Abstract
An aseptic meningitis outbreak occurred in Luoding City of Guangdong, China, in 2012, and echovirus type 30 (ECHO30) was identified as the major causative pathogen. Environmental surveillance indicated that ECHO30 was detected in the sewage of a neighboring city, Guangzhou, from 2010 to 2012 and also in Luoding City sewage samples (6/43, 14%) collected after the outbreak. In order to track the potential origin of the outbreak viral strains, we sequenced the VP1 genes of 29 viral strains from clinical patients and environmental samples. Sequence alignments and phylogenetic analyses based on VP1 gene sequences revealed that virus strains isolated from the sewage of Guangzhou and Luoding cities matched well the clinical strains from the outbreak, with high nucleotide sequence similarity (98.5% to 100%) and similar cluster distribution. Five ECHO30 clinical strains were clustered with the Guangdong environmental strains but diverged from strains from other regions, suggesting that this subcluster of viruses most likely originated from the circulating virus in Guangdong rather than having been more recently imported from other regions. These findings underscore the importance of long-term, continuous environmental surveillance and genetic analysis to monitor circulating enteroviruses.
INTRODUCTION
Enteroviruses (EVs; family Picornaviridae) are small RNA viruses, some of which are associated with several human diseases (1). Based on the similarity of their VP1 nucleotide sequences, EVs are currently grouped as enterovirus species A to J and rhinovirus species A to C (2).
In most cases, human EV infection is generally asymptomatic or causes only mild symptoms; however, EVs can sometimes cause a broad spectrum of clinical illnesses, including aseptic meningitis, acute flaccid paralysis (AFP), acute encephalitis, and hand, foot, and mouth disease (3–5). As most EV infections are asymptomatic, the circulation of EVs cannot be identified without specialized surveillance. Environmental surveillance of EVs provides a supplemental method to elucidate the trend of virus distribution and variation in the corresponding area over a specific period of time (6, 7). Enteroviruses that are shed from affected individuals are most frequently detected in raw sewage samples during environmental surveillance.
Echovirus type 30 (ECHO30), which belongs to the EV-B species, is recognized as the leading cause of viral aseptic meningitis in both children and adults. In the last few decades, repeated outbreaks and nationwide epidemics of ECHO30 have occurred in Europe (8–11), Asia (12–14), and America (15–17). In China, high frequencies of ECHO30 detection in meningitis cases have been documented in the last few years in different coastal provinces, including Zhejiang (18), Jiangsu (14), and Shandong (19, 20). Recently, we reported that an aseptic meningitis outbreak occurred in Luoding City (Guangdong Province, China) in May of 2012, and ECHO30 was identified as the causative pathogen of this outbreak (21). Guangzhou is the capital city of Guangdong Province, and it is adjacent to Luoding City, where the outbreak of meningitis occurred. In environmental surveillance, we previously described the serotype distribution and circulation patterns of non-polio EVs (NPEVs) in sewage collected from Guangzhou from 2009 through 2012 (7). Four strains of ECHO30 were continuously isolated from 2010 through 2012, indicating the virus circulation in the population. However, the relationship between virus strains isolated from the environmental surveillance and the outbreak in the human population has not been identified.
In this study, we investigated the genetic characteristics of ECHO30 that caused an aseptic meningitis outbreak in Luoding City in 2012 and compared them with the ECHO30 isolates identified in raw sewage from 2010 to 2012 in Guangzhou City as well as with the viral isolates from Luoding City sewage that were collected after the outbreak. Sequence alignments and phylogenetic analyses of the entire VP1 gene sequences were performed to present a genetic overview of ECHO30 isolates and shed light on the transmission and evolution of ECHO30 in Guangdong Province, China.
MATERIALS AND METHODS
Ethics statement.
The study was approved by the institutional ethics committee of Center for Disease Control and Prevention of Guangdong Province (Guangdong CDC). This work did not include direct contact with patients or volunteers, and research focused on previously collected samples. Thus, there was no need for ethical approval or informed consent. Patient records were coded and deidentified prior to analysis. No identifying details are included in this article.
Clinical viral isolates collected from the patients of aseptic meningitis.
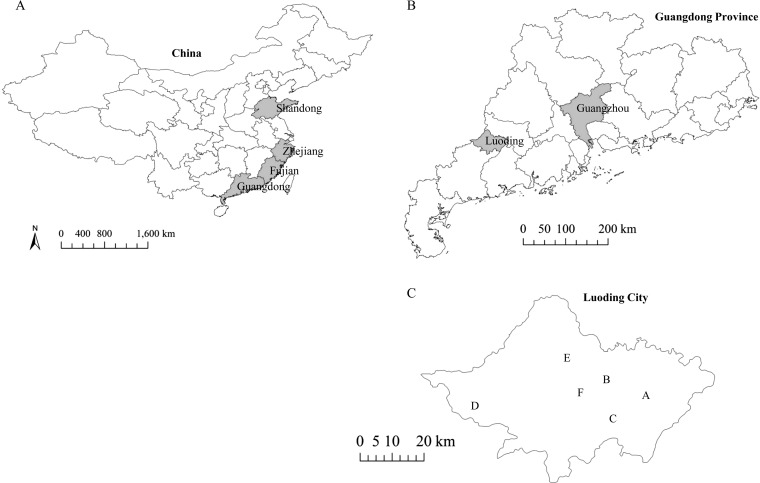
Luoding City is located in the central west region of Guangdong Province and is 200 km from the provincial capital city, Guangzhou (Fig. 1). From May to June 2012, an outbreak of aseptic meningitis in Luoding was reported by the Guangdong Provincial Center for Disease Control and Prevention (21, 22). A total of 121 cerebrospinal fluid (CSF) specimens were collected from patients who presented with aseptic meningitis and analyzed to detect EV based on WHO guidelines (23). Briefly, nucleic acid was first extracted from the collected samples with a QIAamp Viral RNA minikit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. One-step reverse transcription-PCR (RT-PCR) was performed by using a Qiagen OneStep RT-PCR kit with pan-enterovirus primers (PE-F, 5′-TCCGGCCCCTGAATGCGGCTAATCC-3′; PE-R, 5′-ACACGGACACCCAAAGTAGTCGGTCC-3′). Amplification products were analyzed by polyacrylamide gel electrophoresis (PAGE), and positive products were detected at the expected size (114 bp). The EV-positive CSF samples were selected and inoculated on rhabdomyosarcoma (RD) and HEp-2 cell lines for virus isolation.
FIG 1.
Geographic information of ECHO30 sampling. (A) Distribution of ECHO30 outbreaks in China. Four coastal cities with reported ECHO30 outbreaks are indicated in gray. (B) Location of Luoding and Guangzhou City. (C) Clinical samples were collected from six (A to F) different towns in Luoding City. Detailed information for each ECHO30 strain and identifications of the towns are given in Table 1. Maps were created using ArcGis (ArcMap 9.3) with data from the National Administration of Surveying, Mapping and Geoinformation of China.
Environmental viral isolates collected from sewage.
Raw sewage samples were collected monthly from January 2009 to December 2012 from the primary sedimentation tanks at the Liede wastewater treatment plant (WWTP) in Guangzhou City (7). Four samples (1 liter each) were obtained from the inlets of the primary sedimentation tanks on a routine basis each month. The samples were immediately transported to the laboratory, and sample treatment was started within 2 h after arrival. Forty-three sewage samples were also collected from WWTPs 1 and 2 of Luoding City 1 month after the outbreak began. Raw sewage from WWTP 1 of Luoding City is sourced from household sewage and industrial wastewater, and WWTP 2 collects household sewage and hospital wastewater. Viruses in the sewage samples were concentrated and isolated as previously described (7). The 1-liter sewage sample was first concentrated through improved negative-charge filter membrane absorption, and virus was eluted in 10 ml of a 3% beef extract solution (pH 9.6) after sonication and centrifugation (24). Thereafter, 200 μl of each concentrated eluent was used for inoculating the standard monolayer of cells for virus isolation.
Virus isolation.
Enterovirus-positive specimens from the outbreak and the concentrated sewage samples were selected for virus isolation. Human rhabdomyosarcoma (RD) and human laryngeal epidermoid carcinoma (HEp-2) cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and were used for virus isolation with standard procedures (25). Cell culture supernatant was collected as a positive isolate when cytopathic effect (CPE) was observed.
Molecular typing.
For molecular typing, nucleic acid was first extracted from the positive isolates with a QIAamp Viral RNA minikit, and RT-PCR was performed according to the method developed by Nix et al. (26). Briefly, cDNA was synthesized by using a PrimeScript II 1st Strand cDNA synthesis kit (TaKaRa, Dalian, China) with 1 μM each cDNA primer (primers AN32, 5′-GTYTGCCA-3′; AN33, 5′-GAYTGCCA-3′; AN34, 5′-CCRTCRTA-3′; and AN35, 5′-RCTYTGCCA-3′) in a 20-μl system. Following reverse transcription, 2.5 μl of cDNA was then used in the first PCR (PCR1; final volume, 25 μl) by using a Taq PCR master mix kit (Qiagen) with 0.5 μM (each) primers 224 and 222 targeting a highly conserved motif in the VP3 and VP1 genes, respectively (224, 5′-GCIATGYTIGGIACICAYRT-3′; 222, 5′-CICCIGGIGGIAYRWACAT-3′). After the amplification, 2.5 μl of the PCR1 product was used as a template in the second-round PCR with 0.5 μM (each) primers AN88 and AN89 targeting the partial VP1 gene (AN88, 5′-CCAGCACTGACAGCAGYNGARAYNGG-3′; AN89, 5′-TACTGGACCACCTGGNGGNAYRWACAT-3′). The final PCR products were analyzed on 1.2% agarose gels, and the positive products (∼350 to 400 nucleotides [nt]) were purified using a QIAquick PCR purification kit (Qiagen) and sent for sequencing by using primer AN88 or AN89. The sequences were analyzed with the Basic Local Alignment Search Tool (BLAST) server at the National Center for Biotechnology Information (NCBI), and the serotype of each isolate was determined according to a previously described molecular typing method (27). In general, a pending EV was classified as the same serotype as the prototype strain if it had >75% nucleotide identity and >85% amino acid sequence identity in the coding region of the VP1 gene; the pending EVs were classified into different serotypes if they had <70% nucleotide identity and <85% amino acid sequence identity.
Amplification and sequencing of the VP1 gene of ECHO30.
After molecular typing, 27 ECHO30 strains were selected, and viral RNA was extracted and reverse transcribed with random primers. The entire VP1 gene, VP1-F (5′-ACAAGYATYGTGACGCCACCAGA-3′; positions 2331 to 2354, relative to ECHO30 strain Bastianni), and VP1-R (5′-AAGTAYACACCTGTGGWRCACTGGCA-3′; positions 3501 to 3526, relative to the ECHO30 Bastianni strain) were used for PCR amplification. The PCR products were gel purified and then sequenced twice in both directions using the same forward and reverse primers as those used in the PCR.
Sequence analysis.
Full-length VP1 gene sequences (876 nt) were aligned with Clustal W (BioEdit) software. Genetic distances between and within clusters were calculated using the Kimura two-parameter substitution model in the software MEGA (version 6.06). Phylogenetic trees were constructed with MEGA using the maximum-likelihood (ML) method based on entire VP1 gene sequences (28). To assess the robustness of individual nodes on phylogenetic trees, 1,000 bootstrap replicates were performed. The nucleotide sequences of ECHO30 strains were downloaded from the GenBank database (accessed 15 April 2014), and 34 strains from other countries were selected to represent known lineages.
Nucleotide sequence accession numbers.
Sequences obtained in this study have been deposited in the GenBank database under the accession numbers listed in Table 1.
TABLE 1.
Details of ECHO30 strains isolated and sequenced in this study
| GenBank accession no. | Isolate name | Sample type | Collection date (mo-day-yr) | Locationa | Cluster no. in phylogenetic tree |
|---|---|---|---|---|---|
| KM034782 | C3 | CSF | 5-10-2012 | Luoding A | 4 |
| KM034783 | C8 | CSF | 5-11-2012 | Luoding A | 1 |
| KM034787 | C15 | CSF | 5-11-2012 | Luoding A | 3 |
| KM034788 | C16 | CSF | 5-9-2012 | Luoding A | 4 |
| KM034789 | C17 | CSF | 5-17-2012 | Luoding A | 3 |
| KM034796 | C25 | CSF | 5-11-2012 | Luoding A | 4 |
| KM034798 | C29 | CSF | 5-20-2012 | Luoding A | 2 |
| KM034784 | C11 | CSF | 5-13-2012 | Luoding B | 1 |
| KM034785 | C13 | CSF | 5-17-2012 | Luoding B | 1 |
| KM034786 | C14 | CSF | 5-13-2012 | Luoding B | 4 |
| KM034792 | C21 | CSF | 5-6-2012 | Luoding B | 4 |
| KM034793 | C22 | CSF | 5-14-2012 | Luoding B | 1 |
| KM034794 | C23 | CSF | 5-15-2012 | Luoding C | 4 |
| KM034795 | C24 | CSF | 5-11-2012 | Luoding C | 1 |
| KM034790 | C19 | CSF | 5-10-2012 | Luoding D | 2 |
| KM034799 | C33 | CSF | 5-20-2012 | Luoding D | 2 |
| KM034781 | C1 | CSF | 5-17-2012 | Luoding E | 3 |
| KM034791 | C20 | CSF | 5-14-2012 | Luoding E | 2 |
| KM034797 | C26 | CSF | 5-10-2012 | Luoding F | 2 |
| KC897073 | EM161 | CSF | 6-9-2012 | Luoding F | 4 |
| KM034800 | 2113 | Sewage | 5-30-2012 | WWTP 1, Luoding | 3 |
| KM034801 | 2313 | Sewage | 5-30-2012 | WWTP 2, Luoding | 1 |
| KM034802 | 2314 | Sewage | 5-30-2012 | WWTP 2, Luoding | 1 |
| KM034803 | 2412 | Sewage | 5-30-2012 | WWTP 2, Luoding | 4 |
| KM034804 | 5012 | Sewage | 8-18-2010 | Liede WWTP, Guangzhou | |
| KM034805 | 4111 | Sewage | 10-19-2011 | Liede WWTP, Guangzhou | 1 |
| KM034806 | 3221 | Sewage | 7-11-2012 | Liede WWTP, Guangzhou | 1 |
Towns in Luoding are indicated as follows: A, Sulong; B, Luochen; C, Luoping; D, Longwan; E, Fuchen; F, Shengjiang.
RESULTS
Outbreak description.
An aseptic meningitis outbreak in 246 patients occurred in Luoding City from 1 May to 30 June 2012. Seventy-five of the 121 collected CSF samples were EV positive, as identified by real-time PCR. All EV-positive CSF samples were inoculated in RD and HEp-2 cell lines for virus isolation. A cytopathic effect (CPE) was observed in 40 specimens (53.3%); among them, 32 were identified as ECHO30, and 8 were identified as ECHO6. Twenty ECHO30 isolates were randomly chosen to obtain entire VP1 gene sequences. These 20 ECHO30 strains were collected from six different towns in Luoding from 9 to 15 May 2012 (Fig. 1 and Table 1).
Environmental surveillance.
EV environmental surveillance in Guangzhou started in the middle of 2008 (7). During environmental surveillance from January 2009 to December 2012, a total of 947 EV-positive isolates were collected. Molecular typing was successfully conducted on 916 isolates, and the serotypes for the other 31 EV-positive isolates needed to be determined due to the failure of nested PCR amplifications. In total, 17 NPEV serotypes were identified based on the molecular typing method of a 340-bp fragment sequence in the VP1 gene (7). According to the environmental surveillance, ECHO30 was first detected in Guangzhou in August 2010. Then, one and two ECHO30 strains were successfully isolated from sewage samples in 2011 and 2012, respectively (Table 1). All of these ECHO30 strains were isolated from HEp-2 cells. Among them, one virus strain isolated in 2012 was identified as a mixture of ECHO30 and ECHO6, so it was not included for further sequencing in this study.
On 30 May 2012, nearly 1 month after the outbreak began in Luoding, 17 and 26 sewage samples were collected from WWTP 1 and WWTP 2 of Luoding City, respectively. Sewage samples were concentrated and inoculated for virus isolation. Six sewage samples (6/43, 14%) were detected as ECHO30 positive (Table 1). Five ECHO30 strains were successfully isolated from HEp-2 cells, and one viral strain was isolated from RD cells.
Sequence analysis of clinical and environmental ECHO30 isolates in Guangdong.
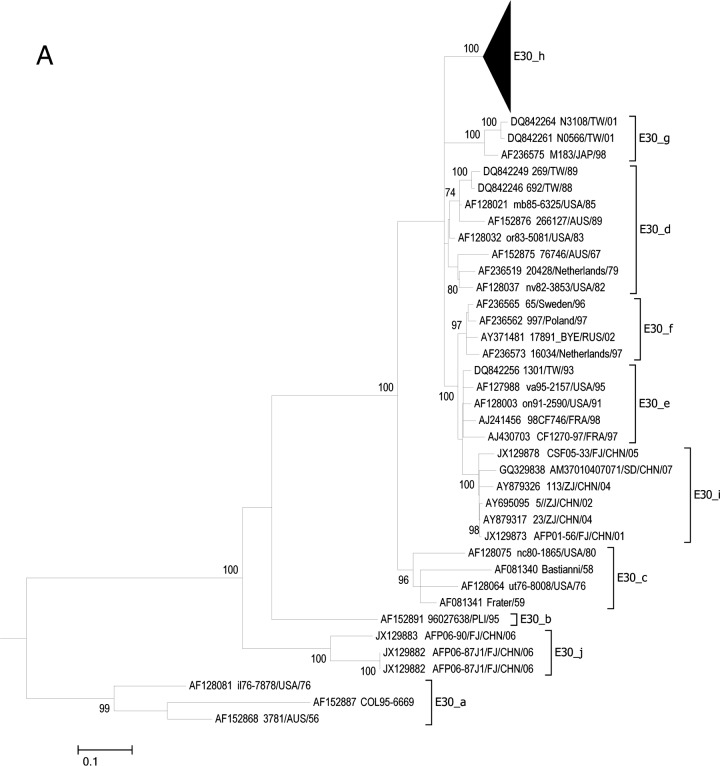
To investigate the genetic relationship between clinical and environmental ECHO30 isolates, 20 ECHO30 clinical strains from the patients and 7 ECHO30 environmental strains (3 from Guangzhou and 4 from Luoding WWTPs) were isolated for VP1 gene sequencing (Table 1). The phylogenetic trees based on entire VP1 gene sequences are shown in Fig. 2. ECHO30 strains could be divided into 10 lineages based on previously established phylogenetic classification criteria (Fig. 2A, E-30_a to E-30_h) (29, 30). Most of the ECHO30 isolates in China clustered into lineage h, except for several strains that were isolated from Zhejiang, Fujian, and Shandong before 2008, which clustered into lineages i and j.
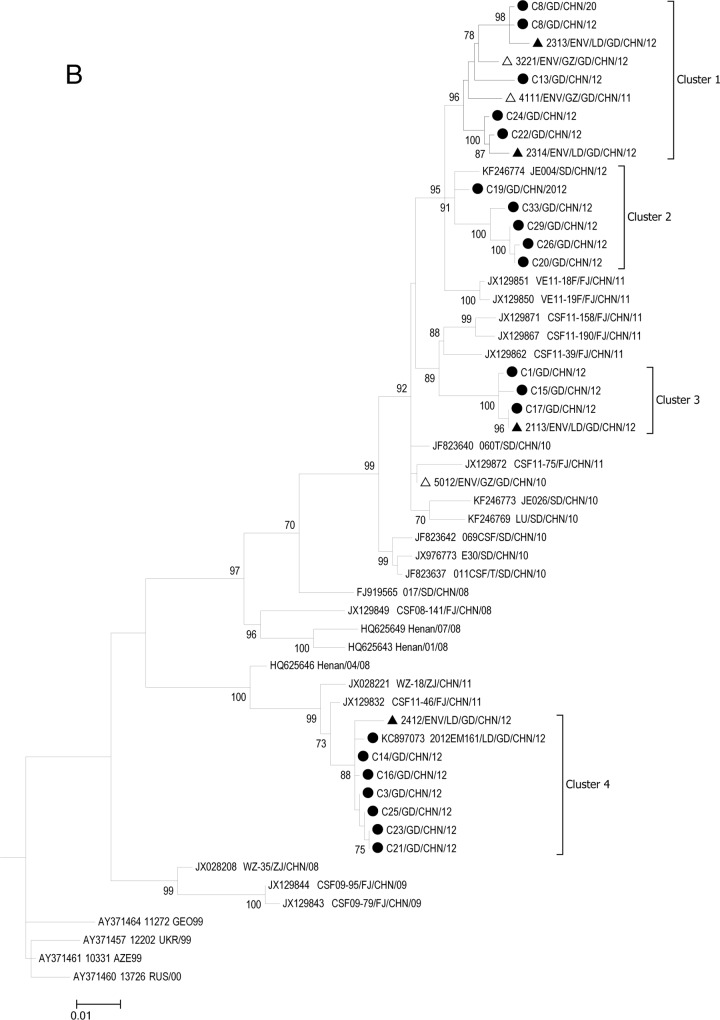
FIG 2.
(A) Phylogenetic relationships of ECHO30 isolates based on the entire nucleotide sequence of the VP1 gene. (B) Phylogenetic relationships of ECHO30 lineage h based on entire nucleotide sequence of the VP1 gene. In panel B, the ECHO30 strains isolated from clinical samples in this study are marked with filled circles. The strains isolated from Luoding and Guangzhou sewage samples are marked with filled and open triangles, respectively. Bars in both panels indicate nucleotide distances as substitutions per site. Only bootstrap values of over 70% are shown.
Twenty-seven viral isolates from the aseptic meningitis outbreak and sewage samples of Guangdong that fell into lineage h could be further divided into four subclusters (clusters 1 to 4) in the phylogenetic tree (Fig. 2B). The bootstrap support value for each cluster was more than 75%. In cluster 1, four clinical strains from three towns in Luoding City clustered together with the environmental strains from both Luoding and Guangzhou sewage samples. In addition, none of the ECHO30 strains from other regions fell into this cluster even when all ECHO30 sequences in the GenBank database were included (Fig. 2B; see also Fig. S1 in the supplemental material). Cluster 2 contained five clinical strains from four towns in Luoding. In clusters 3 and 4, all of the ECHO30 strains that were isolated from the patients clustered together with the environmental strains from sewage samples from Luoding. In contrast to cluster 1, clinical strains in clusters 2, 3, and 4 were clustered with other ECHO30 strains that were recently collected in other Chinese provinces, such as Zhejiang (2011), Shandong (2010 to 2012), and Fujian (2011).
The genetic intra- and intercluster distances were calculated with a Kimura two-parameter substitution model. Consistent with the phylogenetic analysis, the intracluster genetic diversity was greater than that of the intercluster diversity, and the sequences in cluster 4 were more divergent from the sequences in other clusters (genetic distance of ≥0.1) (Table 2). The amino acid alignment showed that the variability in clusters 1, 2, and 3 was produced almost entirely by synonymous changes (see Fig. S2 in the supplemental material). The amino acid differences between clusters 1 to 3 and cluster 4 were identified, suggesting that the outbreak in 2012 was caused by genetically different viruses.
TABLE 2.
Nucleotide distances between and within clusters of ECHO30 isolates in Guangdong
| Cluster no. | No. of base substitutions/sitea |
|||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
| 2 | 0.027 | |||
| 3 | 0.036 | 0.036 | ||
| 4 | 0.101 | 0.102 | 0.1 | |
| Distance within cluster | 0.015 | 0.012 | 0.004 | 0.004 |
The numbers of base substitutions per site from all sequence pairs between and within clusters are shown. Analyses were conducted by using a Kimura two-parameter model.
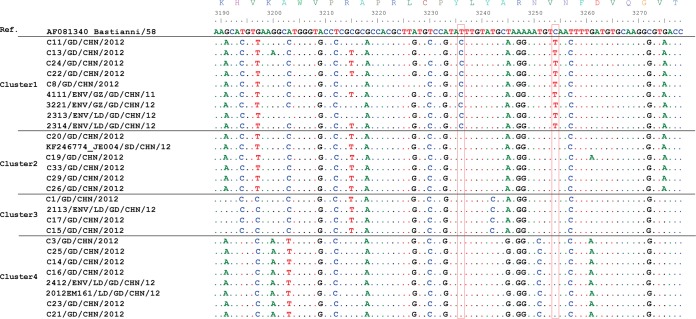
The close relationship between environmental viral isolates and clinical isolates was demonstrated in VP1 nucleotide sequence alignments. Environmental isolates that were collected from the neighboring city (Guangzhou) before the outbreak and from Luoding City after the outbreak share high sequence similarity with the corresponding clinical isolates (nucleotide identity of 98.5% to 100%), except for the strain 5012 collected in Guangzhou City in 2010, which was more closely related to the clinical strain (060T/SD/CHN/10) obtained from Shandong province in the same year (Table 3). The phylogenetic analysis of the VP1 gene suggested that the ECHO30 strains in cluster 1 were separated from the other viral strains. The subsequent nucleotide alignment of the VP1 gene showed that the two nucleotide changes at nt 3236 (T to C) and nt 3254 (C to T) (relative to ECHO30 strain Bastianni) were exclusively observed in virus strains in cluster 1 but not in strains from clusters 2 to 4 (Fig. 3).
TABLE 3.
Comparison of nucleotide and amino acid sequence identities of ECHO30 strains
| Environmental strain | Clinical strain | Nucleotide identity (%) | Amino acid identity (%) |
|---|---|---|---|
| 5012/ENV/GZ/GD/CHN/10 | 060T/SD/CHN/10 | 99.4 | 100 |
| 4111/ENV/GZ/GD/CHN/11 | C24/GD/CHN/2012 | 98.5 | 100 |
| 3221/ENV/GZ/GD/CHN/12 | C8/GD/CHN/2012 | 98.7 | 100 |
| 2113/ENV/LD/GD/CHN/12 | C17/GD/CHN/2012 | 100 | 100 |
| 2313/ENV/LD/GD/CHN/12 | C11/GD/CHN/2012 | 99.4 | 100 |
| 2314/ENV/LD/GD/CHN/12 | C22/GD/CHN/2012 | 99.4 | 99.3 |
| 2412/ENV/LD/GD/CHN/12 | C3/GD/CHN/2012 | 99.4 | 99.6 |
FIG 3.
Alignment of VP1 nucleotide sequences (nucleotide position 3189 to 3278, relative to ECHO30 reference strain Bastianni) of clusters 1 to 4. The significant nucleotide differences between cluster 1 and clusters 2 to 4 were identified at positions 3236 and 3254 (boxed).
DISCUSSION
Environmental surveillance has been proven to be valuable for monitoring circulating EVs in specific communities (6, 7, 31). A surveillance study conducted in Wisconsin from 1994 to 2002 showed that the seasonal and serotype distributions of EVs in sewage were related to those in the affected population. In Wisconsin, the annual peaks of both sewage EV titers and clinical cases occurred in late summer or early fall, and in some years, early spring sewage EVs revealed some of the EVs that would clinically predominate during the following summer. Moreover, most of the EV serotypes that were identified from clinical specimens were also found in sewage samples, and the most commonly detected EV serotypes in sewage were similar to the most commonly detected EV serotypes in clinical samples (31). Compared to Wisconsin, Guangzhou and Luoding cities have high population densities (1,715 people/sq km and 490 people/sq km) but relatively low levels of cleanliness. Therefore, a higher positive rate of EVs and a greater number of serotypes of EVs are observed in sewage samples of Guangzhou City than in Wisconsin (7, 31). Due to a lack of long-term clinical surveillance in Guangdong, we cannot currently compare the prevalence of EVs in sewage samples to that in clinical cases. However, a close phylogenetic relationship between ECHO30 strains isolated from sewage samples and those from outbreaks was demonstrated in this study. The data provided here suggest that sewage surveillance also has a value in monitoring circulating EVs in the communities of cities in developing countries like China.
ECHO30 is one of the most frequently isolated EV serotypes that cause aseptic meningitis. Numerous aseptic meningitis outbreaks that are caused by different lineages of ECHO30 have been reported during the last decade in many countries (15, 21, 32). In Guangdong Province, China, ECHO30 was first isolated from a sewage sample in 2010 through environmental surveillance; subsequently, this virus was continually but not frequently detected in sewage of Guangzhou City (7). Taking into account that similar methods have been used for sewage concentration and virus isolation in cell cultures (as described in the Materials and Methods section) over the years, it is likely that continuous ECHO30 identification is a reflection of the circulation of the virus in this region.
In this study, strains from Guangzhou sewage samples were sequenced and compared with strains from the aseptic meningitis epidemic in adjacent Luoding. Meanwhile, the sewage samples from two major WWTPs in Luoding were also collected, and a high prevalence of ECHO30 (14%) was identified. Previous studies on the molecular epidemiology of ECHO30 and phylogenetic classification were primarily based on the VP1 gene (29, 30, 33). Therefore, phylogenetic analyses of the clinical strains and the environmental strains were also performed by using VP1 gene sequences according to the classification scheme that was established by Bailly et al. (29).
The ECHO30 h lineage represents the primary outbreak virus strain in China, including strains from meningitis outbreaks in Jiangsu Province in 2003 (14), Shandong Province in 2008, and Fujian Province in 2011 (30). Similarly, the ECHO30 strains identified in Guangdong also fell into lineage h but were segregated into four different subclusters (Fig. 2B, clusters 1 to 4). Nine strains collected from Guangdong (five clinical strains and four environmental strains) belonged to cluster 1 and were separated from the ECHO30 strains from other regions (Fig. 2B; see also Fig. S1 in the supplemental material). The sequence alignment also illustrated the coexistence of two site changes on the VP1 gene (T3236C and C3254T) that were exclusively observed in viral strains of cluster 1 (Fig. 3) and not identified in other ECHO30 strains in the GenBank database (data not shown). In addition, the environmental strain 4111 isolated from sewage from Guangzhou in 2011 is the closest strain to the strain from the outbreak in Luoding in 2012 (nucleotide sequence identity of 98.1% to 98.5%). These observations might suggest that ECHO30 strains in cluster 1 are more likely Guangdong local strains and that the cluster 1 viral strain from the meningitis outbreak in 2012 may have been transmitted from the earlier circulating viral strains (4111-like viruses) in Guangdong Province.
The clinical strains were isolated from patients from six different towns in Luoding City without a specific geographic distribution, according to VP1 gene sequences. More interestingly, the various clusters of ECHO30 strains were isolated from the outbreak in a single town (Table 1 and Fig. 1). For example, strains C8, C29, C15, and C3 from town A fell into four different clusters. These observations confirmed the VP1 sequence diversity of ECHO30 strains that were isolated in this outbreak. In addition, the environmental strains isolated from WWTPs in Luoding after the outbreak share high nucleotide sequence similarity with the clinical strains from the epidemic (nucleotide identity of 99.4% to 100%). These analyses indicate that the viral strains that were isolated from both WWTPs of Luoding City after the outbreak represent the predominant ECHO30 clinical strains in this outbreak.
In conclusion, we analyzed the VP1 gene sequences of ECHO30 strains from the aseptic meningitis outbreak in 2012 in Guangdong, China, and the ECHO30 strains isolated from raw sewage before and after the outbreak. One subcluster of ECHO30 clinical strains were closely related with the Guangdong environmental strain isolated before the outbreak but diverged from strains from other regions, suggesting that this subcluster of viruses was likely to have originated from the circulating virus in Guangdong rather than having been recently imported from other regions. In addition, the high nucleotide sequence identities (98.5% to 100%) shared by the clinical strains from the epidemic and the environmental strains from sewage reinforce the value of EV environmental surveillance. In fact, active surveillance on clinical specimens is more efficient than surveillance on sewage for detecting and tracking outbreaks. Some EVs, especially EV-As, might have been missed in environmental surveillance because their growth in cells is lower than that of EV-Bs (7). However, a systematic EV disease surveillance system is absent in China, and clinical specimens are hard to obtain. Moreover, many EV infections are asymptomatic or subclinical, and EV serotypes not detected clinically can be identified from local sewage. Hence, currently sewage surveillance is a practical way to inform us of an epidemic background of the circulating EVs in the community and to provide a warning of possible enteroviral disease outbreaks in China. Since high epidemic activity of ECHO30 has been recently reported in China, ECHO30 has the potential to rapidly spread in the future, and high-quality, continuous environmental surveillance of EVs is warranted.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by Sasagawa Medical Awards in Aid for the Japan-China Cooperation Project, a grant for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare of Japan, the Bill and Melinda Gates Foundation (OPP1039272), and the Guangdong Nature Science Foundation (S2013040015304).
We thank Xiang He for comments on the manuscript and helpful discussions.
We declare that we have no competing interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03200-14.
REFERENCES
- 1.Tapparel C, Siegrist F, Petty TJ, Kaiser L. 2013. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg M, Pallansch MA, Palmenberg AC, Simmonds P, Skern T, Stanway G, Yamashita T, Zell R. 2011. Picornaviridae, p 855–880. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom. [Google Scholar]
- 3.Dourmashkin RR, Dunn G, Castano V, McCall SA. 2012. Evidence for an enterovirus as the cause of encephalitis lethargica. BMC Infect Dis 12:136. doi: 10.1186/1471-2334-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao CD, Yergolkar P, Shankarappa KS. 2012. Antigenic diversity of enteroviruses associated with nonpolio acute flaccid paralysis, India, 2007-2009. Emerg Infect Dis 18:1833–1840. doi: 10.3201/eid1811.111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Xu WB. 2013. Molecular epidemiology of enteroviruses associated with hand, foot, and mouth disease in the mainland of China. Biomed Environ Sci 26:875–876. doi: 10.3967/bes2013.015. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Tao Z, Li Y, Lin X, Yoshida H, Song L, Zhang Y, Wang S, Cui N, Xu W, Song Y, Xu A. 2014. Environmental surveillance of human enteroviruses in Shandong Province, China, 2008 to 2012: serotypes, temporal fluctuation, and molecular epidemiology. Appl Environ Microbiol 80:4683–4691. doi: 10.1128/AEM.00851-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng H, Lu J, Zhang Y, Yoshida H, Guo X, Liu L, Li H, Zeng H, Fang L, Mo Y, Yi L, Chosa T, Xu W, Ke C. 2013. Prevalence of nonpolio enteroviruses in the sewage of Guangzhou city, China, from 2009 to 2012. Appl Environ Microbiol 79:7679–7683. doi: 10.1128/AEM.02058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrerizo M, Echevarria JE, Gonzalez I, de Miguel T, Trallero G. 2008. Molecular epidemiological study of HEV-B enteroviruses involved in the increase in meningitis cases occurred in Spain during 2006. J Med Virol 80:1018–1024. doi: 10.1002/jmv.21197. [DOI] [PubMed] [Google Scholar]
- 9.Mirand A, Archimbaud C, Henquell C, Michel Y, Chambon M, Peigue-Lafeuille H, Bailly JL. 2006. Prospective identification of HEV-B enteroviruses during the 2005 outbreak. J Med Virol 78:1624–1634. doi: 10.1002/jmv.20747. [DOI] [PubMed] [Google Scholar]
- 10.Mladenova Z, Buttinelli G, Dikova A, Stoyanova A, Troyancheva M, Komitova R, Stoycheva M, Pekova L, Parmakova K, Fiore L. 2014. Aseptic meningitis outbreak caused by echovirus 30 in two regions in Bulgaria, May-August 2012. Epidemiol Infect 142:2159–2165. doi: 10.1017/S0950268813003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savolainen C, Hovi T, Mulders MN. 2001. Molecular epidemiology of echovirus 30 in Europe: succession of dominant sublineages within a single major genotype. Arch Virol 146:521–537. doi: 10.1007/s007050170160. [DOI] [PubMed] [Google Scholar]
- 12.Akiyoshi K, Nakagawa N, Suga T. 2007. An outbreak of aseptic meningitis in a nursery school caused by echovirus type 30 in Kobe, Japan. Jpn J Infect Dis 60:66–68. [PubMed] [Google Scholar]
- 13.Faustini A, Fano V, Muscillo M, Zaniratti S, La Rosa G, Tribuzi L, Perucci CA. 2006. An outbreak of aseptic meningitis due to echovirus 30 associated with attending school and swimming in pools. Int J Infect Dis 10:291–297. doi: 10.1016/j.ijid.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhao YN, Jiang QW, Jiang RJ, Chen L, Perlin DS. 2005. Echovirus 30, Jiangsu Province, China. Emerg Infect Dis 11:562–567. doi: 10.3201/eid1104.040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.dos Santos GP, da Costa EV, Tavares FN, da Costa LJ, da Silva EE. 2011. Genetic diversity of echovirus 30 involved in aseptic meningitis cases in Brazil (1998-2008). J Med Virol 83:2164–2171. doi: 10.1002/jmv.22235. [DOI] [PubMed] [Google Scholar]
- 16.Martinez AA, Castillo J, Sanchez MC, Zaldivar Y, Mendoza Y, Tribaldos M, Acosta P, Smith RE, Pascale JM. 2012. Molecular diagnosis of echovirus 30 as the etiological agent in an outbreak of aseptic meningitis in Panama: May-June 2008. J Infect Dev Ctries 6:836–841. doi: 10.3855/jidc.2615. [DOI] [PubMed] [Google Scholar]
- 17.Mistchenko AS, Viegas M, Latta MP, Barrero PR. 2006. Molecular and epidemiologic analysis of enterovirus B neurological infection in Argentine children. J Clin Virol 37:293–299. doi: 10.1016/j.jcv.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Yan JY, Lu YY, Xu CP, Yu Z, Gong LM, Chen Y, Zhang YJ. 2011. Study on the etiological and molecular characteristics of aseptic meningitis epidemic in Zhejiang Province in 2002-2004. Bing Du Xue Bao 27:462–468. (In Chinese.) [PubMed] [Google Scholar]
- 19.Tao Z, Wang H, Li Y, Liu G, Xu A, Lin X, Song L, Ji F, Wang S, Cui N, Song Y. 2014. Molecular epidemiology of human enterovirus associated with aseptic meningitis in Shandong province, China, 2006-2012. PLoS One 9:e89766. doi: 10.1371/journal.pone.0089766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HY, Xu AQ, Zhu Z, Li Y, Ji F, Zhang Y, Zhang L, Xu WB. 2006. The genetic characterization and molecular evolution of echovirus 30 during outbreaks of aseptic meningitis. Zhonghua Liu Xing Bing Xue Za Zhi 27:793–797. (In Chinese.) [PubMed] [Google Scholar]
- 21.Xiao H, Guan D, Chen R, Chen P, Monagin C, Li W, Su J, Ma C, Zhang W, Ke C. 2013. Molecular characterization of echovirus 30-associated outbreak of aseptic meningitis in Guangdong in 2012. Virol J 10:263. doi: 10.1186/1743-422X-10-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao H, Huang K, Li L, Wu X, Zheng L, Wan C, Zhao W, Ke C, Zhang B. 2014. Complete genome sequence analysis of human echovirus 30 isolated during a large outbreak in Guangdong Province of China, in 2012. Arch Virol 159:379–383. doi: 10.1007/s00705-013-1818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. 2003. Guidelines for environmental surveillance of poliovirus circulation. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/hq/2003/who_v&b_03.03.pdf. [Google Scholar]
- 24.Iwai M, Yoshida H, Matsuura K, Fujimoto T, Shimizu H, Takizawa T, Nagai Y. 2006. Molecular epidemiology of echoviruses 11 and 13, based on an environmental surveillance conducted in Toyama Prefecture, 2002-2003. Appl Environ Microbiol 72:6381–6387. doi: 10.1128/AEM.02621-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. 2004. Polio laboratory manual, 4th ed. Document WHO/IVB/04.10. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 26.Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol 73:1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis, version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailly JL, Mirand A, Henquell C, Archimbaud C, Chambon M, Charbonne F, Traore O, Peigue-Lafeuille H. 2009. Phylogeography of circulating populations of human echovirus 30 over 50 years: nucleotide polymorphism and signature of purifying selection in the VP1 capsid protein gene. Infect Genet Evol 9:699–708. doi: 10.1016/j.meegid.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Yang XH, Yan YS, Weng YW, He AH, Zhang HR, Chen W, Zhou Y. 2013. Molecular epidemiology of echovirus 30 in Fujian, China, between 2001 and 2011. J Med Virol 85:696–702. doi: 10.1002/jmv.23503. [DOI] [PubMed] [Google Scholar]
- 31.Sedmak G, Bina D, MacDonald J. 2003. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from Milwaukee, Wisconsin, collected August 1994 to December 2002. Appl Environ Microbiol 69:7181–7187. doi: 10.1128/AEM.69.12.7181-7187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milia MG, Cerutti F, Gregori G, Burdino E, Allice T, Ruggiero T, Proia M, De Rosa G, Enrico E, Lipani F, Di Perri G, Ghisetti V. 2013. Recent outbreak of aseptic meningitis in Italy due to echovirus 30 and phylogenetic relationship with other European circulating strains. J Clin Virol 58:579–583. doi: 10.1016/j.jcv.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 33.McWilliam Leitch EC, Bendig J, Cabrerizo M, Cardosa J, Hyypia T, Ivanova OE, Kelly A, Kroes AC, Lukashev A, MacAdam A, McMinn P, Roivainen M, Trallero G, Evans DJ, Simmonds P. 2009. Transmission networks and population turnover of echovirus 30. J Virol 83:2109–2118. doi: 10.1128/JVI.02109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.