ABSTRACT
The intracellular parasite Toxoplasma gondii infects a wide variety of vertebrate species globally. Infection in most hosts causes a lifelong chronic infection and generates immunological memory responses that protect the host against new infections. In regions where the organism is endemic, multiple exposures to T. gondii likely occur with great frequency, yet little is known about the interaction between a chronically infected host and the parasite strains from these areas. A widely used model to explore secondary infection entails challenge of chronically infected or vaccinated mice with the highly virulent type I RH strain. Here, we show that although vaccinated or chronically infected C57BL/6 mice are protected against the type I RH strain, they are not protected against challenge with most strains prevalent in South America or another type I strain, GT1. Genetic and genomic analyses implicated the parasite-secreted rhoptry effectors ROP5 and ROP18, which antagonize the host’s gamma interferon-induced immunity-regulated GTPases (IRGs), as primary requirements for virulence during secondary infection. ROP5 and ROP18 promoted parasite superinfection in the brains of challenged survivors. We hypothesize that superinfection may be an important mechanism to generate T. gondii strain diversity, simply because two parasite strains would be present in a single meal consumed by the feline definitive host. Superinfection may drive the genetic diversity of Toxoplasma strains in South America, where most isolates are IRG resistant, compared to North America, where most strains are IRG susceptible and are derived from a few clonal lineages. In summary, ROP5 and ROP18 promote Toxoplasma virulence during reinfection.
IMPORTANCE
Toxoplasma gondii is a widespread parasite of warm-blooded animals and currently infects one-third of the human population. A long-standing assumption in the field is that prior exposure to this parasite protects the host from subsequent reexposure, due to the generation of protective immunological memory. However, this assumption is based on clinical data and mouse models that analyze infections with strains common to Europe and North America. In contrast, we found that the majority of strains sampled from around the world, in particular those from South America, were able to kill or reinfect the brains of hosts previously exposed to T. gondii. The T. gondii virulence factors ROP5 and ROP18, which inhibit key host effectors that mediate parasite killing, were required for these phenotypes. We speculate that these results underpin clinical observations that pregnant women previously exposed to Toxoplasma can develop congenital infection upon reexposure to South American strains.
INTRODUCTION
Toxoplasma gondii is an intracellular zoonotic pathogen that chronically infects a wide range of vertebrate species, including sea and land mammals, a variety of birds, and an estimated one-third of the human population. In intermediate hosts, there are two developmental life stages: the fast-dividing tachyzoite stage, which is responsible for acute disease, dissemination, and pathology, and the slow-growing bradyzoite stage, which is responsible for chronic infection and tissue cyst formation. Once infected, most intermediate hosts are chronic carriers for life and develop immunological memory responses that protect the host against reinfection (1) and keep the parasite in its semidormant, chronic stage (2). Members of the cat family are the definitive host and shed infectious oocysts into the environment. Transmission between hosts occurs following ingestion of either tissue cysts or oocysts. Toxoplasma oocysts are initially diploid and undergo one round of meiosis during sporulation in the environment, a characteristic of coccidian parasites (3). Because Toxoplasma can self-mate, the resultant sporozoites are clonal if a cat ingests one parasite strain, but recombinant progeny can be produced when a cat is infected with two or more different strains.
Despite the potential for genetic recombination, this appears to be a rare event on certain continents (4). For example, in North America and Europe, the population structure of T. gondii is remarkably clonal, with four lineages (the clonal or canonical types I, II, and III and type 12) accounting for the majority of isolated strains (4–6). In contrast, T. gondii strains from other parts of the world (often called atypical or exotic strains), and in particular those from South America, lack a clonal population structure and exhibit genetic diversity. Atypical strains can be clustered into haplogroups (HGs) 4 to 15, but most HGs are not clonal (7–11), and there is evidence for increased sexual recombination in South American strains (10, 12). It is unclear why the population structure is linked to geography, but several hypotheses have been put forward, including selective sweeps driven by advantageous whole chromosomes (4), the disappearance of cats in North America during the Great Ice Age extinction event (8), clonal outbreaks due to self-mating in the cat (13), and diversification within the species-rich jungles of South America (14). Given that Toxoplasma is a globally ubiquitous parasite and infection rates can be high in certain locales, T. gondii likely encounters hosts with prior immunity to T. gondii. However, little is known regarding parasite survival within an immune host and whether there are specialized virulence factors that promote reinfection. Because secondary infection may significantly impact the Toxoplasma population structure, we decided to explore the pathogenesis of a panel of T. gondii strains isolated from around the world by infecting chronically infected hosts.
Studies on the pathogenesis of Toxoplasma have mainly focused on primary infection mouse models and Toxoplasma strains isolated from North America and Europe. Type I strains are highly virulent (100% lethal dose [LD100], 1 parasite) but rarely isolated, while types II, III, and 12 are less virulent (LD50s of ~103, ~105, and ~103 parasites, respectively) and frequently observed (5). Using these strains, key virulence factors that inhibit downstream toxoplasmacidal mechanisms elicited by the proinflammatory cytokine gamma interferon (IFN-γ) have been found to explain the relative differences in virulence during primary infection. IFN-γ induces the expression of two classes of GTPases, the immunity-regulated GTPases (IRGs) and guanylate-binding proteins (GBPs), which are important host resistance factors to pathogens that reside in vacuoles, like Toxoplasma, Chlamydia, and Mycobacterium species (15). When Toxoplasma infects a cell previously stimulated with IFN-γ, the absence of regulatory GMS IRGs (16) on the parasitophorous vacuole membrane (PVM) leads to PVM localization of the GKS class of effector IRGs (17) and many GBPs (18), causing PVM destruction and rapid parasite death (19–22). The Toxoplasma rhoptry (ROP) kinases ROP18 and ROP17, the dense granule GRA7, and the ROP5 pseudokinases are secreted into the host cell upon invasion and subsequently traffic back to the PVM, where they cooperatively inhibit the localization and function of IRGs (23–30) and GBPs (21, 31). Differences in virulence between the clonal lineage strains are the result of polymorphisms in the ROP5 (32, 33) and ROP18 effectors (34, 35), with smaller contributions from other genetic loci (36). The type I strains encode virulent alleles of the ROP5 and ROP18 effectors, while the type II and III strains encode combinations of less virulent alleles that render them more susceptible to IRG-mediated killing and therefore less virulent in mice. The few atypical strains that have been studied are extremely virulent in naive mice (37), and most encode combinations of the ROP5/ROP18 alleles that are predicted to confer IRG resistance (26). Whether atypical strains have novel virulence factors is unknown.
The majority of studies on secondary infection have also focused on clonal lineage strains. Whereas naive mice are extremely susceptible to the type I RH strain or high-dose infections with type II strains, chronically infected or vaccinated mice are not (38). In mouse models of chronic infection, T cells and IFN-γ play pivotal roles in preventing reactivation of the dormant form (2, 39). These results mirror clinical observations of HIV/AIDS patients, in whom deterioration of memory T cells correlates with toxoplasmic encephalitis. In mouse vaccination studies, CD8 T cells are the main mediators of protection against lethal secondary challenges (40–43). Protective CD8 T cells require CD4 T cell help (42, 44, 45) and interleukin-12 (IL-12) during priming (46, 47), and protection is dependent on IFN-γ (41) and possibly tumor necrosis factor alpha (TNF-α) and lymphotoxin-alpha (48), but not perforin (49). The importance of cytolysis and inducible nitric oxide synthetase appears to be limited to chronic infection (50–52).
Several studies have aimed to understand how well infection with one strain protects against reinfection with another, i.e., “heterologous immunity.” In regions of endemicity, for example, in Brazil and France, approximately 50 to 80% of the human population has been exposed to the parasite, and multiple exposures are likely the norm. However, because of the genetic diversity in South America and dominance of type II strains in Europe, secondary infections in South America will more often occur with a different strain type. In mouse models of reinfection, a study of 124 isolates from North and Central America, Europe, and the Pacific Islands demonstrated significant heterologous immunity (53). Likewise, vesper mice chronically infected with a type II strain were protected from congenital infection with a type I strain (54). Large parts of the type I and III genome are derived from the type II strain, and there is evidence for genomic introgression of the type II strain into South American strains (8, 55). Heterologous immunity may thus be achieved because immunodominant antigens are encoded by shared genomic regions or because Toxoplasma strains are generally ~99% similar. In contrast, type I-vaccinated rats were not protected against congenital T. gondii infection following oral ingestion of tissue cysts from several strains (56), and in mice chronic infection with certain strains did not generate heterologous immunity to congenital infection (57) or lethal oral challenge with a different strain (58). A few studies have addressed what happens to the challenging strain during heterologous challenge, and those investigators noted that with certain strains and host genetic backgrounds, successful coinfection in the brain can occur (59–61). Thus, the outcome of secondary exposure could be the result of several factors, including the ability of memory cells to recognize the second strain, the type of intermediate host, and unique virulence attributes of the strain causing the secondary infection.
Very little is known about requirements for host immunity to atypical strains. This is important because there is evidence that the severity of human toxoplasmosis is influenced by the parasite genotype and geographic location. Congenitally infected Brazilian children or South American patients with ocular toxoplasmosis present a more severe form of eye disease than those in other locales (62–64), and PCR-based strain typing methods suggest atypical strain infections in Brazil or type I/atypical infections in Europe and North America are associated with more severe ocular toxoplasmosis (65–68). Additionally, severe congenital infection in North America is associated with non-type II strains (69). Importantly for this current study, there have been cases where seropositive healthy women developed acute toxoplasmosis and congenital infection during pregnancy; in one instance when the responsible strain was genotyped it was an atypical strain (70, 71). Whether type I or atypical strains possess unique virulence factors that make them highly virulent in human populations is unknown.
Since most studies on T. gondii strain differences in virulence have focused on primary infection models using the European-North American strains, we asked whether Toxoplasma strains differ in virulence during secondary infection and sampled a broad spectrum of T gondii strains isolated from around the world. Here we present evidence that the majority of Toxoplasma strains are able to kill chronically infected or vaccinated C57BL/6 (B6) mice, including mice vaccinated and subsequently challenged with the same strain type. Toxoplasma expression of the virulent alleles of ROP5 and ROP18 is a requirement for secondary infection virulence and superinfection of chronically infected mice. We hypothesize that superinfection through IRG resistance promotes outcrossing, because two strains would be present in a single rodent meal for the cat. This would make it difficult to maintain a clonal population structure.
RESULTS
The atypical strain MAS is virulent during secondary infection.
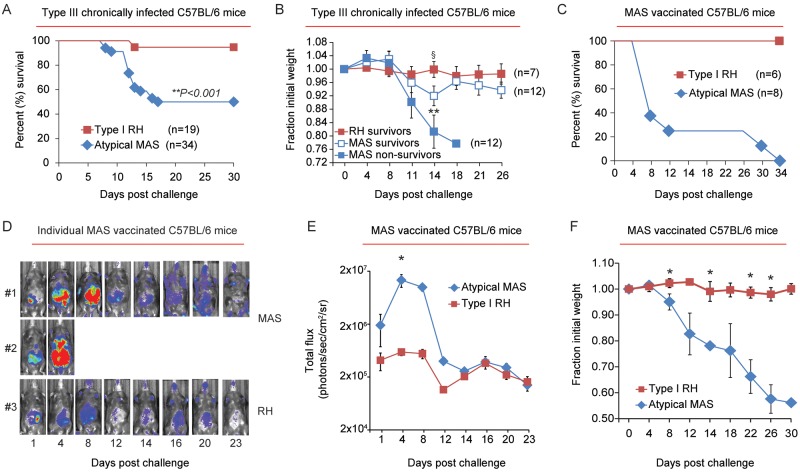
We first explored whether chronic infection conferred protective immunity to secondary infection by working with a nonclonal lineage strain, the atypical strain MAS (haplogroup 4). The MAS strain was originally isolated from a patient with disseminated congenital infection (72) and is highly virulent in naive mice (37). C57BL/6 mice were infected with an avirulent type III strain (CEP) and allowed to progress to chronic infection for 35 to 60 days before secondary infection. While the majority of mice survived a secondary infection (i.e., challenge) with the type I RH strain, as expected, approximately 50% of chronically infected mice succumbed to secondary infection with the atypical strain MAS (Fig. 1A). Moreover, surviving and nonsurviving mice experienced significant weight loss and morbidity during secondary challenge with the MAS strain (Fig. 1B). In contrast, mice challenged with the type I RH strain experienced little weight loss or morbidity following secondary infection.
FIG 1 .
Compromised host immunity to the atypical T. gondii strain MAS. (A) C57BL/6 mice were infected (i.p.) with an avirulent type III strain (CEP) and allowed to progress to chronic infection. Thirty-five to 70 days later, mice were challenged (i.p.) with 5 × 104 tachyzoites of either the atypical MAS or type I RH strains. Results were compiled from 6 to 8 independent experiments, and the percent survival following challenge is plotted for each strain. The number of mice in each group (n) is indicated; **, P value was calculated by a log-rank Mantel-Cox test and was considered significant. (B) The average relative weights ± SEM of mice that did not survive secondary infection with the MAS strain and of those that survived RH and MAS secondary infections are shown; for each measured time point, the fraction of initial weight at the time of challenge (day zero weight = 1) were plotted. Results are cumulative from 5 independent experiments, and the number of mice (n) in each group is indicated. **, P < 0.009; significant by analysis of variance testing; §, P < 0.04 based on two-tailed Student’s t test, comparing the relative weights between survivors of type I RH and MAS secondary challenges. (C) Cumulative survival, combined from 2 experiments, in which C57BL/6 mice were vaccinated with the atypical strain MAS and challenged with either the MAS-Luc or type I RH-Luc strain; the number (n) of mice in each group is indicated and all mice were seropositive prior to challenge (see Materials and Methods). In the absence of vaccination, MAS-infected and type I RH-infected mice died between days 5 and 8 postinjection (data not shown). (D) Representative bioluminescence imaging of 2 individual mice challenged with the MAS-Luc strain and 1 mouse challenged with the RH-Luc strain (from the experiments described for panel C). The relative parasite burden is depicted as a heat map: maximum = 105, minimum = 3 × 103 (photons/s/cm2/sr). MAS-challenged mouse 1 was euthanized on day 27 because it had a poor body condition score (BC, 2−) and was continuing to lose weight; MAS-challenged mouse 2 died on day 5. (E) Average luciferase activity (in photons/s/cm/sr2, ± SEM) for each cohort following challenge with the type I RH-Luc (n = 3) or MAS-Luc (n = 4) strains. Data shown are from a single experiment representative of two experiments. *, P < 0.05 (Student’s t test). (F) Results obtained from the same experiment described in panel C, but the average fraction of initial weight (± SEM) of mice following secondary infection with the MAS and RH strains is shown. *, P < 0.05 (Student’s t test).
Since the clonal strain types (I, II, and III) and atypical strains may express different antigens, we wondered whether strain-specific immunity was generated by the previous immunization regimen. Therefore, C57BL/6 mice were vaccinated with the MAS strain. This was achieved by treating mice with the antiparasitic drugs pyrimethamine and sulfadiazine 1 day after initial infection and giving subsequent “priming” injections with live MAS parasites during drug administration. Two weeks after drug removal, MAS-vaccinated mice survived challenge with the type I RH strain but not the atypical strain MAS (Fig. 1C). MAS-challenged mice were less able to control parasite replication than were type I RH-challenged mice, as evidenced by bioluminescence imaging (luciferase-expressing MAS-Luc and RH-Luc strains) (Fig. 1D and E) and experienced significant weight loss (Fig. 1F). Thus, two different priming conditions that generated protective immunological memory to the type I RH strain failed to do so for the atypical strain MAS.
The CD8 T cell IFN-γ response to the atypical strain MAS is unimpaired.
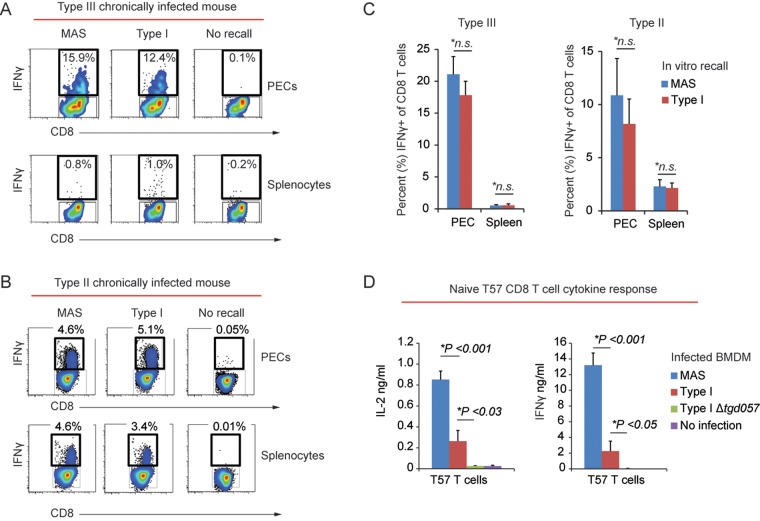
Given the central role of CD8 T cells and IFN-γ in mediating host immunity to secondary Toxoplasma infection (38), we tested whether the CD8 T cell IFN-γ response to the MAS strain was impaired. To this end, peritoneal exudate cells (PECs) and splenocytes from type III chronically infected mice were infected in vitro with either the atypical strain MAS or the type I strain (“recall infection”), and the CD8 T cell IFN-γ response was measured 16 h later by intracellular cytokine staining and fluorescence-activated cell sorting (FACS) analysis. No differences in the frequencies of IFN-γ+ CD8 T cells were observed following recall infections with the various Toxoplasma strains (Fig. 2A). Total amounts of IFN-γ, IL-2, and TNF-α were measured in the supernatants following recall infections, and no differences in cytokine production were observed between MAS and clonal strain type infections (data not shown). We noted that type III chronic infection elicited a robust peritoneal but not splenic CD8 T cell IFN-γ recall response (Fig. 2A and C). In contrast, type II chronic infection elicited a detectable CD8 T cell IFN-γ recall response from both compartments (Fig. 2B and C). Nonetheless, similar frequencies of IFN-γ+ CD8 T cells were also observed from the spleens and PEC compartments of type II chronically infected mice following recall infections with different parasite strains (Fig. 2C). CD8-negative CD3+ T cells, the majority of which are CD4 T cells, were also analyzed in these experiments, and similar results were obtained (data not shown).
FIG 2 .
The CD8 T cell IFN-γ response to the atypical strain MAS is unimpaired. (A) PECs and splenocytes were obtained from an individual C57BL/6 mouse chronically infected with the type III (CEP) strain and were then infected in vitro (i.e., “recall” infections) with the indicated parasite strain (MOI, 0.2). Sixteen hours later, CD8 T cells were analyzed for intracellular IFN-γ by FACS analysis. The percentage of CD8+ (CD3+ CD19−) T cells that were positive for IFN-γ within the indicated gate following recall infection is shown. (B) Results of an experiment similar to that of panel A, except recall infections were performed using PECs and splenocytes from a type II (Pru) chronically infected mouse. (C) Cumulative data showing the average frequency (+ SEM) of IFN-γ+ CD8+ (CD3+ CD19−) T cells from PECs (3 experiments; n = 7 mice) and spleens (1 experiment; n = 3 mice) from type III (CEP) chronically infected C57BL/6 mice and PECs (2 experiments; n = 5 mice) and spleens (2 experiments; n = 6 mice) from type II (Pru) chronically infected C57BL/6 mice. Average frequencies obtained from recall infections with the type I RH strain and MAS strain are plotted. Significant differences in the average frequencies between RH and MAS recall infections were calculated with Student’s t test; *n.s., not significant (P > 0.05). (D) Naive TGD05796–103-specific T57 transnuclear CD8 T cells (77) were cocultured with BMDMs infected with the indicated parasite strain (MOI, 0.2) in triplicate. The average amounts (+ standard deviation) of IFN-γ and IL-2 detected in the supernatants via an ELISA 48 h later are plotted. *, P < 0.05 (Student’s t test). Results are representative of 5 independent experiments.
IFN-γ-producing CD8 T cells from chronically infected mice were further analyzed for CD62L and KLRG1 expression, which in combination can approximate effector memory T cells (TEM; CD62L− CD44+ IL7rα− KLRG1+/−) and central memory CD8 T cell (TCM; CD62L+ CD44+ IL7rα+ KLRG1−) populations. CD8 T cells expressing these markers have different turnover rates (73), homing characteristics (74), priming requirements (46), and T cell receptor (TCR) repertoires (75), and hence they may vary in their responses to the MAS and type I strains, leading to differences in host susceptibility. Although the relative frequency of CD62L+ and/or KLRG1+ IFN-γ+ CD8 T cell PECs varied between individual type III chronically infected mice, at an individual mouse level the frequencies of these populations were equivalent following recall infections with the MAS and clonal parasite strains (see Fig. S1A in the supplemental material). Additionally, IFN-γ+ CD8 T cells from the spleens of type II chronically infected mice had similar IL-7rα hi expression following recall infections with type I and MAS strains (see Fig. S1B). These results suggest that memory CD8 T cells expressing markers that approximate the TEM and TCM memory pools are not initially biased in their IFN-γ response to different T. gondii strains.
In the experiments described above, we analyzed polyclonal memory CD8 T cells with unknown antigen specificities. In the C57BL/6 background (H-2b haplotype), a peptide epitope recognized by CD8 T cells has been identified and is derived from a Toxoplasma protein, TGD057 (TgME49_215980), with unknown function (76). Mice have been cloned from the nucleus of a single Toxoplasma-specific CD8 T cell (“transnuclear mice”) bearing T cell receptor specificity for the TGD05796–103 peptide in complex with the Kb major histocompatibility complex (MHC) class I molecule, and adoptive transfer of CD8 T cells (T57 cells) from these transnuclear mice confers increased host resistance to infection with type II strains (77). Therefore, the naive T57 CD8 T cell cytokine responses to bone marrow-derived macrophages (BMDMs) infected with different parasite strains were measured in an enzyme-linked immunosorbent assay (ELISA), including the response to BMDMs infected with a type I strain in which the TGD057 gene was removed by double homologous recombination (I Δtgd057) (see Fig. S2 in the supplemental material). The T57 IFN-γ and IL-2 responses were 5 to 10 times greater to BMDMs infected with the MAS strain than those infected with the type I strain, and as expected, no response was observed to BMDMs infected with type I Δtgd057 parasites (Fig. 2D). Of note, the TGD05796–103 peptide epitope is conserved between parasite strains. In conclusion, the atypical strain MAS elicits a robust naive and memory CD8 T cell IFN-γ response, suggesting that immunological failure during secondary challenge does not correlate with impaired CD8 T cell recognition or the IFN-γ response to the atypical strain MAS.
Toxoplasma ROP5 and ROP18 are major virulence determinants during secondary infection with atypical strains.
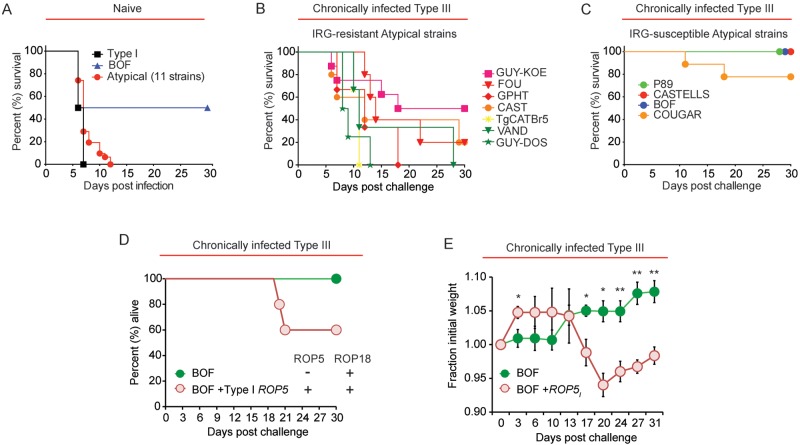
To investigate whether compromised host immunity occurred with other atypical strains, we chronically infected C57BL/6 mice with the avirulent type III strain as described before and then challenged these mice 35 to 65 days later with a panel of atypical strains that are highly virulent in naive mice (Fig. 3A) and can be loosely grouped according to their genotype as follows (haplogroups are in parentheses): CASTELLS (HG 4); GUY-KOE (HG 5); FOU, BOF, and GPHT (HG 6); CAST (HG 7); TgCATBr5 (HG 8); P89 (HG 9); GUY-DOS and VAND (HG 10); COUGAR (HG 11). We noted that atypical strains that resisted IRG-mediated killing in IFN-γ-stimulated mouse embryonic fibroblasts (MEFs; “IRG resistant”) caused between 50% and 100% mortality during secondary infection (Fig. 3B). In contrast, P89, CASTELLS, COUGAR, and BOF, which do not prevent IRG-mediated killing mechanisms in MEFs (“IRG susceptible”) (26), caused 0% mortality (P89, BOF, and CASTELLS) or 22% mortality (COUGAR) during secondary infection (Fig. 3C). In the case of P89 and CASTELLS, these strains do not express ROP18, whereas BOF has the virulent ROP18 allele but has very low expression of ROP5 (26). COUGAR expresses the virulent allele of ROP18 and some members of the ROP5 locus, but possibly because it lacks the ROP5C allele this strain remains sensitive to IFN-γ-mediated killing (26). Thus, there is a positive correlation between atypical Toxoplasma strains that inhibit IRG function through ROP5 and ROP18 and killing of chronically infected mice.
FIG 3 .
T. gondii ROP5- and ROP18-expressing atypical strains cause lethal secondary infection. (A) Naive C57BL/6 mice were infected (i.p.) with 103 tachyzoites of the type I RH strain or 1 of 12 atypical strains: MAS and CASTELLS (HG 4); GUY-KOE (HG 5); FOU, BOF, and GPHT (HG 6); CAST (HG 7); TgCATBr5 (HG 8); P89 (HG 9); GUY-DOS and VAND (HG 10); COUGAR (HG 11). The average survival times of mice infected with the type I RH strain, the atypical BOF strain, or the remaining 11 atypical strains are shown. Two to four individual mice per parasite strain were infected, with the exception of BOF, for which 9 mice were infected. (B) C57BL/6 mice were infected (i.p.) with the avirulent type III (CEP) strain and allowed to progress to chronic infection; 35 to 65 days later, mice were challenged (i.p.) with 5 × 104 tachyzoites of the indicated atypical strains previously shown to inhibit IRGB6 parasitophorous vacuole (PV) coating (IRG resistant) (26). Three to eight individual mice per parasite strain were challenged, and the cumulative survival is plotted. (C) Results of an experiment similar to that in panel B, but atypical strains that are highly susceptible to parasite killing and IRGB6-PV coating in IFN-γ-stimulated MEFs (IRG-susceptible) were used. Four individual mice were challenged with the BOF or P89 strain and 9 mice were challenged with COUGAR. Cumulative survival is plotted. (D) C57BL/6 mice were infected with the type III strain (CEP) and allowed to progress to chronic infection. Seventy days later, mice were challenged (i.p.) with 5 × 104 tachyzoites of either the atypical BOF strain, which expresses ROP18 and very low levels of ROP5 (26), or the BOF strain engineered to express the entire type I ROP5 locus via cosmid integration (BOF + ROP5I). Cumulative survival of 5 mice per parasite strain is plotted. (E) Results were obtained from the same experiment shown in panel D, but the average relative weights (± SEM) of mice following secondary infection with the BOF or BOF + ROP5I strain are shown. *, P < 0.05; **, P < 0.008 (Student’s t test).
To test whether restoration of IRG resistance in an atypical strain can promote virulence during a secondary infection, mice were chronically infected with the type III strain as before and challenged with an atypical BOF strain engineered to express the entire ROP5 locus of the type I strain (BOF+ ROP5I). Compared to the parental strain, BOF+ ROP5I survives significantly better in IFN-γ-stimulated MEFs and inhibits IRGB6-PVM coating (26). Unlike the BOF strain, challenge with the BOF+ ROP5I strain caused host death (40% of the cohort) and morbidity, as evidenced by weight loss (>5% weight loss) during secondary infection (Fig. 3D and E). These results underpin the notion that IRG resistance during a heterologous challenge is an important attribute for parasite virulence.
The Toxoplasma ROP5/SAG3 locus correlates with virulence during secondary infections with F1 progeny derived from clonal lineage strains.
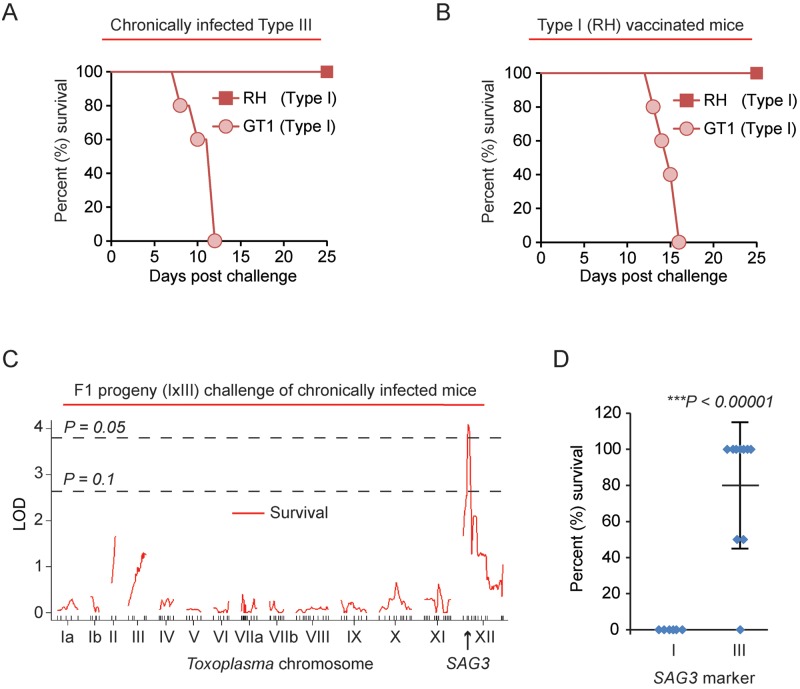
The type I RH strain is highly virulent in naive mice due to expression of the virulent ROP5 and ROP18 alleles (28, 33, 78, 79), but RH was the only IRG-resistant strain that failed to induce morbidity in chronically infected mice (Fig. 1A). This strain has a long laboratory passaging history (80) of at least 50 years prior to the experiments performed in this study, and over time it has accumulated hundreds of mutations and gene expression differences compared to other type I strains (80, 81). Thus, chronically infected mice were challenged with another type I strain called GT1, which was originally isolated from a deceased goat in North America (82). Importantly, the GT1 strain killed type III chronically infected mice (Fig. 4A). To test whether GT1 could also kill mice vaccinated with a type I strain, mice were immunized with the replication-deficient uracil auxotroph type I RH Δompdc Δup vaccine strain (83) and challenged with either the RH or GT1 type I strain. Again, GT1 challenge, but not RH challenge, led to death of vaccinated mice (Fig. 4B) and mice rapidly lost weight prior to death (data not shown).
FIG 4 .
The T. gondii type I ROP5/SAG3 locus is correlated with lethal secondary infection. (A) Type III (CEP) chronically infected C57BL/6 mice (day 37 of chronic infection) were challenged with 5 × 104 tachyzoites of either the RH (n = 2) or GT1 (n = 5) type I strains; cumulative survival is plotted. (B) C57BL/6 mice were vaccinated with 106 tachyzoites of the uracil auxotroph type I RH Δku80 Δompdc Δup vaccine strain (83), and 34 days later mice were challenged with either 5 × 104 tachyzoites of the type I RH (n = 4) strain or 5 × 103 tachyzoites of the type I GT1 strain (n = 5). Cumulative survival is plotted. (C) Type III chronically infected C57BL/6 mice (day 34 of chronic infection) were challenged with 5 × 104 tachyzoites of 16 different F1 progeny derived from a cross between the GT1 (type I) and CTG (type III) strains. The chosen progeny have the type I CS6 marker on Toxoplasma chromosome VIIa, a marker which detects an informative SNP in the ROP18 allele; thus, these F1 progeny express ROP18 and are 100% lethal in naive mice (34). The running LOD score for each of the 176 markers on the various Toxoplasma chromosomes and mouse survival (%) at 26 days after challenge is plotted (red line). The SAG3 marker on Toxoplasma chromosome XII returned the highest LOD score (4.1; P < 0.05). Significance was estimated based on 1,000 permutations at each marker; thresholds of P = 0.05 and P = 0.1 are shown. (D) Effects of the type I and type III SAG3 locus on mouse survival at 26 days. *, significant P value based on Student’s t test.
To determine genetic loci underlying the ability of Toxoplasma to kill an immune host, type III chronically infected C57BL/6 mice were challenged with F1 recombinant progeny derived from a cross between the virulent type I GT1 and the avirulent type III CTG strains (I × III F1 progeny). In previous studies using these progeny, the ROP18 gene was the primary gene responsible for parasite virulence in naive mice (34) and, therefore, we excluded progeny which harbored the avirulent type III ROP18 allele, which is not expressed due to an insertion in its promoter (34, 35). In this screen of 16 F1 I × III progeny (which all are virulent for naive mice), mouse survival significantly correlated with the SAG3 marker (LOD, 4.1; P < 0.05) on Toxoplasma chromosome XII (Fig. 4C), which is the genetic marker closest to the ROP5 locus. Effect plots revealed that type I alleles at the SAG3 locus significantly correlated with mouse mortality during secondary infection (type I allele, 0% ± 0% survival [mean ± standard deviation]; type III allele, 80% ± 35% survival; P < 0.0001) (Fig. 4D; see also Fig. S3 in the supplemental material).
Toxoplasma ROP5 and ROP18 promote superinfection in chronically infected mice.
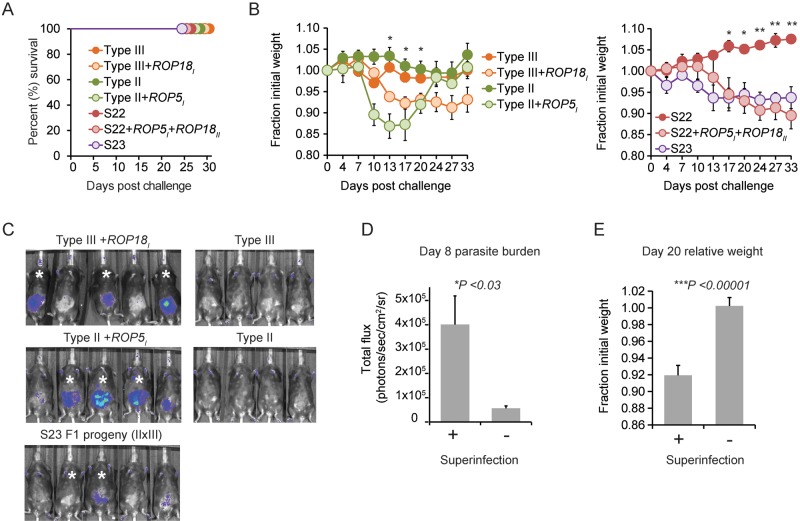
To extend the analysis of the role of Toxoplasma resistance to IRG-mediated killing in virulence during a secondary infection, a panel of ROP5- and/or ROP18-expressing parasite strains was screened for virulence in type III chronically infected mice. First, the IRG-sensitive type II and type III strains engineered to become IRG resistant through ROP5 (II + ROP5I) and ROP18 (III + ROP18I) expression, respectively, induced morbidity during secondary infection but failed to kill chronically infected mice (Fig. 5A and B), even though these strains are virulent in naive mice (26, 84). Second, the highly virulent S23 strain, an F1 progeny derived from a cross between a type II and a type III strain (II × III) that expresses virulent ROP5III and virulent ROP18II alleles (85), induced morbidity during secondary infection but did not kill chronically infected mice (Fig. 5A and B). Finally, the avirulent and IRG-sensitive S22 F1 (II × III) strain engineered to express ROP5I and ROP18II (S22 + ROP5I + ROP18II), making this strain IRG resistant and highly virulent in naive mice (26), induced morbidity but not host death during secondary challenge.
FIG 5 .
T. gondii ROP5 and ROP18 promote superinfection. (A) C57BL/6 mice were infected with the type III strain (CEP) and allowed to progress to chronic infection for 37 to 70 days and then challenged (i.p.) with the avirulent S22 F1 (II × III) progeny strain (n = 5), engineered S22 + ROP5I + ROP18II strain (n = 5), avirulent type III-Luc strain (n = 4), the virulent type III + ROP18I-Luc strain (n = 5); the type II-Luc strain (n = 4) or this strain engineered to express the entire type I ROP5 locus via cosmid integration, II + ROP5I (n = 5); the virulent F1 (II × III) progeny S23-Luc strain. Cumulative survival is plotted. (B) Results obtained from the same experiment described in panel A, but the average relative weights (± SEM) of mice following secondary infection with the indicated parasite strains are plotted. *, P < 0.05; **, P < 0.008 (Student’s t test was used to assess significant differences in the average relative weights between mice challenged with engineered versus corresponding parental control strains). (C) Bioluminescence images of mice challenged with the indicated luciferase-expressing strains on day 8 for the experiments shown in panel A. Relative parasite burden is depicted as a heat map, where the maximum and minimum values were set to 105 and 3 × 103 (photons/s/cm2/sr), respectively. Asterisks indicate individual mice in which the challenging strain was detected in the brain 37 to 46 days after the secondary challenge. (D) Comparison between the average (+ SEM) luciferase activity (in photons/s/cm2/sr) on day 8 after secondary challenge of individual mice that bore evidence for superinfection (indicated with asterisks in panel C) versus mice in which superinfection was not detected. *, significant P value, calculated with a Student’s t test. The parasite strains and mice used for this analysis are those of panel C. (E) Comparison between the average relative weights (+SEM) on day 20 of secondary challenge of mice that bore evidence for superinfection with mice in which superinfection was not detected. ***, significant P value, calculated with a Student’s t test; significant differences between these groups were observed between days 13 and 33 of secondary infection (data not shown). Mice that survived secondary infection and parasite strains used for this analysis are those described in panel A.
Although mice survived challenge with the aforementioned IRG-resistant parasite strains, they each experienced various degrees of sickness, as evidenced by weight loss after challenge. In contrast, challenge with the parental control or IRG-susceptible strains led to no appreciable morbidity or weight loss (Fig. 5B). Importantly, IRG-resistant BOF + ROP5I, S22 + ROP5I + ROP18II, II + ROP5I, and III + ROP18I strains and the virulent S23 strain could be detected in parasite cultures generated from the brains of the challenged survivors (termed “superinfection” here), whereas the parental strains could not be rederived (Table 1). Heterologous brain infections were observed in other challenge experiments, including chronically infected animals challenged with GUY-KOE, but not after challenge with the IRG-susceptible COUGAR or P89 strains. MAS and type I RH strains were rarely rederived from the brains of chronically infected mice; however, both strains are poor at forming cyst walls (37), which perhaps contributes to this phenotype. A positive correlation between day 8 parasite burden, weight loss, and the ability to superinfect was observed (Fig. 5C to E). These results suggest that IRG resistance through ROP5 and ROP18 is required for Toxoplasma to promote morbidity during a heterologous challenge and superinfection, but additional factors are necessary to endow a strain with the ability to kill a chronically infected host.
TABLE 1 .
Superinfection of chronically infected mice challenged with IRG-resistant T. gondii strains
| Challenge straina | ROP18b | ROP5c | Chronic infection strain | Fraction of mice superinfected |
|---|---|---|---|---|
| CEP (III) hxprt- GFP cLUC | N | VIR | CEP (III) hxgprt− | 0/5 |
| CEP (III) GFP cLUC + ROP18I | VIR | VIR | CEP (III) hxgprt− | 3/5d |
| Pru (II) GFP fLUC | VIR | AVIR | CEP (III) hxgprt− | 0/4 |
| Pru (II) GFP fLUC + ROP5I | VIR | AVIR + VIR | CEP (III) hxgprt− | 3/5d |
| RH (I) GFP cLUC | VIR | VIR | CEP (III) hxgprt− | 0/5 |
| S22 (II × III) | N | AVIR | CEP (III) hxgprt− | 0/5 |
| S22 (II × III) + ROP5I + ROP18II GFP | VIR | AVIR + VIR | CEP (III) hxgprt− | 5/5d |
| S22 (II × III) + ROP5I + ROP18II GFP | VIR | AVIR + VIR | Pru (II) hxgprt− GFP fLUC | 2/2e |
| S23 (II × III) GFP cLUC | VIR | VIR | CEP (III) hxgprt− | 2/5d |
| BOF (HG 6) | VIR | N | CEP (III) hxgprt− | 0/5 |
| BOF (HG 6) + ROP5I | VIR | VIR | CEP (III) hxgprt− | 1/3f |
| MAS (HG 4) GFP cLUC | VIR | VIR | CEP (III) hxgprt− | 1/10d |
| GUY-KOE (HG 5) | VIR | VIR | CEP (III) hxgprt− GFP cLUC | 1/1g |
| P89 (HG 9) | N | VIR | CEP (III) hxgprt− GFP cLUC | 0/2 |
| COUGAR (HG 11) | VIR | AVIR? | CEP (III) hxgprt− GFP cLUC | 0/2 |
C57BL/6 mice were chronically infected with either a type III or type II strain (genotype indicated) and challenged with atypical strains (haplogroup indicated in parentheses), clonal strains (strain type indicated in parentheses), F1 progeny derived from a type II, type III cross (II × III), or strains engineered to become IRG resistant through either introduction of the type I ROP5 locus by cosmid integration or transgenic expression of type I or type II ROP18 genes (genotypes are indicated). At 35 to 50 days after secondary challenge, brains from surviving mice were homogenized in PBS and used to inoculate HFF monolayers. The ensuing parasite cultures were then tested for the presence of the challenging strain (superinfection of the brain). Data indicated are the fractions of mice in which the challenging strain was detected (with the number of mice tested as the denominator).
VIR, expression of ROP18, which is equated to possessing the virulent allele from either the type II or type I strain; N, the type III strain does not express ROP18.
VIR, the ROP5 locus of the type I and III strains promotes virulence in naive mice; AVIR, the type II ROP5 alleles are less virulent; N, BOF has very low expression of ROP5; AVIR?, it is unclear why COUGAR is IRG sensitive, but it lacks certain ROP5C isoforms.
GFP-positive parasites were observed in the parasite culture.
RFLP analysis of GRA6 determined that the parasite culture had the S22 GRA6III allele.
Parasite growth was observed in mycophenolic acid-xanthine medium, which selects for parasites that carry a functional HXGPRT gene (e.g., the challenge BOF strain).
GFP-negative parasites were observed in the parasite culture.
DISCUSSION
The results reported here call into question the general assumption that healthy T. gondii-seropositive mice are protected from disease following reexposure and underpin several clinical observations that otherwise healthy seropositive women can develop congenital toxoplasmosis during pregnancy upon secondary infection (70). In these situations, the strain type likely matters. In our model, we searched for Toxoplasma virulence loci that promoted virulence during a secondary infection. The central message from these studies is that evasion of the host’s IFN-γ response through T. gondii virulence factors is central to host and parasite survival during a secondary infection.
Parasite genetic determinants that influence host susceptibility to reinfection.
As observed in naive mouse infection studies, the Toxoplasma ROP5 and ROP18 virulence factors, which antagonize IFN-γ-induced IRGs, are important for parasite virulence during a secondary infection. In nearly every case when Toxoplasma expressed the virulent alleles of ROP5 and ROP18, morbidity ensued in the challenged animals, and this correlated with the parasite burden. Importantly, strain differences in virulence that are not observed in naive animals (because many strains have an LD100 of 1 parasite) can be teased apart in chronically infected mice. An unexpected outcome was the finding that the type I strains GT1 and RH, which have a lethal dose of 1 parasite in naive Mus musculus domesticus mice, differ in virulence in type I-vaccinated or type III chronically infected B6 mice. Genetic linkage analysis using the F1 I × III progeny derived from the type I GT1 and type III CTG strains implicated the type I SAG3/ROP5 locus in mediating GT1 virulence during secondary infection in type III chronically infected B6 mice. The causative mutation underlying this quantitative trait locus (QTL) peak is the subject of ongoing investigation, but this is not the locus responsible for differences in virulence between the GT1 and RH type I strains, since genes in this region were similarly expressed and did not encode nonsynonymous single nucleotide polymorphisms (SNPs) between these two strains (data not shown) (81). Although type III ROP5 gene products are highly functional and able to synergize with ROP18 to antagonize the IRG system (84), the type I ROP5 locus has extra copies of ROP5A and ROP5B (33, 86), perhaps allowing for greater GTPase antagonism in immune mice, which mount an earlier and greater IFN-γ response from the T cell compartment than do naive animals. Additionally, SAG3 is a glycosylphosphatidylinositol-linked surface protein that binds surface glycans (87) on the host cell and is required for virulence of the type I strain (88). SAG3 polymorphisms (5 nonsynonymous SNPs between type I and type III SAG3 alleles [data not shown]) may cause differences in cellular tropisms between strains, resulting in different immune evasion properties. SAG3 and ROP5 are also vaccine antigen candidates (89, 90), and a ROP5-derived peptide epitope in B6 mice was recently described (91). This polymorphic epitope is recognized by CD8 T cells after infection with type II but not type I or III strains, and immunization with dendritic cells loaded with the ROP5 epitope did not confer protection to high-dose challenges with the type II strain. Whether an alternative type III or type I ROP5-derived epitope is recognized by CD8 T cells and confers protection to type I secondary infections is unknown.
In addition to the SAG3/ROP5 locus, it is apparent that other genetic loci are responsible for virulence in vaccinated or chronically infected animals. By analyzing recombinant F1 progeny and various strains engineered to express virulent alleles of ROP5, complete penetrance of the ROP5I locus in causing a lethal secondary infection was not observed. Whether the latter result reflects interference between cosmid-expressed and endogenous ROP5 gene products is unknown. It should be noted that the BOF strain encodes a single ROP5 gene (26) that is expressed at low levels (92). Therefore, the BOF strain may be the best strain to circumvent potential interference between cosmid-expressed and endogenous ROP5 gene products. This may not be the case with the II + ROP5I and S22 + ROP5I + ROP18II strains that express ROP5 from the endogenous ROP5II locus, which has copy number variations and divergent ROP5 isoforms compared to the closely related type I and type III ROP5 isoforms (33). Nonetheless, of the three ROP5I engineered strains, the BOF + ROP5I strain is the most resistant to IRGB6-PVM coating (26) and the only strain that was able to kill some mice during secondary infection. The identity of additional genes that promote virulence in chronically infected mice and whether they are bona fide virulence factors or contain important immune epitopes remain to be determined.
CD8 T cells and IFN-γ are absolute requirements for host immunity to the type I RH and type II ME49 strains (38), and CD8 T cells were able to generate IFN-γ in response to MAS-infected cells (Fig. 2). Whether additional CD8 T cell effector mechanisms, such as cytolysis, or other memory cell types (B and CD4 T cells) are required for host immunity to atypical T. gondii strains remains to be investigated. For example, loss of CD8 T cell polyfunctionality (i.e., the ability to produce more than one cytokine or granzyme) correlates with host susceptibility to chronic infection (93), and perforin-dependent cytolysis is apparently one of these required functions (52). In contrast to chronic infection with ME49 type II strains, the perforin pathway is not required for vaccine-induced protection against RH secondary infection (49). Whether these differences reflect stage-specific (tachzyoite versus bradyzoite) or strain-specific (RH versus ME49) requirements for host immunity remains an open question, especially since most strains are virulent during secondary infection, as reported here. It is clear that some aspect of the memory CD8 T cell response but not the CD4 T cell response is necessary for protection against RH secondary infection (42), even though both cell types make ample amounts of IFN-γ after challenge (data not shown). In addition to their cytolytic activity, which can destroy the host cell that Toxoplasma needs for replication, CD8 T cells express a variety of TNF superfamily members which have dynamic effects on Toxoplasma. For example, parasite death can be triggered following CD40-CD40L interactions (94, 95) and egress following FAS-FASL interactions (96). Additionally, host resistance to infection is promoted by lymphotoxin-alpha (48). A careful dissection of how CD8 T cells restrict Toxoplasma may yield insights into other requirements for host immunity and point to new immune pathways that potentially intersect Toxoplasma virulence factors, in particular those of atypical strains.
Potential effect of superinfection on Toxoplasma population structure.
Although heterologous immunity to ROP5- and ROP18-expressing Toxoplasma strains is achieved in certain mouse genetic backgrounds, “sterile heterologous immunity” may rarely occur. IRG-resistant strains were observed via bioluminescence imaging days after the initial challenge and eventually superinfected the brain of chronically infected mice. These events may have a significant effect on the population structure. Heterologous tissue infection in intermediate hosts could assist parasite sexual recombination in nature by allowing two strains to be present during a single meal consumed by a felid, the definitive host species for Toxoplasma. Mixed infections in the wild have been noted in humans (97) and in sheep (98, 99), and heterologous brain infections have been experimentally verified in laboratory mice (59). Here, we linked these events to allelic combinations of ROP5 and ROP18 that resulted in resistance to IRGs, which can be disadvantageous in naive mice because they die before the infectious tissue cysts are formed, but advantageous in niches where most intermediate hosts are chronically infected, thus facilitating superinfection and the generation of novel parasite genotypes upon feline infection. Although oocysts can infect cats, they are less infectious than tissue cysts and have a much longer (3 to 10 days versus 18 to 30 days) prepatent period (or time to shed oocysts) (100). Thus, heterologous tissue infection in intermediate hosts may be the most efficient means to produce recombinant progeny in nature.
Superinfection may be a major mechanism impacting the population structures observed on the different continents and is likely a consequence of parasite coevolution with local rodents and their IRGs. Unlike the clonal strains of North America and Europe, where the vast majority of isolates are IRG sensitive, most South American strains of the various haplogroups are not clonal and are IRG resistant. The geographical distribution of these phenotypes is the subject of speculation. Certainly, the M. m. domesticus house mouse that is common to North America, Western Europe, and Africa and is highly susceptible to the type I strain has likely shaped the dominance of the IRG-sensitive type II and III strains in these locales. Of course, “IRG sensitive” and “IRG resistance” are terms used to define the interaction between different T. gondii strains and the IRGs of various M. m. domesticus laboratory mouse strains. However, the murine IRG locus is extremely diverse, rivaling that of the MHC, and mouse subspecies from different geographic locations have unique IRG haplotypes that interact with virulent Toxoplasma strains differently (101). For example, the South-Southeast Asian M. m. castaneus subspecies of mice does not die from type I infection, due to polymorphisms in Irgb2-b1, and instead they form a life-long chronic infection (101). Perhaps this is why the type I strain is frequently isolated in Asia (5). Less is known regarding the IRG haplotypes of South American rodents (e.g., capybara, tuco-tuco, guinea pigs), but presumably South American Toxoplasma strains have coevolved with these hosts to achieve maximal chronic infection. Here, we hypothesize that once IRG resistance or optimal IRG antagonism is selected, maintaining a clonal population structure of such strains may be difficult when reexposure to Toxoplasma is common, for example, in South America.
Furthermore, today in North America, approximately half of wild mammals are now chronic carriers for Toxoplasma (6), and the domesticated cat is now highly abundant. If IRG-resistant South America strains invade North America, as is thought to have occurred for the generation of the type I and type III strains (8), then changes in the clonal population structure will likely occur as chronically infected North American hosts become superinfected with South American strains. Cats feeding on those superinfected intermediate hosts may yield strains with enhanced virulence properties, perhaps suited to infect an immune host or a naturally resistant host, like M. m. castaneus, but strains maladapted for other hosts or species irrespective of prior exposure to Toxoplasma.
Toxoplasma virulence factors may also impact human disease.
Finally, given the lack of testing in the United States and the proximity to South America, perhaps closer attention should be paid to the possibility of reexposure to Toxoplasma and whether an individual has consumed food from South American locales. Although humans do not express the expanded small GTPase IRG family that is antagonized by most South American strains, they do express the large 65-kDa IFN-γ-induced GBPs, which are inhibited by virulent versions of ROP5 and ROP18 (31, 102). Furthermore, ROP18 induces ATFβ degradation, a conserved host transcription factor that is required for T. gondii-infected dendritic cells to elicit CD8 T cell activation (78). Whether ROP5/18 alleles promote virulence in humans is unknown; however, type I and atypical strain types have been associated with multiple forms of human toxoplasmosis (62–69). In conclusion, the selective forces that have shaped the Toxoplasma population structure may have the unintended consequence of generating strains that cause severe disease in certain intermediate hosts, and we conclude that T. gondii may be more virulent than previously appreciated.
MATERIALS AND METHODS
Parasite strains.
T. gondii strains were passaged in human foreskin fibroblasts (HFFs; originally obtained from the Boothroyd lab, Stanford University) in Toxo medium (4.5 g/liter d-glucose, l-glutamine in Dulbecco’s modified Eagle’s medium [DMEM; Life Technologies] supplemented with 1% fetal bovine serum [FBS; Omega Scientific] and antibiotics). The following atypical strains were used (haplogroups are shown in parentheses): CASTELLS and MAS (HG 4); GUY-KOE (HG 5); FOU, BOF, and GPHT (HG 6); CAST (HG 7); TgCATBr5 (HG 8); P89 (HG 9); GUY-DOS and VAND (HG 10); COUGAR (HG 11). The clonal types used in this study were type I RH Δhxgprt, RH cLUC (1-1), and RH Δhxgprt Δku80; type II Pru Δhxgprt Δku80, Pru A7 fLUC Δhxgprt::HXGPRT (5-8 B+) (103); and type III CEP hxgprt−, CEP hxgprt− cLUC (C22), CL14 cLUC. The avirulent S22 (ROP5II ROP18III) and virulent S23-cLUC (ROP5II ROP18III) F1 progeny (85), derived from crosses between the type II and III strains (II × III), were also used. F1 progeny from a type I (GT1) × type III (CTG) cross were obtained from BEI Resources and chosen for expression of the type I ROP18 allele inferred from restriction fragment length polymorphism (RFLP) analysis at the CS6 genetic marker within ROP18 (http://toxomap.wustl.edu/IxIII_Typing_Table.html). F1 progeny used to challenge chronically infected mice were E8SF, c295-31, c295-3, B10AF, E7SF, B4SF, c285-13, c295-27, C7SF, P1A5, c295-29, c285-31, C11AF, H7AF, c295-5, and E6AF. The BOF + ROP5I and type II (Pru A7) + ROP5I strains, which express the entire ROP5 locus from the type I strain via integration of a cosmid (LC37), and the S22 +ROP5I (LC37) + ROP18IIHA strain were previously generated (26). The replication-deficient uracil auxotroph vaccine strain RH Δku80 Δompdc Δup::HXGPRT (83) was maintained in medium supplemented with uracil (1 mM).
Generation of luciferase-expressing MAS strain and RH Δtgd057 parasite strain.
A luciferase expressing MAS strain (MAS-LUC; 2C8) was generated by transfecting a linearized dual expression plasmid harboring the click beetle luciferase cassette (cLUC) and GFP (85), FACS sorting of green fluorescent protein-positive (GFP+) parasites, and cloning by limiting dilution. The type I RH Δku80 Δtgd057::HXGPRT strain was generated by homologous recombination via targeting with a linearized pTKO2 “destination” plasmid (103) created by three-way Gateway cloning (Life Technologies). The pTKO2-targeting plasmid consisted of an HXGPRT resistance cassette flanked by 2 kb of the sequence 2,500 to 500 bp upstream of the ATG start site and 2 kb downstream of the stop codon of TGD057 (TGME49_215980). RH Δku80 Δhxgprt parasites were transfected with the targeting plasmid and cloned in selection medium, which was Toxo medium (described above) containing 50 µg/ml mycophenolic acid (MPA) and 50 µg/ml xanthine, as described elsewhere (103). Successful targeting was confirmed based on absence of a PCR product with primers specific for the endogenous TGD057 gene and by a positive PCR product with primers internal to the HXGPRT resistance cassette and primers that flanked the entire targeting region of the TGD057 locus, as described in detail in Fig. S3 in the supplemental material.
Mice and generation of bone marrow-derived macrophages.
Female C57BL/6J (H-2b) mice were purchased from Jackson Laboratories and maintained under specific-pathogen-free conditions. T57 transnuclear mice (from the Ploegh laboratory, MIT) were bred in-house; in short, these mice were generated by somatic cell nuclear transfer of Kb TDG05796–103 tetramer-positive CD8 T cells (B6 background), and thus 90% of peripheral CD3+ T cells are CD8 positive and 80 to 90% of these have endogenously rearranged TCRα (Vα6-4) and β (Vβ13-1) loci with encoded CDR3 regions bearing specificity for Kb-TGD05796–103 (77). BMDMs were obtained by culturing murine bone marrow cells from C57BL/6 mice in DMEM supplemented with 10% FBS, 1× minimal essential medium nonessential amino acids, 1 mM sodium pyruvate (Life Technologies), antibiotics, and 20% L929 conditioned medium. BMDMs were harvested after 7 to 8 days of differentiation (36).
Chronic infection, vaccination, and serotyping.
Parasites were obtained by scraping T-25 flasks containing heavily vacuolated HFFs and sequentially syringe lysing the suspension through 25-gauge and 27-gauge needles. The released parasites were pelleted by spinning at 572 × g for 7 min, washed, and counted in phosphate-buffered saline (PBS). Mice were infected by intraperitoneal (i.p.) injection, and parasite viability of the inoculum was determined in a plaque assay after mouse infections. To induce chronic infection, 104 tachyzoites of the type III strain (CEP) or 5 × 102 tachyzoites of the type II strain (Pru) were injected i.p., and mice were allowed to progress to chronic infection (>30 days postinjection). For vaccination with the atypical MAS strain, mice were given 400 mg/liter (pH 7.2) of sulfadiazine plus 320 µM pyrimethamine (Sigma) in their drinking water 1 day following injection of 105 MAS tachyzoites and kept on drug therapy for 18 days. During this time, mice were boosted two additional times with 6 × 105 live syringe-lysed MAS tachyzoites on day 7 and 4 × 104 MAS parasites on day 14 after the initial injection. After drug removal, mice were placed on normal drinking water for a minimum of 19 days prior to secondary challenge. During this time, mice exhibited no signs of active infection and experienced weight gain, suggesting the overall health of the animals. For vaccination with the RH Δku80 Δompdc Δup::HXGPRT strain, mice were injected with a single dose of 106 tachyzoites.
At least 30 days after vaccination or chronic infection, blood was harvested from mice via the tail vein, collected in tubes containing 5 µl of 0.5 M EDTA, and placed on ice. The blood was pelleted at 5,800 × g for 5 min; blood plasma was collected from the supernatant and stored at −80°C. To determine seroconversion, HFFs were grown on coverslips and infected with the GFP-expressing type I RH (1-1) strain overnight, fixed the next morning with 3% formaldehyde in PBS, permeabilized with 3% bovine serum albumin, 0.2 Triton X-100–0.01% sodium azide, incubated with a 1:100 dilution of collected blood plasma for 2 h at room temperature or overnight at 4°C, washed, and detected with Alexa Fluor 594-labeled secondary antibodies specific for mouse IgG (Invitrogen). “Plasma-stained” parasites were observed by immunofluorescence.
Secondary infections, assessment of parasite viability, and bioluminescence imaging.
Seropositive mice were then challenged with syringe-lysed tachyzoites (5 × 104) and weighed every 3 to 4 days. After the last mouse injection, parasite viability for each strain was determined by a plaque assay in which 100 tachyzoites were plated in HFF monolayers grown in a 24-well plate; 5 to 7 days later plaques were enumerated by microscopy (4× objective). Viability ranged from 20 to 50%. For in vivo bioluminescence imaging, mice were injected i.p. with 300 µg of d-luciferin (Gold Biotechnology) and 5 min later anesthetized with isoflurane (Abbott); 5 min later, photon emission from individual mice was detected with an IVIS Spectrum bioluminescent and fluorescent imaging system (Xenogen). Images were captured with integration times from 10 s to 5 min, depending on the intensity of the bioluminescent signal. Image analysis was performed with Living Image software (Xenogen).
Genetic linkage analysis.
For QTL analysis, 176 informative genetic markers for each of the F1 I × III progeny were obtained from the website http://toxomap.wustl.edu/IxIII_Typing_Table.html, and mouse survival (as a percentage of total mice) at 26 days after secondary infection in type III chronically infected C57BL/6 mice was analyzed with the program R/QTL (Bowman). LOD scores for each marker were calculated. For all LOD scores of >2, 1,000 permutations were performed to obtain the LOD threshold at a P level of ≤0.05, which was considered statistically significant; only the SAG3 marker returned an LOD score of >2 and was significant for this experiment.
Brain superinfection assays.
Brains were dissected from chronically infected mice that survived secondary challenge, rinsed in PBS, placed in 5 ml PBS, and extruded through a 2-inch 21-gauge needle several times (10-ml syringe). The suspension was spun at 572 × g for 7 min and resuspended in 1 ml of PBS. For rederivation, 100 to 200 µl of the brain homogenate was used to inoculate HFF monolayers in a T-25 flask with Toxo medium (described above). One week after inoculation, the T-25 flask was scraped and syringe lysed through a 25-gauge needle, spun for 5 min at 32 × g to pellet the large debris; the supernatant was collected, pelleted with a faster spin of 572 × g for 7 min, and resuspended in Toxo medium, and the entirety was used to inoculate a new HFF monolayer in a T-25 flask. Parasite growth was typically observed between 2 and 3 weeks post-initial inoculation. To determine whether the challenging strain was present in the ensuing parasite culture, several approaches were used: parasite expression of GFP or lack thereof was directly observed in the culture by florescence microscopy; parasites were passed in selection medium containing MPA-xanthine (described above) to test for a functional copy of HXGPRT; parasite genomic DNA was isolated using DNAzol (Invitrogen); RFLP analysis of the GRA6 locus was performed using the primers GRA6FW (5′-ATTTGTGTTTCCGAGCAGGT) and GRA6RV (3′-TCGCCGAAGAGTTGACATAG), and the restriction enzyme MseI, producing unique fragment lengths that identified all three clonal lineages.
Cell isolation, in vitro recall infections, and FACS analysis.
PECs were harvested by injecting 4 ml of PBS plus 3 ml of air into the peritoneal cavity (i.p.) with a 30-gauge needle and shaking and extracting the cells by puncture with a 16-gauge needle. Cells were filtered through 70-µm cell strainer and washed in FACS buffer, 1% FBS–PBS before staining. The spleen or, in the case of T57 mice, the spleen and lymph nodes were dissected and crushed through a cell strainer, pelleted, and incubated in ACK red blood cell (RBC) lysis buffer (NH4Cl at 0.15 M, KHCO3 at 10 mM, EDTA at 0.1 mM) for 5 min at room temperature, washed in FACS buffer, and filtered again through a cell strainer.
For FACS analysis, all preparations were done on ice, and cells were blocked in FACS buffer supplemented with 10% normal hamster serum (Jackson ImmunoResearch) and FcBlock anti-CD16/32 (BD Pharmingen) prior to staining with fluorophore-labeled monoclonal antibodies (MAbs). The following MAbs (1:100 staining dilutions) were purchased from eBioscience unless otherwise stated: anti-CD62L eFluor 450 (MEL-14), anti-CD44 eFluor 450 (IM7), anti-KLRG1–fluorescein isothiocyanate (FITC) (2F1), anti-CD127–phycoerythrin (PE) (A7R34), anti-CD19–PE-Cy5 (1D3), anti-CD8α–allophycocyanin (APC) or anti-CD8α–PE-Cy7 (53-6.7), and anti-CD3ε APC-Cy7 (145-2C11; BD Pharmingen). Cells were washed in FACS buffer prior to analysis on an LSR HTS-2 FACS machine (BD) equipped with BD FACSDIVA software.
For intracellular staining of cells following in vitro recall infections, 6 × 105 PECs or splenocytes per well (96-well plate) were plated in T cell medium (10% FBS in RPMI 1640 with GlutaMAX, antibiotics, 10 mM HEPES, 1 mM sodium pyruvate [Invitrogen], and 1.75 µl β-mercaptoethanol per 500 ml [MP Biomedicals]). Cells were infected with parasites (multiplicity of infection [MOI], 0.2) for 18 to 21 h, and 3 µg/ml brefeldin A (eBioscience) was added for the last 5 h of infection. Tissue culture plates were placed on ice, and cells were harvested by pipetting, washed with FACS buffer, blocked, and stained for surface markers as described above. Cells were then fixed with BD Cytofix/Cytoperm and permeabilized with BD Perm/Wash solution according to the manufacturer’s suggestions (BD Pharmingen). Cells were stained with MAbs labeled with APC or PE that recognized IFN-γ (XMG1.2) at room temperature for 1 h or overnight at 4°C. Cells were then washed once in BD Perm/Wash and once in FACS buffer prior to FACS analysis. All data were compensated and processed with FlowJo software (Treestar).
T57 transnuclear CD8 T cell cytokine secretion.
BMDMs (B6) cells were plated at 2 × 105 cell/well (96-well plate) in BMDM medium (described above) supplemented with 20% L929 conditioned medium. The next day, the medium was removed and cells were infected with parasites in triplicate (MOI, 0.6, 0.2, or 0.08) in T cell medium (see above). Splenocytes and lymph node cells from T57 transnuclear mice were harvested and combined, and RBCs were lysed with ACK lysis buffer. After washing, 5 × 105 cells were added to the infected BMDMs (~2 h from time of infection). Forty-eight hours later, supernatant was removed and stored at −80°C for IL-2 and IFN-γ analysis by ELISA (eBioscience). Viability was inferred via a plaque assay, and wells with equivalent numbers of viable parasites were compared.
Ethics statement.
All mouse work was performed in accordance with the recommendations in the Guide to the Care and Use of Laboratory Animals (104) of the National Institutes of Health. The MIT Committee on Animal Care (assurance no. A-3125-01) approved all protocols, and all efforts were made to minimize unnecessary distress to the animals.
SUPPLEMENTAL MATERIAL
Similar marker expression on IFN-γ+ CD8 T cells following recall infection with different Toxoplasma strains. (A) PECs from type III (CEP) chronically infected C57BL/6 mice were infected (MOI, 0.2) in vitro with the indicated parasite strains for 16 h. The expression of CD62L and KLRG1 on IFN-γ-producing CD8+ (CD3+ CD19−) T cells (top row) and IFN-γ-negative CD8+ (CD3+ CD19−) T cells (bottom row) was analyzed by FACS analysis; the gating strategy is depicted above. Bar graphs plot the percent contribution of each cell population to the total IFN-γ+ or IFN-γ− CD8 T cell PECs observed in each mouse. Results are from 3 individual mice. (B) Splenocytes from type II chronically infected mice were infected (MOI, 0.2) in vitro with the type I RH strain or atypical strain MAS for 16 h; CD19+ (CD8− CD3−) B cells, CD3+ (CD8− CD19−) T cells, and CD8+ (CD3+ CD19−) T cells were analyzed for the expression of IL-7rα (CD127) and intracellular IFN-γ by FACS. Plotted is a representative histogram of the relative CD127 staining intensity on each cell type, and the percentage of cells that fell within the depicted IL-7rα hi gate is shown. Similarly, IFN-γ+ and IFN-γ-negative CD8+ T cells were analyzed for IL-7rα hi expression; a representative histogram and the percentage of CD8 T cells that fell within the IL-7rα hi gate from a representative mouse are shown. Finally, the average percentage (± standard error of the mean) of IFN-γ+ and IFN-γ-negative CD8 T cells from the spleen that were IL-7rα hi was plotted. Result are from 5 mice. n.s., not significant (Student’s t test; P > 0.05). Download
Generation of the type I RH Δtgd057 strain. (A) Scheme depicting the strategy used to obtain the type I (RH) Δtgd057 knockout strain. The 5′ and 3′ flanking regions (FR) of our genes of interest were cloned on both sides of the HXGPRT selection marker. The vector was linearized prior to transfection into RH Δku80 Δhxgprt parasites. Following a double homologous recombination event, the gene of interest was replaced by HXGPRT. The arrows represent the primers (P1 to P8) used to make the construct and verify the gene replacement (P1, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTACACTGCGGAACGACGTGGAGA-3′; P2, 5′-GGGGACAACTTTGTATAGAAAAGTTGGGTGATTTGCCTTCTACAATGCATC-3′; P3, 5′-GGGGACAACTTTGTATAATAAAGTTGCTAACTCCTTCGATCCTCACCTG-3′; P4, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTACGTGTTACCTTGGAAGCTGCA-3′; P5, 5′-ACTGGCGTGTTTCGCCGCAT-3′; P6, 5′-TCTGTCGCCTTTGCCACGCA-3′; P7, 5′-AACATCCAGCGAGTCCTTCC-3′; P8, 5′-GATCCAGACGTCTTCAATGC-3′). (B) To confirm the disruption of TGD057, we performed a PCR with a forward primer upstream of the 5′ flanking region (P7) and a reverse primer within the HXGPRT cassette (P8). A second PCR was executed to confirm the inability to amplify the target gene (P5 and P6). Download
Survival of type III chronically infected C57BL/6 mice to secondary challenge with 16 F1 I × III progeny. C57BL/6 mice were infected with the type III (CEP) strain and allowed to progress to chronic infection. On day 35 of chronic infection, mice were challenged i.p. with 5 × 104 tachyzoites of the indicated F1 I × III progeny derived from a type III (CTG) and type I (GT1) cross. Viability was confirmed by plaque assays, and mice received between 104 and 103 viable parasites. The F1 progeny were chosen based on the presence of a type I allele at the marker CS6, which detects an SNP in ROP18. The F1 progeny used in this experiment were predicted to fully express ROP18, which is not expressed in type III lineage parasite strains, and they are 100% lethal in naive mice (34). The number of animals challenged (n) and the percentage of surviving mice at 26 days after secondary challenge are indicated. QTL analysis detected a significant disease association peak on Toxoplasma chromosome XII at marker SAG3 (LOD, 4.1; P < 0.05); the type I or III SAG3 allele for each F1 progeny is indicated. Download
ACKNOWLEDGMENTS
We thank Sharvan Sehrawat and John Jackson (Ploegh Laboratory, MIT) for providing spleens and lymph nodes for initial screening and subsequent transfer of the T57 mice. We thank David Bzik (Dartmouth) for providing the uracil auxotroph strain.
Footnotes
Citation Jensen KDC, Camejo A, Melo MB, Cordeiro C, Julien L, Grotenbreg GM, Frickel E, Ploegh HL, Young L, Saeij JPJ. 2015. Toxoplasma gondii superinfection and virulence during secondary infection correlate with the exact ROP5/ROP18 allelic combination. mBio 6(2):e02280-14. doi:10.1128/mBio.02280-14.
REFERENCES
- 1.Waldeland H, Frenkel JK. 1983. Live and killed vaccines against toxoplasmosis in mice. J Parasitol 69:60–65. doi: 10.2307/3281275. [DOI] [PubMed] [Google Scholar]
- 2.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149:175–180. [PubMed] [Google Scholar]
- 3.Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd ed. CRC Press, Taylor & Francis Group, Boca Raton, FL. [Google Scholar]
- 4.Sibley LD, Ajioka JW. 2008. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol 62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 5.Shwab EK, Zhu XQ, Majumdar D, Pena HF, Gennari SM, Dubey JP, Su C. 2014. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 141:453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- 6.Dubey JP, Velmurugan GV, Rajendran C, Yabsley MJ, Thomas NJ, Beckmen KB, Sinnett D, Ruid D, Hart J, Fair PA, McFee WE, Shearn-Bochsler V, Kwok OC, Ferreira LR, Choudhary S, Faria EB, Zhou H, Felix TA, Su C. 2011. Genetic characterisation of Toxoplasma gondii in wildlife from North America revealed widespread and high prevalence of the fourth clonal type. Int J Parasitol 41:1139–1147. doi: 10.1016/j.ijpara.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran C, Su C, Dubey JP. 2012. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect Genet Evol 12:359–368. doi: 10.1016/j.meegid.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Minot S, Melo MB, Li F, Lu D, Niedelman W, Levine SS, Saeij JP. 2012. Admixture and recombination among Toxoplasma gondii lineages explain global genome diversity. Proc Natl Acad Sci U S A 109:13458–13463. doi: 10.1073/pnas.1117047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira Ade M, Vitor RW, Gazzinelli RT, Melo MN. 2006. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol 6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé ML, Zhu XQ, Ajioka JW, Rosenthal BM, Dubey JP, Sibley LD. 2012. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A 109:5844–5849. doi: 10.1073/pnas.1203190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parameswaran N, Thompson RC, Sundar N, Pan S, Johnson M, Smith NC, Grigg ME. 2010. Non-archetypal type II-like and atypical strains of Toxoplasma gondii infecting marsupials of Australia. Int J Parasitol 40:635–640. doi: 10.1016/j.ijpara.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann T, Graham DH, Dahl ER, Bahia-Oliveira LM, Gennari SM, Dubey JP. 2004. Variation in the structure of Toxoplasma gondii and the roles of selfing, drift, and epistatic selection in maintaining linkage disequilibria. Infect Genet Evol 4:107–114. doi: 10.1016/j.meegid.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Wendte JM, Miller MA, Lambourn DM, Magargal SL, Jessup DA, Grigg ME. 2010. Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites sarcocystis neurona and Toxoplasma gondii. PLoS Genet 6:e1001261. doi: 10.1371/journal.pgen.1001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins CN, Pimm SL, Joppa LN. 2013. Global patterns of terrestrial vertebrate diversity and conservation. Proc Natl Acad Sci U S A 110:E2602–E2610. doi: 10.1073/pnas.1302251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BH, Shenoy AR, Kumar P, Bradfield CJ, MacMicking JD. 2012. IFN-inducible GTPases in host cell defense. Cell Host Microbe 12:432–444. doi: 10.1016/j.chom.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J. 2013. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog 9:e1003414. doi: 10.1371/journal.ppat.1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, Kaiser F, Zerrahn J, Martens S, Howard JC. 2008. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J 27:2495–2509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degrandi D, Konermann C, Beuter-Gunia C, Kresse A, Würthner J, Kurig S, Beer S, Pfeffer K. 2007. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol 179:7729–7740. doi: 10.4049/jimmunol.179.11.7729. [DOI] [PubMed] [Google Scholar]
- 19.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. 2005. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog 1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. 2006. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DC, Soldati-Favre D, Horie K, Takeda J, Takeda K. 2012. A cluster of interferon-gamma-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Degrandi D, Kravets E, Konermann C, Beuter-Gunia C, Klümpers V, Lahme S, Wischmann E, Mausberg AK, Beer-Hammer S, Pfeffer K. 2013. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci U S A 110:294–299. doi: 10.1073/pnas.1205635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinfeldt T, Könen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, Hunn JP, Howard JC. 2010. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol 8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong YC, Boothroyd JC, Reichmann G, Howard JC. 2010. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol 12:939–961. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fentress SJ, Behnke MS, Dunay IR, Mashayekhi M, Rommereim LM, Fox BA, Bzik DJ, Taylor GA, Turk BE, Lichti CF, Townsend RR, Qiu W, Hui R, Beatty WL, Sibley LD. 2010. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niedelman W, Gold DA, Rosowski EE, Sprokholt JK, Lim D, Farid Arenas A, Melo MB, Spooner E, Yaffe MB, Saeij JP. 2012. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog 8:e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleckenstein MC, Reese ML, Könen-Waisman S, Boothroyd JC, Howard JC, Steinfeldt T. 2012. A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol 10:e1001358. doi: 10.1371/journal.pbio.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA, Sibley LD. 2012. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog 8:e1002992. doi: 10.1371/journal.ppat.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. 2014. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alaganan A, Fentress SJ, Tang K, Wang Q, Sibley LD. 2014. Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc Natl Acad Sci U S A 111:1126–1131. doi: 10.1073/pnas.1313501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW, Macmicking JD, Sibley LD. 2013. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog 9:e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reese ML, Shah N, Boothroyd JC. 2014. The Toxoplasma pseudokinase rop5 is an allosteric inhibitor of the immunity-related GTPases. J Biol Chem 289:27849–27858. doi: 10.1074/jbc.M114.567057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. 2011. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A 108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. 2006. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 35.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen KD, Hu K, Whitmarsh RJ, Hassan MA, Julien L, Lu D, Chen L, Hunter CA, Saeij JP. 2013. Toxoplasma rhoptry kinase ROP16 promotes host resistance to oral infection and intestinal inflammation only in the context of the dense granule protein GRA15. Infect Immun 81:2156–2167. doi: 10.1128/IAI.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fux B, Nawas J, Khan A, Gill DB, Su C, Sibley LD. 2007. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect Immun 75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gigley JP, Bhadra R, Khan IA. 2011. CD8 T cells and Toxoplasma gondii: a new paradigm. J Parasitol Res 2011:243796. doi: 10.1155/2011/243796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Conley FK, Remington JS. 1989. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol 143:2045–2050. [PubMed] [Google Scholar]
- 40.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol 146:286–292. [PubMed] [Google Scholar]
- 41.Gigley JP, Fox BA, Bzik DJ. 2009. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol 182:1069–1078. doi: 10.4049/jimmunol.182.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan KA, Wilson EH, Tait ED, Fox BA, Roos DS, Bzik DJ, Dzierszinski F, Hunter CA. 2009. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infect Immun 77:3894–3901. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki Y, Remington JS. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol 140:3943–3946. [PubMed] [Google Scholar]
- 44.Denkers EY, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. 1996. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med 184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casciotti L, Ely KH, Williams ME, Khan IA. 2002. CD8+ T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect Immun 70:434–443. doi: 10.1128/IAI.70.2.434-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson DC, Matthews S, Yap GS. 2008. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J Immunol 180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 47.Jordan KA, Dupont CD, Tait ED, Liou HC, Hunter CA. 2010. Role of the NF-κB transcription factor c-Rel in the generation of CD8+ T-cell responses to Toxoplasma gondii. Int Immunol 22:851–861. doi: 10.1093/intimm/dxq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlüter D, Kwok LY, Lütjen S, Soltek S, Hoffmann S, Körner H, Deckert M. 2003. Both lymphotoxin-alpha and TNF are crucial for control of Toxoplasma gondii in the central nervous system. J Immunol 170:6172–6182. doi: 10.4049/jimmunol.170.12.6172. [DOI] [PubMed] [Google Scholar]
- 49.Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol 159:1903–1908. [PubMed] [Google Scholar]
- 50.Khan IA, Matsuura T, Kasper LH. 1998. Inducible nitric oxide synthase is not required for long-term vaccine-based immunity against Toxoplasma gondii. J Immunol 161:2994–3000. [PubMed] [Google Scholar]
- 51.Scharton-Kersten TM, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S. 2010. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol 176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith DD, Frenkel JK. 2003. Immunological comparison of 124 isolates of Toxoplasma gondii. Parasitol Res 91:332–337. doi: 10.1007/s00436-003-0886-6. [DOI] [PubMed] [Google Scholar]
- 54.Franco PS, Silva DA, Costa IN, Gomes AO, Silva AL, Pena JD, Mineo JR, Ferro EA. 2011. Evaluation of vertical transmission of Toxoplasma gondii in Calomys callosus model after reinfection with heterologous and virulent strain. Placenta 32:116–120. doi: 10.1016/j.placenta.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Khan A, Böhme U, Kelly KA, Adlem E, Brooks K, Simmonds M, Mungall K, Quail MA, Arrowsmith C, Chillingworth T, Churcher C, Harris D, Collins M, Fosker N, Fraser A, Hance Z, Jagels K, Moule S, Murphy L, O’Neil S, Rajandream MA, Saunders D, Seeger K, Whitehead S, Mayr T, Xuan X, Watanabe J, Suzuki Y, Wakaguri H, Sugano S, Sugimoto C, Paulsen I, Mackey AJ, Roos DS, Hall N, Berriman M, Barrell B, Sibley LD, Ajioka JW. 2006. Common inheritance of chromosome Ia associated with clonal expansion of Toxoplasma gondii. Genome Res 16:1119–1125. doi: 10.1101/gr.5318106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freyre A, Falcón J, Méndez J, Rodriguez A, Correa L, Gonzalez M. 2006. Toxoplasma gondii: partial cross-protection among several strains of the parasite against congenital transmission in a rat model. Exp Parasitol 112:8–12. doi: 10.1016/j.exppara.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Werner H, Janitschke K, Masihi M, Adusu E. 1977. The effect of toxoplasma antibodies after reinfection with T. gondii. III. Communication: investigations on the question of placental transmission of Toxoplasma in immunised pregnant animals. Zentralbl Bakteriol 238:128–142. [PubMed] [Google Scholar]
- 58.Araujo F, Slifer T, Kim S. 1997. Chronic infection with Toxoplasma gondii does not prevent acute disease or colonization of the brain with tissue cysts following reinfection with different strains of the parasite. J Parasitol 83:521–522. doi: 10.2307/3284421. [DOI] [PubMed] [Google Scholar]
- 59.Dao A, Fortier B, Soete M, Plenat F, Dubremetz JF. 2001. Successful reinfection of chronically infected mice by a different Toxoplasma gondii genotype. Int J Parasitol 31:63–65. doi: 10.1016/S0020-7519(00)00151-X. [DOI] [PubMed] [Google Scholar]
- 60.Brandão GP, Melo MN, Caetano BC, Carneiro CM, Silva LA, Vitor RW. 2011. Susceptibility to re-infection in C57BL/6 mice with recombinant strains of Toxoplasma gondii. Exp Parasitol 128:433–437. doi: 10.1016/j.exppara.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Brandão GP, Melo MN, Gazzinelli RT, Caetano BC, Ferreira AM, Silva LA, Vitor RW. 2009. Experimental reinfection of BALB/c mice with different recombinant type I/III strains of Toxoplasma gondii: involvement of IFN-gamma and IL-10. Mem Inst Oswaldo Cruz 104:241–245. doi: 10.1590/S0074-0276009000200017. [DOI] [PubMed] [Google Scholar]
- 62.Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LM, Tan HK, Wallon M, Buffolano W, Stanford MR, Petersen E, European Multicentre Study on Congenital Toxoplasmosis (EMSCOT) . 2008. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis 2:e277. doi: 10.1371/journal.pntd.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dodds EM, Holland GN, Stanford MR, Yu F, Siu WO, Shah KH, Ten Dam-van Loon N, Muccioli C, Hovakimyan A, Barisani-Asenbauer T, International Ocular Toxoplasmosis Research Group . 2008. Intraocular inflammation associated with ocular toxoplasmosis: relationships at initial examination. Am J Ophthalmol 146:856–865.e2. doi: 10.1016/j.ajo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Sauer A, de la Torre A, Gomez-Marin J, Bourcier T, Garweg J, Speeg-Schatz C, Candolfi E. 2011. Prevention of retinochoroiditis in congenital toxoplasmosis: Europe versus South America. Pediatr Infect Dis J 30:601–603. doi: 10.1097/INF.0b013e3182129e70. [DOI] [PubMed] [Google Scholar]
- 65.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis 184:633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- 66.Khan A, Jordan C, Muccioli C, Vallochi AL, Rizzo LV, Belfort R Jr., Vitor RW, Silveira C, Sibley LD. 2006. Genetic divergence of Toxoplasma gondii strains associated with ocular toxoplasmosis, Brazil. Emerg Infect Dis 12:942–949. doi: 10.3201/eid1206.060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Switaj K, Master A, Borkowski PK, Skrzypczak M, Wojciechowicz J, Zaborowski P. 2006. Association of ocular toxoplasmosis with type I Toxoplasma gondii strains: direct genotyping from peripheral blood samples. J Clin Microbiol 44:4262–4264. doi: 10.1128/JCM.01786-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vallochi AL, Muccioli C, Martins MC, Silveira C, Belfort R Jr, Rizzo LV. 2005. The genotype of Toxoplasma gondii strains causing ocular toxoplasmosis in humans in Brazil. Am J Ophthalmol 139:350–351. doi: 10.1016/j.ajo.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 69.McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, Noble AG, Withers S, Swisher CN, Heydemann PT, Sautter M, Babiarz J, Rabiah P, Meier P, Grigg ME, Toxoplasmosis Study Group . 2012. Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009). Clin Infect Dis 54:1595–1605. doi: 10.1093/cid/cis258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elbez-Rubinstein A, Ajzenberg D, Dardé ML, Cohen R, Dumètre A, Yera H, Gondon E, Janaud JC, Thulliez P. 2009. Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J Infect Dis 199:280–285. doi: 10.1086/595793. [DOI] [PubMed] [Google Scholar]
- 71.Valdès V, Legagneur H, Watrin V, Paris L, Hascoët JM. 2011. Congenital toxoplasmosis due to maternal reinfection during pregnancy. Arch Pediatr 18:761–763. doi: 10.1016/j.arcped.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Dardé ML, Bouteille B, Pestre-Alexandre M. 1992. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J Parasitol 78:786–794. doi: 10.2307/3283305. [DOI] [PubMed] [Google Scholar]
- 73.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui W, Kaech SM. 2010. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev 236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baron V, Bouneaud C, Cumano A, Lim A, Arstila TP, Kourilsky P, Ferradini L, Pannetier C. 2003. The repertoires of circulating human CD8(+) central and effector memory T cell subsets are largely distinct. Immunity 18:193–204. doi: 10.1016/S1074-7613(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 76.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, Ploegh HL, Yap GS. 2010. Differential regulation of effector- and central-memory responses to Toxoplasma gondii infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog 6:e1000815. doi: 10.1371/journal.ppat.1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirak O, Frickel EM, Grotenbreg GM, Suh H, Jaenisch R, Ploegh HL. 2010. Transnuclear mice with predefined T cell receptor specificities against Toxoplasma gondii obtained via SCNT. Science 328:243–248. doi: 10.1126/science.1178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto M, Ma JS, Mueller C, Kamiyama N, Saiga H, Kubo E, Kimura T, Okamoto T, Okuyama M, Kayama H, Nagamune K, Takashima S, Matsuura Y, Soldati-Favre D, Takeda K. 2011. ATF6β is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med 208:1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD. 2011. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci U S A 108:9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan A, Behnke MS, Dunay IR, White MW, Sibley LD. 2009. Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryot Cell 8:1828–1836. doi: 10.1128/EC.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang N, Farrell A, Niedelman W, Melo M, Lu D, Julien L, Marth GT, Gubbels MJ, Saeij JP. 2013. Genetic basis for phenotypic differences between different Toxoplasma gondii type I strains. BMC Genomics 14:467. doi: 10.1186/1471-2164-14-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dubey JP. 1980. Mouse pathogenicity of Toxoplasma gondii isolated from a goat. Am J Vet Res 41:427–429. [PubMed] [Google Scholar]
- 83.Fox BA, Bzik DJ. 2010. Avirulent uracil auxotrophs based on disruption of orotidine-5′-monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun 78:3744–3752. doi: 10.1128/IAI.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim D, Gold DA, Julien L, Rosowski EE, Niedelman W, Yaffe MB, Saeij JP. 2013. Structure of the Toxoplasma gondii ROP18 kinase domain reveals a second ligand binding pocket required for acute virulence. J Biol Chem 288:34968–34980. doi: 10.1074/jbc.M113.523266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. 2005. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun 73:695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adomako-Ankomah Y, Wier GM, Borges AL, Wand HE, Boyle JP. 2014. Differential locus expansion distinguishes Toxoplasmatinae species and closely related strains of Toxoplasma gondii. mBio 5(1):e01003-13. doi: 10.1128/mBio.01003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacquet A, Coulon L, De Nève J, Daminet V, Haumont M, Garcia L, Bollen A, Jurado M, Biemans R. 2001. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol 116:35–44. doi: 10.1016/S0166-6851(01)00297-3. [DOI] [PubMed] [Google Scholar]
- 88.Dzierszinski F, Mortuaire M, Cesbron-Delauw MF, Tomavo S. 2000. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol 37:574–582. doi: 10.1046/j.1365-2958.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- 89.Caetano BC, Bruña-Romero O, Fux B, Mendes EA, Penido ML, Gazzinelli RT. 2006. Vaccination with replication-deficient recombinant adenoviruses encoding the main surface antigens of Toxoplasma gondii induces immune response and protection against infection in mice. Hum Gene Ther 17:415–426. doi: 10.1089/hum.2006.17.415. [DOI] [PubMed] [Google Scholar]
- 90.Zheng B, Lu S, Tong Q, Kong Q, Lou D. 2013. The virulence-related rhoptry protein 5 (ROP5) of Toxoplasma gondii is a novel vaccine candidate against toxoplasmosis in mice. Vaccine 31:4578–4584. doi: 10.1016/j.vaccine.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 91.Grover HS, Chu HH, Kelly FD, Yang SJ, Reese ML, Blanchard N, Gonzalez F, Chan SW, Boothroyd JC, Shastri N, Robey EA. 2014. Impact of regulated secretion on antiparasitic CD8 T cell responses. Cell Rep 7:1716–1728. doi: 10.1016/j.celrep.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melo MB, Nguyen QP, Cordeiro C, Hassan MA, Yang N, McKell R, Rosowski EE, Julien L, Butty V, Dardé ML, Ajzenberg D, Fitzgerald K, Young LH, Saeij JP. 2013. Transcriptional analysis of murine macrophages infected with different Toxoplasma strains identifies novel regulation of host signaling pathways. PLoS Pathog. 9:e1003779. doi: 10.1371/journal.ppat.1003779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhadra R, Gigley JP, Weiss LM, Khan IA. 2011. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1–PDL-1 blockade. Proc Natl Acad Sci U S A 108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ogolla PS, Portillo JA, White CL, Patel K, Lamb B, Sen GC, Subauste CS. 2013. The protein kinase double-stranded RNA-dependent (PKR) enhances protection against disease cause by a non-viral pathogen. PLoS Pathog 9:e1003557. doi: 10.1371/journal.ppat.1003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. 2006. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest 116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Persson EK, Agnarson AM, Lambert H, Hitziger N, Yagita H, Chambers BJ, Barragan A, Grandien A. 2007. Death receptor ligation or exposure to perforin trigger rapid egress of the intracellular parasite Toxoplasma gondii. J Immunol 179:8357–8365. doi: 10.4049/jimmunol.179.12.8357. [DOI] [PubMed] [Google Scholar]
- 97.Aspinall TV, Guy EC, Roberts KE, Joynson DH, Hyde JE, Sims PF. 2003. Molecular evidence for multiple Toxoplasma gondii infections in individual patients in England and Wales: public health implications. Int J Parasitol 33:97–103. doi: 10.1016/S0020-7519(02)00230-8. [DOI] [PubMed] [Google Scholar]
- 98.Boughattas S, Ayari K, Sa T, Aoun K, Bouratbine A. 2014. Survey of the parasite Toxoplasma gondii in human consumed ovine meat in Tunis City. PLoS One 9:e85044. doi: 10.1371/journal.pone.0085044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ajzenberg D, Bañuls AL, Tibayrenc M, Dardé ML. 2002. Microsatellite analysis of Toxoplasma gondii shows considerable polymorphism structured into two main clonal groups. Int J Parasitol 32:27–38. doi: 10.1016/S0020-7519(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 100.Dubey JP. 2006. Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Vet Parasitol 140:69–75. doi: 10.1016/j.vetpar.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 101.Lilue J, Müller UB, Steinfeldt T, Howard JC. 2013. Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. Elife 2:e01298. doi: 10.7554/eLife.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Virreira Winter S, Niedelman W, Jensen KD, Rosowski EE, Julien L, Spooner E, Caradonna K, Burleigh BA, Saeij JP, Ploegh HL, Frickel EM. 2011. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One 6:e24434. doi: 10.1371/journal.pone.0024434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. 2011. Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.National Research Council 2011. Guide to the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Similar marker expression on IFN-γ+ CD8 T cells following recall infection with different Toxoplasma strains. (A) PECs from type III (CEP) chronically infected C57BL/6 mice were infected (MOI, 0.2) in vitro with the indicated parasite strains for 16 h. The expression of CD62L and KLRG1 on IFN-γ-producing CD8+ (CD3+ CD19−) T cells (top row) and IFN-γ-negative CD8+ (CD3+ CD19−) T cells (bottom row) was analyzed by FACS analysis; the gating strategy is depicted above. Bar graphs plot the percent contribution of each cell population to the total IFN-γ+ or IFN-γ− CD8 T cell PECs observed in each mouse. Results are from 3 individual mice. (B) Splenocytes from type II chronically infected mice were infected (MOI, 0.2) in vitro with the type I RH strain or atypical strain MAS for 16 h; CD19+ (CD8− CD3−) B cells, CD3+ (CD8− CD19−) T cells, and CD8+ (CD3+ CD19−) T cells were analyzed for the expression of IL-7rα (CD127) and intracellular IFN-γ by FACS. Plotted is a representative histogram of the relative CD127 staining intensity on each cell type, and the percentage of cells that fell within the depicted IL-7rα hi gate is shown. Similarly, IFN-γ+ and IFN-γ-negative CD8+ T cells were analyzed for IL-7rα hi expression; a representative histogram and the percentage of CD8 T cells that fell within the IL-7rα hi gate from a representative mouse are shown. Finally, the average percentage (± standard error of the mean) of IFN-γ+ and IFN-γ-negative CD8 T cells from the spleen that were IL-7rα hi was plotted. Result are from 5 mice. n.s., not significant (Student’s t test; P > 0.05). Download
Generation of the type I RH Δtgd057 strain. (A) Scheme depicting the strategy used to obtain the type I (RH) Δtgd057 knockout strain. The 5′ and 3′ flanking regions (FR) of our genes of interest were cloned on both sides of the HXGPRT selection marker. The vector was linearized prior to transfection into RH Δku80 Δhxgprt parasites. Following a double homologous recombination event, the gene of interest was replaced by HXGPRT. The arrows represent the primers (P1 to P8) used to make the construct and verify the gene replacement (P1, 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTACACTGCGGAACGACGTGGAGA-3′; P2, 5′-GGGGACAACTTTGTATAGAAAAGTTGGGTGATTTGCCTTCTACAATGCATC-3′; P3, 5′-GGGGACAACTTTGTATAATAAAGTTGCTAACTCCTTCGATCCTCACCTG-3′; P4, 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTACGTGTTACCTTGGAAGCTGCA-3′; P5, 5′-ACTGGCGTGTTTCGCCGCAT-3′; P6, 5′-TCTGTCGCCTTTGCCACGCA-3′; P7, 5′-AACATCCAGCGAGTCCTTCC-3′; P8, 5′-GATCCAGACGTCTTCAATGC-3′). (B) To confirm the disruption of TGD057, we performed a PCR with a forward primer upstream of the 5′ flanking region (P7) and a reverse primer within the HXGPRT cassette (P8). A second PCR was executed to confirm the inability to amplify the target gene (P5 and P6). Download
Survival of type III chronically infected C57BL/6 mice to secondary challenge with 16 F1 I × III progeny. C57BL/6 mice were infected with the type III (CEP) strain and allowed to progress to chronic infection. On day 35 of chronic infection, mice were challenged i.p. with 5 × 104 tachyzoites of the indicated F1 I × III progeny derived from a type III (CTG) and type I (GT1) cross. Viability was confirmed by plaque assays, and mice received between 104 and 103 viable parasites. The F1 progeny were chosen based on the presence of a type I allele at the marker CS6, which detects an SNP in ROP18. The F1 progeny used in this experiment were predicted to fully express ROP18, which is not expressed in type III lineage parasite strains, and they are 100% lethal in naive mice (34). The number of animals challenged (n) and the percentage of surviving mice at 26 days after secondary challenge are indicated. QTL analysis detected a significant disease association peak on Toxoplasma chromosome XII at marker SAG3 (LOD, 4.1; P < 0.05); the type I or III SAG3 allele for each F1 progeny is indicated. Download