Abstract
Background:
A number of plants have been used in Indian system of medicine such as ayurveda, unani and siddha, but most of these plants were not explored properly. Sphaeranthus amaranthoides (SA) Burm.f., is one such plant used as an energizer in siddha.
Objective:
To evaluate the anticancer effect of chloroform extract of the whole plant of SA Burm.f. against Ehrlich ascites carcinoma bearing Swiss albino mice.
Materials and Methods:
The anticancer effect of chloroform extract was investigated in Swiss albino mice bearing Ehrlich ascites carcinoma at two different dose levels. Acute toxicity studies were also performed to determine the safety of the extract. Mice injected with Ehrlich ascites carcinoma cells were treated with the extract of doses 200 and 400 mg/kg body weight and standard 5-fluorouracil 20 mg/kg body weight for 15 consecutive days. Animals were sacrificed on day 15 for determination of anticancer activity by evaluating tumor volume, nonviable and viable tumor cell count and hematological parameters.
Results:
Mice treated with the extract showed a significant decrease in tumor volume and viable cell count and an increase in nonviable cell count and mean survival time. The hematological parameters were also found to be restored to a normal level.
Conclusion:
The results indicate that the chloroform extract was producing anticancer activity comparable with that of the standard 5-fluorouracil.
Keywords: Antitumor, ehrlich ascites carcinoma, Sphaeranthus amaranthoides Burm.f
INTRODUCTION
Cancer is the leading cause of mortality next to cardiovascular disorders. It causes around 6 million death per year, and this number may increase up to 11.5 million by 2030 worldwide.[1] More than 60% of currently used anti-cancer agents are obtained from natural sources, like plants, micro-organisms, and marine-organisms. Molecules obtained from natural sources have played and continue to play a main role in the invention of leads for the development of conventional drugs for the treatment of a number of human diseases.[2]
Sphaeranthus amaranthoides (SA) Burm.f., (Tamil-Sivakaranthai) a small procumbent herb belonging to the family Asteracea, was used to cure skin diseases, eczema, acne dermatitis, and also removes kapha, vata and piles. The flowers of the plant produce cooling and tonic effect. Seeds and root are used as stomachic and anti-helminthic.[3] Powdered leaf is used for the treatment of urethral discharges and jaundice. The plant was reported to have biological activities such as analgesic, sedative and anti-inflammatory activity.[4] The plant was also active against a number of bacteria.[5,6] To investigate the potential role of SA Burm.f. as an anticancer agent, in this study the chloroform extract of the whole plant was tested against Ehrlich's ascites carcinoma (EAC) in Swiss albino mice.
MATERIALS AND METHODS
The entire plant of SA Burm. f. was collected from Thuthukudi District of Tamil Nadu in the month of September 2010 and was identified and authenticated by Mr. V. Chelladurai, Retired research officer, Botany, CCRAS, Government of India. A voucher specimen of the plant was preserved in the department for future studies. Air-dried plant material was extracted with solvents of increasing polarity such as petroleum ether, chloroform, ethyl acetate and methanol in succession using soxhlet extraction apparatus. After extraction, the solvents were removed under reduced pressure and stored in a vacuum desiccator until use. The chloroform extract was selected for in vivo anticancer studies as it showed a good anti-proliferative activity in MTT assay. Preliminary phytochemical studies showed the presence of flavonoids, tannins and alkaloids in the chloroform extract.
Acute toxicity study
Acute toxicity studies were performed as per OECD 423 guidelines. Swiss albino mice (18 ± 2 g) (male, n = 3), maintained at a temperature of 22°C ± 2°C, fed with standard pelleted feed and water ad libitum was used for the study. The animals were kept fasting for overnight provided only with water. Then, the extract was administered orally by gastric intubation and observed for 14 days for toxic symptoms like behavioral change, locomotion, convulsion, and mortality.
Anticancer studies
Ehrlich's ascites carcinoma cell line was procured from National Centre for Cell Science (Pune, India). Cells were grown in minimum essential medium supplemented with 15% Fetal Bovine serum and penicillin/streptomycin (100 IU, 100 mg/ml, respectively) at 37°C in 5% CO2. Experiment was performed in 60 mm petri plates with cells seeded at a density of 200,000 cells/plate and incubated at 37°C with humidified atmosphere till cells reached confluence.[7]
In vitro cytotoxicity assay
Pre-confluent EAC cells were seeded in 96-well plates at a density of 8,000 cells/200 μl/well. Cells were treated with different concentrations of chloroform extract after 24 h following plating and incubated at 37°C for one day. At 20 h following drug exposure, the cells were incubated at 37°C with 5 mg/ml MTT for 4 h. At the end of the experiment, the medium was removed, and the insoluble formazan product was dissolved in dimethyl sulfoxide (200 μL) and kept at least 15 min in the dark. MTT reduction was quantified by measuring the absorbance at 570 nm and 630 nm in Thermo Scientific Multiscan ultraviolet spectrophotometer, USA. The assay was performed in triplicates.[8]
Determination of Ehrlich's ascites carcinoma viability by trypan blue staining
Ehrlich's ascites carcinoma cells were harvested after reaching confluence and washed with PBS, followed by centrifugation at 2500 rpm for 5 min. The cell pellet was suspended in 1 ml of fresh culture medium. The cell suspension was mixed with an equal volume of trypan blue (4 mg/ml) in the ratio 1:1 and incubated for 5 min at 37°C. The estimation of the total number of viable cells was done using hemocytometer chamber.[9] Percentage of viable cells were calculated by the formula,
Percentage viable cells= [1.00 −(Number of trypan blue stainedcells/Totalcells)] ×100
Tumor induction and test drug exposure
Briefly, 0.1 ml (irrespective of body weight) of EAC cells was implanted into the peritoneal cavity of each recipient mouse to develop tumor except the negative control for the development of ascites tumor.[10] The entire process was approved by the Institutional Animal Ethical Committee IAEC/XXXIII/SRU/251/2013. Animals were distributed into five groups of ten mice of similar weight.
Group 1: Negative control (no tumor) - received vehicle
Group 2: Positive control (tumor) - received vehicle
Group 3: Received 5-fluoro uracil 20 mg/kg body weight ip
Group 4: Received extract at dose 200 mg/kg body weight per orally
Group 5: Received extract at dose 400 mg/kg body weight per orally.
After 24 h following induction, vehicle/standard drug/test drug was administered for a period of 14 days. Body weight was recorded once in 3 days throughout the experimental period. Following treatment, blood was collected through retro-orbital plexus for analysis of hematological parameters. Further, half of the animals were sacrificed and liver and spleen tissues were collected for histopathology evaluation.[11,12]
Tumor volume
After dissection of the mice, the ascetic fluid from the peritoneal cavity was collected in a measuring tube and the volume was noted. It was then centrifuged at 1000 rpm for 5 min and the packed cell volume was determined.
Viable/nonviable tumor cell count
The cells of the ascetic fluid were stained with trypan blue dye during which only the nonviable cell took up the dye. Therefore, the colored cells were nonviable, and the others were viable. Both the viable and nonviable cells were counted.
Increase in life span
All the animals were monitored carefully, and the time of death of each mouse was noted. Percentage increase in lifespan was calculated using the formula:
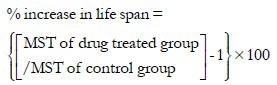
Where MST is mean survival time, which can be calculated by adding the time in the day of the first death and final death and dividing by two.
Effect on body weight
All the animals were weighed on the day of inoculation and then during the postinoculation period once in 3 days. The percentage increase in weight was calculated using the formula:
% Increase in weight = [(animal weight on respective day/animal weight on day-0)-1]× 100
Hematological studies
Comparison was made between groups of mice on the fourteenth day after transplantation, to find the effect of the extract on the hematological parameter of EAC-bearing mice. Blood was collected from the retro-orbital plexus of each mouse under ether anesthesia. Determination of total white blood cell (WBC) count, red blood cell (RBC) count, and hemoglobin content was done.
Statistical analysis
Statistical analysis was performed by one-way ANOVA followed by Tukey post-hoc analysis using SPSS computer package (SPSS version 16).
RESULTS AND DISCUSSION
In vitro cytotoxicity assay
In vitro cytotoxicity assay of chloroform extract of SA had shown that there was a significant increase in percentage inhibition with increase in concentration. More than 50% of the cells were inhibited by <3 μg of chloroform extract. By increasing the concentration of chloroform extract to 15 μg, around 82% of the cell were inhibited, showing the cytotoxic nature of the extract against EAC cell lines [Figure 1].
Figure 1.
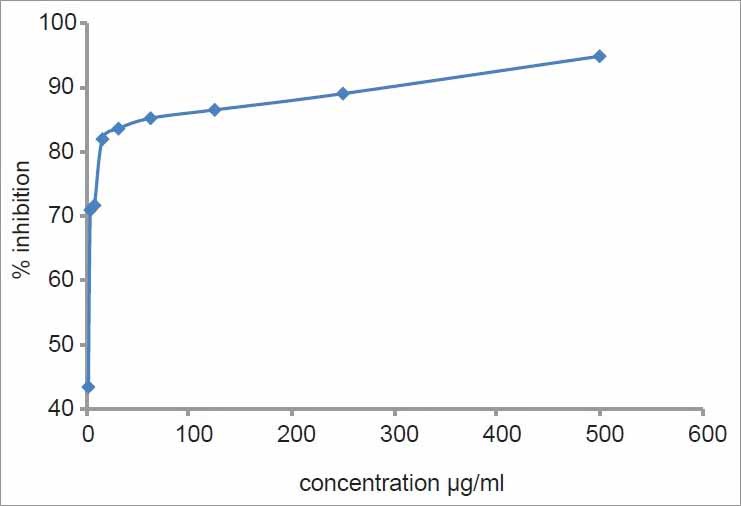
Cytotoxicity of chloroform extract against Ehrlich's ascites carcinoma cell line
In the tumor control mice, the hematological parameters were found to be significantly altered on day 15 when compared to the normal group. There was an increase in total WBC count with a reduction in RBC count and hemoglobin content. The extract treated group showed a significant effect on increasing life span and retrieved all the parameters to the normal level.
In vivo anticancer activity Weight variation
Significant weight gain of about 62% was observed in tumor inoculated mice by day 15. Administration of 5-fluorouracil significantly reduced weight gain as compared with that of EAC control. The group treated with the extract of 400 mg/kg concentration also shown a reduction in weight gain.
Survival time parameter
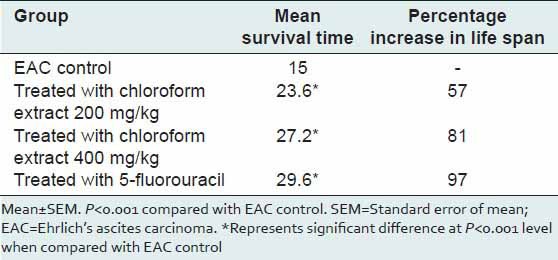
Reliable criteria for judging the value of any anti-cancer agent is the prolongation of life span of animals.[13] The effect of the extract on survival of tumor-bearing mice is shown in Table 1. The mean survival time was prolonged by the administration of the standard drug, 5-fluorouracil. Even the extract significantly prolonged the mean survival time (P < 0.001) at both the concentration when compared to control. The increase in lifespan of extract treated animals was found to be more than 50%. By convention, a 25% increase in lifespan is considered as possible anticancer activity of a test compound.
Table 1.
Antitumor activity of Sphaeranthus amaranthoides extract with regard to mean survival time and increase in life span
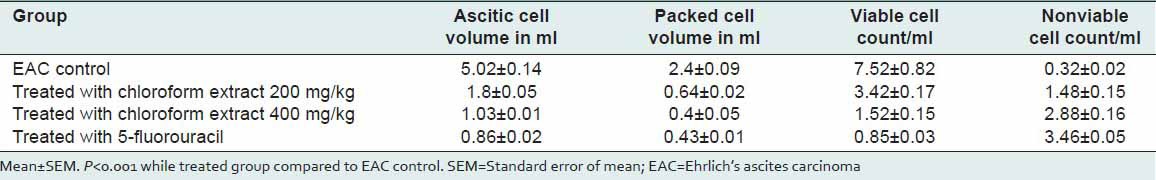
Treatment with extract reduced the tumor-viable count, the ascitic cell volume and packed cell volume in a significant manner (P < 0.001) as compared to that of EAC control group. Further, nonviable tumor cell count was increased significantly when compared with the EAC control [Table 2].
Table 2.
Antitumor activity of Sphaeranthus amaranthoides extract with regard to tumor volume, viable tumor cells count and nonviable tumor cells count (mean±SEM, n=5)
Hematological parameters
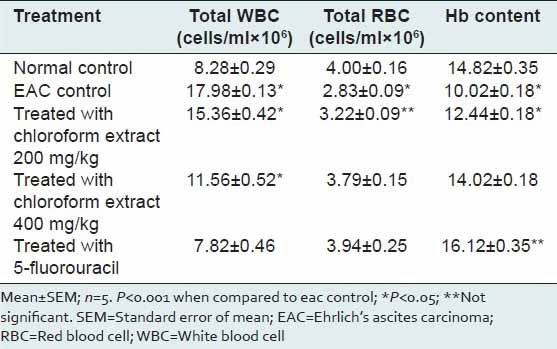
Table 3 shows the effect of the extract on hematological parameters. Most chemotherapeutic agents induce collateral effects. Direct myelotoxicity is often observed in patients undergoing anticancer treatments. Hence, the evaluation of toxicity can be accomplished by blood count evaluation.[14] Induction of tumor increased significantly (P < 0.05) the total number of WBC by almost two times. However, the effect was reversed significantly by 5-fluorouracil administration. The extract at both the concentration significantly (P < 0.05) reversed the tumor-induced rise in total counts of WBC. However, the effect was less when compared with the standard. There was a significant decrease in RBC and Hemoglobin due to tumor induction. This was significantly (P < 0.05) reversed towards normal by standard drug and the tested extract.
Table 3.
Effect of Sphaeranthus amaranthoides extract on hematological parameters of Swiss albino mice
CONCLUSION
It was found that the treatment with the drug enhanced the nonviable cell count and decreased the viable cell count. It may be due to the absorption of extract by viable cells that leads the lysis of the cells through the activation of macrophages or some cytokinin production in the peritoneal cavity. These effects were almost comparable to 5-flurouracil – the standard drug used in the study.
The prolongation of life span (ILS) by treatment with the extract was found to be comparable to that of the standard drug 5-flurouracil. These findings imply that the extract might be having some anticancer principles. Further, possible mechanism of the chloroform extract for anticancer effect should be explored.
Inoculation of EAC in the mice caused an increase in body weight, ascites, and the mice became inactive and slow. From the in vivo cancer model (Ehrlich ascitic carcinoma model), it was inferred that the extract significantly (P < 0.05) reversed the tumor-induced changes in the parameters examined namely % increase in body weight, % increase in lifespan, viable tumor cell count, and hematological parameters (total WBC, RBC, and hemoglobin count).
Anemia and myelosuppression are the major problem encountered in cancer chemotherapy.[15] The reduction in RBC and hemoglobin percentage in tumor-bearing mice may be due to iron deficiency or due to hemolytic or myelopathic conditions.[16] Treatment with the extract reversed the blood count and hemoglobin content near to normal values. This indicates that the extract possess a significant anti-cancer activity. The antioxidants present in the plant may be responsible for this effect.[17]
Footnotes
Source of Support: GATE young faculty research grant from the management of Sri Ramachandra University.
Conflict of Interest: None declared.
REFERENCES
- 1.Dashora N, Sodde V, Bhagat J, Kirti SP, Labo R. Antitumor activity of Dendrophoe falcate against Ehrlich ascites carcinoma in swiss albino mice. Pharm Crops. 2011;2:1–7. [Google Scholar]
- 2.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni KM. Bombay: Popular Prakashan; 1976. Indian Materia Medica. [Google Scholar]
- 4.Shankarananth V, Senthil Kumar A, Ramesh S, Lenin JT, Prema S. Evaluations of pharmacological activity of Sphaeranthus amaranthoides Burm. Plant Arch. 2009;9:321–4. [Google Scholar]
- 5.Sumithra P, Selvaraj T, Shanthi S. Antibacterial and phytochemical screening of Sphaeranthes amaranthoides (L. Burm) against human pathogenic bacteria. J Pharm Res. 2011;4:2206–8. [Google Scholar]
- 6.Thanigavelan V, Lakshmanakumar V, Kaliyamurthi V, Kumar MP, Victor R. Pharmacological study of a siddha holistic herb sivakaranthai – Sphaeranthus amaranthoides burm for analgesic and anti-inflammatory activities. J Appl Pharm Sci. 2012;02:95–101. [Google Scholar]
- 7.Fan Y, Liu C, Huang Y, Zhang J, Cai L, Wang S, et al. Dipyrithione induces cell-cycle arrest and apoptosis in four cancer cell lines in vitro and inhibits tumor growth in a mouse model. BMC Pharmacol Toxicol. 2013;21(14):54. doi: 10.1186/2050-6511-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 9.Hashim YZ, Phirdaous A, Azura A. Screening of anticancer activity from agarwood essential oil. Pharmacognosy Res. 2014;6:191–4. doi: 10.4103/0974-8490.132593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devi PU, Rao BS, Solomon FE. Effect of plumbagin on the radiation induced cytogenetic and cell cycle changes in mouse Ehrlich ascites carcinoma in vivo. Indian J Exp Biol. 1998;36:891–5. [PubMed] [Google Scholar]
- 11.Eckhardt AE, Malone BN, Goldstein IJ. Inhibition of Ehrlich ascites tumor cell growth by Griffonia simplicifolia I lectin in vivo. Cancer Res. 1982;42:2977–9. [PubMed] [Google Scholar]
- 12.Babu TD, Kuttan G, Padikkala J. Cytotoxic and anti-tumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. J Ethnopharmacol. 1995;48:53–7. doi: 10.1016/0378-8741(95)01284-k. [DOI] [PubMed] [Google Scholar]
- 13.Docie JV, Lewis SM. 2nd ed. London: J and A Churchill; 1958. Practical Hematology. [Google Scholar]
- 14.Flávia CR, Thalita CT, Vany PF, Elaine MS, Geovanni DC, Andréa TC, et al. Effect of Arrabidaea chica extracts on the Ehrlich solid tumor development. Braz J Pharmacogn. 2012;22:364–73. [Google Scholar]
- 15.Marklund SL, Westman NG, Lundgren E, Roos G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, and glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982;42:1955–61. [PubMed] [Google Scholar]
- 16.Price VE, Greenfield RE. Anemia in cancer. Adv Cancer Res. 1958;5:199–290. doi: 10.1016/s0065-230x(08)60413-3. [DOI] [PubMed] [Google Scholar]
- 17.Pandey G, Madhuri S. Some medicinal plants as natural anticancer agents. Pharmacogn Rev. 2009;3:259–63. [Google Scholar]


