Abstract
Objective:
To evaluate the anticancer potential of seeds of Nigella sativa using MCF and HepG2 cell lines along with its mechanism of action.
Materials and Methods:
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and acridine orange/ethidium bromide nuclear staining technique were selected to evaluate anticancer potential and mechanism of action of test extract.
Results:
Aqueous extract of N.sativa at a test dose of 180 mg and 300 mg was identified to be the best as anticancer agent against MCF and HepG2 cell lines among different solvent test extract where doxorubicin and cisplatin were employed as standard references.
Discussion:
Further study including separation and characterization of active principles in the aqueous extract shall prove beneficial.
Keywords: Anti-neoplastic, HepG2, human breast adenocarcinoma cells, Nigella sativa seeds, Nuclear staining
INTRODUCTION
Controlled proliferation of living cells is the secret of growth and development in living organisms when once body loses control over the cell growth becomes a major problem for mankind and termed as cancer. It is considered as second major cause of death after myocardial infarction as millions of people die every year despite tremendous amount of efforts and economy is placed for the development of possible cure to it.[1]
Breast cancer and liver cancer are the second and fifth leading cause of death from cancer, in men and women in the United States. In Britain as many as 20% of cases of cancer were breast cancer, as well as causing the most deaths in women who falls in the age group of 35–55 years. The hospital data indicate that breast cancer ranked first among other cancers in women[1] and hepatic cancer is the fifth most life threatening type of cancer with the incidence and mortality rates of 554,344 and 536,904 cases globally.[1] In India, breast cancer is the second most common type of cancer. A survey carried by Indian Council of Medical Research for a period of 23 years has revealed that the incidences of breast cancers have doubled in last few years. As much as 100,000 new cases of breast cancer are being detected every year.[2] Cancer is a condition in which cells have lost normal growth control mechanisms, which results in a unusual, fast and control less growth of cells, which is a neoplastic disease derived from the parenchyma or connective tissues.
Totally, 4.2 million new cancer cases which accounts for about 39% of new cases worldwide, were diagnosed among 3.2 billion persons about 48% of the world's population, living in the developing countries in Asia.[3] About 22.9% cases in India, 21.97% cases in USA, 26.0% cases in China, 26.08% cases in European union, 54.41% cases in African region, 25.6% cases in American region were identified as cancer cases. In the year 2008; the incidence of cancer in USA was 182,000 and in India was 115,000. Cancer cases in India seem to be less in comparison to USA, but it is equivalent to 2/3rd of cancer burden of USA.[4] The number of deaths because of cancer in India may climb up to 106,124 by 2015 1,23,634 by 2020. The cancer incidence is the highest in Christian and Parsi community whereas lowest in Hindu and Muslim community.[5]
Various kinds of anticancer treatments are available including surgery, chemotherapy, radiation therapy but none is free of unwanted effects like bone marrow depression, secondary infections, nausea, vomiting, internal bleeding, etc. Hence, it has become a need to explore other forms of medicine to derive active ingredients that shall treat cancer with least or no side effects.[6]
MATERIALS AND METHODS
Chemicals
Petroleum ether (Merck Specialities. Pvt. Ltd. Mumbai. India.) was used for the preparation of pet ether extract designated as Pet. ether extract of N.sativa (PNS); chloroform (NICE Chemicals Pvt. Ltd., India) was used to prepare chloroform extract and designated as Chloroform extract of N.sativa (CNS); ethanol (analytical CSC reagent, Changshu Yangyuan Chemicals Pvt. Ltd., China) was used to prepare ethanolic extract and designated as Ethanolic extract of N.sativa (ENS) and distilled water was used to prepare aqueous extract designated as Aqueous extract of N.sativa (ANS). Trypan blue, triton X-100, dimethyl sulfoxide culture grade, sodium bicarbonate, streptomycin (100 μg/ml), amphotericin – B (5 μg/ml) and penicillin (100 IU/ml) antibiotic mixture, ethylenediamine tetraacetic acid (EDTA), Dulbecco's phosphate buffered saline (DPBS) modified (DPBS without cationic elements), trypsin, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) kit, doxorubicin (IC50 study) and cisplatin (nuclear staining).
Media
Dulbecco's modified Eagles medium (DMEM), fetal bovine serum (FBS), serum free media.
Cell lines
MCF (human breast adenocarcinoma cells), HepG2 (hepatocellular carcinoma cells). All the cell lines were procured from National Centre for Cell Sciences (NCCS), Pune.
Plant material
The seeds of Nigella sativa were obtained from the medicinal gardens of Raghavendra Institute of Pharmaceutical Education and Research, in the month of January 3 months after sowing. After shade drying the seeds were authentified by Dr. C. Sudhakar, Professor, Department of Botany, Sri Krishna Devaraya University, Anantapur.
Preparation of extract
The different solvents successive extractions of seeds of N. sativa were carried out through cold maceration in an earthen pot. A volume of 200 g of fine powder of seeds was soaked into 1000 ml of each solvent successively for 7 days, menstrum was removed under reduced pressure and the extract was preserved for further use.
Preparation of Dulbecco's modified Eagles medium media
Eleven grams of DMEM powder was added in 1 l of distilled water and was shaken till solution without sediment was formed. Sodium bicarbonate was added to maintain pH around neutral and also to supply CO2 to the medium. The solution was then filtered to prevent any solid substances through filtration assembly. About 10% FBS and 1 ml of each antibiotic solution were added to the preparation.
The cultures were viewed under microscope to make sure that the cultures are free from bacterial and fungal contamination. Cells layers were washed with phosphate buffer saline (PBS) bearing volume half equivalent that of culture medium. EDTA was added to the culture cells, and the flask was circularly rotated to distribute the added substance equally and incubated for another 5–10 min in the incubator. The cells were resuspended in a small volume of fresh serum. 50–100 μl of preparation was removed to perform cell count. Desired number of cells was removed from the flask and study was carried out.
The extracts of “N. sativa” were selected to evaluate against the MCF-7 (human breast adenocarcinoma cells) and HepG2 (hepatocellular carcinoma cells) cell lines for its anticancer potency in uncontrolled dividing cells. Well-defined MTT in vitro micro culture assay was selected for the study. The extract with good anticancer potency among the selected extracts was further subjected to nuclear staining assay also.
Micro culture tetrazolium (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay
HepG2 and MCF-7 cell lines were procured from NCCS, Pune, India. The cells were cultured in minimum essential growth medium that is, DMEM medium supplemented with 10% heat inactivated FBS including streptomycin (100 μg/ml), amphotericin – B (5 μg/ml) and penicillin (100 IU/ml) in a humidified atmosphere of 5% CO2 at 32°C until confluent. The cells were subjected to trypsinization of the cells with trypsin phosphate versene glucose solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS) the stock cultures were grown in 25 cm2 flat bottle and the studies were carried out in 96 well microtiter plates.[7]
Micro culture tetrazolium (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay
Cells were plated in 96 well flat bottom microtiter plate at a density of 10,000 cells per well and cultured for 24 h at 37°C in 5% CO2 atmosphere to allow cell adhesion. After 24 h, when partial monolayer was formed, medium was removed, and cells were treated with different concentration of standard drug (doxorubicin) and sample compounds for 48 h. Microscopic examination was carried out, and observations recorded every 24 h. After the treatment, the solutions in the wells were discarded, and 50 μl of freshly prepared MTT (2 mg/ml, prepared in PBS) was added to each well. The plates were shaken gently and incubated for 3 h at 37°C in 5% CO2 atmosphere. After 3 h, the supernatant was removed, and the formazan crystals formed in the cells were solubilized by addition of 50 μl of iso-propanol. Finally, the absorbance was read using a micro-plate reader (Bio-Tek, ELX-800 MS) at a wavelength of 540 nm.[7]
The percentage growth was calculated using the formula below:

Percentage growth inhibition versus concentration graph was plotted and from curve IC50 (concentration of drug required to kill 50% of cells in exponentially growing cultures after a 48 h and exposure to the drug) values can be calculated.
Nuclear staining (acridine orange/ethidium bromide) assay
Totally, 50,000 MCF-7 cells were seeded in each well of 24 well plates with culture medium containing 10% FBS. After 24 h, cells were treated with drugs for 48 h. The plates were incubated at 37°C in 5% CO2 atmosphere. After exposure of cells with drugs below CTC50 values, cells were fixed with 1 ml of methanol (90%) at −20°C for 20 min. The methanol was removed and air-dried. Fixed cells were washed with ice cold PBS 2–3 times. Plate was washed with PBS 2–3 times and 200 μl of acridine orange/ethidium bromide (10 μg/ml in PBS pH-7.4) was added and incubated at 37°C for 20 min. The plate was washed thrice with PBS and observed under fluorescent microscope for any nuclear changes.[8]
RESULTS
Different solvents extracts of seeds of N. sativa was subjected to test using two different cell lines to determine their IC50 value. Later, the study was designed to carry out nuclear staining to determine the mechanism of action of the most potent extract of all, tested for their anticancer potency.
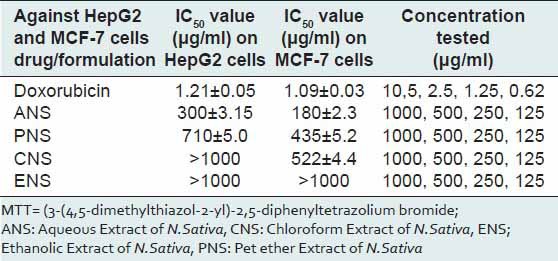
The IC50 values reveal that aqueous extract of N. sativa produced anticancer activity at a lowest concentration (MCF cell lines 180 μg/ml and HepG2 cell lines 300 μg/ml) among all the four extracts screened, the results obtained were very close to the standard drug selected Doxorubicin and the nuclear staining studies has shown that aqueous extract of N. sativa has successfully produced inhibition of growth of MCF cell lines with an equivalent potency as that of the standard cisplatin. Cells with green illuminating light like appearance show the apoptotic stage of cell cycle. The drug causes the self-death of cancer cells, which is considered as the beneficial side of anticancer application of the test agent [Figures 1-4].
Figure 1.
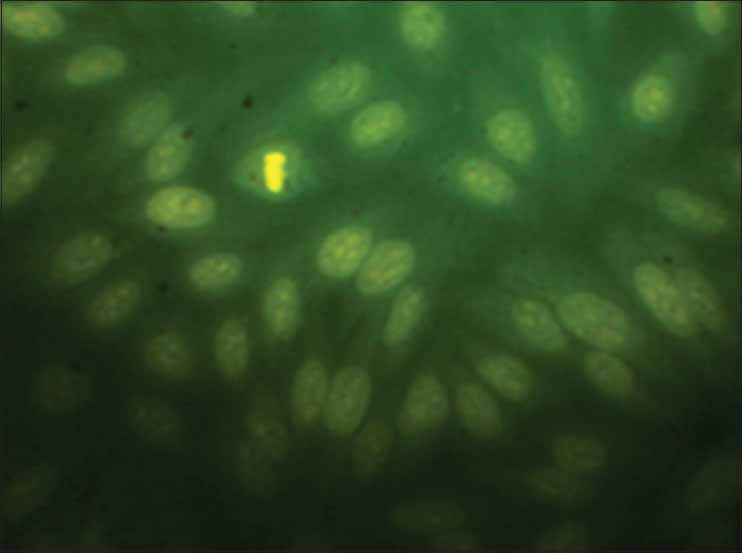
MCF cells without standard or test substances
Figure 4.
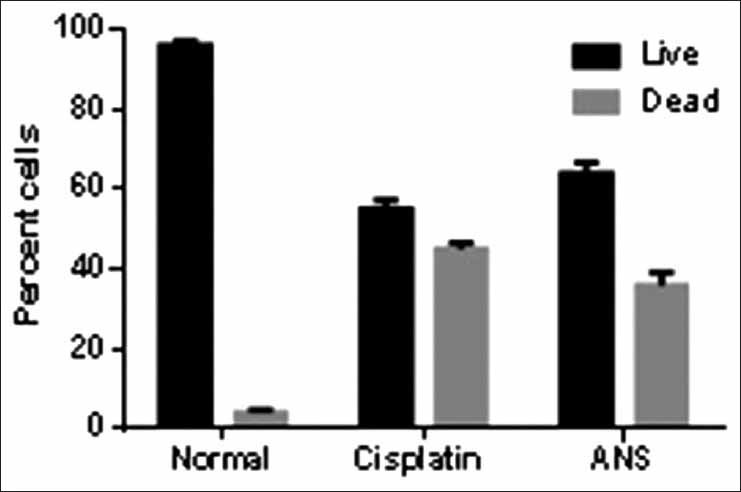
Comparative graphical representation of effect of standard and test drug on the MCF cell lines
Figure 2.
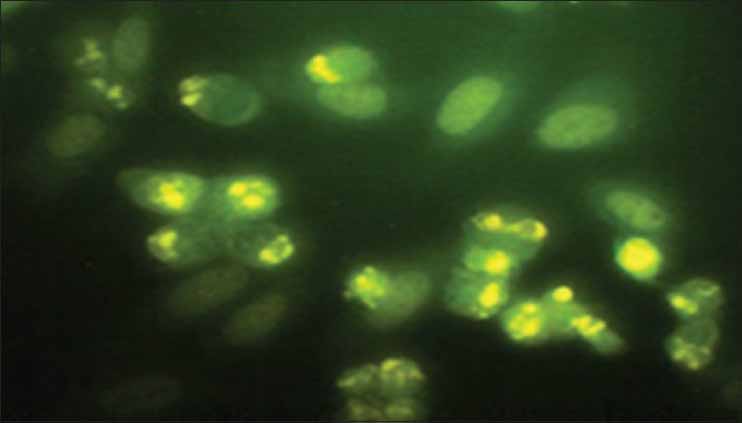
Cisplatin treated (standard drug)
Figure 3.
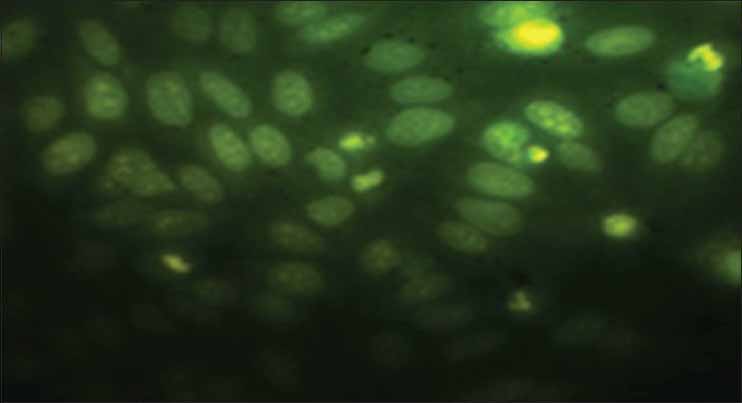
Autonomic nervous system treated (test drug)
DISCUSSION
The preliminary phytochemical study of extracts of N. sativa has revealed the presence of Volatile oils, fixed oils, steroids, flavonoids, carbohydrates, proteins, flavonoids, alkaloids, glycosides, tannins, saponins, etc., basing upon their chemistry and nature. A work proposed by Sharma et al.,[9] explains that the pet ether extract gave negative result when tested for the presence of flavonoids whereas, the present study has revealed the presence of flavonoids in the pet ether extract as well. On contrary, similar work proposed by Ishtiaq et al.[10] explained the presence of flavonoids in alcoholic and chloroform extracts and the present work explains the absence of flavonoids in alcoholic and chloroform extracts.
After completion of the study, it is successfully established that the ANS is the most potent anticancer test extract subjected to the test in this study. The ANS at different strengths ranging from 125 to 1,000 mg were put to the test against MCF and HepG2 cell lines. A curve was plotted using concentration to absorbance, and IC50 value was calculated from the graph [Figures 1-4].
Nuclear staining has revealed the Aqueous extract of N.sativa at a concentration of 50 mg/ml has produced noteworthy cell growth inhibition. The cells appearing as small illuminating fragments over dark green background shall be considered as apoptotic cells.
One probable mechanism which may better explain the activity of Aqueous extract of N.sativa is that the organic extract is a good source of elemental calcium, magnesium etc., and if the calcium concentration is triggered high inside the cell it definitely kick starts the cell death process through necrosis or apoptosis. On the other hand, it may develop changes intracellularly that further triggers suicidal enzymes that in turn kills the cell through apoptosis.[11]
A study published by Raval et al. 2010, on anticancer activity of seeds of N. sativa on three different cell lines has shown that the methanolic extract has produced noteworthy anticancer potency at 13.70 mg/ml in HL-60 cell lines (human promyelocytic leukemia cells) and at 28.31 mg/ml in U-397 cell lines[12] (monocyte cell line). Whereas in our study ethanolic extract of seeds of N. sativa has shown cell inhibitory potency at a concentration of more than 1000 mg/ml in both MCF and HepG2 cell lines.
Dilshad et al. 2012, has reported that the methanolic extracts of seeds of N. sativa on MDA-MB-231 (human breast cancer cell lines) has shown anti cell proliferative activity at 2.5–5 mg/ml, whereas in our study ethanolic extract was not much active.
A review work published by Al-Khalaf et al. 2013 has suggests that seeds of N. sativa is active as anti-proliferative against ehrlichascitis carcinoma, Daltons lymphoma ascitis, and sarcoma – 180 cells at a concentration of 1.5, 3 and 4.5 mg respectively.[13] The extracts ability to produce anti-neoplastic activity may be attributed to the fact that the active principles are directly inhibiting the DNA synthesis, whereas in our study it revealed that the death of uncontrolled proliferating cells is mainly because of necrosis or apoptosis.
The studies conclude that the extracts of seeds of N. sativa are heavily active against various cancer cell lines in vitro. Methanolic extract being more potent in case of literature survey whereas our study has revealed that the ethanolic extract was the least potent and aqueous extract was observed to be most potent against uncontrolled proliferating cells. The said anti-neoplastic activity of seeds of N. sativa may be attributed to the presence of flavonoids that are potentially beneficial for human survival.[13]
Reasons for the difference in the study results are primarily the seeds of N. sativa, which were used in our study was naturally grown product of medicinal gardens and in other reviewed studies the seeds were procured commercially from the market, secondarily the research work was carried out at geographically different regions so there might be a possible chance of developmental difference expressed in terms of chemical constituents, thirdly different cell lines were selected in the various reviewed studies which is also may be another reason for different in the outcome of the studies.
The study has proved that the cell death may be because of apoptosis or necrosis, whereas in published work it is confirmed that the cell death was because of DNA damage. Hence, a further in sighted study into this topic is required so as in the future the principle active ingredients shall be separated and studied for the same study [Table 1].
Table 1.
MTT assay: IC50 values of doxorubicin and sample compounds against HepG2 and MCF-7 cells
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. World Health Organization; 2001. [Last accessed on 2014 Apr 10]. Cancer incidence, mortality and survivalby site for 14 regions of the world. Global Programme on Evidence for Health Policy Discussion; pp. 3–49. Available from: http://www.who.int/evidence . [Google Scholar]
- 2.Imran A, Wani WA, Saleem K. Cancer scenario in India with future perspectives. Cancer Ther. 2011;8:56–70. [Google Scholar]
- 3.Murthy NS, Mathew A. Cancer epidemiology, prevention and control. Curr Sci. 2004;86:518–27. [Google Scholar]
- 4.Globocon 2008 Data: Number of Cases of Breast Cancer in India. Breast Cancer Incidence Comparision: INDIA – US. [Last accessed on 2014 Apr 10]. Available from: http://www.breastcancerindia.net/bc/statistics/stat_global.htm .
- 5.Dhillon PK. South Asia Network for Chronic Diseases, Public Health Foundation of India. Breast Cancer Factsheet. [Last accessed on 2014 Apr 10]. Available from: http://www.saned.org/breast%20cancer%20factsheet%2003.11.11.pdt .
- 6.Ekowati H, Prasasti E, Rastuti U. The active fraction from Nigella sativa and its activity against T47D cell line. Indones J Chem. 2011;11:217–22. [Google Scholar]
- 7.Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] Assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999;65:3727–9. doi: 10.1128/aem.65.8.3727-3729.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawara A, Nathan CF. A simple method for counting adherent cells: Application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983;56:261–8. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- 9.Sharma NK, Ahirwar D, Gupta S, Jhade D. Pharmacognostic standardization, physico and phytochemical evaluation of Nigella sativa linn. Seed. Int J Pharm Sci Res. 2011;2:713–8. [Google Scholar]
- 10.Ishtiaq S, Ashraf M, Hayat MQ, Asrar M. Phytochemical analysis of Nigella sativa and its antibacterial activity against clinical isolates identified by ribotyping. Int J Agric Biol. 2013;15:1151–6. [Google Scholar]
- 11.Dilshad A, Abulkhair O, Nemenqani D, Tamimi W. Antiproliferative properties of methanolic extract of Nigella sativa against the MDA-MB-231 cancer cell line. Asian Pac J Cancer Prev. 2012;13:5839–42. doi: 10.7314/apjcp.2012.13.11.5839. [DOI] [PubMed] [Google Scholar]
- 12.Raval BP, Shah TG, Patel JD, Patel BA, Patel RK, Suthar MP. Potent anticancer activity of Nigella sativa seeds. Arch Appl Sci Res. 2010;2:52–6. [Google Scholar]
- 13.Al-Khalaf MI, Ramadan KS. Antimicrobial and anticancer activity of Nigella sativa oil – Review. Aust J Basic Appl Sci. 2013;7:505–4. [Google Scholar]


