Abstract
Context:
Oxidative stress is a major determinant in carcinogenesis and serum total antioxidant capacity (TAC) indirectly reflects the level of oxidative stress. Although oral cancer is the third most frequent cancer in Indian population, there are no standard noninvasive marker for early detection and monitoring therapeutic response in oral cancer patients.
Aims:
This study was carried out to investigate implications of serum TAC in oral cancer by evaluating pre- and post-operative levels in oral cancer patients.
Settings and Designs:
It was a prospective single blinded study.
Subjects and Methods:
Serum TAC was measured using ferric-reducing antioxidant power assay. Pre- and post-operative values were estimated and compared in 30 oral cancer patients who underwent surgery.
Statistical Analysis Used:
Paired t-test was used to compare pre- and post-operative values.
Results:
Compared to the normal value, both pre- and post-operative serum TAC were significantly low in oral cancer patients. However, there was no statistically significant difference between pre- and postoperative levels.
Conclusions:
In this study, low mean serum TAC was detected in oral cancer patients. However, its diagnostic and prognostic significance in oral cancer needs further investigation.
Keywords: Cancer marker, oral cancer, squamous cell carcinoma, total antioxidant capacity
INTRODUCTION
In India, oral cancer is the third most common cancer in both sexes accounting for 69,820 new cases, and 47,653 cancer related deaths per year.[1] It is the major cause of cancer related mortality in Indian population owing to the distinct demographic profile and lifestyle.[2] Indigenous practices of tobacco consumption in various forms are directly related high incidence of oral cancer in men. However, it is preventable by simple lifestyle modification and early detection. Oral submucous fibrosis, leukoplakia and erythroplakia are precancerous conditions that often precede the development of oral cancer with a long latent period and are amenable to therapy.[3,4] Although the main diagnostic tool for oral cancer is histopathology, a noninvasive, simple, and cost-effective investigation is more likely to facilitate detection of oral cancer in early stage.
Serum total antioxidant capacity (TAC) is one of the several cancer biomarkers, which are being evaluated in different neoplastic conditions.[5,6,7,8] Reactive oxygen and nitrogen species are responsible for DNA damage and other cellular alterations, which ultimately results in cancer.[9,10,11] Both endogenous and dietary antioxidants protect cellular microenvironment from these oxidative damages and thus prevent cancer. A low serum TAC has been reported to have a strong association with various cancers.[5,6,7] However, there are inadequate published data to validate the significance of serum TAC in oral cancer patients as a cancer biomarker in Indian population. This study was carried out to investigate implications of serum TAC in oral cancer.
SUBJECTS AND METHODS
A prospective study was carried out in Department of Otorhinolaryngology in a tertiary care hospital in South India from October, 2009 to July, 2011. Ethical clearance was obtained from the Ethics Committee of the Institute (K. S. Hegde Medical Academy, Karnataka, registered on September, 2009 and approved on October, 2009).
Single blinding was carried out by concealing the serum TAC levels of oral cancer patients from pathologists and the identification of cases and controls from laboratory personnel. Thirty patients with histopathologically confirmed oral malignancies, who were admitted and underwent surgical therapy during the study period and had given consent to participate in this study, were included in the study group. Informed consent was obtained from all participating individuals. Metastatic lesions of bone, liver and lung were ruled out by X-ray chest and ultrasonography of the abdomen. Contrast enhanced computed tomography was employed to diagnose the lesions that appeared to be metastasis in chest X-ray and abdominal ultrasound. Oral cancer staging was done as per American Joint Committee on Cancer (AJCC 2002) staging system. Serum TAC was estimated preoperatively and postoperatively (3 weeks after surgery) for all patients. Serum TAC was estimated by determined by ferric-reducing antioxidant power assay.[9] It is a simple, inexpensive, automated colorimetric assay for assessing antioxidant capacity of biological fluids. The ability of plasma to reduce ferric compound is the principle of this method. It measures the change in absorbance when the intense blue color of ferric tripyridyltriazine complex is reduced to its ferrous form by plasma antioxidants.
Statistical analysis of clinical and laboratory data was done in SPSS statistical software version 17.0 (International Business Machines Corporation (IBM), USA). Paired t-test was used to compare pre- and post-operative values of serum TAC. All P < 0.05 were considered statistically significant.
RESULTS
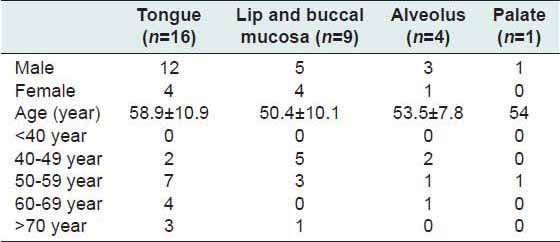
The mean age of patients was 55.5 ± 10.5 years. Among these cancer patients, 21 were male and 9 female. The mean ages of male and female patients were 57.1 ± 11.4 and 51.7 ± 7.2 years respectively. No oral precancerous lesions were noted during the study. The most common site of malignancy was tongue (n = 16, 53.3%), followed by lip and buccal mucosa, alveolus and palate [Table 1]. The postoperative period was uneventful without any complication. In 26 cases (n = 86.6%), surgical wound healed within 2 weeks and for remaining four patient it took 3 weeks. Radiation therapy of 55–65 grays was administered postoperatively to stage III (n = 6) and stage IV (n = 4) cancer patients. One patient with stage III oral carcinoma received both postoperative chemotherapy and radiation therapy as adjuvant treatment. The patients enrolled in the study were followed up for a period of 1-year. There was no mortality during this period. However, we could not estimate serum TAC again because of economic constraints and lack of patient compliance.
Table 1.
Site-wise distribution of oral carcinoma
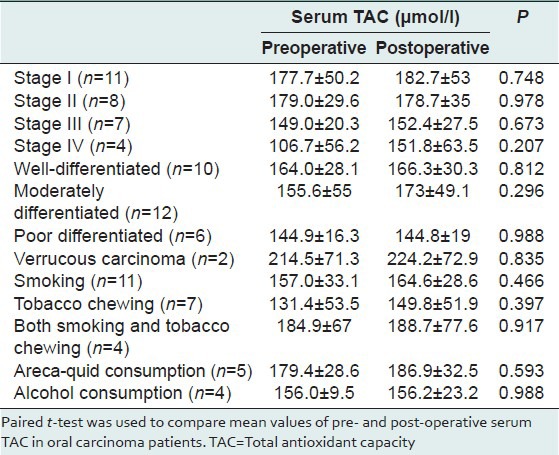
We found the mean value of serum TAC in oral cancer patients was161.9 ± 46.1 μmol/l. The pre- and post-operative mean value of serum TAC in these patients were 161.9 ± 46.1 and 170.5 ± 45.1 μmol/l respectively (two tailed P = 0.315). Among these cases, early stage carcinoma (stages I and II) was more common (n = 19, 63.3%). In histology, four different types of squamous cell carcinoma (SCC) were detected viz. well-differentiated (n = 10), moderately differentiated (n = 12), poorly differentiated (n = 6) and verrucous carcinoma (n = 2). The pre- and post-operative serum TAC showed variation in different stages and histological types of cancer [Table 2]. In our study, the habits of smoking (n = 11), tobacco chewing (n = 7), both smoking and tobacco-chewing (n = 4), areca-quid (n = 5) and alcohol consumption (n = 4) were common among oral cancer patients.
Table 2.
Comparison of pre- and post-operative serum TAC in oral carcinoma patients
DISCUSSION
Antioxidants are cyto-protective chemicals that prevent the oxidative damage caused by free radicals.[9] Reactive oxygen and nitrogen species are oxygen and nitrogen derived free radicals are generated naturally as by-products of cellular metabolism. Under physiological conditions, the free radicals are rapidly neutralized by antioxidants. In oxidative stress, these free radicals are not counterbalanced adequately and remain in excess to cause damage by reacting with nucleic acids, proteins, polyunsaturated fatty acids and carbohydrates. Oxidative damage is implicated in the pathogenesis of several medical disorders viz. atherosclerosis, cancer, arthritis and neurodegenerative disorders.[9] DNA damage and anomalous DNA repair mechanisms are key factors in the pathogenesis of cancer. Hence, investigators have evaluated the association between a low antioxidant level and various malignant and premalignant conditions.
Oral cancer is strongly related to tobacco use and its associated oxidative damage.[12,13,14] Tobacco smoke contains free radicals that induce oxidative damage. p53 mutations frequently reported in tobacco related cancers.[12] The distribution of oral cancer in various countries correlates well with the habit of tobacco consumption.[4,15] India contributes a major portion of global oral cancer burden which may be attributed to the large population and common practices like intake of tobacco in various forms. The second commonest malignancy in men after lung cancer in India is oral carcinoma.[1,16] According to Globocan 2008, the estimated age-standardized incidence and mortality rates in Indian men were 45,445 and 31,102/year.[1] We found oral cancer was more in male patients with a male: Female ratio of 2.3:1. This is in accordance with other studies where investigators have documented a male preponderance in oral cancer.[2,17] In India, due to lack of diagnostic infrastructure oral cancers are frequently diagnosed at advanced stage.[3] At their first presentation, the mean age of patients in this study was 55.5 ± 10.5 years. The mean age of males (57.1 ± 11.4 years) was higher than female patients (51.7 ± 7.2 years).
A major proportion of oral malignancy occurs among smokers and tobacco chewers. We found 73.3% (n = 22) patients had habit of tobacco. Among them, 11 were smoker, 7 were tobacco chewer, and 4 had both these habits. In this study, 4 patients were chronic alcoholic, and 5 had this habit of areca-quid. Similar risk factors were reported in various other studies.[17,18] National Sample Survey Organization data from India have shown that the tobacco consumption has decreased documented an overall drop in incidence of carcinoma of the oral cavity in urban as well as a rural community in India.[2] This may be attributed to a reduction in the use of tobacco and the beneficial effect of healthcare education. However, oral cancer rate in rural women was reported to be constant.
In general, an oral cavity cancer often follows premalignant lesions.[4] Oral submucus fibrosis and leukoplakia typically herald oral cancer and respond well to therapy. Leukoplakia is the most common type having 0.2–5.2% prevalence and 0.13–10% malignant transformation rates in Indian population.[3] However, no oral precancerous lesions were detected during our study. Among different types of oral SCC, moderately differentiated carcinoma was 40% (n = 12). Oral verrucous carcinoma is a relatively uncommon tumor. It is a special form of well-differentiated SCC that is locally invasive and has a low potential to metastasize to remote sites.[19] These lesions may also develop from leukoplakia. In this study, 6.6% (n = 2) cases were attributed to verrucous carcinoma. Mehrotra et al. studied 759 oral biopsies and found 35.1% were benign, 16.7% premalignant and 39.9% malignant lesions in North India.[17] Among the malignant lesions, well-differentiated carcinoma was the commonest (22.6%). Similar findings also reported elsewhere.[18] In the present study, tongue and buccal mucosa were the most common sites of malignant lesions [Table 1]. This finding is in keeping with other studies.[17,18] Tongue is reported to be the commonest site of involvement in oral cancer in Indian population.[2]
We have analyzed the pre- and post-operative serum TAC in different stages and histological types of oral carcinoma [Table 2]. There was no statistically significant association in pre- and post-operative serum TAC (P > 0.05). Sener et al. reported a significantly lower-levels of TAC in patients with breast cancer in comparison to controls.[20] Habitual consumptions of alcohol, cigarettes, or areca-quids have been implicated in the pathogenesis of oral carcinoma.[13] Furthermore, tobacco and areca-quids were reported to reduce the serum TAC.[21] We analyzed pre- and post-operative serum TAC in patients with smoking, tobacco, areca-quid and alcohol habits and found marginal increase in serum TAC from the preoperative level after surgery in all patients irrespective of the nature of their habits. Although the results were not statistically significant (P > 0.05), patients with tobacco habits had the minimum mean value of serum TAC (131.4 ± 53.5 μmol/l).
Several investigators also evaluated the role dietary antioxidant capacity in different malignancies and reported that dietary antioxidant capacity was associated with a reduction in the risk of gastric and colorectal cancer.[8,22] Antioxidant capacity in diseased tissue was also evaluated in different studies since it is more likely to reflect the association with malignant changes better than serum TAC. However, the findings showed wide variation different malignancies. Korde et al. found depletion of TAC in serum and tissues of oral carcinoma patients when compared to controls.[5] Whereas, Kocot et al. reported no significant difference in mean TAC value in colorectal cancer tissue from the mean value in healthy tissue.[7] The normal range of serum TAC for healthy adults is 300–460 μmol/l.[23] We found the mean value of serum TAC in our patients was substantially lower than the normal value. This implies that serum TAC could have potential diagnostic value in oral carcinoma. Lack of significant difference in pre- and post-operative serum TAC may be attributed to the fact that low serum TAC is the predisposing factor rather than the pathological effect of cancer. Consequently, it may not change drastically even if the cancer is treated. Small sample size was a limitation of the study. No power analysis was done. Because of cost constraints serum TAC could not be evaluated in a larger patient group, which could have affected the findings or might result in the lack of statistical significance.
CONCLUSION
In this study, a lower mean serum TAC was observed in oral cancer patients. However, lack of significant difference in pre- and post-operative serum TAC was noticed in different stages and histological types of oral carcinoma. The results of this study indicate the need for future research on diagnostic implications of serum TAC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. [Last accessed on 2014 Jun 21]. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. Available from: http://www.globocan.iarc.fr . [Google Scholar]
- 2.Elango JK, Gangadharan P, Sumithra S, Kuriakose MA. Trends of head and neck cancers in urban and rural India. Asian Pac J Cancer Prev. 2006;7:108–12. [PubMed] [Google Scholar]
- 3.Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4:29–35. [Google Scholar]
- 4.Shah M, Telang S, Raval G, Shah P, Patel PS. Serum fucosylation changes in oral cancer and oral precancerous conditions: Alpha-L-fucosidase as a marker. Cancer. 2008;113:336–46. doi: 10.1002/cncr.23556. [DOI] [PubMed] [Google Scholar]
- 5.Korde SD, Basak A, Chaudhary M, Goyal M, Vagga A. Enhanced nitrosative and oxidative stress with decreased total antioxidant capacity in patients with oral precancer and oral squamous cell carcinoma. Oncology. 2011;80:382–9. doi: 10.1159/000329811. [DOI] [PubMed] [Google Scholar]
- 6.Ching S, Ingram D, Hahnel R, Beilby J, Rossi E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J Nutr. 2002;132:303–6. doi: 10.1093/jn/132.2.303. [DOI] [PubMed] [Google Scholar]
- 7.Kocot J, Kielczykowska M, Dabrowski W, Pilat J, Rudzki S, Musik I. Total antioxidant status value and superoxide dismutase activity in human colorectal cancer tissue depending on the stage of the disease: A pilot study. Adv Clin Exp Med. 2013;22:431–7. [PubMed] [Google Scholar]
- 8.Serafini M, Jakszyn P, Luján-Barroso L, Agudo A, Bas Bueno-de-Mesquita H, van Duijnhoven FJ, et al. Dietary total antioxidant capacity and gastric cancer risk in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2012;131:E544–54. doi: 10.1002/ijc.27347. [DOI] [PubMed] [Google Scholar]
- 9.Badarinath A, Rao KM, Chetty CM, Ramkanth S, Rajan T, Gnanaprakash K. A review on in-vitro antioxidant methods: Comparisions, correlations and considerations. Int J PharmTech Res. 2010;2:1276–85. [Google Scholar]
- 10.Hu ML. Dietary polyphenols as antioxidants and anticancer agents: More questions than answers. Chang Gung Med J. 2011;34:449–60. [PubMed] [Google Scholar]
- 11.Abdolsamadi HR, Goodarzi MT, Mortazavi H, Robati M, Ahmadi-Motemaye F. Comparison of salivary antioxidants in healthy smoking and non-smoking men. Chang Gung Med J. 2011;34:607–11. [PubMed] [Google Scholar]
- 12.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–51. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 13.Bose KS, Gokhale PV, Dwivedi S, Singh M. Quantitative evaluation and correlation of serum glycoconjugates: Protein bound hexoses, sialic acid and fucose in leukoplakia, oral sub mucous fibrosis and oral cancer. J Nat Sci Biol Med. 2013;4:122–5. doi: 10.4103/0976-9668.107275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addala L, Pentapati CK, Reddy Thavanati PK, Anjaneyulu V, Sadhnani MD. Risk factor profiles of head and neck cancer patients of Andhra Pradesh, India. Indian J Cancer. 2012;49:215–9. doi: 10.4103/0019-509X.102865. [DOI] [PubMed] [Google Scholar]
- 15.Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667–75. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra R, Singh M, Kumar D, Pandey AN, Gupta RK, Sinha US. Age specific incidence rate and pathological spectrum of oral cancer in Allahabad. Indian J Med Sci. 2003;57:400–4. [PubMed] [Google Scholar]
- 18.Iype EM, Pandey M, Mathew A, Thomas G, Sebastian P, Nair MK. Oral cancer among patients under the age of 35 years. J Postgrad Med. 2001;47:171–6. [PubMed] [Google Scholar]
- 19.Alkan A, Bulut E, Gunhan O, Ozden B. Oral verrucous carcinoma: A study of 12 cases. Eur J Dent. 2010;4:202–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Sener DE, Gönenç A, Akinci M, Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct. 2007;25:377–82. doi: 10.1002/cbf.1308. [DOI] [PubMed] [Google Scholar]
- 21.Bose KS, Vyas P, Singh M. Plasma non-enzymatic antioxidants-vitamin C, E, beta-carotenes, reduced glutathione levels and total antioxidant activity in oral sub mucous fibrosis. Eur Rev Med Pharmacol Sci. 2012;16:530–2. [PubMed] [Google Scholar]
- 22.La Vecchia C, Decarli A, Serafini M, Parpinel M, Bellocco R, Galeone C, et al. Dietary total antioxidant capacity and colorectal cancer: A large case-control study in Italy. Int J Cancer. 2013;133:1447–51. doi: 10.1002/ijc.28133. [DOI] [PubMed] [Google Scholar]
- 23.Toescu V, Nuttall SL, Martin U, Nightingale P, Kendall MJ, Brydon P, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci (Lond) 2004;106:93–8. doi: 10.1042/CS20030175. [DOI] [PubMed] [Google Scholar]


