Abstract
The growing use of fluorescent biosensors to directly probe the spatiotemporal dynamics of biochemical processes in living cells has revolutionized the study of intracellular signaling. In this review, we summarize recent developments in the use of biosensors to illuminate the molecular details of G-protein-coupled receptor (GPCR) signaling pathways, which have long served as the model for our understanding of signal transduction, while also offering our perspectives on the future of this exciting field. Specifically, we highlight several ways in which biosensor-based single-cell analyses are being used to unravel many of the enduring mysteries that surround these diverse signaling pathways.
Keywords: Biosensor, Cell Signaling, Fluorescence Resonance Energy Transfer (FRET), G Protein, Signal Transduction, Live-cell Imaging
Introduction
All cells rely on signal transduction to communicate extracellular information to the intracellular machinery. In particular, G-protein signaling controls a multitude of diverse cellular functions, including responses to hormonal signals and environmental stimuli such as light and odor. It is estimated that ∼1000 human genes encode G-protein-coupled receptors (GPCRs),2 the initiators of G-protein signaling (1). Understanding G-protein signaling is therefore essential to unravel important signaling processes and to determine how the disruption of these processes can lead to disease. G-protein signaling begins with the activation of a GPCR by a corresponding ligand, which induces a conformational change in the receptor that transduces the external signal into the cell. This conformational change results in the recruitment and activation of heterotrimeric G-proteins, composed of Gα, Gβ, and Gγ subunits (Gαβγ). Specifically, the GPCR acts as a guanine nucleotide exchange factor (GEF) and converts the Gα subunit into its GTP-bound activated state. Once activated, Gα dissociates from the Gβγ dimer to activate various downstream effectors. Different Gα isoforms are known to associate with different GPCRs and/or effectors; thus, the specific downstream effects depend in part on the particular isoform that is activated. These effectors generate second messengers that both amplify the initial signal and modulate various downstream targets. G-protein signaling is regulated by the intrinsic GTPase activity of Gα, as well as by arrestin, which promotes GPCR internalization and mediates additional signaling pathways (2).
This classical view of G-protein signaling, revealed through years of painstaking biochemical study, has long served as a model for our understanding of intracellular signal transduction. However, recent studies are providing a more nuanced understanding of G-protein signaling through the use of single-cell analyses powered by fluorescent biosensors. These genetically encoded molecular tools offer a rapid and dynamic readout that enables the detection of biochemical activities within the native environment of a living cell and provide a unique platform for visualizing temporal and spatial information that traditional biochemical approaches often fail to capture. Such spatial and temporal information is crucial to our understanding of signaling dynamics and the complex interplay between different signaling cascades.
In this review, we first provide a brief summary of common biosensor design strategies. We then describe various studies that have used fluorescent biosensors and single-cell analyses to probe different aspects of G-protein signaling, specifically focusing on novel insights into GPCR activation, heterotrimeric G-protein dynamics, and second messenger production. Finally, we discuss some of our thoughts regarding the application of these techniques as well as the future of single-cell analyses of G-protein signaling.
Fluorescent Biosensors for Single-cell Analyses
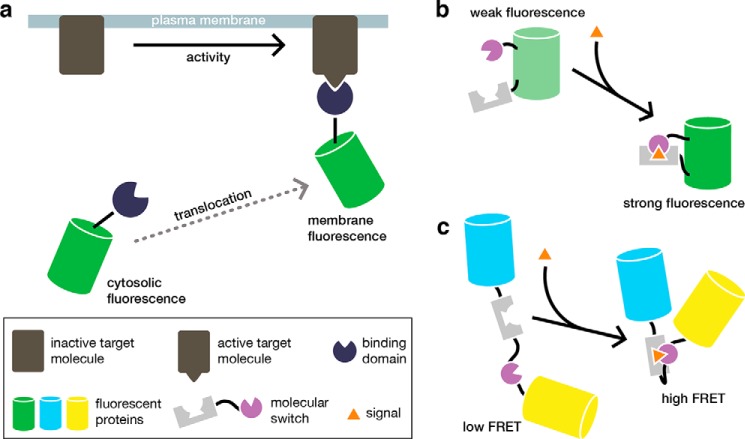
Biosensors are engineered constructs that couple the detection of a biochemical event to an optical signal. Genetically encoded fluorescent biosensors in particular have greatly enhanced the study of biochemical processes in living cells (see Ref. 3). These modular tools are typically composed of a sensing unit that detects a specific biochemical activity and induces an observable change in the fluorescent signal from a reporting unit. For example, many proteins change their localization in response to the appearance or disappearance of binding partners in particular subcellular regions. Translocation-based biosensors, which combine a binding domain with a single fluorescent protein (FP), are thus able to report on the presence of specific molecules through the redistribution of fluorescence (Fig. 1a). The sensing unit can also comprise a molecular switch that modulates the fluorescent properties of the reporting unit. Generally, molecular switches are derived from proteins or protein fragments whose conformation changes in response to specific input signals. Inserting a molecular switch into a single FP results in a biosensor whose fluorescence intensity changes in response to an activity of interest (Fig. 1b). Alternatively, a molecular switch can be coupled to a pair of FPs that are capable of undergoing FRET (Fig. 1c), which involves the non-radiative transfer of excited state energy from a donor fluorophore to a nearby acceptor (4). These basic designs comprise a diverse molecular toolkit for visualizing a myriad of signaling events in cells. Furthermore, because these biosensors can be directly expressed in and targeted throughout the cell, they are particularly powerful tools for monitoring biochemical processes at specific subcellular locations, which has proven invaluable for studying signaling dynamics.
FIGURE 1.
Standard approaches for biosensor design. a, translocation-based biosensors are generated by fusing a fluorescent protein to a protein or protein domain that specifically recognizes a molecule of interest. Here, GFP is fused to a binding domain that specifically recognizes the active conformation of a target protein located in the plasma membrane. The target protein becomes activated in response to an upstream signal, which induces the biosensor to translocate to the membrane and results in the redistribution of the fluorescent signal from the cytosol to the plasma membrane. b, in this example of an intensity-based biosensor, a molecular switch is inserted within a fluorescent protein such as GFP. The molecular switch consists of two protein fragments that associate in response to a specific input signal, which leads to a conformational change in the molecular switch and an increase in the fluorescence intensity of the biosensor. c, molecular switches are also used to generate FRET-based biosensors. Here, the molecular switch consists of two protein fragments fused in a single polypeptide that is sandwiched between two proteins capable of undergoing FRET. In response to the input signal, the molecular switch undergoes a conformational change that alters the distance and relative orientation of the fluorescent proteins, thereby resulting in a FRET change.
Visualizing GPCR Signaling Behavior
GPCRs are a major family of plasma membrane receptors that are characterized by seven-transmembrane domains and classified according to their known structure and function. Understanding how these receptors transduce signals is essential to resolving the specificity and interplay of their downstream effects. In the classical model, ligand binding to a GPCR induces a conformational change in the receptor that activates Gα, which then dissociates from the receptor to activate effectors. Under prolonged ligand signaling, GPCRs are phosphorylated by G-protein-coupled receptor kinases (GRKs), leading to the recruitment of arrestin. GPCRs are then internalized via endocytosis, inactivated, and subsequently recycled back to the plasma membrane or targeted for degradation in the lysosome or proteasome. Biosensors have been applied in three general approaches to visualize GPCR signaling, mirroring the different steps in GPCR activation: 1) examining ligand-induced conformational changes; 2) monitoring Gαβγ coupling; and 3) monitoring arrestin binding.
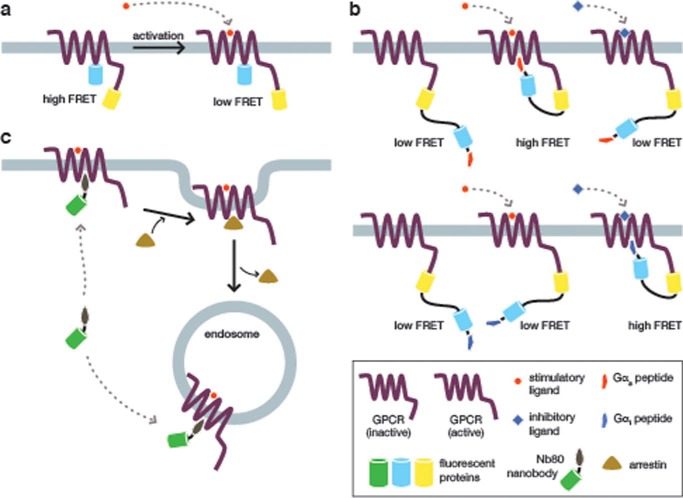
The first approach depends on conformational changes in the ligand-bound GPCR. Several biosensors have been developed to monitor GPCR activation and explore the functional effects of GPCR conformational dynamics. Vilardaga et al. (5) developed a pair of GPCR biosensors, based on the α2-adrenergic receptor (α2-AR) and the parathyroid hormone receptor (PTHR), which were used to determine the unique kinetics of each receptor (∼40 ms and ∼1 s, respectively). These biosensors were generated by inserting CFP into the third intracellular loop of each receptor, with YFP fused to the C terminus, such that ligand-induced conformational changes in the receptor cause a change in FRET (Fig. 2a). Unfortunately, the intramolecular FP adds bulk that can adversely interfere with downstream signaling. This same group created a modified sensor to mitigate this issue by using FlAsH (fluorescein arsenical hairpin binder), a small-molecule dye that only fluoresces when bound to a specific peptide sequence. Hoffman et al. (6) replaced the FP in the intercellular loop of α2-AR with the binding motif for FlAsH and fused CFP to the C terminus of the receptor to make an improved GPCR biosensor. The reduced bulk improved the biosensor response 5-fold without changing the observed kinetics or inhibiting downstream signaling. These early GPCR biosensors set the stage for further studies of GPCR signaling.
FIGURE 2.
GPCR activation dynamics revealed using biosensors. a, a FRET-based biosensor for measuring GPCR activation dynamics. Vilardaga et al. (5) generated a pair of biosensors based on the α2-AR and the PTHR, in which CFP was inserted within the third intracellular loop and YFP was fused to the C terminus of the full-length receptor. In both biosensors, receptor activation leads to a conformational change that decreases the FRET between the two fluorescent proteins. When expressed in cells, these biosensors made it possible to directly visualize the ligand-induced conformational dynamics of these two receptors with millisecond precision. b, a family of biosensors illuminates ligand-specific conformational changes and G-protein coupling. In a recent study, Malik et al. (14) generated a panel of biosensors consisting of the β2-AR fused to YFP, a flexible linker, and CFP. Each sensor also contained a fragment from a particular Gα protein at the far C terminus. In cells expressing the biosensor variant containing a Gαs fragment (upper panel), only a corresponding “stimulatory” agonist was able to activate the biosensor and elicit a FRET response. Conversely, only an “inhibitory” agonist was able to elicit a FRET change in cells expressing the Gαi-fused biosensor (lower panel), thus demonstrating that different conformational changes induced by specific ligands can link a single receptor to diverse downstream pathways. c, detecting endogenous GPCR activation in endosomes. Most GPCR biosensors report on the behavior of exogenously expressed GPCR constructs. Thus, to investigate endogenous GPCR dynamics, Irannejad et al. (18) fused GFP to a nanobody that specifically binds the active conformation of the β2-AR. This probe decorates the plasma membrane in stimulated cells but is displaced following the binding of arrestin during GPCR internalization. However, this translocation-based biosensor was subsequently observed to label the resulting endosomes, indicating that the β2-AR is still active in these compartments.
Multiple experimental studies have demonstrated that GPCRs adopt distinct conformations in response to different ligands, leading to the hypothesis that different downstream signaling pathways are coupled to specific GPCR conformations (7–13). Recently, Malik et al. (14) created a series of biosensors that contain the GPCR-binding domain of different Gα subunits to study ligand-specific conformational changes and subsequent differences in downstream effects. These biosensors contain full-length β2-AR followed by YFP, a flexible linker, CFP, and a C-terminal fragment from a particular Gα subunit (14) (Fig. 2b). Gα proteins have been shown to bind a cytosolic groove on activated GPCRs, and the C terminus of Gα is important for transducing signals between GPCRs and Gαβγ. By creating multiple biosensors, each containing a unique Gα subunit, it is possible to probe the link between a specific Gα and GPCR activation induced by different ligands. For example, the authors found that treating β2-AR with one of two inhibitory ligands enhanced the binding of Gαi over Gαs. These results confirm previous studies that established Gαi as being responsible for inhibitory GPCR signaling and Gαs as being responsible for stimulatory GPCR signaling. Furthermore, structural studies have shown that a highly conserved (E/D)RY motif in β2-AR forms an “ionic lock” with neighboring residues when cells are treated with an inhibitory ligand, and mutating certain residues in this motif has been shown to cause constitutive GPCR signaling (15–17). Using the aforementioned biosensors, the authors found that mutating either Glu/Asp or Arg increased the association of Gαs to β2-AR; however, as with previous studies, only the Glu/Asp mutations induced the increased cAMP response caused by Gαs signaling. Using these modified FRET-based GPCR biosensors, this group was able to directly visualize the links between specific ligand-induced conformational changes and the downstream effects controlled by specific Gα proteins.
Classically, endocytosis is thought to result in the inactivation and recycling of GPCRs; however, the question of whether GPCRs are inactive in endosomes has recently been debated. To examine this question directly and determine whether GPCRs could remain active in endosomes, Irannejad et al. (18) developed a translocation-based biosensor that specifically binds the β2-AR in its active conformation to probe endogenous GPCR activation (Fig. 2c). This biosensor comprises GFP fused to a nanobody, Nb80, that specifically binds activated β2-AR after isoprenaline treatment. The nanobody is competed off by arrestin binding, which leads to receptor endocytosis. This group observed that GFP-tagged Nb80 translocated to the plasma membrane upon β2-AR activation and then again to endosomes once the receptor was internalized, revealing that at least a subset of endogenous β2-AR is active in endosomes. The biosensor used in this study binds endogenous β2-AR, thereby circumventing concerns regarding artifacts due to exogenous GPCR biosensor expression and providing valuable insights into endosomal GPCR activity.
GPCR activity can also be monitored via the dissociation of Gαβγ or by the binding of arrestin. One question that has remained unclear in the field is whether Gαβγ associates with GPCRs prior to receptor activation or whether they are only recruited to the receptor after its conformational change. Nobles et al. (19) used a bimolecular FRET sensor to study the interaction between Gαβγ (Gαo or Gαs, Gβ1, and Gγ2) and multiple receptors (α2-AR, muscarinic acetylcholine receptor M4, A1 adenosine receptor, and D2S dopamine receptor). Each component was tagged with either CFP or YFP, and the FRET responses were monitored between different combinations of G-protein and receptor. The authors used these biosensors to demonstrate that specific G-proteins tend to “precouple” with specific receptors. For example, α2-AR precouples with Gαo but not with Gαs, whereas the known Gαs receptor prostacyclin precouples with Gαs but not with Gαo. This precoupling model conflicts with other studies that instead suggest a diffusion-controlled model for the interaction between GPCRs and heterotrimeric G-proteins. These studies used FRET biosensors for PTHR and α2A-AR but did not observe any precoupling (12, 20). The reason for these discrepancies is unclear, and further studies are thus needed to resolve the nature of G-protein-receptor coupling.
The binding of arrestin to GPCRs is another indicator of GPCR activity, specifically long-term GPCR activity leading to endocytosis. Arrestin is recruited to phosphorylated GPCRs and is necessary for receptor endocytosis via clathrin-coated pits. Violin et al. (21) developed a bimolecular FRET reporter of arrestin binding to study the specificity of GRKs, which are the enzymes responsible for phosphorylating GPCRs and hence recruiting arrestin. The authors fused CFP to the β2-AR and YFP to β-arrestin and found that the recruitment of β-arrestin to β2-AR can act as an indicator of endogenous and exogenous GRK activity. This study also revealed a high degree of redundancy in GRK specificity, with the amount of GRK activity being proportional to the kinetics of the arrestin-receptor interaction, leading the authors to conclude that the regulation of GRK, and subsequent GRK regulation of GPCRs, is a mechanism to control the length of GPCR activation. Krasel et al. (22, 23) also used FP-fused proteins to study the interaction between β2-AR and β-arrestin. These FRET studies, which use the same biosensor design described above, reveal the kinetics of β-arrestin binding to the receptor, as well as the reliance of this interaction on GRK activity. The authors also found that the C terminus of the receptor aids in β-arrestin binding and subsequent receptor internalization, which they suggest may occur through the recruitment of other proteins to aid in internalization.
A more recent study used multiple FRET biosensors to study interactions between PTHR and both arrestin and Gαβγ (24). For some GPCRs, such as the β2-AR, Gαβγ and arrestin are generally thought to bind sequentially. However, studies have suggested that Gαβγ and arrestin can bind PTHR together. Previous work by Feinstein and colleagues (12, 25) showed that, unlike β2-AR, PTHR induced prolonged cAMP signaling. PTHR-arrestin complexes were specifically associated with this prolonged cAMP signaling, a finding that calls into question the classical model of G-protein signaling and recycling. To understand how PTHR signaling and β2-AR signaling differ with regard to prolonged cAMP signaling, Wehbi and colleagues (27) used multiple FRET biosensors, which were described previously (reviewed in Ref. 26) to study the interactions between PTHR, arrestin, and Gαβγ by tagging PTHR, Gαs, Gβγ, and arrestin with CFP or YFP and monitoring FRET between pairs of tagged proteins. Using this approach, arrestin and Gβγ were found to associate with each other and simultaneously bind the PTHR. This arrangement prolonged cAMP production (as previously observed) (12, 25) through continued Gαs activation from within endosomes, thereby revealing the mechanism underlying prolonged cAMP signaling in the PTHR pathway. Prolonged cAMP signaling, based on receptor internalization via arrestin, was also shown to be the main downstream signaling difference between two agonists of the V2 receptor (V2R) (27).
Monitoring the Dynamics of Heterotrimeric G-proteins
Following GPCR activation, Gαβγ carries the signal to various downstream effectors. Classically, Gαβγ dissociates from the GPCR and disassembles after the GPCR activates Gα by exchanging its bound GDP for GTP. There are multiple isoforms of the Gα subunit, each of which is involved in specific downstream signaling (28). For instance, Gαs induces adenylyl cyclase (AC) to produce cAMP, whereas Gαi inhibits AC activity. Biosensors that focus on Gαβγ are thus important tools for studying the diversity and specificity of G-protein signaling. FRET biosensors can be used to monitor Gαβγ activity through the disassembly of the subunits or through the association of a G-protein subunit with an effector, which forms the next step of the G-protein signaling pathway.
Janetopoulos et al. (29) created the first FRET biosensor for Gαβγ signaling based on the classical understanding of the dissociation of Gα from the Gβγ dimer by fusing YFP and CFP to Gβ and Gαs, respectively. This group observed a transient, decreasing FRET response in Dictyostelium discoideum upon GPCR activation, corresponding to the dissociation of the heterotrimeric components. Bünemann et al. (30) then modified this design to study the dissociation of the Gαi family of inhibitory G-proteins by fusing YFP to Gαi, and by fusing CFP to three different regions of the Gβγ dimer (Fig. 3a). Interestingly, two of the three resulting biosensors produced increasing FRET responses, which is contrary to the expected response if the heterotrimer subunits were dissociating from each other. This implies a continued interaction between the tagged subunits upon GPCR activation and questions the classical view of Gαβγ disassembly upon the activation of G-protein signaling, at least for Gαi. These results suggest that, unlike the dissociation observed with Gαs, Gαi proteins undergo a conformational rearrangement but do not fully dissociate. These conclusions are supported by fluorescence recovery after photobleaching (FRAP) experiments that show the same dissociation or continued association of different Gα subunits after GPCR activation (31).
FIGURE 3.
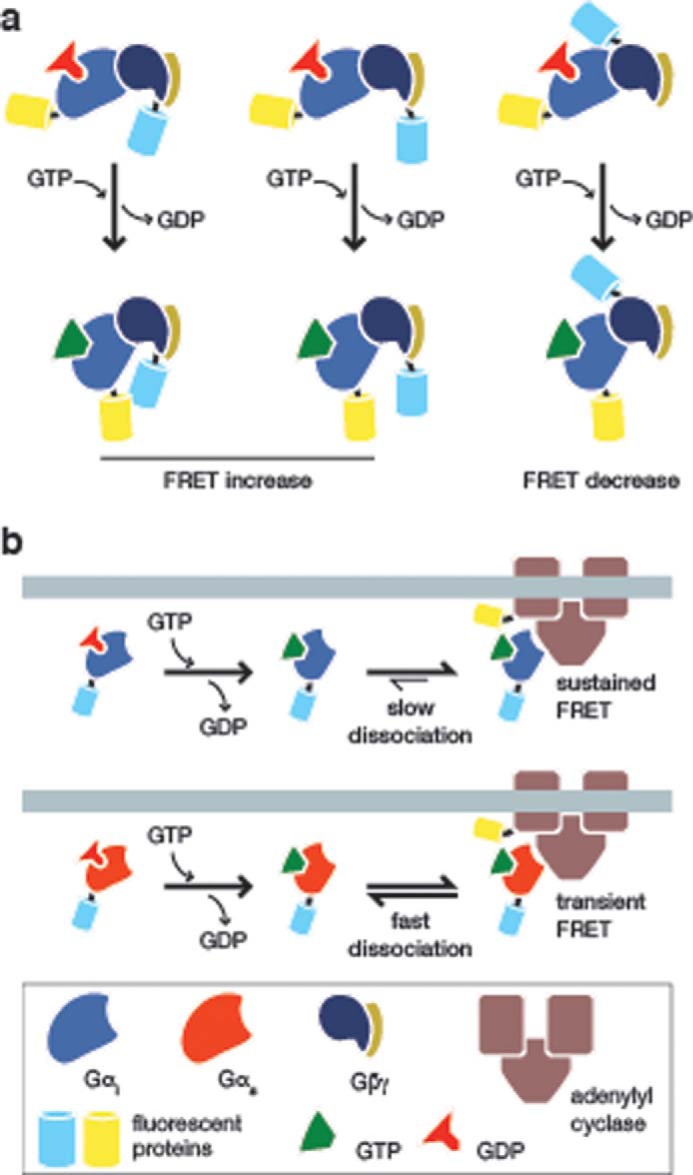
Visualizing heterotrimeric G-protein signaling. a, conformational rearrangement upon activation of the Gi heterotrimer. To test the dissociation of Gi upon activation by a GPCR, Bünemann et al. (30) generated a series of biosensors by fusing YFP to Gαi and CFP to three different positions on the Gβγ dimer. Although one of these biosensors produced a FRET decrease upon GPCR stimulation (right panel), the other two both produced increasing FRET responses (left and middle panels), suggesting that Gi proteins undergo a conformational rearrangement upon activation and do not fully dissociate, as in the case of the Gs heterotrimer. b, monitoring Gαi interaction with adenylyl cyclase. Milde et al. (35) recently examined the interaction between Gαi and adenylyl cyclase by fusing CFP to Gαi1 and YFP to AC5. The activation of Gαi1 by GPCR signaling results in its interaction with AC5, leading to a FRET increase (upper panel). However, when compared with Gαs (lower panel), Gαi1 was observed to dissociate very slowly from AC5, which the authors suggested may be a mechanism for the self-regulation of AC5 activity in cells. For clarity, Gβγ is not shown.
Using another Gαβγ dissociation biosensor, Kataria et al. (32) recently studied the specific role of the protein resistant to inhibitors of cholinesterase 8 (Ric8), a molecular chaperone with putative non-receptor GEF activity, in D. discoideum chemotaxis (see Ref. 33 for a discussion of Ric8 regulation of heterotrimeric G-proteins). The chemoattractant cAMP is known to activate Gαβγ and the monomeric G-protein Ras, which are necessary for coordinating chemotaxis. In this study, Ric8 was found to be necessary for chemotaxis when there is a shallow cAMP gradient. Using a bimolecular sensor composed of Gα2-CFP and Gβ-YFP, the authors studied the dissociation of the heterotrimeric subunits, and hence the activation of G-protein signaling, in response to cAMP agonist stimulation in wild-type and ric8-null cells. In cells lacking Ric8, the total FRET change was less than half that observed in wild-type cells, and the response was slower. These results, together with biochemical data, indicated that Ric8 acts as a GEF to reactivate inactive Gα2-containing G-proteins and sustain G-protein signaling without continued receptor signaling. This sustained Gα signaling results in downstream effects on Ras that lead to concentrated Ras activity at the leading edge of a migrating cell.
Heterotrimeric G-proteins are the main link between GPCRs and downstream effects, with different Gα subunit isoforms able to activate different effectors. Thus, a more complete picture of the interactions between Gαβγ isoforms and various effectors will improve our understanding of how different Gα subunits shape the diversity of G-protein signaling. To this end, a number of biosensors have been developed to probe the interactions between Gαβγ components and downstream effectors. For example, Sadana et al. (34) previously used a FRET probe to study the dynamics of the interaction between Gαs and adenylyl cyclase 5 (AC5). However, it was unclear how the dynamics of Gαi differed from those of Gαs. Recently, Milde et al. (35) reported the development of a FRET biosensor to monitor the association between Gαi and AC5 (Fig. 3b). This bimolecular sensor contains YFP fused to the N terminus of AC5 and CFP attached to Gαi1. Upon activation of Gαi1 by the α2a-AR, the authors observed rapid interaction between Gαi1 and AC5. Surprisingly, this response was followed by the slow dissociation of Gαi1 from AC5, which differed from the dynamics of the Gαs-AC5 interaction and was not altered by increasing the intrinsic GTPase activity of Gαi1. The authors concluded that the slow deactivation kinetics of AC5-bound Gαi1 can delay the reassembly of the Gαβγ complex and hence may be a mechanism for AC5 self-regulation.
Tracking Second Messenger Dynamics
Second messengers are small molecules whose intracellular levels are tightly regulated by the cell to amplify and propagate signals to diverse downstream effectors. In the context of G-protein signaling, these can include cAMP, inositol 1,4,5-trisphosphate, diacylglycerol, and Ca2+, and fully understanding G-protein signaling dynamics requires knowing how these different second messenger pools are generated. For example, Chakir et al. (36) used biosensors to study cAMP production in response to β2-AR stimulation in canine heart cells with synchronous or dyssynchronous heart failure and to investigate the mechanism of cardiac resynchronization therapy. Using a FRET biosensor that contains a known cAMP-binding protein sandwiched between CFP and YFP, this group found that cardiac resynchronization therapy improves stimulatory G-protein signaling in both synchronous and dyssynchronous heart failure models. Specifically, cells from treated canines produced more cAMP because the inhibitory Gαi subunit was inhibited by regulators of G-protein signaling (RGS2 and RGS3). Because Gαi is inhibited, β2-AR signaling is biased to the Gαs pathway, which activates AC and increases cAMP production, thereby restoring normal physiological conditions.
Similarly, Verma et al. (37) used a Ca2+ biosensor to measure the effect of various agonists and GRKs on D1-D2 heteromer dopamine receptor signaling. The homo-oligomers of the dopamine receptors have been well studied, but the dynamics of the D1-D2 hetero-oligomer are not well characterized. A FRET-based Ca2+ probe, composed of the Ca2+-binding protein calmodulin fused to the calmodulin-binding peptide M13 and flanked by CFP and YFP, was used in this study to measure intracellular Ca2+ levels in response to a D1-specific agonist, a D1-D2-specific activating agonist, and GRK knockdown. This group found that the binding of a D1 agonist, even without full activation, is sufficient to induce an increase in Ca2+ that can be attenuated by GRK activity.
Conclusions and Future Perspectives
Single-cell analyses using fluorescent biosensors are clearly a powerful method to study signaling dynamics in pathways such as G-protein signaling. The genetic encodability and modular nature of biosensors provide a convenient way to create sensors for different processes by fusing FPs to appropriate sensing units. However, biosensors are not without faults. FPs can disrupt the natural functions of the sensing unit due to their added bulk, whereas the sensing unit itself can perturb the natural signaling system. For example, a biosensor that contains a catalytically inactive protein as the sensing unit may buffer the natural ligand pool and affect native signaling (38). However, these effects can be reduced by using enhanced biosensors, thus lowering the required biosensor concentrations, as well as by performing the proper controls. The drawbacks of biosensors are also balanced by their principle advantage in single-cell analysis: the ability to directly observe living cells in real time and collect both temporal and spatial information on biochemical activities.
Single-cell analyses also pose an interesting dilemma, in that they can reveal dynamics that are rare or hidden at the population level but may also reveal a wide range of behaviors across a population, making it difficult to tease out the relevant dynamics. This natural heterogeneity can hinder our understanding of signaling dynamics, but it can also contain functional information. Studies have presented different functional models for how noise in a population is created and how it can affect function (39, 40). For instance, stochastic differences may contribute to the ability of a subpopulation of cells to exceed a threshold and differentiate, which is an important event in many processes, including development and immune cell differentiation. Single-cell analyses are something of a mixed blessing in this respect as they can provide valuable information that is lost in population experiments, but the natural heterogeneity of a signal may hinder the understanding of its function unless the role of the heterogeneity can also be determined.
In addition to monitoring signaling dynamics, it is also possible to perturb specific biochemical processes at the single-cell level by using optogenetics or chemically inducible dimerization (CID) to directly manipulate cellular processes with spatiotemporal precision. Combining biosensor-based single-cell analyses with these novel tools offers new opportunities to expand our understanding of signaling dynamics in general and G-protein signaling in particular. Masseck et al. (41) provide an excellent overview of current optogenetic methods and tools to study G-protein signaling, particularly at the GPCR level. Optogenetics encompasses techniques in which light is used to control the activity of specific proteins. Opsins, a class of GPCRs, are ideal for optogenetics because these light-sensing receptors can be experimentally activated by light. Optogenetics is therefore readily applicable to the study of G-protein signaling.
A recent study selected opsins based on their ability to be activated by specific wavelengths of light that do not overlap with those necessary for fluorescent biosensor use (42). The authors found three distinct opsins that meet this criterion and enable the specific activation of GPCRs coupled to Gαs, Gαq, or Gαi while simultaneously using FRET biosensors to measure downstream effects. The three opsins developed are: a blue variant of rhodopsin (termed bOpsin) to activate Gαi/o signaling; melanopsin to activate Gαq signaling; and a fusion construct between a jellyfish GPCR domain that binds Gαs and the fluorophore component of bOpsin (termed CrBlue). When these opsins were activated by an optical input, fluorescently tagged heterotrimeric G-protein subunits translocated into the cytosol, indicating successful activation of the receptor and G-protein signal transduction. Beyond combining the optical activation of G-protein signaling with the imaging of downstream activity, the authors demonstrated that the light-activated receptors could be activated at distinct cellular locations while activity was measured throughout the cell. These studies present a powerful tool to activate distinct GPCRs at specific, confined locations in the membrane, which the authors used to direct the growth of neurites. When the optical input was directed at the edge of a neurite and steadily moved away, lamellipodia were observed to expand in the direction of the optical input, concomitant with the retraction of a distal neurite. The ability to locally activate GPCRs opens new possibilities for ways to control and study G-protein signaling dynamics.
CID is another method of directing protein activity that utilizes the induced dimerization or association of specific protein domains. For example, the FK506-binding protein (FKBP) and FKBP12-rapamycin-binding domain (FRB) proteins dimerize in the presence of rapamycin, which is a useful tool for directing the association or translocation of proteins (43). To study the specificity of heterotrimeric G-protein signaling, Putyrsky et al. (44) used this system to tether different components of the Gαβγ heterotrimer to the plasma membrane. They then observed the downstream effect by measuring intracellular cAMP and Ca2+. FKBP was fused to Gαq or Gαs subunits or to Gγ (remained associated with Gβ), and FRB was targeted to the membrane. Upon rapamycin treatment, the G-protein subunits translocated to the plasma membrane and induced downstream signaling independent of receptor activation. This study demonstrates a new technology for selectively studying G-protein signaling diversity controlled by distinct heterotrimeric G-proteins, as well as wide-ranging applications for studying other aspects of G-protein signaling.
Optogenetics and CID are effective methods to directly control different aspects of protein behavior that, together with fluorescent biosensors, provide a promising new way to probe the dynamics of G-protein signaling. Future studies utilizing these molecular tools to control, perturb, and analyze G-protein signaling at the single-cell level will enable far more comprehensive and detailed studies of the spatiotemporal dynamics of G-protein signaling, including GPCR activation, heterotrimeric G-protein activity, and downstream functions.
Acknowledgments
We thank members of the Zhang laboratory for helpful discussions, in particular Fabian Hertel for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK073368 and DP1CA174423 (to J. Z.). This is the first article in the Thematic Minireview series “Cell Biology of G Protein Signaling.”
- GPCR
- G-protein-coupled receptor
- GEF
- guanine nucleotide exchange factor
- GRK
- G-protein-coupled receptor kinase
- α2-AR
- α2-adrenergic receptor
- β2-AR
- β2-adrenergic receptor
- PTHR
- parathyroid hormone receptor
- FP
- fluorescent protein
- FKBP
- FK506-binding protein
- AC
- adenylyl cyclase
- FlAsH
- fluorescein arsenical hairpin binder
- CID
- chemically inducible dimerization.
REFERENCES
- 1. Lefkowitz R. J. (2004) Historical review: A brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 25, 413–422 [DOI] [PubMed] [Google Scholar]
- 2. DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) β-Arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 3. Newman R. H., Fosbrink M. D., Zhang J. (2011) Genetically encodable fluorescent biosensors for tracking signaling dynamics in living cells. Chem. Rev. 111, 3614–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell R. E. (2009) Fluorescent-protein-based biosensors: modulation of energy transfer as a design principle. Anal. Chem. 81, 5972–5979 [DOI] [PubMed] [Google Scholar]
- 5. Vilardaga J.-P., Bünemann M., Krasel C., Castro M., Lohse M. J. (2003) Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 21, 807–812 [DOI] [PubMed] [Google Scholar]
- 6. Hoffmann C., Gaietta G., Bünemann M., Adams S. R., Oberdorff-Maass S., Behr B., Vilardaga J.-P., Tsien R. Y., Ellisman M. H., Lohse M. J. (2005) A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Methods 2, 171–176 [DOI] [PubMed] [Google Scholar]
- 7. Gether U., Lin S., Ghanouni P., Ballesteros J. A., Weinstein H., Kobilka B. K. (1997) Agonists induce conformational changes in transmembrane domains III and VI of the β2 adrenoceptor. EMBO J. 16, 6737–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahsai A. W., Xiao K., Rajagopal S., Ahn S., Shukla A. K., Sun J., Oas T. G., Lefkowitz R. J. (2011) Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 7, 692–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghanouni P., Steenhuis J. J., Farrens D. L., Kobilka B. K. (2001) Agonist-induced conformational changes in the G-protein-coupling domain of the β2 adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 98, 5997–6002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen A. D., Guarnieri F., Rasmussen S. G. F., Asmar F., Ballesteros J. A., Gether U. (2001) Agonist-induced conformational changes at the cytoplasmic side of transmembrane segment 6 in the β2 adrenergic receptor mapped by site-selective fluorescent labeling. J. Biol. Chem. 276, 9279–9290 [DOI] [PubMed] [Google Scholar]
- 11. Seifert R., Wenzel-Seifert K., Gether U., Kobilka B. K. (2001) Functional differences between full and partial agonists: evidence for ligand-specific receptor conformations. J. Pharmacol. Exp. Ther. 297, 1218–1226 [PubMed] [Google Scholar]
- 12. Ferrandon S., Feinstein T. N., Castro M., Wang B., Bouley R., Potts J. T., Gardella T. J., Vilardaga J.-P. (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiner S., Ambrosio M., Hoffmann C., Lohse M. J. (2010) Differential signaling of the endogenous agonists at the β2-adrenergic receptor. J. Biol. Chem. 285, 36188–36198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik R. U., Ritt M., DeVree B. T., Neubig R. R., Sunahara R. K., Sivaramakrishnan S. (2013) Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells. J. Biol. Chem. 288, 17167–17178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ballesteros J. A., Jensen A. D., Liapakis G., Rasmussen S. G. F., Shi L., Gether U., Javitch J. A. (2001) Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 [DOI] [PubMed] [Google Scholar]
- 16. Rovati G. E., Capra V., Neubig R. R. (2007) The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol. Pharmacol. 71, 959–964 [DOI] [PubMed] [Google Scholar]
- 17. Wacker D., Fenalti G., Brown M. A., Katritch V., Abagyan R., Cherezov V., Stevens R. C. (2010) Conserved binding mode of human β2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J. Am. Chem. Soc. 132, 11443–11445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Irannejad R., Tomshine J. C., Tomshine J. R., Chevalier M., Mahoney J. P., Steyaert J., Rasmussen S. G. F., Sunahara R. K., El-Samad H., Huang B., von Zastrow M. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nobles M., Benians A., Tinker A. (2005) Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc. Natl. Acad. Sci. U.S.A. 102, 18706–18711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hein P., Frank M., Hoffmann C., Lohse M. J., Bünemann M. (2005) Dynamics of receptor/G protein coupling in living cells. EMBO J. 24, 4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Violin J. D., Ren X.-R., Lefkowitz R. J. (2006) G-protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J. Biol. Chem. 281, 20577–20588 [DOI] [PubMed] [Google Scholar]
- 22. Krasel C., Bünemann M., Lorenz K., Lohse M. J. (2005) β-Arrestin binding to the β2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J. Biol. Chem. 280, 9528–9535 [DOI] [PubMed] [Google Scholar]
- 23. Krasel C., Zabel U., Lorenz K., Reiner S., Al-Sabah S., Lohse M. J. (2008) Dual role of the β2-adrenergic receptor C terminus for the binding of β-arrestin and receptor internalization. J. Biol. Chem. 283, 31840–31848 [DOI] [PubMed] [Google Scholar]
- 24. Wehbi V. L., Stevenson H. P., Feinstein T. N., Calero G., Romero G., Vilardaga J.-P. (2013) Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc. Natl. Acad. Sci. U.S.A. 110, 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feinstein T. N., Wehbi V. L., Ardura J. A., Wheeler D. S., Ferrandon S., Gardella T. J., Vilardaga J.-P. (2011) Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 7, 278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lohse M. J., Nuber S., Hoffmann C. (2012) Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol. Rev. 64, 299–336 [DOI] [PubMed] [Google Scholar]
- 27. Feinstein T. N., Yui N., Webber M. J., Wehbi V. L., Stevenson H. P., King J. D., Hallows K. R., Brown D., Bouley R., Vilardaga J. P. (2013) Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem. 288, 27849–27860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neves S. R., Ram P. T., Iyengar R. (2002) G protein pathways. Science 296, 1636–1639 [DOI] [PubMed] [Google Scholar]
- 29. Janetopoulos C., Jin T., Devreotes P. (2001) Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291, 2408–2411 [DOI] [PubMed] [Google Scholar]
- 30. Bünemann M., Frank M., Lohse M. J. (2003) Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Digby G. J., Lober R. M., Sethi P. R., Lambert N. A. (2006) Some G protein heterotrimers physically dissociate in living cells. Proc. Natl. Acad. Sci. U.S.A. 103, 17789–17794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kataria R., Xu X., Fusetti F., Keizer-Gunnink I., Jin T., van Haastert P. J. M., Kortholt A. (2013) Dictyostelium Ric8 is a nonreceptor guanine exchange factor for heterotrimeric G proteins and is important for development and chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 110, 6424–6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tall G. G. (2013) Ric-8 regulation of heterotrimeric G proteins. J. Recept. Signal Transduct. Res. 33, 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadana R., Dascal N., Dessauer C. W. (2009) N terminus of type 5 adenylyl cyclase scaffolds Gs heterotrimer. Mol. Pharmacol. 76, 1256–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Milde M., Rinne A., Wunder F., Engelhardt S., Bünemann M. (2013) Dynamics of Gαi1 interaction with type 5 adenylate cyclase reveal the molecular basis for high sensitivity of Gi-mediated inhibition of cAMP production. Biochem. J. 454, 515–523 [DOI] [PubMed] [Google Scholar]
- 36. Chakir K., Depry C., Dimaano V. L., Zhu W.-Z., Vanderheyden M., Bartunek J., Abraham T. P., Tomaselli G. F., Liu S.-B., Xiang Y. K., Zhang M., Takimoto E., Dulin N., Xiao R. P., Zhang J., Kass D. A. (2011) Gαs-biased β2-adrenergic receptor signaling from restoring synchronous contraction in the failing heart. Sci. Transl. Med. 3, 100ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verma V., Hasbi A., O'Dowd B. F., George S. R. (2010) Dopamine D1-D2 receptor heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J. Biol. Chem. 285, 35092–35103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyawaki A. (2003) Visualization of the spatial and temporal dynamics of intracellular signaling. Dev. Cell 4, 295–305 [DOI] [PubMed] [Google Scholar]
- 39. Eldar A., Elowitz M. B. (2010) Functional roles for noise in genetic circuits. Nature 467, 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shalek A. K., Satija R., Adiconis X., Gertner R. S., Gaublomme J. T., Raychowdhury R., Schwartz S., Yosef N., Malboeuf C., Lu D., Trombetta J. J., Gennert D., Gnirke A., Goren A., Hacohen N., Levin J. Z., Park H., Regev A. (2013) Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498, 236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masseck O. A., Mark M. D., Herlitze S. (2014) Use of optogenetic approaches to control intracellular signaling of G protein-coupled receptors. in G-Protein Coupled Receptor Genetics: Research and Methods in the Post-Genomic Era (Stevens C. W., ed), pp. 149–160, Humana Press, Totowa, NJ [Google Scholar]
- 42. Karunarathne W. K. A., Giri L., Kalyanaraman V., Gautam N. (2013) Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension. Proc. Natl. Acad. Sci. U.S.A. 110, E1565–E1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeRose R., Miyamoto T., Inoue T. (2013) Manipulating signaling at will: chemically-inducible dimerization (CID) techniques resolve problems in cell biology. Pflugers Arch. 465, 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Putyrski M., Schultz C. (2011) Switching heterotrimeric G protein subunits with a chemical dimerizer. Chem. Biol. 18, 1126–1133 [DOI] [PubMed] [Google Scholar]