Abstract
Background:
Sperm dysfunction is one of the main causes of male infertility. Petroselinum crispum (P. crispum) is a member of umbelliferae family that contains different vitamins and minerals and has numerous therapeutic properties. The aim of this study was to evaluate P. crispum effect on sperm parameters, testis tissue and serum nitric oxid levels in mice.
Materials and Methods:
Hydroalcoholic extract of P. crispum was prepared and administered intraperitoneally (0,100, 150 and 200 mg/kg) to 40 mice, which were divided into four groups (n = 10), one control group and three experimental groups, for 14 consequent days. The sperm parameter such as motility, sperm count, morphology, and seminiferous tubules diameter, and weight of prostate and testis, and serum nitric oxide levels were analyzed.
Results:
P. crispum administration (100, 150 and 200 mg/kg) significantly increased mean percentage of sperm motility, testis and prostate weight and serum nitric oxide compared to the control group (P < 0.05). However, no significant effect was reported for different doses of P. crispum extract on sperm parameters.
Conclusion:
Administrating hydroalcoholic extract of P. crispum has positive effects on some reproductive parameters.
Keywords: Mice, nitric oxide, Petroselinum crispum, sperm parameters, testis
INTRODUCTION
In recent years, more attention has been paid to the study of the effect of various plants on the reproduction of laboratory mammals by which precious information has been acquired.[1] P. crispum is a species of petroselinum in the family Apiaceae native to the central Mediterranean region which are used in food, pharmaceutical, and cosmetics industries.[2] Shi et al. reported numerous properties for P. crispum such as antimicrobial, anticoagulant, antimenorrhagia, antihepatic fibrosis, and antioxidant.[3] A previous study by Petrolini et al. indicated antibacterial potential of P. crispum against bacteria that cause urinary tract infections[4] and Kreydiyyeh et al. showed the effect of this plant on nose bleeding, hematoma, skin blemishes, halitosis, ear ache, and otitis.[5] Other properties of P. crispum include regulating blood pressure, treating eczema and sexual dysfunctions, and relieving pain.[6] Scientific research in the field of P. crispum has led to the discovery of an ascorbic acid, carotenoids, flavonoids, coumarin, different terpenoic compounds, phenylpropanoids, tocopherol, and various minerals such as iron.[7] Infertility and its associated problems are important issues in the couple lives and sperm dysfunction is a major cause of infertility in men.[8] About 40% of infertility problems are associated with men.[9] Hafez indicated that infertility in males is mostly due to sperm cells dysfunctions such as low sperm cells count, immaturity, abnormality, and lack of motility.[10] Chemotherapy drugs, antibiotics, radiations, stress, pollution, and inadequate intake of vitamins are from among the factors that affect infertility.[11] It has been shown that these factors can decrease count of sperm, by producing free radicals and germinal cells oxidation in the testis tissue.[12,13] Zhou et al. showed that in fertile individuals, sperm motility levels especially progressive sperms is directly related to the ability of fertilization.[14] Food supplements containing the leaf of P. crispum significantly increase antioxidant level.[15] Thus, based on aforementioned information about P. crispum properties, the aim of the present work was to examine the effect of hydroalcoholic extract of P. crispum on sperm parameters such as motility, count, morphology, testis tissue, and serum nitric oxide levels in mice.
MATERIALS AND METHODS
Extract preparation
In this experimental study, P. crispum plant was prepared from the local store and its impurities were removed. The plant was then dried, grinded, powdered, and added to ethanol (400 cc) in the proportion of 1 to 4. The obtained solution was preserved in the hot water bath 35°C in darkness. Then, the solution was gradually poured onto Buchner funnel filter paper and was cleared by a vacuum pump. It was then transferred to the rotary machine to be concentrated and separated of the extra solvent. The separation process was continued until the concentrated extract was obtained. The obtained extract was dissolved in distilled water and administered intraperitoneally (per kilogram of body weight) to the mice.[16]
Animals
Forty BALB/c male mice with the weight range of 25-30 grams were purchased from Tehran Razi Institute. The animals were kept under controlled laboratory conditions of 22 ± 2°C, 12/12 dark/light cycles with free access to sufficient food and water.[6] The animals were randomly assigned to 4 groups (n = 10). The control group and experimental groups received the hydroalcoholic extract of P. crispum (100, 150 and 200 mg/kg) intraperitoneally for 14 consequent days.[17,18] All animals were cared and used in accordance with the International Guiding Principle for Biomedical Research Involving Animals at Medical Faculty of Kermanshah University, Iran (Certificate No. 91238).
Preparation of samples and analysis of testes and prostate weight
The animals were anesthetized, 24 hours after the last injection. Blood was taken from the heart and preserved at the temperature of 37°C for 30 minutes to remove the serum. The samples were then centrifuged (1000 cycles per second) for 15 minutes. The obtained serums were separated and kept in the temperature of -20°C to analyze nitric oxide. The right testes and prostate gland were separated and weighed separately. Then, the right testes were preserved in 10% neutral buffered formalin.[15]
Nitric oxide assay
NO concentration in the blood serum was determined with the Greiss method. The Greiss reagent is made up of a 1% solution of sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihycrochloride in distilled water. Sample serums were collected and kept at -20° C. The protein and phenol red of the serum were deleted using zinc sulfate (6 mg/400 μl). Sodium nitrite (0.1 M) was used for the standard curve, and increasing concentrations of sodium nitrite (5, 10, 25, 50, 75, and 100 μM) were prepared. The Greiss solution was added to all microplates, containing sodium nitrite and blood serum and was read by an ELISA reader (stat fax 100. USA) in 540 nm and 630 nm filters.[19]
Evaluation of the sperms’ characteristics
The cauda epididymis was separated and segmented, in DMEM/F12 medium containing 5% FBS, which had previously been balanced in incubator. It was then put in incubator with temperature of 37°C and 5% CO.[17] The obtained suspension was used for analyzing sperm parameters (motility, count, viability, and morphology).
Progressive sperm motility
Progressive sperm motility was assessed in four levels according to certain criteria: (a) quick progressive motility in direct line, (b) slow progressive motility in direct or indirect line, (c) no progressive motility and (d) no motility.[20]
Sperm count
To count the sperms, after putting the sperm suspension on Neubauer's chamber, the sperms on the four corners of the central square were counted by the optic microscope (magnification 400X).[21]
Sperm morphology
To examine sperm morphology, smear was prepared from the samples and was stained and investigated by the Papanicohaou staining method.[22]
Histological analyses
After testes fixation by formalin, the histological process including dehydration, clearing, and embedding was carried out. The microscopic sections (5 μm) were prepared and H and E staining method was used. The seminiferous tubules diameter was measured by Motic camera and software (Moticam 2000, Spain). Seminiferous tubules average diameter (μm) was determined for each testis.[17]
Statistical test
One-way analysis of variance (ANOVA) and Tukey tests were applied on the data to perform statistical analysis and compare experimental groups with the control group. P < 0.05 was considered significant.
RESULTS
In this study, increase of P. crispum extract dose revealed no significant increase in the sperm count, normal morphology, and seminiferous tubules diameter in the treated groups compared 125 to the control group [Figures 1 and 2]. However, it significantly increased the viability of 126 sperms in all treated groups in comparison with the control group (P < 0.05).
Figure 1.
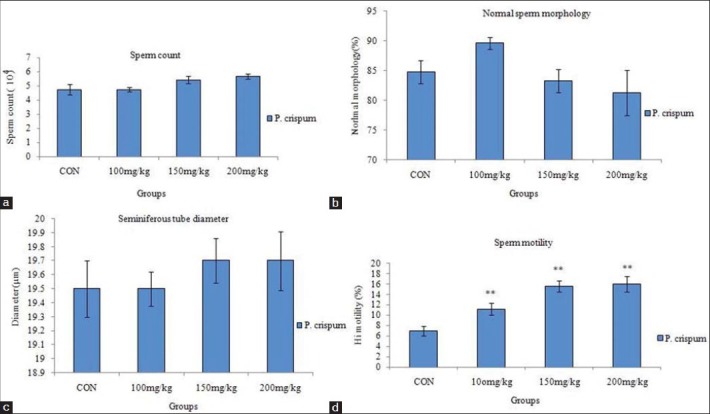
Effect of different concentrations of P. crispum extract on sperm count. (a) Sperm morphology. (b) Seminiferous tubules diameter. (c) Sperm quick progressive motility. (d) Compared to the control group P < 0.001**
Figure 2.
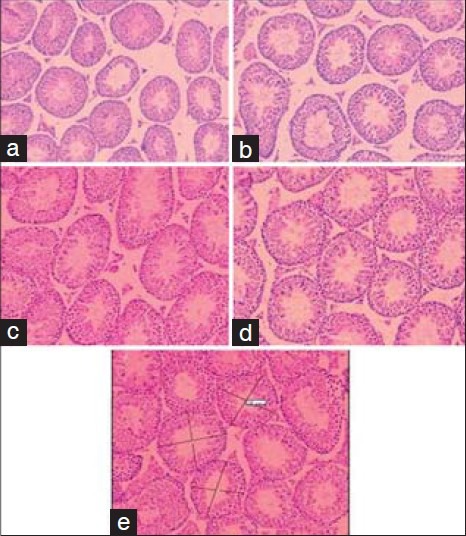
Effect of different concentrations of P. crispum extract on the testis histological. (a) Control, (b) P. crispum (100 mg/kg), (c) P. crispum (150 mg/kg), (d) P. crispum (200 mg/kg) (×40), (e) Motic camera and software (×10)
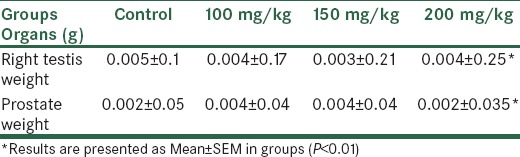
The higher doses of P. crispum extract caused a significant increase in the testis and prostate weight in all treated groups in comparison with the control group (P < 0.05) [Table 1].
Table 1.
Comparison of the testis and prostate weight in different groups
The mean of sperm motility (quick progressive motility) increased significantly (P < 0.05) in all treated groups in comparison with the control group [Figure 1].
Further, the mean of nitric oxide in blood serum decreased significantly (P < 0.05) in the 100 mg/kg group compared to the control group. In other groups of the study, however, this decline was not significant despite nitric oxide [Figure 3].
Figure 3.
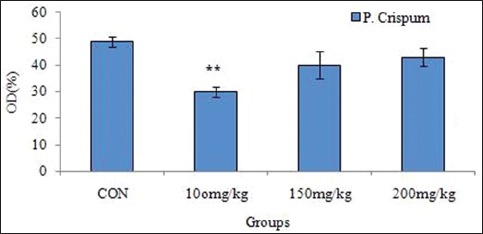
Effect of different concentrations of P. crispum extract on nitric oxide level in blood serum compared to the control group, P < 0.01**
DISCUSSION
In the present study, the effects of P. crispum hydroalcoholic extract on sperm parameters (motility, count and morphology), testis tissue, serum nitric oxide level, and testis and prostate weight in mice were studied. Nowadays, plant extracts have been largely taken into consideration and their positive and negative impact on various organs and tissues of the body have been identified. One of the target tissues of plant extracts are reproductive tissues, such as testis and sperm parameters. Sperm motility is introduced as an important factor in the success of natural and experimental fertilization. In fertile individuals, sperm motility levels especially progressive sperms are directly related to the ability of fertilization.[14] Researchers believe that increasing the free radicals causes the loss of epithelial cells, which can destroy cytoplasmic bridges and consequently decrease sperm count and motility levels and increase sperm deformities.[23,24] It seems that antioxidant properties of P. crispum can improve the sperm quality by increasing the expression of anti-oxidant genes.[3] The findings obtained in this study was in line with results of the study conducted by Wong et al.,[25] in which they investigated the relative peroxidative and anti-oxidant effects of P. crispum. Further, the findings of the present research indicated a significant relationship between the P. crispum extract and sperm motility. It seems that glutathione peroxidase increases as a result of existing flavonoid antioxidants in P. crispum extract that resulted in an increase of motile sperms.[2,20] Glutathione peroxidase enzyme affects the sperm function by preventing the sperm membrane per-oxidation and consequently improving sperm motility.[6] Moreover, the results of the study conducted by Ozsoy-Sacan et al. on the effects of P. crispum extract on the liver of diabetic mice indicated that the antioxidants present in P. crispum can induce positive effects against the toxic effects of diabetes on the liver, which confirms the findings of the present study.[26] Atig et al. showed that lack of glutathione per-oxid may decrease the fertility capacity, which is in line with our findings.[15] It seems that the increase of testis and prostate weight is due to the presence of high levels of vitamin A in P. crispum extract, which is considered as a growth factor.[27] Vitamin A, by converting into retinoid, stores the fat as triglyceride in the body and increases the weight.[12] The findings of the present study are in line with those of Lasnitzki et al. which indicated keratinization of the prostate epithelium can be prevented when vitamin A is added to the medium.[11] Sertoli cells are one of the major components of seminiferous tubules and their number is greatly linked to total sperm production. Sertoli cells take care of germ cells during their maturity period. Modulating the function of all hormone stimuli which regulate spermatogenesis process, Sustentocytes provide a completely regulated environment that results in germ cells maturation from spermatogony to mature sperm.[28] Since P. crispum plant contains various minerals such as iron, vitamins A, B and C, and vitamin A is known for proliferation of epithelial cells,[7] it seems that the parietal cells of sperm tubules in groups receiving the extract are rapidly proliferation. Therefore, the diameter of seminiferous tubules increased after treatment with P. crispum extract. Patil et al.[29] showed that both Bacopa monniera and P. crispum are potent antioxidants that reduce the oxidative stress induced by D-galactose and can increase diameter of seminiferous tubules which is in line with our findings. In the present study, the mean of nitric oxide level in blood serum decreased significantly in the 100 mg/kg group comparing to the control group. Nitric oxide and signal pathways of 3’, 5’-cyclic Guanosine monophosphate (cGMP) as an important cascade signal are found in many mammalian cells such as sertoli and germinal cells in the testis tissue.[30] Nitric oxide plays a pivotal role in blood circulation regulation in the reproductive system and previous studies have reported the increase in nitric oxide expression along with apoptosis in germinal cells.[31] It seems that metabolites of vitamin A inhibit nitric oxide production by macrophages in mice and human.[32] This study's findings confirm the results of Foghi et al., that increasing the nitric oxide level may play an important role in the apoptosis process in germinal cells and spermatogenesis process.[33] It seems that increasing the viability of sperms is due to decreasing the reactive oxygen species (ROS) in the medium via P. crispum extract treatment.[12] The results of Shi et al. indicated that the continuous decline of active and live cells can be associated with increase of ROS in the medium, which is in line with the results of the present study.[3]
CONCLUSION
Based on the results of this study, it seems that the hydroalcoholic extract of P. crispum can affect some reproductive indices such as weight of testis and prostate, sperm motility and decrease nitric oxide level in blood serum. However, further studies are required to shed light on the mechanisms of these compounds in the reproductive system.
ACKNOWLEDGMENT
We sincerely and gratefully thank the, Kermanshah University of Medical Sciences for financial support of this project (No. 91238).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Handelsman DJ. Hormonal male contraception. Int J Androl. 2000;23:8–12. doi: 10.1046/j.1365-2605.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 2.Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–23. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Shi TY, Chen G, Huang X, Yuan Y, Wu X, Wu B, et al. Effects of reactive oxygen species from activated leucocytes on human sperm motility, viability and morphology. Andrologia. 2012;44(Suppl 1):696–703. doi: 10.1111/j.1439-0272.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- 4.Petrolini FV, Lucarini R, de Souza MG, Pires RH, Cunha WR, Martins CH. Evaluation of the antibacterial potential of Petroselinum crispum and Rosmarinus officinalis against bacteria that cause urinary tract infections. Braz J Microbiol. 2013;44:829–34. doi: 10.1590/S1517-83822013005000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreydiyyeh SJ, Usta J. Diuretic effect and mechanism of action of Parsley. J Ethnopharmacol. 2002;79:353–7. doi: 10.1016/s0378-8741(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 6.Khosrowbeygi A, Zarghami N, Deldar Y. Correlation between sperm quality parameters and seminal plasma antioxidants status. Iran J Reprod Med. 2004;2:58–64. [Google Scholar]
- 7.Breininger E, Beorlegui NB, O’Flaherty CM, Beconi MT. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology. 2005;63:2126–35. doi: 10.1016/j.theriogenology.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrios F, Filipponi D, Pellegrini M, Paronetto MP, Di Siena S, Geremia R, et al. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123:871–80. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- 10.Hafez DA. Effect of extracts of ginger goots and cinnamon bark on fertility of male diabetic rats. J Am Sci. 2010;6:940–7. [Google Scholar]
- 11.Chung SS, Choi C, Wang X, Hallock L, Wolgemuth DJ. Aberrant distribution of junctional complex components in retinoic acid receptor alpha-deficient mice. Microsc Res Tech. 2010;73:583–96. doi: 10.1002/jemt.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol. 2008;46:446–75. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 13.Vora SR, Patil RB, Pillai MM. Protective effects of Petroselinum crispum (Mill) Nyman ex A.W. Hill leaf extract on D-galactose-induced oxidative stress in mouse brain. Indian J Exp Biol. 2009;47:338–42. [PubMed] [Google Scholar]
- 14.Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod. 2008;78:537–45. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atig F, Raffa M, Ali HB, Abdelhamid K, Saad A, Ajina M. Altered antioxidant status and increased lipid per-oxidation in seminal plasma of tunisian infertile men. Int J Biol Sci. 2012;8:139–49. doi: 10.7150/ijbs.8.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ, Jr, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16:1922–30. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 17.Lotfi N, Khazaei M, Shariatzadeh SM, Mehranjani MS, Ghanbari A. The effect of Cannabis sativa hydroalcoholic extract on sperm parameters and testis histology in rats. Int J Morphol. 2013;31:82–6. [Google Scholar]
- 18.Al-Howiriny T, Al-Sohaibani M, El-Tahir K, Rafatullah S. Prevention of experimentally-induced gastric ulcers in rats by an ethanolic extract of “Parsley” Petroselinum crispum. Am J Chin Med. 2003;31:699–711. doi: 10.1142/S0192415X03001405. [DOI] [PubMed] [Google Scholar]
- 19.Khazaei M, Roshankhah S, Ghorbani R, Chobsaz F. Sildenafil effect on nitric oxide secretion by normal human endometrial epithelial cells cultured in vitro. Int J Fertil Steril. 2011;5:142–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C, Wang C, Gao SQ, Kong TT, Chen L, Li XF, et al. Effects of permethrin, cypermethrin and 3-phenoxybenzoic acid on rat sperm motility in vitro evaluated with computer-assisted sperm analysis. Toxicol In Vitro. 2010;24:382–6. doi: 10.1016/j.tiv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Ohtani K, Yamazaki S, Kubota H, Miyagawa M, Saegusa J. Comparative investigation of several sperm analysis methods for evaluation of spermatotoxicity of industrial chemical: 2-bromopropane as an example. Ind Health. 2004;42:219–25. doi: 10.2486/indhealth.42.219. [DOI] [PubMed] [Google Scholar]
- 22.Zare Z, Eimani H, Mohammadi M, Mofid M, Dashtnavard H. The effect of orally administered l-carnitine on testistissue, sperm parameters and daily sperm production in adult mice. Yakhteh Med J. 2010;11:382–9. [Google Scholar]
- 23.Aziz N, Saleh RA, Sharma RK, Lewis-Jones I, Esfandiari N, Thomas AJ, Jr, et al. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril. 2004;81:349–54. doi: 10.1016/j.fertnstert.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Kolarovic J, Popovic M, Zlinská J, Trivic S, Vojnovic M. Antioxidant activities of celery and parsley juices in rats treated with doxorubicin. Molecules. 2010;15:6193–204. doi: 10.3390/molecules15096193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong PY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–15. [Google Scholar]
- 26.Ozsoy-Sacan O, Yanardag R, Orak H, Ozgey Y, Yarat A, Tunali T. Effects of parsley (Petroselinum crispum) extract versus glibornuride on the liver of streptozotocin-induced diabetic rats. J Ethnopharmcol. 2006;104:175–81. doi: 10.1016/j.jep.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 27.Yanardað R, Bolkent S, Tabakoðlu-Oðuz A, Ozsoy-Saçan O. Effects of Petroselinum crispum extract on pancreatic B cells and blood glucose of streptozotocin-induced diabetic rats. Biol Pharm Bull. 2003;26:1206–10. doi: 10.1248/bpb.26.1206. [DOI] [PubMed] [Google Scholar]
- 28.Brinster RL. Male germline stem cells: From mice to men. Science. 2007;316:404–5. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patil RB, Vora SR, Pillai MM. Spermatogenic activity of dietary antioxidant in oxidatively stressed mice. J Cell Tissue Res. 2008;8:1519–24. [Google Scholar]
- 30.O’Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP. Inducible nitric oxide synthase in the rat testis: Evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod. 2000;63:1285–93. doi: 10.1095/biolreprod63.5.1285. [DOI] [PubMed] [Google Scholar]
- 31.Mruk DD, Sarkar O, Mathur PP. Nitric Oxide-cGMP signaling: Its role in cell junction dynamics during spermatogenesis. Curr Med Chem Immunol Endocr Metab Agents. 2008;8:28–35. [Google Scholar]
- 32.Rajaraman V, Nonnecke BJ, Franklin ST, Hammell DC, Horst RL. Effect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J Dairy Sci. 1998;81:3278–85. doi: 10.3168/jds.S0022-0302(98)75892-8. [DOI] [PubMed] [Google Scholar]
- 33.Foghi K, Novin MG, Jabbari ZM, Najaf T, Heidari MH, Yasoori AR. Immuno-histochemical localization of endothelial nitric oxide synthase in testicular cells of men with non-obstructive azoospermia. Iran J Reprod Med. 2011;9:277–80. [PMC free article] [PubMed] [Google Scholar]


