Abstract
Background:
Global prevalence of metabolic syndrome (MS) and diabetes is increasing, but the reference ranges for MS indices have yet to be established for sub-Saharan African countries. As part of the international research collaboration agenda for Prediabetes and Cardiovascular Complications Study (PACCS), a pilot study was conducted in one of the Ndokwa communities of Nigeria in 2013.
Aim:
The study was to obtain preliminary indication of prevalence and reference values of MS in the rural communities of a low-mid income country.
Materials and Methods:
Seventy-four volunteer participants were recruited, after public lectures in high schools and churches in the community. Body mass index (BMI), blood pressure and waist circumference (WC), blood glucoselevel, and lipid profile were measured. Percentage prevalence MS was determined using commonest three criteria (Third Adult Treatment Panel (ATP III) 2001, International Diabetes Federation (IDF) 2005, and World Health Organization (WHO) 1999).
Results:
When individual indices of MS are considered separately; the males seem healthier than females. However, the prevalence of high-density lipoprotein (HDL) cholesterol was higher in males than in females. Equal 3% prevalence of MS was seen in both genders using the WHO standard. Other criteria show prevalence of 8% females and 11% males (ATP III), 5% females and 8% males (IDF 2005 European), and 14% females and 17% males (IDF 2005 Ethnic).
Conclusion:
The prevalence of MS is higher in males than females; and relative to ATP III 2001 criteria, either the IDF 2005 European may underestimate MS, or the ethnic specific could overestimate the prevalence. Hence, it is important to define the criteria to be used.
Keywords: Cardiovascular disease, Diabetes, Diabetes prevention, Low-mid income communities, Public health, Prediabetes and cardiovascular complications study (PACCS)
Introduction
Metabolic syndrome (MS) is a risk factor for the development of diabetes mellitus (DM) and cardiovascular disease (CVD) complications and the development to overt diabetes passes through subclinical prediabetic states that most times coexist with one or more metabolic disorders such as hypertension, dyslipidemia, and/or obesity.[1] MS involves related and connected factors that increase the risk of coronary heart disease (CHD), CVDs, and DM type 2 (DMT2). The principal features include dyslipidemia (elevated triglycerides and apolipoprotein B (apoB)-containing lipoproteins and low high-density lipoproteins (HDL), hypertension, and dysregulated glucose hemostasis. Abdominal obesity and/or insulin resistance (IR) are also core manifestations of the syndrome. The definition is growing to include chronic proinflammatory and prothrombic states, nonalcoholic fatty liver disease, and sleep apnea. There is still no universally accepted pathogenic mechanism or clearly defined diagnostic criteria.[2] Perhaps, this explains the various criteria used and the need to define the criteria used in studying or diagnosing this syndrome.
The 2009 Atlas report of the International Diabetes Federation (IDF) on the prevalence rates of diabetes showed that diabetes prevalence is much more prominent in the developing regions of the world and more than 438 million people will be living with diabetes by the year 2030[3,4] citing IDF 2004, 2006 and WHO 2000 reported noted that 70% of world diabetes cases are resident in the developing countries. A prevalence of 13-30% in developing countries and approximately 30-35% in developed countries for MS has been reported.[5,6]
An area that needs studying is the prevalence of chronic disorders in young adult populations in rural areas with limited health services. Thus, the Prediabetes and Cardiovascular Complications Study (PACCS), including this preliminary study, focuses on the Ndokwa local government area of Nigeria. Health disparity issues between the poor and the rich, the urban and rural dwellers, and ethnic/racial minority and majority groups are factors that influence the prevalence of chronic diseases. Knowledge, attitude, and practice (KAP) gaps of individuals in these communities also predispose to the risk of developing diabetes and CVDs. Education of communities at risk can become a cost-effective public health strategy. It has been shown that self-care among individuals with type 2 diabetes improved glycemic control and reduced complications.[7,8] Therefore, health education and counseling is an indispensable component of the management strategies of CVD and DM risk factors.
Assessment of prediabetes and MS is a useful tenet to determine the level of prevalence of these risk factors and this can be achieved through screening and identifying the knowledge and perceptions of the communities towards these risk factors. These will allow advice and the desirable management protocols to be instituted. Part of the objectives of this pilot data is to gather data on prevalence of MS and prediabetes in the Ndokwa region with a view to develop program for prevention.[8,9]
Materials and Methods
Ethics approval was obtained from Human Research Ethics Committee of Novena University and the Local Government Ministry of Health at Kwale, Delta State Nigeria. This work was the first pilot from PACCS.[10] The mixed secondary/high (Abbi Grammar School) institution was contacted through the principal for a public lecture and recruitment of participants among the staff and students. Churches in the community were also contacted through their respective pastors. In all cases public lectures were followed by provision of information sheets as well as consent forms and questionnaires. Seventy-four volunteers, of equal number (37) of each gender, complied/responded, and were included in the pilot screening. Exclusion criteria were age less than 18years or greater than 60 years, if the subject had previous diagnosis of diabetes, and the inability to provide contacts for future follow-up should screening for MS and prediabetes be positive.
CardioChek® point of care testing (POCT) equipment was used to measure blood glucose level and lipid profile according to manufacturer's instructions. Glucose was measured by the glucose oxidase/peroxidase reaction.[11] Cholesterol and HDL-cholesterol (in very low-density lipoprotein (VLDL) and LDL depleted plasma) by the cholesterol esterase and oxidase/peroxidase reactions, while triglycerides was by the lipoprotein lipase/glycerol kinase/glycerophosphate and oxidase/peroxidase reactions.[12] Specimens were fresh capillary whole blood collected by finger prick. All the biochemistry blood tests were performed following fasting by the subjects. Two participants initially presented with high blood glucose level that was interpreted as diabetes and on the consideration of concerns and merits of POCT,[13,14,15] the participants were invited for repeat screening and are scheduled for follow-up. Blood pressure was determined using digital meter (Omron®) meter. Anthropometric measures included height and waste circumference (WC), which were measured by tape and weight by Precision Scale. The waist was defined as the midway between iliac crest and coastal margin (lower rib); while the hip circumference was defined as being the widest circumference over the buttocks and below the iliac crest.[16]
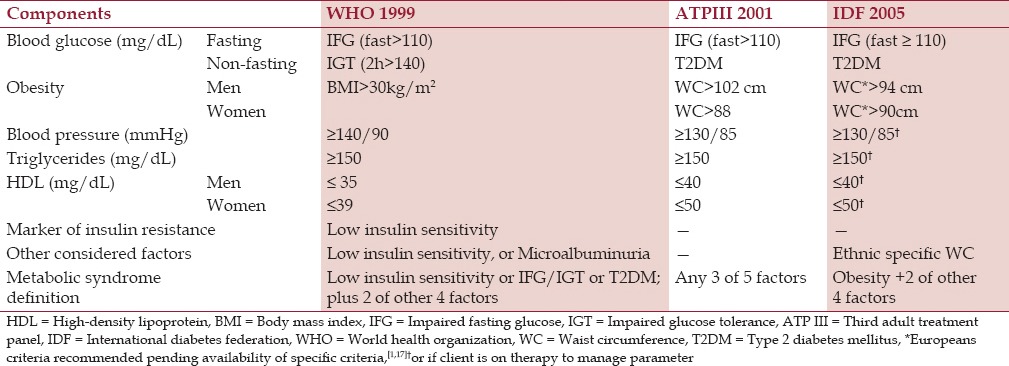
MS in this study is defined according to three criteria as indicated in Table 1. Given that European criteria is recommended pending availability of specific criteria,[1,17] ethnic specific waist circumference (WC) as other considered factor in IDF 2005 criteria was presumed from the mean values to be obtained. This study was limited by inability to test for insulin sensitivity and microalbuminuria. Screening of the participants from Abbi Grammar School occurred in the school, while those recruited through the Churches took place at the privately owned St Mathias Hospital, Abbi.
Table 1.
The three different criteria for diagnosis of metabolic syndrome used in this study
Results
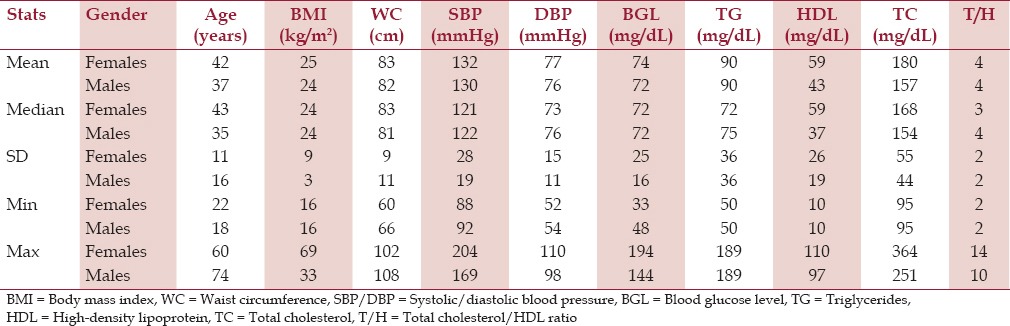
The study population has, on average, a healthy body mass index (BMI) of 24 kg/m2, WC of 82 cm, blood pressure of 131/76 mmHg, blood glucose level of 96 mg/dL, and total cholesterol (TC)/HDL ratio of 4.0. Descriptive statistics for the female and male groups are presented in Table 2.
Table 2.
Descriptive statistics
Evaluation of data shows that more females are worse in almost all indices than males, except in blood pressure and HDL [Figure 1]. Multivariate statistical comparison of the female versus male group show a statistically significant difference (multivariate analysis of variance (MANOVA): P < 0.001). The prevalence of MS according to the three different criteria show some disparity in outcome between the WHO 1999 system compared to the other two definitions [Figure 2].
Figure 1.
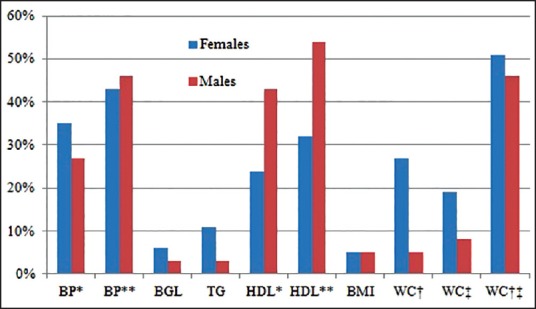
Prevalence (%) of individual risk factors of metabolic syndrome. *WHO, **ATPIII and IDF, †ATPIII, ‡IDF European, †‡IDF ethnic. ATP III = Third adult treatment panel, IDF = International diabetes federation, WHO = World health organization, BP = Blood pressure, BGL = Blood glucose level, TG = Triglyceride, HDL = High-density lipoprotein, BMI = Body mass index, WC = Waist circumference
Figure 2.
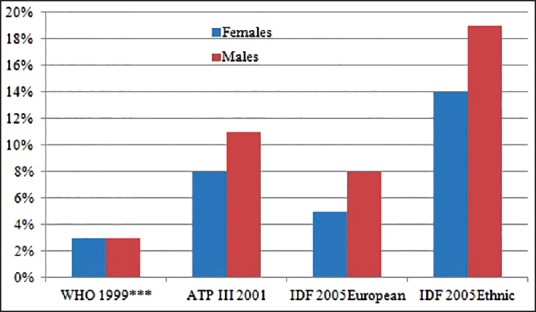
Prevalence of metabolic syndrome according to the three different criteria. ***BMI >25 kg/m2
It should be noted that IDF in Figure 2 include evaluation based on the IDF European standard. What is new in this pilot result is the attempt “to develop the WC cut point for sub-Saharan African community”. Hence, the IDF Ethnic in this pilot report is based on ‘mean’ WC of the studied population substituted for “> 102 cm in men and 88cm in women” of Europeans. We acknowledge that the best approach to develop reference values is by running a population study to establish ‘mean ± 2 standard deviation (SD)’ range. In this circumstance, we want to establish reference range of WC in the community; in order to authoritatively substitute for “>102 cm in men and 88cm in women” of Europeans.
Discussion
A POCT was chosen in this study because the machine is portable; specimen collection is relatively not traumatic to participants, and fast turnaround time of results. Such factors make POCT ideal for screening, especially in rural communities. Using the three criteria, the results from this pilot study show that abnormal levels of the individual parameters of MS such as blood glucose, triglyceride levels, and WC; as well as high blood pressure (WHO definition), are more prevalent in females compared to males. Therefore, when these particular indices are considered separately; more females than males seem to be at risk of CVD. However, the prevalence of abnormal HDL cholesterol as well as high blood pressure (ATP III and IDF definitions) is less in females than in males [Figure 1].
The observation from this preliminary study indicate that the WHO 1999 criteria identified the least, but equal prevalence (3%) in females and males. The ATPIII 2001 and IDF 2005 criteria identified more MS in males than in females [Figure 2]. Our study shows prevalence of 8% in females and 11% in males (ATPIII 2001 criteria), 5% in females and 8% in males (IDF2005 European criteria), and 14% females and 17% males (IDF 2005 Ethnic criteria), and 14% males and 17% females (IDF 2005 Ethnic criteria). These figures are comparatively lower than for instance in Seychelles, another low-mid income country from African region where higher prevalence levels (25-30%) of MS at the age of 25-64 years; and using the ATP, WHO, and IDF definitions the prevalence of 24, 25, and 25.1% in men and 32.2, 24.6, and 35.4% in women, respectively, were reported.[18]
The study on Spanish adult workers using IDF and ATP III diagnostic criteria reports that in adult Spanish males and females, the presence of MS with adjusted global prevalence was 12.4% using ATP III criteria and 16.5% using the IDF criteria; and prevalence in males was always higher than in females.[5] The European standard of IDF identified more MS than the ATPIII, as the ethnic specific standard of IDF in this study did. Results from the Spanish study are comparable to ours, but we are mindful of the differences in settings and these include urban in the Spanish study, while our study was rural based. The influences of other factors such as physical and socioeconomic factors, which are different, are not established.
A study in a rural African population, Ghana, reports the overall prevalence of MS by the IDF and ATP III criteria were 35.9 and 15.0%, respectively. The study further noted alarming female preponderance by both criteria and that the triad of central obesity, high blood pressure, and low HDL cholesterol were most responsible for the syndrome in this rural population.[19] In evaluating the various criteria for MS, it has been noted that the IDF is closest to ATPIII in that it includes the same variables, but it differs in that central obesity is an essential component. Also the waist measurement is set at a lower level than in ATP III and it is ethnic-specific. In a study of adults over 20 years, in Chennai, a city in Southern India, MS was in 23.2% by the WHO criteria, 18.3% by ATP III criteria, and 25.8% by the IDF criteria. The WHO criteria marked out a much higher population for coronary artery disease (CAD) risk compared to ATP III and IDF criteria in males but not in females. Although the prevalence of MS varies according to the definition used, the study identified a greater number in males,[20] which further corroborate with our observation presented in Figure 2.
Our data [Figure 2] show that ATPIII criteria pick up MS that is otherwise missed by WHO classification. While this may be argued as due to our acknowledged study limitation, it is noteworthy that nowadays, following the general consensus, the two most widely used definitions of MS are those of the ATPIII and the IDF.[5,16] Using these criteria, a prevalence of 13-30% of MS in developing countries and approximately 30-35% in developed countries is usually found.[5,6] It has been noted that the WHO definition is better suited as a research tool, whereas the National Cholesterol Education Program (NCEP) ATP III definition was more useful for clinical practice.[21] Clinicians prefer simple tools to assess patients and improve their management and it is generally agreed that the NCEP ATPIII definition is simpler for practice. It requires only a fasting assessment of blood glucose, whereas the WHO definition can require an oral glucose tolerance test. Further the WHO definition can also require assessment of IR and this is a relatively complicated test.[21,22] There is no single test that can directly diagnose IR in clinical practice;[23,24] and it is irrational to recommend such test where basic laboratory services are either inaccessible or unaffordable.
The prevalence of 6% in females and 3% in males for prediabetes in our study was lower than 10% of people in the age group 25 years and above reported in Pakistan are suffering from prediabetes and in the same article they noted that prevalence of prediabetes was 23.4% among South Asians.[25] The findings of the present study are still lower than the observation of Sabir et al.,[26] who found that in the adult Fulanis of Northern Nigeria there was a high prevalence of prediabetes using impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) as indices. They found prevalence rates of IGT and IFG were 8 and 6.9%, respectively, and noted that the relative high prevalence of IGT was similar to IDF estimates of about 8% prevalence for IGT in Nigeria. A nationwide survey in Bangladeshi reported that 23% have prediabetes.[27]
These higher prevalence reports indicate that larger sample sized cross-sectional study is required to confirm our observation regarding the prediabetes in Ndokwa community of Nigeria. This pilot study is PACCS’ first preliminary investigation in the rural community of Nigeria; and it is envisaged to expand the study longitudinal with more samples population and vertical with follow-ups. POCT may be best suited in a rural setting in a developing community to screen for diabetes and MS. This study is mindful that other parameters that contribute to diabetes or MS were not studied, but the observations are significant.
Conclusion
The results show that prevalence of prediabetes MS may be low in the Ndokwa region of Nigeria by comparison with rural communities elsewhere. However, it appears females are at greater risk than males to develop diabetes; whereas males seem more at risk of MS perhaps due to HDL cholesterol and stricter definition of blood pressure. Relative to ATPIII 2001 criteria, either the IDF2005 European standard may underestimate MS, or the IDF ethnic specific could overestimate the prevalence. It is therefore important to define the criteria to be used.
Acknowledgement
This work was made possible by support with point-of-care resources for the screening provided by Charles Darwin University, Australia. The work is been supported by the Director of Friends Laboratory Obiaruku; the Principal of Abbi Grammar School, Abbi; Ndokwa West local government of Nigeria. The Nigerian High Commissioner to Australia also supported this work during the logistics of courier delivery of the point-of-care resources for the screening. The continuity of this work is now being coordinated through the Department of Public and Community Health, Novena University; Delta State, Nigeria.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Babu A, Fogelfeld L. Metabolic syndrome and prediabetes. Dis Mon. 2006;52:55–144. doi: 10.1016/j.disamonth.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Ezenwaka C, Eckel J. Prevention of diabetes complications in developing countries: Time to intensify self-management education. Arch Physiol Biochem. 2011;117:251–3. doi: 10.3109/13813455.2011.602692. [DOI] [PubMed] [Google Scholar]
- 5.Tauler P, Bennasar-Veny M, Morales-Asencio JM, Lopez-Gonzalez AA, Vicente-Herrero T, De Pedro-Gomez J, et al. Prevalence of premorbid metabolic syndrome in Spanish adult workers using IDF and ATP 111 diagnostic criteria: Relationship with cardiovascular risk factor. PLoS One. 2014;9:e89281. doi: 10.1371/journal.pone.0089281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–75. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Heisler M, Piette JD, Spencer M, Kieffer E, Vijan S. The relationship between knowledge of recent HbA1c values and diabetes care understanding and self-management. Diabetes Care. 2005;28:816–22. doi: 10.2337/diacare.28.4.816. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–97. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M, Jones R, Freeman C, Woods HB, Gillett M, Goyder E, et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med. 2013;30:3–15. doi: 10.1111/dme.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwose EU, Richards RS, Digban K, Bwititi PT, Ennis G, Yee KC, et al. Cardiovascular risk assessment in prediabetes and undiagnosed diabetes mellitus study: International collaboration research overview. N Am J Med Sci. 2013;5:625–30. doi: 10.4103/1947-2714.122303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polymer Technology Systems Inc, PTS PANELS™ Glucose Test Strips for use with CardioChek™ Brand Analyzers. PS-002580E Rev. 3/10/05. 2005 [Google Scholar]
- 12.Polymer Technology Systems Inc, PTS PANELS Lipid Panel Test Strips for use with CardioChek P•A™ Analyzer. PS-002575 E Rev. 4/09/05. 2005 [Google Scholar]
- 13.Vucic Lovrencic M, Radisic Biljak V, Bozicevic S, Pape-Medvidović E, Ljubić S. Validation of Point-of-Care glucose testing for diagnosis of type 2 diabetes. Int J Endocrinol 2013. 2013 doi: 10.1155/2013/206309. 206309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewat N, Mc D, Taylor D, Macdonald E. Pilot study of random finger prick glucose testing as a screening tool for type 2 diabetes mellitus in the emergency department. Emerg Med J. 2009;26:732–3. doi: 10.1136/emj.2008.067041. [DOI] [PubMed] [Google Scholar]
- 15.National Diabetes Information Clearinghouse. Comparing tests for diabetes and prediabetes: A quick reference guide. [Accessed April 16, 2014]. NIH Publication No 14-78502014. at http://diabetes.niddk.nih.gov/dm/pubs/comparingtests/
- 16.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 17.Parikh RM, Mohan V. Changing definitions of metabolic syndrome. Indian J Endocrinol Metab. 2012;16:7–12. doi: 10.4103/2230-8210.91175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelliny C, William J, Riesen W, Paccaud F, Bovet P. Metabolic syndrome according to different definitions in a rapidly developing country of the African region. Cardiovasc Diabetol. 2008;7:27. doi: 10.1186/1475-2840-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gyakobo M, Amoah AG, Martey-Marbell DA, Snow RC. Prevalence of the metabolic syndrome in a rural population in Ghana. BMC Endocr Disord. 2012;12:25. doi: 10.1186/1472-6823-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deepa M, Farooq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATPIII and IDF definitions in Asian Indians: The Chennai Urban Rural Epidemiology Study (CURES-34) Diabetes Metab Res Rev. 2007;23:127–34. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 21.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 22.Eckel RH, Alberti K, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375:181–3. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- 23.American Association for Clinical Chemistry. Insulin resitance. Lab Tests Online 2012. [Accessed June 28, 2014]. at http://www.labtestsonline.org.au/understanding/conditions/insulin-resistance/start/1 .
- 24.Olatunbosun ST. Insulin resistance workup. Medscape. 2013. [Accessed June 28, 2014]. at http://emedicine.medscape.com/article/122501-workup#aw2aab6b5b3 .
- 25.Shaikh S, Hanif G, Kashif, Humera M. Frequency of prediabetes and influence of various risk factors on the development of prediabetes: A tertiary care hospital experience. Int J Diabetes Dev Ctries. 2011;31:65–9. [Google Scholar]
- 26.Sabir A, Ohwovoriole A, Isezuo S, Fasanmade O, Abubakar S, Iwuala S. Type 2 diabetes mellitus and its risk factors among the rural Fulanis of Northern Nigeria. Ann Afr Med. 2013;12:217–22. doi: 10.4103/1596-3519.122689. [DOI] [PubMed] [Google Scholar]
- 27.Akter S, Rahman MM, Abe SK, Sultana P. Prevalence of diabetes and prediabetes and their risk factors among Bangladeshi adults: A nationwide survey. Bull World Health Organ. 2014;92:204–13. doi: 10.2471/BLT.13.128371. 13A. [DOI] [PMC free article] [PubMed] [Google Scholar]


