Abstract
Background:
Migration, expansion and survival of endothelial cells that are an important cellular component of blood vessels plays an important role in the induction of tumor growth. Kisspeptins (kp), peptides that bind to coupled-G protein receptor (GPR54), inhibit each step of metastatic cascade include invasion, migration and homing, angiogenesis, survival and proliferation. In this study we investigated effects of kisspeptin-10, the most potent member of kisspeptin family, on Migration and proliferation of endothelial cells that are necessary for angiogenesis and tumor metastasis.
Materials and Methods:
We compared migration of Human Umbilical Vein Endothelial Cells (HUVECs) were treated with 10-100 or 500 nM kp-10 for 24 hours and no treated cells using an in vitro trans membrane migration assay and HUVEC proliferation of treated endothelial cells with 10-100 or 500 nM kp-10 for 48 hours and no treated cells was measured by MTT Cell Proliferation Assay Kit. Analysis of data was performed using the Kruskal-Wallis test followed by the Mann-Whitney test.
Results:
Migration and proliferation of endothelial cells were increased at lower concentration of kp-10 specially at 100 nM while higher concentration reduced both migration and proliferation.
Conclusion:
Our data showed that different concentrations of kp-10 have distinct effects on migration and proliferation of endothelial cells.
Keywords: Endothelial cell, kisspeptin-10, migration, proliferation
INTRODUCTION
Angiogenesis, formation of new blood vessels from pre-existing vessels, is an essential process in normal development and a key component in processes such as wound healing, inflammation as well as tumor growth and metastasis.[1,2] Angiogenesis is a complex multistep process including proliferation, migration and tube formation of endothelial cells.[3] Migration, expansion and survival of endothelial cells that are an important cellular component of blood vessels, constitute the basic functional network of angiogenesis that plays an important role in the induction of tumor growth.[4]
One of the possible candidate suppressor genes that may plays a major role in metastatic cascade is kiss-1 which originally has identified by it's altered expression in metastatic melanoma.[5,6] In clinical studies loss of kiss-1 expression is associated with poor prognosis of several malignancies including melanoma,[7] urinary bladder,[8] breast cancer,[9] ovarian, stomach[10] and esophageal[11] cancers. It has identified that kiss-1 encodes Kisspeptin with 145 residue and several shorter products resulting naturally proteolytic of primary product (kp-54, kp-14, kp-13, kp-10) which kisspeptin-10, shortest and the most active product of kiss-1, is a 10 residue peptide. All kisspeptins bind to coupled-G protein receptor, GPR54 that result in regulation of hypothalamic-pituitary-gonad axis and placentation, as well as inhibition of cell motility, proliferation, invasion, chemotaxis and metastasis. However, exact mechanism of their anti metastatic function is unclear.[12,13]
Since the migration and proliferation of endothelial cells are necessary for angiogenesis and tumor metastasis, we aimed to investigate the effect of kisspeptin-10 on this processes.
MATERIALS AND METHODS
Mouse kp-10, sequence TATASPGLAT-NH2 with purity > 95% was purchased from Anaspec (USA), stored at –20˚C and used to prepare working dilutions 10-100 and 500 nM in PBS immediately before each experiments.
Human Umbilical Vein Endothelial Cells (HUVECs) (Cell bank of Pasteur Institute, Iran) were cultured in M199 (Gibco, USA) medium supplemented with 10% fetal bovine serum, penicillin 100IU/ml and streptomycin 100 μg/ml (Invitrogen, USA) and were incubated at 37˚C with 5% CO2 and 95% O2 concentration. Medium changed every day and the third passage cells after reaching 80% confluency were used for experiments.[1]
B16F10 melanoma cells (Cell bank of Pasteur Institute, Iran) were cultured in DMEM containing with 10% FBS,1g/L glucose, 1% L-glutamine, 1% penicillin-streptomycin at 37˚C in a CO2 atmosphere.[14]
The in vitro migration assay was performed in the transwell inserts with an 8-μm pore size that membrane was uncoated (SPL, Germany). HUVECs were treated by PBS (Phosphate buffered saline) with 10-100 or 500 nM kp-10 (Anaspec, USA) for 24 hours and one other group of cells was not treated with kp-10 as control, then 1×104 cells in 200 μl serum-free medium were seeded into the top chamber and 4×104 melanoma cells in 500 μl medium were added to the lower chamber as a chemoattractant. After 24 hours incubation at 37˚C, non-migrated cells were removed from the top of membrane, whereas cells on the bottom face of membrane were resuspended in medium and counted in 1minute by flow cytometry (BD CaliburTM (Becton Dickinson)). Results were obtained by two experiments.[15,16]
HUVEC proliferation was measured by MTT Cell Proliferation Assay Kit (Invitrogen, USA). Briefly, 5×103 cells/well were seeded in a 96-well plate and after the cells attached, they were treated with 1-100 or 500 nM kp-10 for 48 hours and one other group of cells was not treated. Then MTT reagent added to the wells and after incubation for 4 hours, the absorbance each well measured at 570 nm. Experiments were performed in triplicate.[15]
Data were obtained from two or three experiments. All data were presented as mean ± SEM and were analyzed for significant differences between control and experimental groups at P < 0.05 by SPSS 16.0 using the Kruskal-Wallis test followed by the Mann-Whitney test.
RESULT
We have evaluated the effect of kp-10 on migration of HUVEC. To assess migration cells were seeded in serum-free media containing kp-10 and allowed to migrate across an uncoated Transwell membrane for 24 hours. HUVECs showed decreased migration ability at higher concentration of kisspeptin-10, but migration at lower concentration was increased specially at 100 nM kp-10 (P <0.05) [Figure 1].
Figure 1.
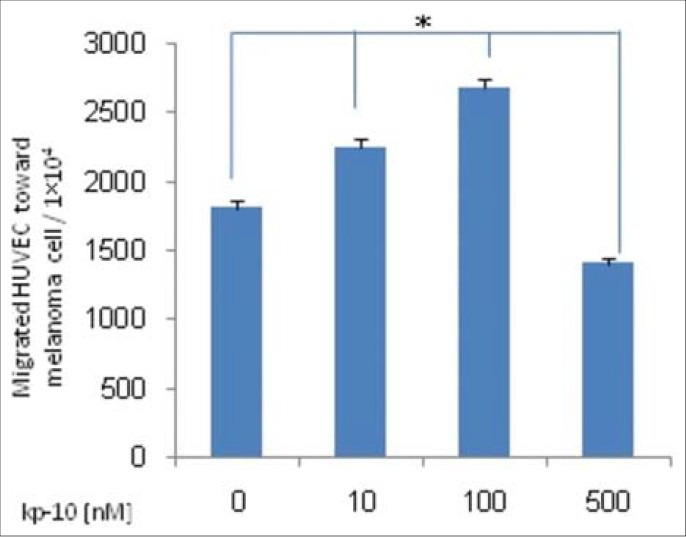
HUVEC migration assay. After 24 hours incubation of treated HUVECs with 10-100 or 500 nM kp-10 and no treated cells, number of migrated cells across membrane counted by flow cytometry. Results are indicative two experiments. Kp-10 significantly decreased migration of HUVEC at higher concentration but migration was increased at lower concentration. Results are compared between groups by Kruskal-Wallis test (*P < 0.05)
We have examined the effect of kp-10 on endothelial cell proliferation with MTT Cell Proliferation Assay Kit that indicated Kp-10 decreased proliferation at higher concentration, but HUVEC proliferation at lower concentration was increased specially at 100 nM kp-10 (P < 0.05) [Figure 2].
Figure 2.
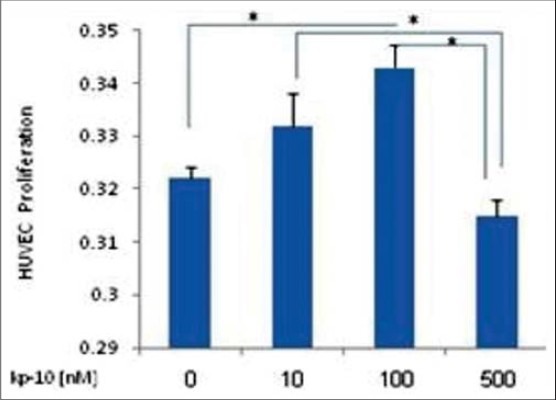
HUVEC proliferation assay. 5000 HUVECs were seeded at 96-well plate and after 48 hours treatment with 10-100 or 500 nM kp-10 and no treated cells, incubated with MTT reagent and absorbance measured at 570 nm. Kp-10 inhibits proliferation of HUVEC at the highest concentration. Result are indicative three experiments and are compared between groups by Kruskal-Wallis test (*P < 0.05)
DISCUSSION
In this study we investigate the effect of kp-10 on migration and proliferation of endothelial cells and we have shown that kp-10 inhibited cell migration and proliferation at higher concentration but it increased both processes at lower concentration.
Metastasis is the criteria of malignancy and a significant definite prognostic mark in more of the patients with malignant tumors.[10] Cell migration is a key component of the metastatic process for both tumor cells and stromal cells recruited in pre metastatic niche including myofibroblasts, hematopoietic and endothelial progenitor cells. So far, only a small number of molecules involved in metastasis process were discovered and among them recently, kisspeptin (also known as kiss-1 protein) identified as a promising metastasis suppressor. It specifically targets spread of tumor cells to distant organs and is involved with each step of metastatic cascade include invasion, migration and homing, angiogenesis, survival and proliferation in a new microenvironment. Although, the mechanism of metastasis suppressor kiss-1 and GPR54 receptor is not still quite described.[12]
Suppression of cell invasion and metastasis by transfection kiss-1cDNA into metastatic cancer cell lines and suppression of metastasis in nude mice provide a definite role for kiss-1 in metastatic process.[10] Some studies reported that kp-10 reduced cell migration in response to cellular stimuli in a dose-dependent manner. Kisspeptin-10 at 1 and 10 μM concentrations inhibit migration and invasion of MDA-MB-231 and MCF-7 cells.[17] As well as 1 and 10 μM metastin in PANC-1 cells result in decreased cell migration.[18] In another study, it has been reported that kp-10 at a dose window of 10-9 to 10-11 M reduced breast cancer cell migration.[13] Furthermore, Ramaesh, has shown that kp-10 significantly inhibited angiogenesis in placental vessels at 1nM to 1μM concentrations.[3] In another study, Cho et al. have reported that kp-10 significantly inhibits angiogenesis at 10-1 to 10 μM concentrations.[2]
Although, another study has reported that kisspeptin-10 stimulate invasion of MDA-MB231 and Hs578T breast cancer cells, so that maximum of invasion and migration was observed by 10 to 100nM kp-10.[19] In addition, there are some studies which expressed that kiss-1 inhibit cell proliferation.[10,17,18] Although, some studies reported that kisspeptin-10 inhibit cell migration without effect on proliferation.[2,20] So, it seems that there is a distinctive dose dependent pattern effect of kisspeptin on cell migration and proliferation. Furthermore, it seems that KISS1/KISS1R signaling could be cell-type-specific[21,22] and due to difference in study model, distinct results can be obtained.
CONCLUSION
Our data showed that kp-10 can differently affect on migration and proliferation of endothelial cell in various concentrations.
ACKNOWLEDGMENTS
This study was funded by Isfahan University of Medical Sciences (IUMS).
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, et al. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS One. 2009;4:e8283. doi: 10.1371/journal.pone.0008283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SG, Yi Z, Pang X, Yi T, Wang Y, Luo J, et al. Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor angiogenesis by suppressing Sp1-mediated VEGF expression and FAK/Rho GTPase activation. Cancer Res. 2009;69:7062–70. doi: 10.1158/0008-5472.CAN-09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaesh T, Logie JJ, Roseweir AK, Millar RP, Walker BR, Hadoke PW, et al. Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology. 2010;151:5927–34. doi: 10.1210/en.2010-0565. [DOI] [PubMed] [Google Scholar]
- 4.Dejana E. Endothelial cell-cell junctions: Happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 5.Welch DR, Chen P, Miele ME, McGary CT, Bower JM, Stanbridge EJ, et al. Microcell-mediated transfer of chromosome 6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene. 1994;9:255–62. [PubMed] [Google Scholar]
- 6.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 7.Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res. 2001;61:7422–5. [PubMed] [Google Scholar]
- 8.Sanchez-Carbayo M, Capodieci P, Cordon-Cardo C. Tumor suppressor role of KiSS-1 in bladder cancer: Loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am J Pathol. 2003;162:609–17. doi: 10.1016/S0002-9440(10)63854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–7. [PubMed] [Google Scholar]
- 10.Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–72. doi: 10.1002/ijc.20357. [DOI] [PubMed] [Google Scholar]
- 11.Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–83. doi: 10.1158/1078-0432.ccr-1519-02. [DOI] [PubMed] [Google Scholar]
- 12.Navenot JM, Fujii N, Peiper SC. Activation of Rho and Rho-associated kinase by GPR54 and KiSS1 metastasis suppressor gene product induces changes of cell morphology and contributes to apoptosis. Mol Pharmacol. 2009;75:1300–6. doi: 10.1124/mol.109.055095. [DOI] [PubMed] [Google Scholar]
- 13.Olbrich T, Ziegler E, Türk G, Schubert A, Emons G, Gründker C. Kisspeptin-10 inhibits bone-directed migration of GPR54-positive breast cancer cells: Evidence for a dose-window effect. Gynecol Oncol. 2010;119:571–8. doi: 10.1016/j.ygyno.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–8. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Yusenko MV, Kovacs G. Lack of KISS1R expression is associated with rapid progression of conventional renal cell carcinomas. J Pathol. 2011;223:46–53. doi: 10.1002/path.2764. [DOI] [PubMed] [Google Scholar]
- 16.Huang YL, Qiu RF, Mai WY, Kuang J, Cai XY, Dong YG, et al. Effects of insulin-like growth factor-1 on the properties of mesenchymal stem cells in vitro. J Zhejiang Univ Sci B. 2012;13:20–8. doi: 10.1631/jzus.B1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SG, Li D, Stafford LJ, Luo J, Rodriguez-Villanueva M, Wang Y, et al. KiSS1 suppresses TNFalpha-induced breast cancer cell invasion via an inhibition of RhoA-mediated NF-kappaB activation. J Cell Biochem. 2009;107:1139–49. doi: 10.1002/jcb.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, et al. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Zajac M, Law J, Cvetkovic DD, Pampillo M, McColl L, Pape C, et al. GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS One. 2011;6:e21599. doi: 10.1371/journal.pone.0021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhillo WS, Savage P, Murphy KG, Chaudhri OB, Patterson M, Nijher GM, et al. Plasma kisspeptin is raised in patients with gestational trophoblastic neoplasia and falls during treatment. Am J Physiol Endocrinol Metab. 2006;291:E878–84. doi: 10.1152/ajpendo.00555.2005. [DOI] [PubMed] [Google Scholar]
- 21.Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. Intracellular signaling pathways activated by kisspeptins through GPR54: Do multiple signals underlie function diversity? Peptides. 2009;30:10–5. doi: 10.1016/j.peptides.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Cho SG, Wang Y, Rodriguez M, Tan K, Zhang W, Luo J, et al. Haploinsufficiency in the prometastasis Kiss1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer Res. 2011;71:6535–46. doi: 10.1158/0008-5472.CAN-11-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]


