION
Streptococcus thermophilus is a major dairy starter traditionally used in combination with Lactobacillus delbrueckii subsp. bulgaricus or Lactobacillus helveticus for the manufacture of yogurt and cheeses (1, 2). S. thermophilus is a GRAS (“generally recognized as safe”) species, and >1021 live cells are ingested annually. For long-term growth in milk, S. thermophilus forms a unique carbon metabolic system to utilize lactose efficiently and to produce energy for its growth (3, 4). At the same time, S. thermophilus is a fastidious microorganism, requiring exogenous sources of amino acids or peptides for optimal growth. Since milk is poor in these low-molecular-weight compounds, the growth of this organism depends largely on a proteolytic system to hydrolyze the casein in milk (5). Accordingly, S. thermophilus has evolved a well-developed nitrogen pathway for de novo biosynthesis of amino acids and has acquired cell envelope proteinase to enable it to adapt to the milk environment (6–8). Finally, the carbon and amino acid metabolisms and their interconversion facilitate the growth of S. thermophilus. Fortunately, all bacteria have evolved additional layers of control to coordinate the use of nutrients by using global regulators (9, 10). CodY, a protein highly conserved in the low-GC group of Gram-positive bacteria, belongs to a unique family of regulatory proteins; it helps bacteria to adapt to conditions of poor nutrient availability and also controls the catabolic pathways, environmental response, and virulence of strains (11–16). The effect of CodY is stimulated by its interaction with either of two ligands, GTP or branched-chain amino acids (BCAA). However, the mechanism of CodY regulation is species dependent (17–20).
Analysis of the annotated genome sequences identified a conserved gene designated codYSt (S. thermophilus codY). Here we focused on the properties and metabolic regulation of the global regulator CodYSt in S. thermophilus ST2017. The carbon and amino acid metabolism regulatory networks were elucidated by transcriptome analysis, and the regulatory mechanism of CodYSt was investigated by electrophoretic mobility shift assays (EMSA) and isothermal titration calorimetry (ITC). Furthermore, we determined that CodYSt coordinated the interconversion of the carbon and amino acid metabolisms through glutamate dehydrogenase to enable adaptation to the milk environment.
MATERIALS AND METHODS
Bacterial strains, culture, and transformation conditions.
The S. thermophilus strains and plasmids used in this study are listed in Table 1. S. thermophilus strains were routinely cultured at 37°C in M17 broth (Oxoid) supplemented with 1.0% lactose or sterile milk (Yili, China). Where appropriate, S. thermophilus cultures were supplemented with erythromycin at a concentration of 2.5 μg/ml. The complete chemically defined medium (CDM) was adapted from that described by Letort and Juillard (21). Escherichia coli strains were grown in Luria-Bertani (LB) broth with shaking at 37°C. Transformants were selected by plating onto LB agar containing erythromycin at 250 μg/ml or ampicillin at 100 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli DH5α | Host strain for general cloning | 36 |
| E. coli BL21 | Expression host | Novagen |
| S. thermophilus ST2017 | Wild-type strain isolated from fermented dairy products | Preserved in the laboratory |
| S. thermophilus ST2017ΔcodY | ΔcodY mutant strain | This study |
| S. thermophilus ST2017ΔcodY-codYexp | codYSt overexpression strain | This study |
| S. thermophilus ST2017ΔgdhA | gdhA inactive in S. thermophilus ST2017 | This study |
| S. thermophilus ST2017ΔcodYΔgdhA | gdhA inactive in S. thermophilus ST2017ΔcodY | This study |
| Plasmids | ||
| pG+host9 | Ermr; pWV01 replicon (temp sensitive) | 22 |
| pG+host-Up-Down | Ermr; codYSt knockout vector | This study |
| pG+host-gdhAin | Ermr; vector with inactive gdhA | This study |
| pSEC | Cmr; nisin-inducible expression vector | 37 |
| pSEC-codY | codYSt under the control of the nox promoter | This study |
Construction of the codYSt mutant in S. thermophilus ST2017.
Chromosomal deletion mutants were constructed in S. thermophilus by plasmid integration and two-crossover homologous recombination using the method described by Biswas et al. (22). To construct the integration vector, the 1,135-bp Up fragment was PCR amplified from the chromosomal DNA with primers UpF (5′-ATGGTCGACTATCCTCTTTGGACAGC) and UpR (5′-CTTCCAAGCTTTCTACTGAGCGTTG), and the 1,214-bp Down fragment was PCR amplified with primers DownF (5′-TCGTAAGCTTGGTATGAAAGGCAC) and DownR (5′-GGGAATTCTAGCACCTTCTGGCAAC). The Up fragment and Down fragment were digested with SalI and HindIII and with HindIII and EcoRI, respectively, and were subsequently inserted into plasmid pBluescript II SK(+). Then the SalI/EcoR I fragment was ligated into the temperature-sensitive vector pG+host9, resulting in the knockout plasmid pG+host-Up-Down.
Plasmid pG+host-Up-Down was introduced into S. thermophilus ST2017 by electroporation (23). The transformants were first selected at 28°C (permissive temperature) with 2.5 μg/ml of erythromycin. To obtain single-crossover integrants, appropriate diluted cultures were plated onto LM17 medium at 42°C (nonpermissive temperature) with the selective pressure of erythromycin. Cultures were plated onto the same medium at the same temperature without erythromycin in order to count the rate of integration. One clone of the erythromycin-resistant integrant was propagated at 28°C without erythromycin to stimulate the second-crossover integration. The cultures were transferred several times, and culture was extended over 50 generations. Dilutions of the cultures were plated at 42°C without erythromycin to select the second-crossover integrants eliminating the excised vector. As a control, the same diluted cultures were plated with erythromycin to calculate the percentage of plasmid excision. The clones in the plates without erythromycin were plated in the presence or absence of erythromycin to select the Erms clones (two single-crossover integrants). Specialized primers were used for PCR amplification to verify the integrants.
Construction of a codYSt overexpression strain in S. thermophilus ST2017ΔcodY.
To construct the codYSt overexpression vector, the engineered promoter of the NADH oxidase gene (24) was PCR amplified using primers NoxF (5′-GTTAGATCTAGAAACTATGTGGCAAGC) and NoxR (5′-ACTAATAGGTCTCCTTTAAATG), and the codYSt gene was PCR amplified using primers codYF (5′-CATTTAAAGGAGACCTATTAGTATGGCAAATTTGCTTGATA) and codYR (5′-ATGCTCGAGTTATTCGTATTCTTTCAA). The two fragments were fused by overlapping PCR and were then inserted into plasmid pSEC, yielding the expression vector pSEC-codY. Finally, the vector was introduced into S. thermophilus ST2017ΔcodY to construct the overexpression strain S. thermophilus ΔcodY-codYexp.
Construction of the gdhA mutant in S. thermophilus ST2017 and S. thermophilus ST2017ΔcodY.
To construct the integration vector, the 735-bp fragment in the middle of the gdhA gene was PCR amplified from the genomic DNA by using primers gdhAin F (5′-AGTGAATTCTCAAAACAGCCACGAG) and gdhAin R (5′-GAGTCGACACATGTCACAGCTTTAGC). The fragment was digested with SalI and EcoRI and was inserted into pG+host9, resulting in the inactive plasmid pG+host-gdhAin. Plasmid pG+host-gdhAin was introduced into S. thermophilus ST2017 and S. thermophilus ST2017ΔcodY. The transformants were first selected at 28°C (permissive temperature) with 2.5 μg/ml of erythromycin. To produce the gdhA mutant, appropriate diluted cultures were plated onto LM17 medium at 42°C (nonpermissive temperature) with the selective pressure of erythromycin. Specialized primers were used for PCR amplification to verify the integrants.
Illumina-based RNA sequencing and transcriptome analysis.
Overnight cultures of the S. thermophilus ST2017 and ST2017ΔcodY strains were diluted in fresh LM17 medium (1:50) and were grown at 42°C. Cells were harvested by centrifugation (10,000 × g, 1 min, 4°C) until the optical density at 600 nm (OD600) reached 0.4. To isolate the RNA, cell pellets were first resuspended in Tris-EDTA (TE) buffer containing 5 mg/ml lysozyme and then incubated for 10 min at 30°C. Total RNA was isolated according to the manufacturer's instructions (Total RNA Midi kit; Omega, USA). To remove rRNA, a Ribo-Zero rRNA removal kit (Epicentre, Madison, WI, USA) was used according to the manufacturer's instructions. The mRNA was fragmented ultrasonically, and fragmented mRNAs were converted into a transcriptome-sequencing (RNA-seq) library by using the mRNA-seq library construction kit (Illumina Inc., San Diego, CA, USA). RNA sequencing was performed on an Illumina HiSeq 2000 platform (Illumina, San Diego, CA, USA) using a standard paired-end protocol. Clean reads were first obtained from the raw reads by filtering dirty reads, which contained adapters, low-quality bases, or unknown bases, and then imported into the DEGseq package. The MARS model in the DEGseq package was used to calculate the expression levels of each gene in S. thermophilus ST2017 and S. thermophilus ST2017ΔcodY (25). The false discovery rate (FDR) was used to determine the threshold of the P value. We identified differentially expressed genes as those that were significant at an FDR of <0.001, a P value of <0.05, and a ≥2-fold normalized change.
qPCR.
Transcriptome-sequencing results were verified via quantitative PCR (qPCR) analysis. Total RNA was extracted using the Bacterial RNA kit (Omega, USA). Then 1 μg of the total-RNA sample was used to synthesize cDNA according to the kit instructions (PrimeScript RT-PCR kit; TaKaRa, Japan). qPCR was carried out by using a TaKaRa SYBR Premix Ex Taq kit in the Roche LightCycler 480 system according to the manufacturer's instructions. After qPCR, relative mRNA expression was normalized to the constitutive expression of the 16S rRNA housekeeping gene and was calculated by the comparative threshold cycle (CT) method (2−ΔΔCT method). For the 26 selected genes of interest, qPCR was performed at least in duplicate on RNAs purified from 2 independently grown cultures.
Purification of the CodYSt protein and gel mobility shift assay.
The codYSt gene was PCR amplified with primers CodYF (5′-TTGGATCCATGGCAAATTTGCTTGATAA) and CodYR (5′-ATGCTCGAGTTATTCGTATTCTTTCAA). The PCR product was cloned into the BamHI/XhoI sites of pETPPA, a vector in which a gene encoding an N-terminally six-histidine-tagged, PreScission protease (PPA) cleavage site-linked version of S. thermophilus CodYSt is under the control of the T7 promoter. Escherichia coli strain BL21(DE3) harboring pETPPA-CodYSt was grown in 500 ml of LB broth containing ampicillin until the OD600 reached 0.4. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.4 mM, and the temperature was lowered to 16°C for the induction of CodYSt overexpression. After 24 h of induction, the cells were pelleted by centrifugation at 8,000 × g and 4°C. One hundred milliliters of binding buffer (20 mM Tris-HCl, 500 mM NaCl, 20 mM imidazole [pH 8.0]) was added to the pellets. Cells were disrupted by sonication, and cellular debris was removed by centrifugation at 10,000 × g and 4°C. For the purification of CodYSt, a 1-ml HisTrap FF column (GE Healthcare) was prepared according to the manufacturer's instructions. Following application of the cleared bacterial lysate and extensive washing, purified CodYSt was eluted by using PreScission protease digestion overnight. Protein samples were stored at 4°C until required.
The gel mobility shift assay was performed essentially as described previously (26), with slight modifications. Briefly, the 253-bp upstream region of the gdhA gene (ortholog of STND_0425) was PCR amplified with primers PgdhAF (5′-TTTCAAACAAATTCAGCTTCAAG) and PgdhAR (5′-TGTTGTCATATCAATGTTTCCTT). One-hundred-nanogram DNA probes were added to 20-μl reaction mixtures containing the binding buffer and purified CodYSt protein at a concentration of 0, 25, 50, 75, 100, 150, 200, 300, or 500 ng per reaction. The binding buffer was composed of 10 mM Tris-HCl (pH 7.5), 5% (vol/vol) glycerol, 100 mM KCl, and 1 mM dithiothreitol. To confirm specific protein-DNA interactions, replicates were carried out with 10 μg sheared salmon testis DNA. Immediately after incubation for 30 min at room temperature, samples were loaded onto a 5% nondenaturing polyacrylamide gel. Gel electrophoresis was performed at 100 V for 60 min in 0.5× Tris-acetate-EDTA (TAE) buffer. The binding complex was visualized by ethidium bromide staining.
Isothermal titration calorimetry.
To determine the Kd (dissociation constant) value and the thermodynamic parameters of the interaction of CodYSt with the CodYSt box (ATTGACAATTTTCTGATAATTCTGTAACCT [the CodYSt box within the livJ promoter region is underlined]) in S. thermophilus ST2017, the protein was titrated with the CodYSt box in a MicroCal iTC200 system (GE Healthcare) equipped with a 300-μl sample cell and a 40-μl syringe. The purified protein was thoroughly dialyzed at 4°C against Tris-HCl buffer (100 mM Tris-HCl, 100 mM KCl [pH 7.5]). The protein solution (50 μM, relating to the CodY dimer) was titrated at 30°C with a double-stranded DNA (dsDNA) solution containing 5 μM CodYSt box or CodYSt box mutant (ATTGACCATTTTATGCTCATTCTGTAACCT [the CodYSt box mutant is underlined]) (13 injections of 2 μl after the first injection of 0.5 μl), using a time interval of 120 s between injections. The heat effects from a blank experiment (injection of CodYSt into dialysis buffer) were subtracted before the titration curves were fit to a nonlinear least-squares function. From these curve fits, Kd, the change in binding enthalpy (ΔH), and the binding stoichiometry were determined. The change in Gibbs free energy (ΔG) and the change in entropy (ΔS) were calculated using the equations ΔG = RT ln Kd and ΔG = ΔH − TΔS, where R is the universal molar gas constant and T is the temperature (in kelvins).
Data analysis.
The data were analyzed for statistical significance by using the paired Student t test. P values of <0.05 indicated statistical significance.
RNA-seq data accession number.
All RNA-seq data from both conditions have been deposited in the Sequence Read Archive under accession number SRP051756.
RESULTS
Demonstration of a functional CodYSt protein in S. thermophilus ST2017.
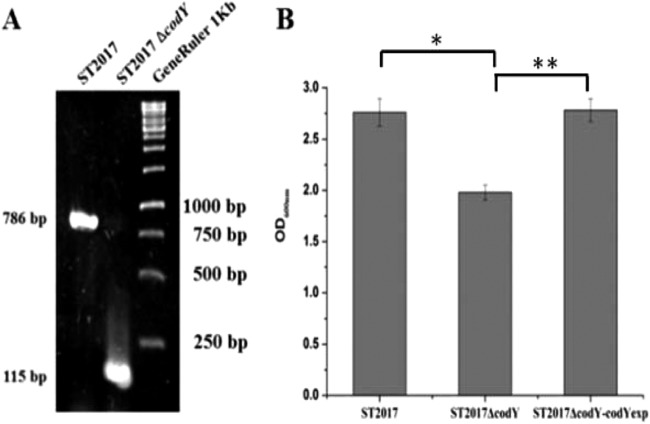
Analysis of the annotated S. thermophilus genome sequences identified a conserved gene, designated codYSt. Comparison of CodYSt to the CodY protein sequences of Streptococcus pneumoniae (CodYSp) and Bacillus subtilis (CodYBs) revealed that at the amino acid level, CodYSt has 67% sequence similarity to CodYSp and 53% sequence similarity to CodYBs. In particular, the C terminus contained a conserved helix-turn-helix motif, which mediated the binding of CodY protein to DNA. To investigate the functions of CodY in S. thermophilus ST2017, the pG+host system was used to knock out the codYSt gene in this strain. As a result, the putative codYSt mutant generated (ST2017ΔcodY) showed a 115-bp PCR fragment with specific primers for the integrated codYSt gene, in contrast to a 786-bp DNA fragment from wild-type ST2017, suggesting that a 671-bp internal region in the codYSt gene was deleted in the ST2017ΔcodY mutant (Fig. 1A). The sequencing results further confirmed the deletion of the codYSt gene in the S. thermophilus ST2017 genome.
FIG 1.
Functional CodY protein in S. thermophilus ST2017. (A) PCR validation of the codY mutant. (B) Final biomass levels of S. thermophilus ST2017, ST2017ΔcodY, and ST2017ΔcodY-codYexp. Asterisks indicate statistically significant differences (*, P < 0.001; **, P < 0.01) between the values obtained for the ΔcodY mutant strain and those obtained for wild-type (ST2017) and complemented (ΔcodY-codYexp) cells.
To evaluate the effect of the codYSt gene on growth, the growth curves of S. thermophilus strains ST2017, ST2017ΔcodY, and ST2017ΔcodY-codYexp in LM17 medium were compared. Figure 1B shows that knockout of the codYSt gene resulted in a decrease in the growth of S. thermophilus ST2017, whereas normal growth was restored by overexpression of the codYSt gene in S. thermophilus ST2017ΔcodY. The results revealed that CodYSt is a functional protein in S. thermophilus ST2017.
Function of the CodYSt regulon.
It has been reported that CodY in low-G+C Gram-positive bacteria regulates genes involved in a wide variety of metabolic pathways and cellular processes. To identify the genes of the CodYSt regulon in S. thermophilus ST2017, we compared the transcriptional profile of S. thermophilus ST2017 with that of its mutant ST2017ΔcodY by transcriptome analysis. The genes that showed a 2-fold or greater change in the transcript level with a P value of <0.05 are listed in Table 2. The transcriptome analysis showed that CodYSt functions mainly as a transcriptional repressor, since 59 of the 86 differentially expressed genes were found to be upregulated in the codYSt mutant, whereas the rest were activated by CodYSt. These 86 regulated genes were assigned to different groups based on function, as follows. (i) The first group comprises amino acid biosynthesis and transport genes. The genes involved in the biosynthesis of BCAA, tryptophan, cysteine, aspartic acid, serine, and threonine were repressed. The guanosine hydratase gene (hutU), which acts during the histidine degradation process, was the sole gene activated by CodYSt in amino acid metabolism. (ii) The second group comprises genes involved in the Embden-Meyerhof-Parnas (EMP) pathway and the Leloir pathway, including the glyceraldehyde-3-phosphate dehydrogenase and phosphate mutase genes. (iii) In the third group, the glutamate dehydrogenase gene (gdhA) was significantly derepressed in the codYSt mutant. (iv) The fourth group consists of a number of genes, including orthologs of STND_0202 and -0203, orthologs of STND_1235, STND_1233, and STND_1232, and an ortholog of STND_0733, that encode ABC transporters or ion channels and were derepressed by CodYSt. (v) The fifth group comprises genes that respond to environmental stresses. The DnaK and GroESL chaperone operons were significantly upregulated in the codYSt mutant. The osmotic-stress-related ortholog of STND_0113, the ortholog of STND_0135, and the oxidative-stress-related ortholog of STND_1346 were downregulated in the codYSt mutant. (vi) The sixth group included the two-component system TCS01 (orthologs of STND_0306 and -0307), involved in environmental stress response. (vii) In the seventh group, the transcription of the hypoxanthine guanine de novo IMP synthesis operon was activated by CodYSt, while the transcription of the uracil synthesis operon was repressed. (viii) The eighth group comprises genes encoding hypothetical proteins. In conclusion, the global regulator CodYSt was involved in the regulation of various metabolic processes, especially in amino acid metabolism. At the same time, CodYSt also regulated genes responding to environmental stresses. The reliability of the RNA sequence data was also tested by checking the consistency of expression levels within each cluster and performing additional measurements by qPCR (see Fig. S1 in the supplemental material).
TABLE 2.
Functions and changes in expression of the genes regulated by CodYSt in S. thermophilus ST2017
| Locus (gene) | Assignment | ST2017/ST2017ΔcodY log2 (normalized fold change) | Presence of CodYSt box |
|---|---|---|---|
| leuA | 2-Isopropylmalate synthase | −2.44 | |
| leuB | 3-Isopropylmalate dehydrogenase | −2.32 | |
| leuC | 3-Isopropylmalate dehydratase, large subunit | −3.08 | |
| leuD | 3-Isopropylmalate dehydratase, small subunit | −3.14 | |
| ilvA | Threonine dehydratase biosynthetic | −1.58 | + |
| ilvB | Acetolactate synthase, catalytic subunit | −2.26 | + |
| ilvH | Acetolactate synthase, small subunit | −2.65 | |
| ilvC | Ketol-acid reductoisomerase | −2.61 | |
| livJ | Branched-chain amino acid ABC transporter substrate binding protein | −3.34 | + |
| livH | Branched-chain amino acid ABC transporter permease protein | −3.87 | |
| livM | ABC-type transport system, permease component | −3.23 | |
| livG | ABC-type branched-chain amino acid transport, ATPase component | −2.54 | |
| livF | ABC-type branched-chain amino acid transport, ATPase component | −2.51 | |
| bcaT | Branched-chain amino acid aminotransferase | −2.06 | + |
| trpA | Tryptophan synthase, alpha chain | −2.79 | |
| trpB | Tryptophan synthase, beta chain | −1.13 | |
| trpF | Anthranilate isomerase | −1.81 | |
| trpC | Indole-3-glycerol phosphate synthase | −1.62 | |
| trpD | Anthranilate phosphoribosyltransferase | −2.53 | |
| trpG | Anthranilate synthase glutamine amidotransferase, component II | −1.38 | |
| trpE | Anthranilate synthase, component I | −1.25 | + |
| argH | Argininosuccinate lyase | −1.93 | |
| argG | Argininosuccinate synthase | −1.58 | |
| cysM2 | Cysteine synthase | −2.80 | |
| metB2 | Cystathionine gamma lyase | −2.66 | |
| cysE2 | Serine acetyltransferase, putative | −3.36 | |
| lysC | Aspartokinase | −2.92 | + |
| thrB | Homoserine kinase | −1.55 | + |
| thrC | Threonine synthase | −1.75 | + |
| hutU | Urocanate hydratase | 2.77 | |
| gdhA | Glutamate dehydrogenase GdhA | −1.74 | + |
| Ortholog of STND_0811 | Chorismate mutase | −2.88 | + |
| purC | Phosphoribosylaminoimidazole-succinocarboxamide synthase | 1.91 | |
| purL | Phosphoribosylformylglycinamidine synthase | 2.00 | |
| purF | Amidophosphoribosyltransferase | 1.71 | |
| purM | Phosphoribosylformylglycinamidine cyclo-ligase | 1.57 | |
| purN | Phosphoribosylglycinamide (GAR) formyltransferase | 2.2 | |
| purH | Phosphoribosylaminoimidazolecarboxamide formyltransferase | 1.61 | |
| purD | Phosphoribosylamine-glycine ligase | 1.25 | |
| purE | Phosphoribosylaminoimidazole carboxylase carboxyltransferase | 1.74 | |
| purK | Phosphoribosylaminoimidazole carboxylase II | 1.65 | |
| pyrK | Dihydroorotate dehydrogenase, electron transfer subunit | −2.66 | |
| pyrDb | Dihydroorotate dehydrogenase B | −1.53 | |
| pyrF | Orotidine 5′-phosphate decarboxylase | −2.77 | |
| pyrE | Orotate phosphoribosyltransferase PyrE | −1.22 | |
| Ortholog of STND_0202 | ABC transporter permease protein | −1.94 | |
| Ortholog of STND_0203 | ABC-type transport system, ATPase component | −2.17 | |
| Ortholog of STND_0733 | Mn2+ and Fe2+ transporter of the NRAMP family | −1.07 | + |
| Ortholog of STND_1235 | Predicted membrane protein | −2.33 | + |
| Ortholog of STND_1233 | Predicted membrane protein | −2.16 | |
| Ortholog of STND_1232 | ATPase component of ABC transporter | −1.24 | |
| hrcA | Heat-inducible transcription repressor HrcA | −1.54 | |
| grpE | Putative Hsp70 cofactor GrpE protein | −1.06 | |
| dnaK | Hsp70-like protein | −1.13 | |
| Ortholog of STND_0135 | Mechanosensitive ion channel (MscS) | 1.53 | |
| groES | 10-kDa chaperonin | −1.10 | |
| Ortholog of STND_0307 | Sensor protein | −1.73 | |
| Ortholog of STND_0658 | CRISPR-associated endonuclease, Csn1 family | −3.49 | |
| Ortholog of STND_0862 | Transcription regulator, GntR family | 1.40 | |
| Ortholog of STND_1346 | Glutamate–cysteine ligase | 1.54 | |
| Ortholog of STND_1572 | Universal stress protein UspA | 2.22 | |
| Ortholog of STND_1784 | Transcriptional regulator | 2.30 | |
| Ortholog of STND_1785 | Permease | 1.20 | |
| Ortholog of STND_0113 | Enzyme of poly-gamma-glutamate biosynthesis | 2.15 | |
| Ortholog of STND_0114 | Cytochrome c oxidase, monoheme subunit | 2.65 | |
| Ortholog of STND_0115 | Glycerol-3-phosphate dehydrogenase NAD(P)+ | 1.94 | |
| Ortholog of STND_0182 | Putative uncharacterized protein | −3.01 | |
| Ortholog of STND_0394 | Hypothetical protein | 1.50 | |
| Ortholog of STND_0393 | Hypothetical protein | 2.31 | |
| Ortholog of STND_0392 | Hypothetical protein | 1.12 | |
| Ortholog of STND_0611 | Methenyltetrahydrofolate cyclohydrolase | −1.47 | + |
| Ortholog of STND_0612 | Predicted sugar kinase | −1.80 | |
| Ortholog of STND_0663 | Hypothetical protein | 1.16 | |
| Ortholog of STND_0664 | Hypothetical protein | 1.96 | |
| Ortholog of STND_0782 | Formate–tetrahydrofolate ligase 2 | 2.24 | |
| Ortholog of STND_1004 | Phosphoglycerate mutase family protein | −1.65 | |
| Ortholog of STND_1003 | Phosphoglycerate mutase family protein | −1.76 | |
| Ortholog of STND_1007 | Phosphatase YbjI | −3.22 | |
| Ortholog of STND_1008 | Hypothetical protein | −4.52 | |
| Ortholog of STND_1212 | NADP-dependent glyceraldehyde-3-phosphate dehydrogenase GapN | −1.50 | + |
| Ortholog of STND_1301 | Hypothetical protein | −1.57 | |
| Ortholog of STND_1308 | Conserved protein | −2.06 | + |
| Ortholog of STND_1569 | Amidase nicotinamidase-like protein | −4.11 | |
| Ortholog of STND_1636 | Peptide chain release factor 2 | 1.90 | |
| Ortholog of STND_1658 | Hydrolase, haloacid dehalogenase-like family | 1.44 | |
| Ortholog of STND_1689 | Hypothetical protein | −2.42 |
CodYSt coordinates the flux of carbon and amino acid metabolisms.
S. thermophilus has a complete metabolic pathway from pyruvate to α-oxoglutarate, and the metabolite α-oxoglutarate stands at the crossroads between carbon metabolism and amino acid metabolism. α-Oxoglutarate, one of the substrates of glutamate dehydrogenase, provides the de novo carbon skeleton for glutamate. Moreover, as a product of amino acid metabolism, α-oxoglutarate can be the entry point into the central metabolism. The transcriptome data and qPCR analysis showed that the glutamate dehydrogenase gene (gdhA; ortholog of STND_0425) was significantly repressed by CodYSt (see Table S2 in the supplemental material). We also determined the glutamate dehydrogenase activities (27) of S. thermophilus ST2017 and the codYSt mutant. We found that deletion of codYSt led to an increase in enzyme activity from 190 U to 330 U, while overexpression of codYSt in S. thermophilus ST2017ΔcodY decreased enzyme activity to 110 U (Fig. 2). These results indicated that CodYSt directly inhibited the expression of the gdhA gene. EMSA analysis further confirmed that CodYSt played a direct role in the binding of the promoter of gdhA (ortholog of STND_0425) (see Fig. S2 in the supplemental material), suggesting that CodYSt may affect gdhA transcription.
FIG 2.
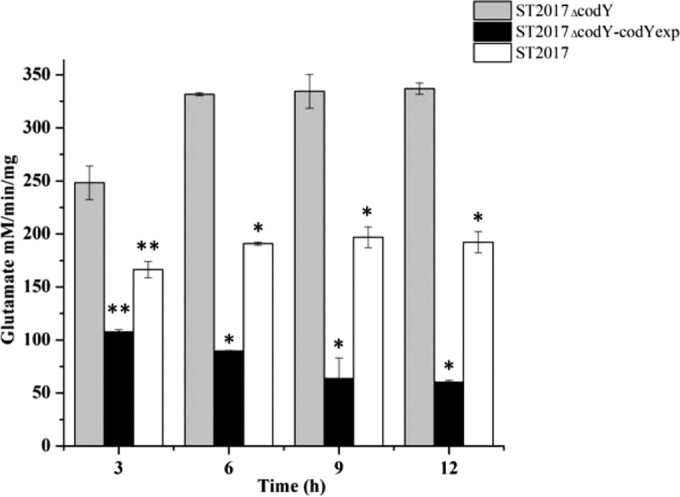
Assay of glutamate dehydrogenase activity in S. thermophilus ST2017, ST2017ΔcodY, and ST2017ΔcodY-codYexp. Asterisks indicate statistically significant differences (*, P < 0.001; **, P < 0.01).
To determine the role of glutamate dehydrogenase in carbon and amino acid interconversion, the gdhA gene was inactivated in S. thermophilus ST2017 and ST2017ΔcodY, yielding the gdhA mutant strains ST2017ΔgdhA and ST2017ΔcodYΔgdhA. Furthermore, we compared the growth of S. thermophilus ST2017, ST2017ΔcodY, ST2017ΔgdhA, and ST2017ΔcodYΔgdhA under carbon- or nitrogen-limiting conditions. Here, chemically defined medium (CDM) supplemented with exogenous histidine, cysteine, proline, and methionine was adapted to maintain the growth of S. thermophilus ST2017, with nitrogen as its growth constraint. When the lactose concentration in CDM increased from 0.5% to 1.0%, the cell density (OD600) of S. thermophilus ST2017 increased from 0.768 ± 0.011 to 1.048 ± 0.061, while that of ST2017ΔcodY increased from 0.822 ± 0.017 to 1.381 ± 0.023. The biomass levels of the gdhA mutant strains ST2017ΔgdhA and ST2017ΔcodYΔgdhA in CDM with 0.5% lactose were lower, with OD600 values of 0.305 ± 0.034 and 0.531 ± 0.041, respectively. When the lactose concentration increased to 1.0%, the biomass levels of both mutants increased slightly, to 0.328 ± 0.009 and 0.581 ± 0.104, respectively (Fig. 3A). The results suggested that under nitrogen-limiting conditions, S. thermophilus ST2017 could convert a carbon source into amino acids for bacterial growth through glutamate dehydrogenase, and the deletion of codYSt resulted in an increase in the percentage of the carbon source converted into amino acids. When the link between the carbon and nitrogen metabolic pathways was blocked (by an inactive gdhA gene), the growth of mutant strains was severely decreased. On the other hand, under carbon-limiting conditions (CDM containing 0.1% lactose and 0.25% or 0.5% tryptone), the OD600 values of S. thermophilus ST2017 and ST2017ΔcodY reached 1.340 ± 0.067 and 1.324 ± 0.017, respectively, when the concentration of tryptone increased from 0.25% to 0.5%, while the growth of strains ST2017ΔgdhA and ST2017ΔcodYΔgdhA increased only to OD600 values of 1.188 ± 0.004 and 1.164 ± 0.044, respectively (Fig. 3B). These results confirmed that the capacity for converting nitrogen to carbon was reduced in ΔgdhA mutants, consequently limiting bacterial growth.
FIG 3.
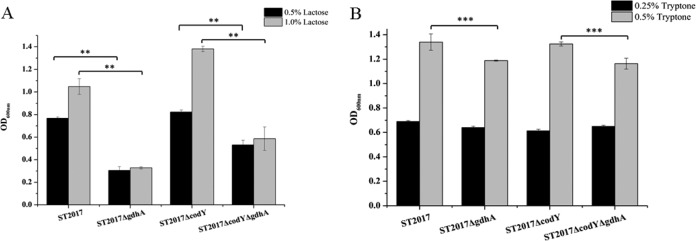
Final biomass levels of S. thermophilus ST2017, ST2017ΔcodY, ST2017ΔgdhA, and ST2017ΔcodYΔgdhA under nitrogen-limiting conditions (A) or carbon-limiting conditions (B). Asterisks indicate statistically significant differences (**, P < 0.01; ***, P < 0.05).
From the results presented above, we concluded that glutamate dehydrogenase catalyzed the reversible interconversion of α-oxoglutarate and glutamate, and the Michaelis-Menten constant (Km) of glutamate dehydrogenase was determined. Interestingly, glutamate dehydrogenase catalyzed the conversion of α-oxoglutarate to glutamate with a Km of 0.131 ± 0.030 mM, while it catalyzed the degradation of glutamate to α-oxoglutarate with a Km of 1.170 ± 0.088 mM. The Km of the conversion of α-oxoglutarate to glutamate was much lower than that of glutamate to α-oxoglutarate. This metabolic advantage forced the carbon into nitrogen metabolism and effectively utilized the lactose in milk. To confirm this hypothesis, we compared the growth of S. thermophilus ST2017, ST2017ΔcodY, ST2017ΔgdhA, and ST2017ΔcodYΔgdhA in milk. Figure 4 shows that the inactive gdhA gene significantly reduced the cell densities of S. thermophilus ST2017 and ST2017ΔcodY, from 6 × 109 CFU/ml and 2 × 109 CFU/ml to 9 × 108 CFU/ml and 8 × 108 CFU/ml, respectively. The results suggested that S. thermophilus ST2017 could convert the rich carbon source into amino acids for bacterial growth through glutamate dehydrogenase in milk, and they further confirmed the important role of glutamate dehydrogenase in the adaptation of S. thermophilus to the milk environment.
FIG 4.
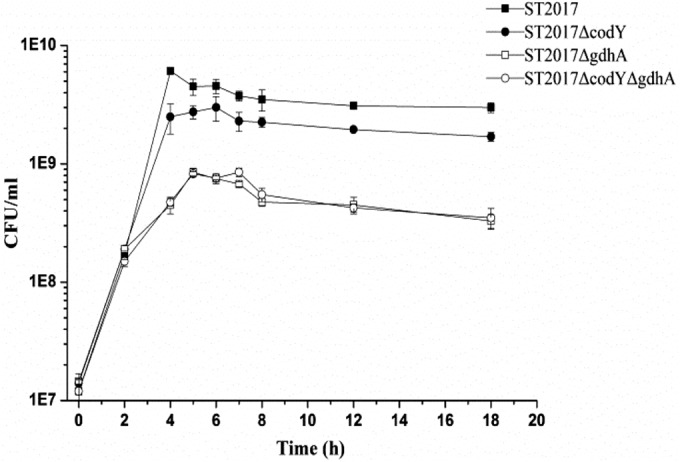
Growth curves of S. thermophilus ST2017, ST2017ΔcodY, ST2017ΔgdhA, and ST2017ΔcodYΔgdhA in milk.
The CodYSt box in S. thermophilus ST2017.
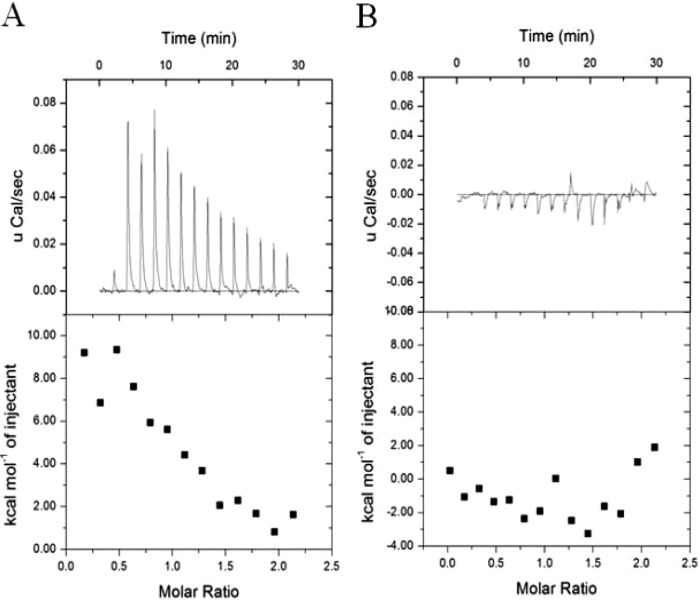
EMSA were performed to confirm the direct interaction between CodYSt and the promoters of regulated genes. The promoters of both downregulated genes (purC [ortholog of STND_0027] and the orthologs of STND_0135, STND_1346, and STND_1572) and upregulated genes (hrcA [ortholog of STND_0119], the ortholog of STND_0202, groES [ortholog of STND_0204], livJ [ortholog of STND_0352], and the ortholog of STND_0658) were retarded in the presence of 200 ng CodYSt protein, while no shifted band was observed with the promoter of the control gene (ortholog of STND_0099) (Fig.5). This result showed that the global regulator CodYSt could regulate the genes directly. In Lactococcus lactis, B. subtilis, Streptococcus pneumoniae, and Listeria monocytogenes, a conserved 15-bp palindromic sequence (AATTTTCWGAAAATT) has been shown to serve as a high-affinity binding site for CodY (12). In an attempt to distinguish direct and indirect effects of CodYSt on gene expression in the transcriptome data of S. thermophilus ST2017, we selected the upstream regions of the 31 repressed gene clusters that were upregulated in the codYSt mutant strain. Sixteen of the 31 gene clusters were shown to contain at least a putative CodYSt box [AA(T/A)(A/T)TTCTGA(A/C)AATT]. Interestingly, two putative CodYSt boxes were also found in the promoter region of the ortholog of STND_0733 (see Table S1 in the supplemental material). To further confirm the role of the CodYSt box in S. thermophilus ST2017, the interaction of CodYSt with DNA fragments containing the 15-bp conserved motif was monitored by ITC analysis. The results indicated that CodYSt and the conserved motif had a significant integration effect. In contrast, the mutated conserved motif lost binding affinity for the CodYSt protein, indicating that the conserved motif played a crucial role in the identification of the CodYSt protein (Fig. 6). Furthermore, the results revealed that BCAA could effectively enhance the affinity by reducing the dissociation constant (Kd) from 780 nM to 8.33 nM and reducing ΔH from 11.43 ± 1.01 to −5.69 ± 0.33 kcal/mol. Surprisingly, the increase in the stoichiometry of binding to 3 indicated that in the presence of BCAA, CodYSt could effectively bind to the conserved motif at a low concentration (Table 3); this result was in agreement with the EMSA results (see Fig. S2 in the supplemental material).
FIG 5.
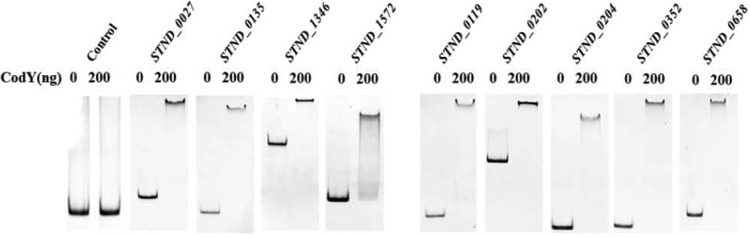
Gel mobility shift assays for the binding of CodYSt to the promoter regions of differentially expressed genes. The promoter regions of CodYSt-regulated genes (orthologs of STND_0027, STND_0135, STND_1346, STND_1572, STND_0119, STND_0202, STND_0204, STND_0352, and STND_0658) were PCR amplified, and the promoter region of the ortholog of STND_0099 was used as a control. One hundred nanograms of each promoter region was incubated with CodYSt protein, and binding was analyzed by nondenaturing PAGE.
FIG 6.
Affinity of binding of CodYSt to the CodYSt box. Shown are results of isothermal titration calorimetry experiments titrating S. thermophilus CodYSt with the CodYSt box (A) and a CodYSt box mutant (B). (Top) Raw data; (bottom) data fitted using a one-site model.
TABLE 3.
Thermodynamic parameters of the binding of the CodYSt box to CodYSt at 30°C
| Motif | Thermodynamic parameter of CodYSt binding |
|||
|---|---|---|---|---|
| Stoichiometry | Kd (nM) | ΔH (kcal/mol) | ΔS (cal/mol/degree) | |
| CodYSt box | 1 | 780 | 11.43 ± 1.01 | 61.5 |
| CodYSt box containing BCAA | 3 | 8.33 | −5.69 ± 0.33 | 18.2 |
DISCUSSION
S. thermophilus is the major dairy starter traditionally used for the manufacture of yogurt and cheeses. However, how S. thermophilus regulates the metabolic pathways to adapt to the milk environment is still unclear. In low-GC-group Gram-positive bacteria, the CodY regulon has been identified as an important mechanism of environmental adaptation (11, 12, 14, 15). Experimental evidence from the knockout and complementation of the codYSt gene demonstrated that S. thermophilus ST2017 has a functional CodYSt protein. In order to elucidate the role of CodYSt, transcriptome analysis to identify the extent of the CodYSt regulon in S. thermophilus ST2017 was carried out. A total of 86 genes organized into 45 apparent transcriptional units were differently expressed in the codYSt mutant and thus are thought to be controlled by CodYSt. The size of the regulon is comparable to those identified in B. subtilis (16), S. pneumoniae (13), and L. lactis (12, 28). Therefore, the global regulator CodYSt plays an important role in the regulation of cellular processes in S. thermophilus.
Regulatory network controlled by CodYSt.
S. thermophilus is closely related to L. lactis, but it is even more closely related to other streptococcal species (29, 30). The comparative genomics analysis highlights its relatedness to pathogenic species but also reveals that the most important determinants of pathogenicity either are absent or are present as pseudogenes (30–32). Comparative genomics also revealed that the dairy streptococcus species has followed an evolutionary path divergent from that of pathogenic species due to its adaptation to the milk environment (33).
As reported previously, CodY in L. lactis controls all pathways for nitrogen supply by modulating de novo amino acid biosynthesis and the assimilation of peptides, including their transport and further degradation by peptidases. However, the role of CodY in nitrogen supply is significantly less in S. thermophilus than in L. lactis, since CodYSt controls only the genes involved in amino acid biosynthesis and transport, including BCAA, tryptophan, cysteine, aspartic acid, serine, and threonine, and none of the peptide transporters and peptidases are regulated by CodYSt. We suppose that S. thermophilus is not able to utilize the casein in milk due to the lack of extracellular protease and finally loses control of the proteolytic system. Rather, S. thermophilus strengthens the regulation of the synthesis and transport of amino acids and controls the intracellular amino acid level effectively. BCAA biosynthesis genes are among those most tightly regulated by CodYSt. BCAA are the intracellular effectors activating CodY repression in S. thermophilus, L. lactis, and B. subtilis. Thus, CodY exerts feedback control on the pathway involved in the biosynthesis of BCAA (16, 28).
S. thermophilus has evolved a well-developed de novo pathway of amino acid biosynthesis and a unique carbon metabolic system to efficiently utilize lactose for its adaptation to the milk environment. The transcriptome data showed that the global regulator CodYSt controlled not only amino acid metabolism but also lactose utilization processes. Unlike the global regulator CcpA (34), CodYSt does not control the expression of the lactose operon directly. Instead, CodYSt represses the transcription of phosphomutase genes (orthologs of STND_1003 and STND_1004) and the glyceraldehyde-3-phosphate dehydrogenase gene (gapN) to control the carbon flux. Normally, lactose is decomposed into glucose and galactose by β-galactosidase in S. thermophilus; glucose is catabolized via the glycolytic pathway, and part of the galactose is used for the synthesis of extracellular polysaccharides, while the rest is exported to the extracellular environment by lactose permease. Our results suggested that derepression of phosphomutase genes (orthologs of STND_1003 and STND_1004) led to an increase in galactose utilization and improved the yield of extracellular polysaccharides and lactic acid as well (data not shown).
The whole-genome sequence analysis showed that S. thermophilus contains a complete metabolic pathway from lactose to glutamate. Glutamate dehydrogenase catalyzes the reversible interconversion of α-oxoglutarate to glutamate and controls the interconversion of carbon metabolism and nitrogen metabolism. Milk is a nutrient-rich and stable environment containing 4.6% lactose and 3.3% casein. Due to the weak activity or absence of extracellular protease in S. thermophilus, the nitrogen source became a major limiting factor in the optimal growth of this organism. Our results demonstrate that glutamate dehydrogenase effects the conversion from a carbon source to amino acids in milk, thus conferring optimal growth on S. thermophilus in milk.
It has been reported that deletion of the codY gene led to low transcription levels of a number of genes in Streptococcus pyogenes and B. subtilis (15, 20). In S. thermophilus ST2017, some genes followed the same pattern, including the purine biosynthetic operon, the poly-gamma-glutamate biosynthetic operon (orthologs of STND_0113 to -0115), a mechanosensitive ion channel gene (ortholog of STND_0135), a glutathione synthetase gene (ortholog of STND_1346), and a number of hypothetical protein genes (Table 2). Surprisingly, transcription of both the purCLFMNH and purDEK operons was reduced 4-fold in the codYSt mutant. The products of these genes catalyze the de novo synthesis of IMP, the precursor of GTP and ATP. This suggests a link between CodYSt and the synthesis of GTP and ATP within the cells. Poly-gamma-glutamate and the mechanosensitive ion channel protein are involved in the osmotic stress response. Glutathione synthetase (encoded by the ortholog of STND_1346) catalyzes the synthesis of glutathione, which is related to bacterial resistance to reactive oxygen species. Therefore, we suppose that S. thermophilus ST2017 responds to environmental stresses via the global regulator CodYSt.
Mechanism of regulation by CodYSt in S. thermophilus ST2017.
To find out whether the genes differentially expressed in the codYSt mutant were under the direct or indirect control of CodYSt, the upstream regions of a number of regulated genes were tested for their abilities to interact with purified CodYSt (Fig. 5). CodYSt protein could bind to the promoters of both downregulated and upregulated genes. Surprisingly, only those genes that are derepressed in the codYSt mutant led to the identification of a conserved inversely repeated motif, AA(T/A)(A/T)TTCTGA(A/C)AATT, which shows a high degree of homology to the canonical CodY box (AATTTTCWGAAAATT) in L. lactis and B. subtilis (12, 20). ITC analysis proved that this conserved motif, designated the CodYSt box, had a crucial role in the identification of CodYSt in S. thermophilus ST2017. Further analysis shows that multiple copies of the CodYSt box are present in the intergenic regions of some genes, and we suppose that the presence of multiple CodYSt binding sites strengthens the control of the target genes by CodYSt. Interestingly, the absence of a putative CodYSt box in genes downregulated in the codYSt mutant suggests that these genes may be under the direct control of a regulator that is regulated by CodYSt, or that CodYSt may be able to function as a transcriptional activator in another way.
Since it has been shown that BCCA and GTP are the effectors of CodY in B. subtilis, while the activity of lactococcal and streptococcal CodY proteins seems to be modulated solely by BCAA and not by GTP (13, 19, 35), we performed gel mobility shift assays to determine the effects of BCCA and GTP on the efficiency of the binding of CodYSt to target regions in S. thermophilus ST2017. It was possible to demonstrate that only BCAA stimulated CodYSt binding; the effect of GTP was insignificant. The mechanism of CodY in S. thermophilus ST2017 is similar to those in L. lactis and S. pneumoniae (12, 15). Sequence similarity analysis of CodY proteins suggests that mutations in L. lactis, S. pneumoniae, and S. thermophilus Phe40 and Ser129 were related to failure to respond to GTP (18), and our results prove the hypothesis in S. thermophilus.
In summary, we have identified the CodYSt regulon in S. thermophilus ST2017; we show the important roles of CodYSt in regulating carbon and nitrogen metabolism; and we further reveal that CodYSt could control the interconversion of carbon and amino acids through glutamate dehydrogenase as an important mechanism of adaptation to the milk environment.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Emmanuelle Maguin for the gift of the pG+host9 plasmid.
This work was supported by the HI-Tech Research and Development Program of China (grant 2011AA100902), the National Natural Science Foundation of China (grant 31271905), and the National Sci-Tech Support Program of China (grant 2012BAD39B01-6).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03361-14.
REFERENCES
- 1.Fox PF, McSweeney PL, Cogan TM, Guinee TP. 2004. Cheese: chemistry, physics and microbiology: general aspects, 3rd ed, vol 1 Elsevier Academic, Oxford, United Kingdom. [Google Scholar]
- 2.Tamime A, Deeth H. 1980. Yogurt technology and biochemistry. J Food Prot 43:939–977. [DOI] [PubMed] [Google Scholar]
- 3.Gunnewijk MG, Poolman B. 2000. HPr(His∼P)-mediated phosphorylation differently affects counterflow and proton motive force-driven uptake via the lactose transport protein of Streptococcus thermophilus. J Biol Chem 275:34080–34085. doi: 10.1074/jbc.M003513200. [DOI] [PubMed] [Google Scholar]
- 4.van den Bogaard P, Hols P, Kuipers O, Kleerebezem M, de Vos W. 2004. Sugar utilisation and conservation of the gal-lac gene cluster in Streptococcus thermophilus. Syst Appl Microbiol 27:10–17. doi: 10.1078/0723-2020-00258. [DOI] [PubMed] [Google Scholar]
- 5.Garault P, Le Bars D, Besset C, Monnet V. 2002. Three oligopeptide-binding proteins are involved in the oligopeptide transport of Streptococcus thermophilus. J Biol Chem 277:32–39. doi: 10.1074/jbc.M107002200. [DOI] [PubMed] [Google Scholar]
- 6.Delorme C, Bartholini C, Bolotine A, Ehrlich SD, Renault P. 2010. Emergence of a cell wall protease in the Streptococcus thermophilus population. Appl Environ Microbiol 76:451–460. doi: 10.1128/AEM.01018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garault P, Letort C, Juillard V, Monnet V. 2000. Branched-chain amino acid biosynthesis is essential for optimal growth of Streptococcus thermophilus in milk. Appl Environ Microbiol 66:5128–5133. doi: 10.1128/AEM.66.12.5128-5133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neviani E, Giraffa G, Brizzi A, Carminati D. 1995. Amino acid requirements and peptidase activities of Streptococcus salivarius subsp. thermophilus. J Appl Microbiol 79:302–307. [DOI] [PubMed] [Google Scholar]
- 9.Sonenshein AL. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol 5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- 10.Titgemeyer F, Hillen W. 2002. Global control of sugar metabolism: a Gram-positive solution. Antonie Van Leeuwenhoek 82:59–71. doi: 10.1023/A:1020628909429. [DOI] [PubMed] [Google Scholar]
- 11.Bennett HJ, Pearce DM, Glenn S, Taylor CM, Kuhn M, Sonenshein AL, Andrew PW, Roberts IS. 2007. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol Microbiol 63:1453–1467. doi: 10.1111/j.1365-2958.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- 12.Guédon E, Sperandio B, Pons N, Ehrlich SD, Renault P. 2005. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology 151:3895–3909. doi: 10.1099/mic.0.28186-0. [DOI] [PubMed] [Google Scholar]
- 13.Hendriksen WT, Bootsma HJ, Estevão S, Hoogenboezem T, de Jong A, de Groot R, Kuipers OP, Hermans PW. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J Bacteriol 190:590–601. doi: 10.1128/JB.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemos JA, Nascimento MM, Lin VK, Abranches J, Burne RA. 2008. Global regulation by (p)ppGpp and CodY in Streptococcus mutans. J Bacteriol 190:5291–5299. doi: 10.1128/JB.00288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malke H, Steiner K, McShan W, Ferretti J. 2006. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol 296:259–275. doi: 10.1016/j.ijmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, Sonenshein AL. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J Bacteriol 185:1911–1922. doi: 10.1128/JB.185.6.1911-1922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handke LD, Shivers RP, Sonenshein AL. 2008. Interaction of Bacillus subtilis CodY with GTP. J Bacteriol 190:798–806. doi: 10.1128/JB.01115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levdikov VM, Blagova E, Joseph P, Sonenshein AL, Wilkinson AJ. 2006. The structure of CodY, a GTP- and isoleucine-responsive regulator of stationary phase and virulence in gram-positive bacteria. J Biol Chem 281:11366–11373. doi: 10.1074/jbc.M513015200. [DOI] [PubMed] [Google Scholar]
- 19.Petranovic D, Guédon E, Sperandio B, Delorme C, Ehrlich D, Renault P. 2004. Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol Microbiol 53:613–621. doi: 10.1111/j.1365-2958.2004.04136.x. [DOI] [PubMed] [Google Scholar]
- 20.Shivers RP, Sonenshein AL. 2004. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol 53:599–611. doi: 10.1111/j.1365-2958.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- 21.Letort C, Juillard V. 2001. Development of a minimal chemically defined medium for the exponential growth of Streptococcus thermophilus. J Appl Microbiol 91:1023–1029. doi: 10.1046/j.1365-2672.2001.01469.x. [DOI] [PubMed] [Google Scholar]
- 22.Biswas I, Gruss A, Ehrlich SD, Maguin E. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol 175:3628–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fontaine L, Boutry C, de Frahan MH, Delplace B, Fremaux C, Horvath P, Boyaval P, Hols P. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J Bacteriol 192:1444–1454. doi: 10.1128/JB.01251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo T, Kong J, Zhang L, Zhang C, Hu S. 2012. Fine tuning of the lactate and diacetyl production through promoter engineering in Lactococcus lactis. PLoS One 7:e36296. doi: 10.1371/journal.pone.0036296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Feng Z, Wang X, Zhang X. 2010. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 26.Pagels M, Fuchs S, Pané-Farré J, Kohler C, Menschner L, Hecker M, McNamarra PJ, Bauer MC, von Wachenfeldt C, Liebeke M. 2010. Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Mol Microbiol 76:1142–1161. doi: 10.1111/j.1365-2958.2010.07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treberg JR, Brosnan ME, Brosnan JT. 2010. The simultaneous determination of NAD(H) and NADP(H) utilization by glutamate dehydrogenase. Mol Cell Biochem 344:253–259. doi: 10.1007/s11010-010-0549-8. [DOI] [PubMed] [Google Scholar]
- 28.den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J Biol Chem 280:34332–34342. doi: 10.1074/jbc.M502349200. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell TJ. 2003. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat Rev Microbiol 1:219–230. doi: 10.1038/nrmicro771. [DOI] [PubMed] [Google Scholar]
- 30.Tettelin H. 2004. Streptococcal genomes provide food for thought. Nat Biotechnol 22:1523–1524. doi: 10.1038/nbt1204-1523. [DOI] [PubMed] [Google Scholar]
- 31.Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, Lai HS, Lin SP, Qian Y, Jia HG, Najar FZ, Ren Q, Zhu H, Song L, White J, Yuan X, Clifton SW, Roe BA, McLaughlin R. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci U S A 98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoskins J, Alborn WE Jr, Arnold J, Blaszczak LC, Burgett S, DeHoff BS, Estrem ST, Fritz L, Fu DJ, Fuller W, Geringer C, Gilmour R, Glass JS, Khoja H, Kraft AR, Lagace RE, LeBlanc DJ, Lee LN, Lefkowitz EJ, Lu J, Matsushima P, McAhren SM, McHenney M, McLeaster K, Mundy CW, Nicas TI, Norris FH, O'Gara M, Peery RB, Robertson GT, Rockey P, Sun PM, Winkler ME, Yang Y, Young-Bellido M, Zhao G, Zook CA, Baltz RH, Jaskunas SR, Rosteck PR Jr, Skatrud PL, Glass JI. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol 183:5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hols P, Hancy F, Fontaine L, Grossiord B, Prozzi D, Leblond-Bourget N, Decaris B, Bolotin A, Delorme C, Ehrlich SD, Guédon E, Monnet V, Renault P, Kleerebezem M. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol Rev 29:435–463. doi: 10.1016/j.femsre.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 34.van den Bogaard PT, Kleerebezem M, Kuipers OP, de Vos WM. 2000. Control of lactose transport, β-galactosidase activity, and glycolysis by CcpA in Streptococcus thermophilus: evidence for carbon catabolite repression by a non-phosphoenolpyruvate-dependent phosphotransferase system sugar. J Bacteriol 182:5982–5989. doi: 10.1128/JB.182.21.5982-5989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guédon E, Serror P, Ehrlich SD, Renault P, Delorme C. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol 40:1227–1239. doi: 10.1046/j.1365-2958.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 37.Le Loir Y, Azevedo V, Oliveira SC, Freitas DA, Miyoshi A, Bermudez-Humaran LG, Nouaille S, Ribeiro LA, Leclercq S, Gabriel JE, Guimaraes VD, Oliveira MN, Charlier C, Gautier M, Langella P. 2005. Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb Cell Fact 4:2. doi: 10.1186/1475-2859-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.