Abstract
Background:
Copper (Cu) is essential both for its role in antioxidant enzymes, like Cu/zinc (Zn) superoxide dismutase (SOD) and ceruloplasmin, as well as its role in lysyl oxidase, essential for the strength and integrity of the heart and blood vessels. With such a central role in cardiovascular health, Cu has been generally overlooked in the debate over improving our cardiovascular health. Cu deficiency has produced many of the same abnormalities present in cardiovascular disease. It seems almost certain that Cu plays a large role in the development of this killer disease, not because of its excess in the diet, but rather its deficiency.
Aim:
This study was undertaken to investigate the cardiovascular effects of Cu deficiency on the activity of SOD in patients with type 2 diabetes mellitus (T2DM) with and without diabetic nephropathy.
Materials and Methods:
Fifty-five patients with T2DM were recruited in this study which were divided into two subgroups based on the presence of microalbuminuria, the first group (microal buminuric group, n = 31) had a microalbuminuria between 30 and 299 μg/mg. The second group (normoal buminuric group, n = 29) had an albumin level less than 30 μg/mg. The two diabetic groups were compared to the control group (n = 37).
Results:
The results of our study showed a significant reduction in the levels of SOD enzyme associated with an increased urinary Cu excretion in microalbuminuric group compared to the control group at P < 0.05.
Conclusions:
The current study illustrates that the regulation of the blood concentrations of Cu may be a potential therapeutic target for prevention and treatment of diabetic nephropathy.
Keywords: Copper, Microalbuminuria, Superoxide dismutase, Type 2 diabetes mellitus
Introduction
The ratio of copper (Cu) to other dietary components (e.g., zinc (Zn), iron, sulfate, and molybdenum) may be as important as the actual Cu levels in the diet.[1] Cu/Zn ratios may be important to the adequate metabolism of cholesterol, with low ratios resulting in hypercholesterolemia.[1,2,3] Previous studies have focused on acute severe Cu deficiency, which is relatively rare in humans and animals on typical varied diets. Marginal chronic deficiency, however, is much more common. The determination of Cu needs and marginal deficiency is complicated by the fact that while Cu deficiency does not necessarily lower the level of Cu-dependent enzymes, it does significantly lowers their activity.[3]
Superoxide dismutase (SOD) functions as an antioxidant by catalyzing the conversion of superoxide radicals (free radicals or reactive oxygen species (ROS)) to hydrogenperoxide, which can subsequently be reduced to water by other antioxidant enzymes.[4,5] Superoxide radicals may react with other ROS such as nitricoxide to form highly toxic species like peroxynitrite, in addition to its direct toxic effects.[4,5] Peroxy nitrite reacts with the tyrosine residues in proteins resulting with the nitrotyrosine production in plasma proteins, which is considered as an in direct evidence of peroxynitrite production and increased oxidative stress.[6] Although nitrotyrosine was not detectable in the plasma of the healthy controls, nitrotyrosine was found in the plasma of all type 2 diabetic patients (type 2 diabetes mellitus (T2DM)) examined. Previous studies correlated plasma nitrotyrosine values with plasma glucose concentrations and found a significant positive correlation.[7,8] Furthermore, exposure of endothelial cells to high glucose level leads to an augmented production of superoxide anion, which may quench nitric oxide level resulting in impaired endothelial functions, vasodilation, and delayed cell replication.[9] Alternatively, superoxide can be dismutated to a much more reactive hydrogen peroxide, which through the Fenton reaction can then lead to a highly toxic hydroxyl radical formation.[10,11]
Two forms of SOD contain Cu: i) Cu/Zn-SOD is found within most cells of the body, including red blood cells, and ii) extracellular (EC)-SOD is a Cu-containing enzyme found in high levels in the lungs and low levels in blood plasma.[5,12,13] Almost all of the Cu in our bodies is bound either to transport proteins (ceruloplasmin and Cu-albumin), storage proteins (metallothioneins), or Cu containing enzymes.[14,15] Intracellular metallothionine normally stores little Cu providing protection from the harmful effects of free Cu.[14,15] Ceruloplasmin may function as an antioxidant in two different ways: By binding to Cu, ceruloplasmin prevents free Cu ions from catalyzing oxidative damage. The other way is through the oxidation of ferrous iron by ceruplasmin, facilitating iron load into its transport protein, transferrin, and preventing free ferrous ions from participating in harmful free radical generating reactions.[16]
Major reason for the decreased SOD activity is the glycosylation of Cu/Zn-SOD which has been shown to lead to enzyme inactivation both in vivo and in vitro.[17] Also Cu/Zn-SOD cleavage and release of Cu++ in vitro resulted intransition metalcatalyzed ROS formation.[17,18] Erythrocyte Cu/Zn-SOD activity correlated inversely with indices of glycemic control in DM patients.[17,18] However, red cell Cu/Zn-SOD activity has also been found to be decreased in DM patients.[17,18] Glycation may decrease cell-associated EC-SOD, which could predispose to oxidative damage. Earlier reports found decreased red cell Cu/Zn-SOD activity in DM patients with retinopathy compared to DM patients without microvascular complications and nondiabetic control subjects.[19]
Materials and Methods
Study protocol and participants
This study was approved by the Scientific and Ethics Committee of the College of Medicine, Al-Nahrain University. Informed consent was obtained from all participants. Ninety-two participants were recruited for this study (55 participants with T2DM and 37 normal control subjects). T2DM was diagnosed as per the World Health Organization (WHO) definition.[20] Type 2 diabetic patients (n = 55) were divided according to the urine protein (albumin) excretion measured in μg/mg creatinine [Table 1] into:
Table 1.
Demographic and clinical data of the participants included in the study
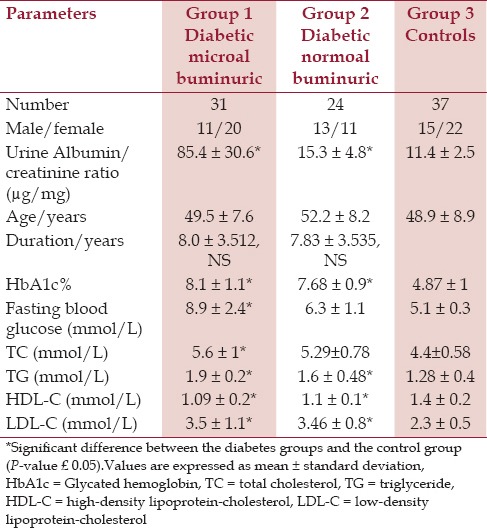
Patients with albumin-creatinine ratio that is equal to 30-299 μg/mg were considered to have microal buminuria (n = 31).
Patients with albuminexcretion less than 30 μg/mg creatinine were considered normoal buminuric (n = 24).
All patients were recruited from the outpatient diabetes clinic at Al-Kadhymia Teaching Hospital. The exclusion criteria included: Patients with any recent medical illness; impaired thyroid or renal function; diagnosis of renal disease; and treatment with estrogen, glucocorticoids, or other drugs except oral hypoglycemic and/or beta blocker antihypertensive drugs. All patients included in the study were nonsmokers; none were taking antioxidant supplements or drugs with known antioxidant activity. The mean duration of diabetes was (7.96 ± 3.45 years).
The control group consisted of 37 healthy, age- and gender-matched subjects (48.92 ± 8.9 years). The control group consisted of participants with no known medical history and with no family history of diabetes or nephropathy.
Blood samples
A total of 10 ml of venous blood samples were collected from each subject in the study after 10-12 h fasting. Two milliliters were collected into ethylene diaminetetraa cetic acid (EDTA) containing tubes for glycated hemoglobin (HbA1c) assay. The remaining 8 ml were centrifuged at 3,000 rpm for 10 min after about 30 min from the time of blood collection. Sera were separated for measurement of serum creatinine, serum lipids and serum SOD. The sera were stored at -80°C. All assays were obtained by running duplicates for the test, control, and the standard.
Urine samples
Random morning urine specimens were obtained from each subject in the study, to quantify albuminuria, creatinine, Cu, and albumin to creatinine ratio. No urine preservatives were used; the samples were stored in appropriate containers and were kept at the refrigerator until the time of measurements.
Parameters of the study
A. Methods applied in urine: A micro method was employed for the determination of urinary protein based upon the coprecipitation of protein and Ponceau S dye by trichloracetic acid (TCA), dissolution of the precipitate in dilute alkali, and spectrophotometric determination of the dye in alkaline solution.[21] Urinary creatinine was estimated by the BioMerieux assay kit based on the method of Bartels et al.[22]
B. Methods applied in blood: Serum creatinine was estimated by the BioMerieux assay kit based on the method of Bartels et al.[22] Cu was measured by flame atomic absorption spectrophotometer. A stock standard concentration of Cu (50 mol/L) was prepared and subsequent dilutions were made to obtain a calibration curve. Urine samples were diluted (1:10) by deionized water and measured directly against an aqueous standard made from certified standard solution. Cu hallow cathode lamps were used at wavelength of 324.75 nm. These solutions were aspirated directly into air-acetylene flame.
Serum lipids were measure using BioMerieux assay kits for total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
Serum SOD was measured using the SOD assay kit-water soluble tetrazolium salt (Dojindo Molecular Technologies, Rockville, MD, USA).[23]
Glycated hemoglobin (HbA1c) samples were by VariantTM HbA1c program, which is intended for the determination of HbA1c in human whole blood using ion-exchange high performance liquid chromatography (HPLC).
Statistical analysis
Data are expressed as mean ± standard deviation of mean. Statistical significance was determined by ANOVA test followed by unpaired Student's t-test and Pearson's correlation (r) to test correlation of regression. P-values equal or lower than 0.05 were considered statistically significant.
Results
All groups were closely age-matched, and the two diabetes groups were well-matched for duration of disease [Table 1].
Microabuminuric diabetic group shows a significant increase in the mean urinary Cu/creatinine ratio when compared with controls (P < 0.05), while normoal buminuric diabetic patients shows an insignificant increase, P > 0.05 in mean urine Cu/creatinine ratio when compared with controls [Table 2].
Table 2.
Urinary copper excretion and serum superoxide dismutase enzyme levels in microal buminurics, normoal buminurics, and control subjects
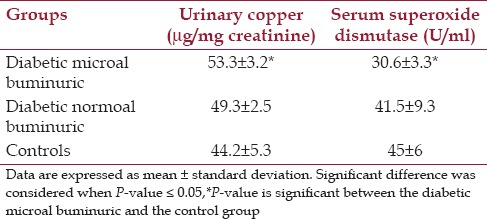
Serum SOD was significantly decreased in the diabetes microal buminuric group compared to the control group (P < 0.05), such a significant correlation was not seen with the diabetes normoal buminuric group [Figure 1 and Table 2].
Figure 1.
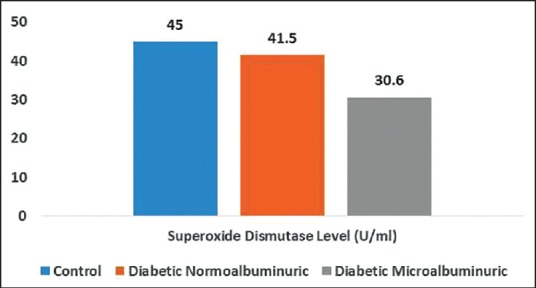
Mean values of serum superoxide dismutase enzyme among the three groups of the study
Discussion
Studies showed a reduction in erythrocyte SOD and catalase (CAT) activities in subjects with impaired glucose tolerance (IGT), early hyperglycemia, and type 2 DM patients.[24] However, other studies showed that the activities of these enzymes were within normal range in T2DM patients in poor glycemic control.[25] EC-SOD activity was found to be similar in T1DM patients despite some what higher plasma EC-SOD levels.[26] Red cell Cu/Zn-SOD activity was similar in T1DM and T2DM patients compared to normal subjects,[27,28,29] irrespective of microvascular complications. Leukocyte SOD activity was similar between type 2DM patients and healthy control subjects, despite increased lipidperoxidation and decreased ascorbate levels.[30]
While the role of adequate Cu levels in maintaining cardiovascular health is well-established, there are still inconsistent data about the correlation of Cu with persisting hyperglycemia. Some studies showed an elevation of serum Cu, while other studies showed a significant reduction of Cu in diabetes.[31,32] While this may sound confusing, recent research has helped to explain this paradox.[31,32,33,34]
Many researchers have considered this elevation of serum Cu to play a role in the pathogenesis of cardiovascular disease, although other researchers have strongly disagreed with this hypothesis. An animal study, however, seems to have explained this relationship between Cu levels and cardiovascular disease. This study examined the effects of diet-induced atherosclerosis on the Cu levels and status of numerous tissues.[35] It was found that serum Cu levels increase significantly, while aorta and liver Cu levels decrease significantly, in rats with experimental atherosclerosis. So instead of assuming that these elevated Cu levels contribute to the formation of atherosclerosis, these researchers examined the effects of increasing the dietary Cu levels in these animals. Administration of additional Cu resulted in a further increase in serum Cu, a significant decrease in serum cholesterol, and an increase and normalization in aorta and liver Cu levels.[35] However, instead of increasing the incidence of atherosclerosis, additional Cu significantly decreased the incidence of atherosclerosis in the aorta and coronary arteries. Further, it has been shown that excess dietary cholesterol causes cardiovascular disease by lowering the absorption of Cu, an effect that is preventable by increasing the Cu level in the diet.
The mechanism of the link between microal buminuria and cardiovascular mortality is still unclear. However, increased urinary albumin loss has been postulated to be a marker of a generalized increase in vascular permeability, which might predispose to greater penetration into the arterial wall of atherogenic lipoprotein particles.[36,37,38]
Urinary Cu concentrations significantly increased only in microal buminuric patients, similar results were found by[39], but with macroal buminuric diabetic patients. In diabetic patients with advanced nephropathy, urinary Cu excretion may be due to dissociations from both Cu-albumin and ceruloplasmin-Cu complexes filtered through the damaged glomerulus. Overloading of urinary Cu to damaged renal tubules may play some roles in the progression of nephropathy in patients with advanced nephropathy.[39]
Conclusion
Cellular concentrations of Cu must be maintained somewhere below toxicity but above nutrient deficiency. Therefore, regulation of the blood concentrations of Cu may be a potential therapeutic target for prevention and treatment of diabetic nephropathy. However, there is still a lot to know about the mechanism of Cu homeostasis at the cellular level. If reduction of serum Cu can be shown to have a protective effect against oxidative stress in DM, this may have a direct impact on the use of Cu chelators as a safe therapeutic modality in diabetes.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Bellof G, Most E, Pallauf J. Concentration of copper, iron, manganese and zinc in muscle, fat and bone tissue of lambs of the breed German Merino Landsheep in the course of the growing period and different feeding intensities. J Anim Physiol Anim Nutr. 2007;91:100–8. doi: 10.1111/j.1439-0396.2006.00648.x. [DOI] [PubMed] [Google Scholar]
- 2.Grammer TB, Kleber ME, Silbernagel G, Pilz S, Scharnagl H, Lerchbaum E, et al. Copper, ceruloplasmin, and long-term cardiovascular and total mortality (the Ludwigshafen Risk and Cardiovascular Health Study) Free Radic Res. 2014;48:706–15. doi: 10.3109/10715762.2014.901510. [DOI] [PubMed] [Google Scholar]
- 3.Roughead ZK, Johnson LK, Hunt JR. Dietary copper primarily affects antioxidant capacity and dietary iron mainly affects iron status in a surface response study of female rats fed varying concentrations of iron, zinc and copper. J Nutr. 1999;129:1368–76. doi: 10.1093/jn/129.7.1368. [DOI] [PubMed] [Google Scholar]
- 4.Jendryczko A, Tomala J, Janosz P. Effects of two low-dose oral contraceptives on erythrocyte superoxide dismutase, catalase and glutathione peroxidase activities. Zentralbl Gynakol. 1993;115:469–72. [PubMed] [Google Scholar]
- 5.Kharb S. Activity of extracellular superoxide dismutase in gestational diabetes. Res J Obstet Gynecol. 2010;3:1–4. [Google Scholar]
- 6.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–57. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 7.Horvath EM, Magenheim R, Kugler E, Vacz G, Szigethy A, Levardi F, et al. Nitrative stress and poly (ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009;52:1935–43. doi: 10.1007/s00125-009-1435-3. [DOI] [PubMed] [Google Scholar]
- 8.Mohammad BI, Hadi NR, Jawad HM, Jamil DA, Al-Aubaidy HA. Improve markers of oxidative stress and coagulation parameters in response to atorvastatin therapy. Br J Pharm Res. 2014;4:1242–52. [Google Scholar]
- 9.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 10.Clerici G, Slavescu C, Fiengo S, Kanninen TT, Romanelli M, Biondi R, et al. Oxidative stress in pathological pregnancies. J Obstet Gynaecol. 2012;32:124–7. doi: 10.3109/01443615.2011.637139. [DOI] [PubMed] [Google Scholar]
- 11.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10:339–52. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 12.Ferns GA, Lamb DJ, Taylor A. The possible role of copper ions in atherogenesis: The Blue Janus. Atherosclerosis. 1997;133:139–52. doi: 10.1016/s0021-9150(97)00130-5. [DOI] [PubMed] [Google Scholar]
- 13.Jamil DA, Al-Aubaidy HA, Al-Wasiti EA, Al Bayati M. Evaluating markers of oxidative stress in managing gestational diabetes mellitus: A cross sectional study in Iraq. Br J Med Med Res. 2014;4:3870–7. [Google Scholar]
- 14.Li X, Cai L, Feng W. Diabetes and metallothionein. Mini Rev Med Chem. 2007;7:761–8. doi: 10.2174/138955707781024490. [DOI] [PubMed] [Google Scholar]
- 15.Nakazato K, Tomioka S, Nakajima K, Saito H, Kato M, Kodaira T, et al. Determination of the serum metallothionein (MT)1/2 concentration in patients with Wilson's disease and Menkes disease. J Trace Elem Med Biol. 2014;28:441–7. doi: 10.1016/j.jtemb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MA, Fischer JG, Kays SE. Is copper an antioxidant nutrient? Crit Rev Food Sci Nutr. 1992;32:1–31. doi: 10.1080/10408399209527583. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW, Nam SM, Kim YN, Yoo DY, Choi JH, Jung HY, et al. exercise ameliorates diabetes-induced increases in lipid peroxidation and decreases in Cu, Zn-superoxide dismutase levels in the hippocampus of Zucker diabetic fatty rats. J Vet Sci. 2014 doi: 10.4142/jvs.2015.16.1.11. (in print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, et al. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. North Am J Med Sci. 2013;5:213–9. doi: 10.4103/1947-2714.109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings PE, McLaren M, Scott NA, Saniabadi AR, Belch JJ. The relationship of oxidative stress to thrombotic tendency in type 1 diabetic patients with retinopathy. Diabet Med. 1991;8:860–5. doi: 10.1111/j.1464-5491.1991.tb02125.x. [DOI] [PubMed] [Google Scholar]
- 20.Geneva: WHO; 1999. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. [Google Scholar]
- 21.Pesce MA, Strande CS. A new micromethod for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1973;19:1265–7. [PubMed] [Google Scholar]
- 22.Bartels H, Bohmer M, Heierli C. Serum creatinine determination without protein precipitation. Clin Chim Acta. 1972;37:193–7. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 23.Ukeda H, Maeda S, Ishii T, Sawamura M. Spectrophotometric assay for superoxide dismutase based on tetrazolium salt 3’-1-(phenylamino)-carbonyl-3, 4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate reduction by xanthine-xanthine oxidase. Anal Biochem. 1997;251:206–9. doi: 10.1006/abio.1997.2273. [DOI] [PubMed] [Google Scholar]
- 24.Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabet Med. 1996;13:715–9. doi: 10.1002/(SICI)1096-9136(199608)13:8<715::AID-DIA172>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Peuchant E, Delmas-Beauvieux MC, Couchouron A, Dubourg L, Thomas MJ, Perromat A, et al. Short-term insulin therapy and normoglycemia. Effects on erythrocyte lipid peroxidation in NIDDM patients. Diabetes Care. 1997;20:202–7. doi: 10.2337/diacare.20.2.202. [DOI] [PubMed] [Google Scholar]
- 26.Adachi T, Yamada H, Yamada Y, Morihara N, Yamazaki N, Murakami T, et al. Substitution of glycine for arginine-213 in extracellular-superoxide dismutase impairs affinity for heparin and endothelial cell surface. Biochem J. 1996;313(Pt 1):235–9. doi: 10.1042/bj3130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krolak B, Kaminski K. Activity of superoxide dismutase (SOD) and its isoenzyme activities in newborn children and adult women and men. Ginekol Pol. 1992;63:404–9. [PubMed] [Google Scholar]
- 28.Lang I, Deak G, Muzes G, Pronai L, Feher J. Effect of the natural bioflavonoid antioxidant silymarin on superoxide dismutase (SOD) activity and expression in vitro. Biotechnol Ther. 1993;4:263–70. [PubMed] [Google Scholar]
- 29.Winterbourn CC, Peskin AV, Parsons-Mair HN. Thiol oxidase activity of copper, zinc superoxide dismutase. J Biol Chem. 2002;277:1906–11. doi: 10.1074/jbc.M107256200. [DOI] [PubMed] [Google Scholar]
- 30.Akkus I, Kalak S, Vural H, Caglayan O, Menekse E, Can G, et al. Leukocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase and serum and leukocyte vitamin C levels of patients with type II diabetes mellitus. Clin Chim Acta. 1996;244:221–7. doi: 10.1016/0009-8981(96)83566-2. [DOI] [PubMed] [Google Scholar]
- 31.Salmonowicz B, Krzystek-Korpacka M, Noczynska A. Trace elements, magnesium, and the efficacy of antioxidant systems in children with type 1 diabetes mellitus and in their siblings. Adv Clin Exp Med. 2014;23:259–68. doi: 10.17219/acem/37074. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Zhou Q, Liu G, Tan Y, Cai L. Analysis of serum and urinal copper and zinc in Chinese northeast population with the prediabetes or diabetes with and without complications. Oxid Med Cell Longev 2013. 2013 doi: 10.1155/2013/635214. 635214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basaki M, Saeb M, Nazifi S, Shamsaei HA. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res. 2012;148:161–4. doi: 10.1007/s12011-012-9360-6. [DOI] [PubMed] [Google Scholar]
- 34.Qazzaz M, Abdul-Ghani R, Metani M, Husein R, Abu-Hijleh AL, Abdul-Ghani AS. The antioxidant activity of copper(II) (3,5-diisopropyl salicylate)4 and its protective effect against streptozotocin-induced diabetes mellitus in rats. Biol Trace Elem Res. 2013;154:88–96. doi: 10.1007/s12011-013-9697-5. [DOI] [PubMed] [Google Scholar]
- 35.Rayssiguier Y, Gueux E, Bussiere L, Mazur A. Copper deficiency increases the susceptibility of lipoproteins and tissues to peroxidation in rats. J Nutr. 1993;123:1343–8. doi: 10.1093/jn/123.8.1343. [DOI] [PubMed] [Google Scholar]
- 36.Al-Bayati MA, Jamil DA, Al-Aubaidy HA. The association of oxidized high density lipoprtotein and oxidized non-high density lipoprotien with the development of microalbuminuria in diabetic nephropathy. World Journal of Pharmacy and Pharmaceutical Sciences. 2014;3:49–59. [Google Scholar]
- 37.Popov AV, Ketlinski SA, Tararak EM. Historadioautographic and biochemical studies on the transport of atherogenic lipoproteins into the rabbit aortic wall. Paroi Arterielle. 1975;3:61–9. [PubMed] [Google Scholar]
- 38.Torzewski M, Suriyaphol P, Paprotka K, Spath L, Ochsenhirt V, Schmitt A, et al. Enzymatic modification of low-density lipoprotein in the arterial wall: A new role for plasmin and matrix metalloproteinases in atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24:2130–6. doi: 10.1161/01.ATV.0000144016.85221.66. [DOI] [PubMed] [Google Scholar]
- 39.Ito S, Fujita H, Narita T, Yaginuma T, Kawarada Y, Kawagoe M, et al. Urinary copper excretion in type 2 diabetic patients with nephropathy. Nephron. 2001;88:307–12. doi: 10.1159/000046013. [DOI] [PubMed] [Google Scholar]


