Background: Mammalian hearts express hormones such as natriuretic peptides.
Results: Cardiomyocytes also express the cholecystokinin gene at the protein level with unique precursor processing.
Conclusion: Cardiac expression of procholecystokinin is cell-specific, which differentiates the expression from that of intestinal endocrine cells and cerebral neurons.
Significance: Cardiomyocytes express not only natriuretic peptides but also known peptides in intestinal endocrine cells and cerebral neurons.
Keywords: Gene Expression, Heart, Heart Failure, Hormone, Natriuretic Peptide, ANP, BNP, Cholecystokinin, Gut Hormone, Peptide Identification
Abstract
Heart muscle cells produce peptide hormones such as natriuretic peptides. Developing hearts also express the gene for the classic intestinal hormone cholecystokinin (CCK) in amounts similar to those in the intestine and brain. However, cardiac expression of peptides other than natriuretic peptides has only been suggested using transcriptional measures or methods, with the post-translational phase of gene expression unaddressed. In this study, we examined the cardiac expression of the CCK gene in adult mammals and its expression at the protein level. Using quantitative PCR, a library of sequence-specific pro-CCK assays, peptide purification, and mass spectrometry, we demonstrate that the mammalian heart expresses pro-CCK in amounts comparable to natriuretic prohormones and processes it to a unique, triple-sulfated, and N-terminally truncated product distinct from intestinal and cerebral CCK peptides. Isoprenaline rapidly stimulated cardiac CCK gene expression in vitro and in vivo, which suggests that the cardiac-specific truncated pro-CCK may have pathophysiological relevance as a new marker of heart failure. The suggestion is confirmed by measurement of plasma from heart failure patients.
Introduction
Heart muscle cells produce peptide hormones. The best known are the natriuretic peptides (1). In addition, parathyroid hormone-like protein (2), endothelin (3), and relaxin (4) have been reported in cardiac myocytes, but molecular data on the cardiomyocyte synthesis of these hormones are lacking. Fetal mouse hearts also express mRNA for the intestinal hormone cholecystokinin (CCK)3 in amounts similar to those found in the developing small intestine and central nervous system (5), where the known hormonal and transmitter-active CCK peptides are synthesized. In this study, we examined the cardiac expression of the CCK gene in adult mammals and expression at the protein level. Using quantitative PCR, a library of sequence-specific pro-CCK assays, peptide purification, and mass spectrometry, we demonstrate that the mammalian heart expresses pro-CCK and processes it to a unique, triple-sulfated, and N-terminally truncated form distinct from intestinal and cerebral CCK peptides. Measurement of the cardiac-specific form in plasma provides valuable information for patients with stable heart failure.
EXPERIMENTAL PROCEDURES
RT-PCR
We used RT-PCR for quantitation of single-gene expression. The protocols followed for RNA extraction, cDNA generation, and RT-PCR analyses using the LightCycler FastStart DNA Master SYBR Green kit (Roche Applied Science) have been reported previously (6, 7). The following sense and antisense primers were used in this study: mouse CCK, 5′-CTGAAGGTGCTGTCCCAGAT-3′ and 5′-GTTCTTTTGTGAGGCCTTGG-3′; mouse atrial natriuretic peptide (ANP), 5′-CCTGTGTACAGTGCGGTGTC-3′ and 5′-CCTAGAAGCACTGCCGTCTC-3′; mouse β2-microglobulin, 5′-AGTATGTTCGGCTTCCCATTCTC-3′ and 5′-CGGCTTGTATGCTATCCAGAAAA-3′; rat CCK, 5′-CGCAGTGCTGAGGACTACG-3′ and 5′-CACCAGAGGGAAACATTGCC-3′; rat brain natriuretic peptide (BNP), 5′-GCAGAAGCTGCTGGAGCTGA-3′ and 5′-GATCCGGAAGGCGCTGTCT-3′; rat GAPDH, 5′-GTCGGTGTGAACGGATTTG-3′ and 5′-CTTGCCGTGGGTAGAGTCAT-3′; pig CCK, 5′-CAAAAGGTAGACGGCGAGTCC-3′ and 5′-CCGGCCAAAATCCATCCAGC-3′; pig gastrin, 5′-GCCAACAGCAGCACACCTGC-3′ and 5′-GCCGATCCAGCTCATGTGGC-3′; pig BNP, 5′-GTGCTCCTGCTCCTGTTCTT-3′ and 5′-TCCCAGGCTTCTGTGAGG-3′; pig β-actin, 5′-TGGACATCAGGAAGGACCTC-3′ and 5′-ACATCTGCTGGAAGGTGGAC-3′; and pig GAPDH, 5′-TGGAAATCCCATCACCATCT-3′ and 5′-TTCATGCCCATCACAAACAT-3′. All samples were assayed in duplicate, and the specificity of the PCRs was evaluated by DNA sequencing of the PCR products.
Immunoassays
Porcine pro-BNP was quantified with a radioimmunoassay based on the N terminus of porcine pro-BNP (6, 8). For measurement of porcine pro-CCK, we used a radioimmunoassay based on antibody 1564, was raised against a mid-region epitope in porcine pro-CCK and which binds this sequence sidewise (amino acids 55–60) (9, 10). Moreover, pro-CCK was measured with an immunoassay based on antibody 3208, which was raised against the glycine-extended forms of CCK, i.e. pro-CCK(75–82) (11). Prior to measurement with antibody 3208, the extracts were incubated in two separate enzymatic steps with trypsin and carboxypeptidase B to free the C-terminal epitope. Carboxyamidated bioactive CCK peptides were measured using antiserum 92128, which requires amidated and sulfated CCK and which does not cross-react with the homologous gastrin peptides (12).
Gel Chromatography
For evaluation of the pro-CCK-derived peptides in tissue extracts, we applied 1–2-ml tissue extract to a 1000 × 10-mm glass column packed with Sephadex G-50 Superfine (GE Healthcare). A 0.05 m barbital buffer containing 0.1% bovine albumin was used as eluent at a flow rate of 4 ml/h (4 °C). The buffer was supplemented with 0.02 m NaCl to minimize protein binding. Void and total volumes were determined by eluting 125I-albumin and 22Na, respectively.
Pro-CCK Purification from Porcine Heart
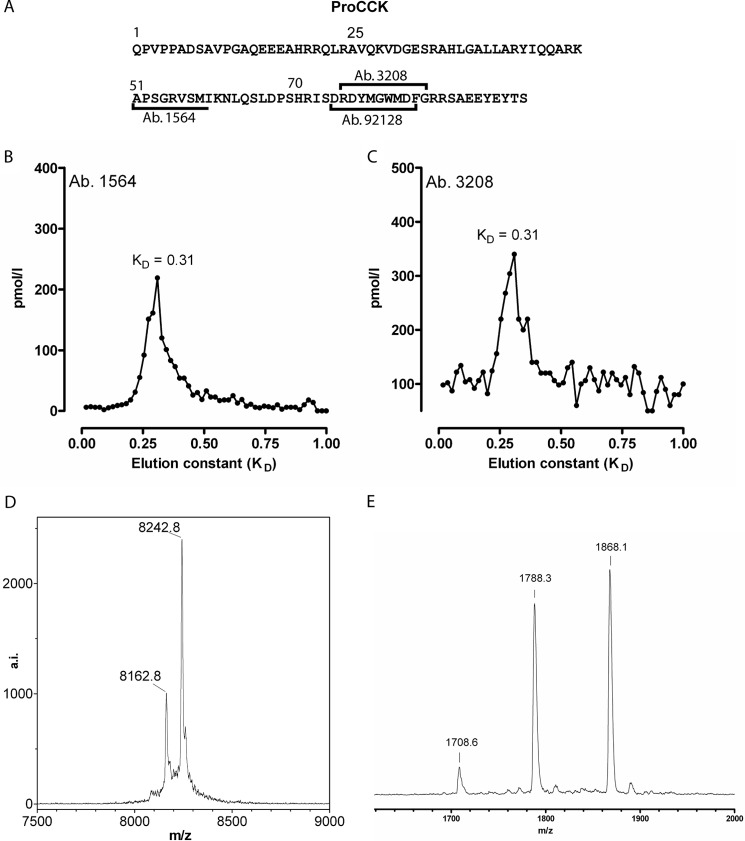
Porcine hearts were obtained from a local abattoir. One-hundred hearts were rapidly divided into atrial and ventricular samples and frozen on dry ice. The atrial samples were pooled and extracted with 0.5 m acetic acid. The extract was purified in six HPLC steps monitored by the antibody 1564 immunoassay: step 1, preparative C4 column (22 × 250 mm); steps 2 and 3, C4 column (4.6 × 250 mm); step 4, phenyl column (4.6 × 200 mm); step 5, C4 column (4.6 × 250 mm); and step 6, C8 column (4.6 × 250 mm). For steps 1–4 and 6, gradients of acetonitrile containing 0.1% TFA were used, whereas for step 5, 10 mm ammonium acetate was used. Mass spectroscopy was performed with a Bruker Autoflex II system in linear mode. The spectrum shown in Fig. 2E was obtained in linear negative mode, i.e. conditions that best preserve tyrosyl sulfation.
FIGURE 2.
A, primary sequence of porcine pro-CCK with the epitopes used for measurement indicated. B and C, gel chromatography elution profiles of pro-CCK using two different antibodies directed at different epitopes within the pro-CCK structure. D, molecular mass of the identified pro-CCK. E, double sulfation of the C-terminal pro-CCK fragment.
Isoprenaline Infusion
To test whether cardiac CCK expression is acutely affected by adrenaline, we stimulated rats and anesthetized pigs with isoprenaline. Isoprenaline (25 mg/kg) was first administered intraperitoneally to male Sprague-Dawley rats (∼250 g, n = 5). In parallel, control rats were injected with isotonic saline. The rats were then left for 5 h and killed by decapitation. The experimental timespan and isoprenaline dose were chosen from previous reports on isoproterenol bolus infusion in rats (13). After decapitation, cardiac and duodenal samples were obtained and snap-frozen in liquid nitrogen. The porcine experiments were performed on Danish mixed-breed pigs weighing 30–35 kg (n = 4 for each group). The pigs were anesthetized with midazolam (5 mg/10 kg) and a mixture of xylazine, ketamine, and methadone. Anesthesia was maintained with propofol (480 mg/h) and fentanyl (150 μg/h). A transducer was inserted into the internal carotid artery to register pulse and blood pressure. Heart rate was monitored by continuous electrocardiogram. Isoprenaline (5 mg/ml) was mixed in a 5% glucose solution and administered by an infusion pump into a peripheral vein. The infused dose was continuously titrated to a heart rate of 172 ± 6 beats/min. The pigs were killed 2 h after initiation of infusion with pentobarbital, and transmural cardiac tissue samples (∼1 g) were obtained, immediately frozen, and stored at −80 °C.
Newborn Piglets
Hearts from newborn piglets (n = 10) were collected at term (14). Transmural samples were collected from each chamber and stored at −80 °C for later pro-CCK and pro-ANP measurement in the regional samples.
Myoblast Cell Line
The rat cell line H9c2 was derived from embryonic cardiac myoblasts. The cell line was purchased from the European Collection of Cell Cultures and cultured according to standard protocols (15). Cells were washed, split into wells (1 × 106 cells/well), and stimulated with medium containing 0.1 or 0.01 μm isoprenaline. The cells were collected 4 h after stimulation, and BNP and CCK transcriptional expression was quantitated by RT-PCR.
Myocardial Hypoxia
Myocardial hypoxia activates the natriuretic peptide genes via the transcription factor hypoxia-inducible factor 1α pathway (16). We have reported previously that acute myocardial hypoxia increases the BNP mRNA contents in porcine heart muscle (8). Using this model, we tested the acute effects of severe myocardial hypoxia on cardiac CCK mRNA and peptide tissue contents. In brief, 14 pigs were anesthetized with a mixture of zolazepam, tiletamine, xylazine, ketamine, and methadone. The pigs were intubated and ventilated with 1 liter of oxygen/min and 2 liters of atmospheric air/min. The anesthesia was maintained with isoflurane (3%) and intravenous Haldid (400 μg/h). Amiodarone (300 mg) was administered intravenously before sternotomy to minimize later arrhythmic complications. After sternotomy, the left internal mammary artery was mobilized and anastomosed to the left ascending interventricular vein. Ligation bands were placed without closing around the left anterior descending interventricular artery. The left anterior descending artery was closed, and blood flow from the anastomosed left internal mammary artery to the interventricular vein was established. In this way, a limited arterial blood supply to the anterior ventricular wall was ensured. The intramyocardial oxygen tension was continuously monitored with a Revoxode pO2 probe (Licox CMP Instruments). The central venous pressure and heart rate were also monitored continuously, and dobutamine was administered when necessary to maintain normal hemodynamics. Four pigs developed ventricular fibrillation and were excluded from the study. Control pigs (n = 4) underwent the same procedures, except that the anastomosis between the mammary artery and the interventricular vein was omitted, and the left anterior descending artery was not closed. The pigs were killed with pentobarbital 2.2 ± 0.2 h after induction of myocardial hypoxia. The experimental animal protocol was approved by the local ethics committee for animal research. Transmural biopsies (0.5–1.0 g) were collected immediately after death from the normoxic inferior left ventricular wall, from a hypoxic region adjacent to the pO2 probe in the anterior left ventricular wall, and from the appendage of the left atrium. The biopsies were immediately frozen in liquid nitrogen and stored at −80 °C. Blood was collected before induction of myocardial hypoxia and 2 h after in Na2-EDTA (1.5 mg/ml)-containing tubes, and the plasma was stored at −80 °C.
Heart Failure Patients
The study included 186 outpatients followed at a specialized heart failure clinic. The original design of the study has been described in detail previously (17). Patients were referred with known or suspected systolic heart failure. If systolic heart failure was confirmed, the patients were offered admission to the clinic. Systolic heart failure was defined as a left ventricular ejection fraction of ≤45% by two-dimensional echocardiography. A two-dimensional Doppler echocardiogram was obtained, and data were entered into a designated database. Blood samples were taken under fasting conditions. Plasma N-terminal pro-BNP, CCK, and pro-CCK were analyzed. Patients were followed up for a median of 2.6 years. All patients were followed regarding mortality, which was confirmed using the Danish Civil Register System. The study and obtained information were approved by the central ethics committee, and all patients provided written informed consent. Baseline characteristics were analyzed using Student's t test and the χ2 test as appropriate. Survival analysis was carried out using Kaplan-Meier statistics (with multivariable survival models) using Cox regression analyses. For Kaplan-Meier analyses, patients were grouped according to the median of either the CCK or pro-CCK plasma concentration, and in the following Cox regression analysis, pro-CCK concentrations were entered after logarithmic conversion (ln pro-CCK) due to skewness of the data.
RESULTS
Cardiac Tissue Contains Pro-CCK
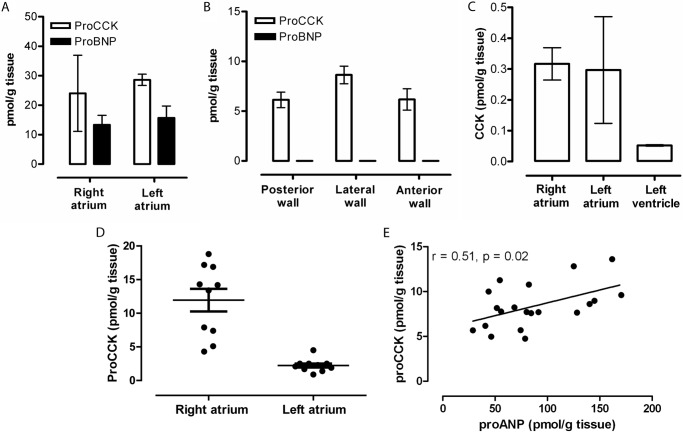
The content of pro-CCK in tissue extracts from the heart chambers of healthy pigs was measured using a species-specific immunoassay for porcine pro-CCK and its products. The right and left atrial chambers, e.g. the appendages, contained pro-CCK in concentrations superseding those of the pro-BNP (Fig. 1A), which is known to be stored in the atrial myocyte granules (6). Ventricular tissue extracts also contained significant amounts of pro-CCK, where the current dogma dictates the absence of natriuretic peptides, as also confirmed in the present examination (Fig. 1B). The known hormonal CCK peptides in the brain and intestine, i.e. α-amidated and tyrosyl-sulfated CCK-58, CCK-33, CCK-22, and CCK-8, were detected only in negligible trace amounts (Fig. 1C), indicating that only little pro-CCK in the normal heart is processed to α-amidated CCK peptides, which regulate gallbladder emptying, pancreatic enzyme secretion, and pancreatic growth and which act as potent neurotransmitters in the central and peripheral nervous systems (7). We then examined pro-CCK expression in cardiac chambers from newborn piglets. The cardiac pro-CCK contents were predominantly localized in the right atria compared with the left atria (Fig. 1D). In addition, the ventricular concentrations of pro-CCK correlated with the pro-ANP concentrations (r = 0.51, p = 0.02) (Fig. 1E).
FIGURE 1.
Pro-CCK protein contents in neutral and acidic extracts of normal pig heart. A, atrial pro-CCK and pro-BNP contents. B, ventricular concentrations. C, trace amounts of amidated CCK. D and E, atrial pro-CCK contents compared with pro-ANP in neonatal pig hearts, where the hemodynamic strain on the right atrium dominates. Pro-CCK was measured with an immunoassay specific for porcine pro-CCK(51–59), CCK was measured with an assay specific for amidated CCK (antibody 92128), and pro-ANP and pro-BNP were measured by porcine-specific in-house analyses.
The Cardiac Processing of Pro-CCK Is Unique
To identify the structure of pro-CCK in the porcine heart, atrial acidic and neutral extracts were subjected to gel chromatography. Using two sequence-specific immunoassays for measurement of pro-CCK or fragments (Fig. 2A), we found a major peak corresponding to a large fragment (Fig. 2, B and C). To identify the molecular structure, we purified the fragment, and the substance was identified by mass spectrometry as having a total mass of 8242 Da, which corresponds to fragment 25–94 of the 94-amino acid pro-CCK with O-sulfation at three tyrosyl residues, Tyr-76, Tyr-90, and Tyr-92 (Fig. 2, A and D). The purified peptide was then subjected to digestion with endoproteinase Asp-N, and mass spectrometry of the HPLC-purified fragments confirmed the identity. One fragment corresponded to the C-terminal flanking peptide 82–94 with two O-sulfated tyrosyl residues (Tyr-90 and Tyr-92). Under the mass spectrometry conditions used, the sulfated residues are labile, giving rise to three species containing zero, one, and two sulfated residues, respectively (Fig. 2E).
Myocyte Expression of the CCK Gene Is Stimulated by Isoprenaline
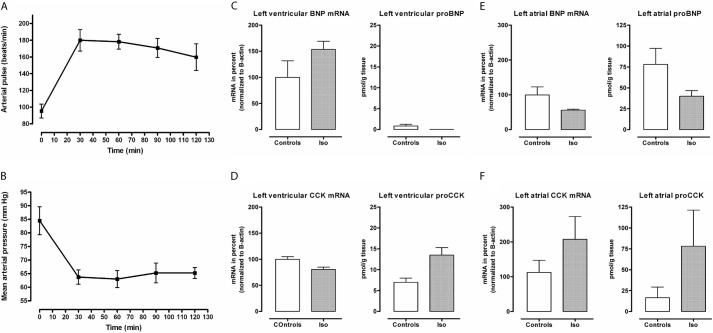
The CCK mRNA contents were examined in a rat myoblast cell line (H9c2). After stimulation with isoprenaline for 4 h, the CCK mRNA contents increased by >3-fold from baseline (p = 0.09), whereas the BNP mRNA contents increased by 2-fold (p = 0.41) (Fig. 3A). Taken together, the in vitro data suggest a transcriptional regulation mediated by isoprenaline, which fits with the known regulation of the CCK promoter. To further examine the regulation of cardiac CCK gene expression, rats were injected intraperitoneally with isoprenaline. The duodenal CCK mRNA did not change after 5 h (Fig. 3B). In contrast, the cardiac CCK mRNA increased by ∼5-fold (Fig. 3C), which supports the in vitro data from the myoblast cell line. Notably, cardiac myocytes express receptors for isoprenaline, whereas CCK-expressing intestinal cells do not. To extend the rat findings, anesthetized pigs were continuously infused with isoprenaline titrated to a heart rate of ∼175 beats/min with a concomitant decrease in mean arterial pressure (Fig. 4, A and B). Left ventricular BNP mRNA increased in vivo (Fig. 4C), whereas ventricular CCK mRNA did not (Fig. 4D). However, the tissue concentrations of pro-CCK in ventricular samples increased by 2-fold. The atrial response to isoprenaline infusion showed a paradoxical down-regulation of both BNP mRNA and pro-BNP contents (Fig. 4E), whereas the atrial CCK mRNA and pro-CCK concentrations increased markedly (Fig. 4F). Taken together, the data show that isoprenaline, an adrenergic agonist, rapidly stimulates cardiac CCK expression.
FIGURE 3.
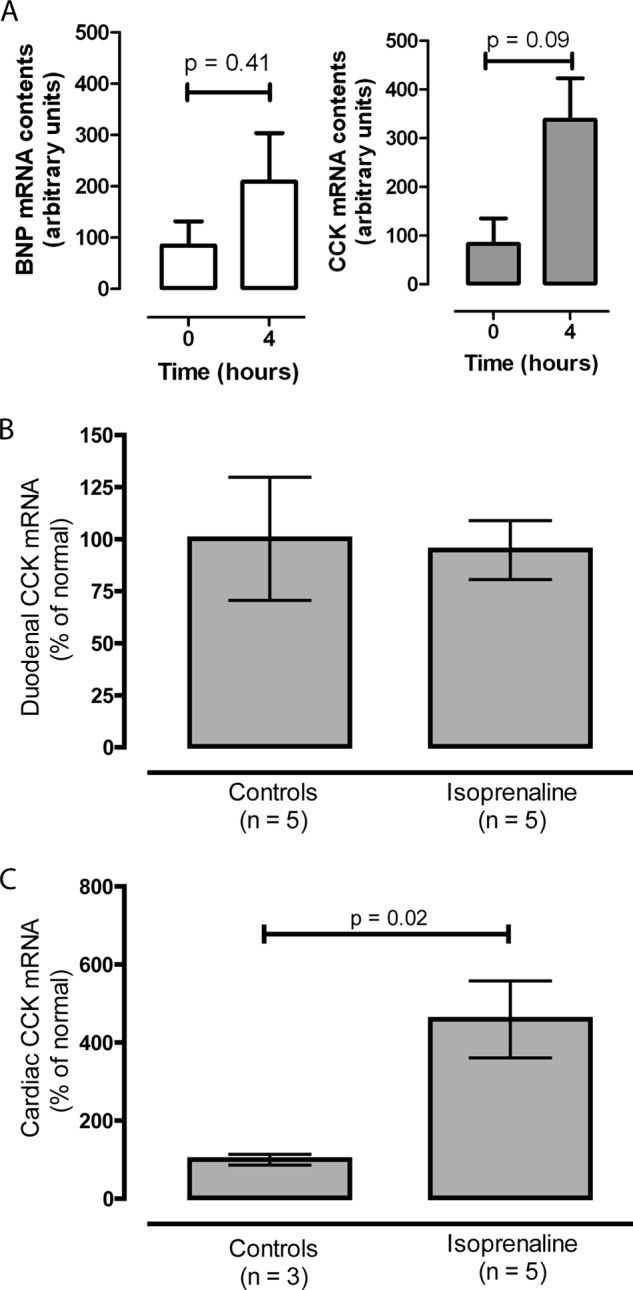
A, effect (4 h) of isoprenaline on cardiac (H9c2) BNP and CCK mRNA expression in culture. B and C, effect of in vivo isoprenaline stimulation on rat duodenal and cardiac CCK mRNA contents, respectively (n = 5).
FIGURE 4.
A and B, hemodynamic effects of isoprenaline (Iso) infusion in anesthetized pigs (n = 4). C and D, isoprenaline effects on left ventricular BNP and CCK mRNA and protein contents compared with control animals (n = 4). E and F, concomitant BNP and CCK mRNA and protein contents in left atrial samples compared with controls.
Myocardial Hypoxia Does Not Affect CCK Gene Expression
Myocardial hypoxia is an important stimulus for regulating cardiac gene expression. We previously reported that BNP and VEGF mRNA contents increase in hypoxic compared with normoxic ventricular samples (8). In samples from hypoxic pig hearts, however, the CCK mRNA contents did not change over a period of 2 h (data not shown). These in vivo data therefore suggest that hypoxia per se does not affect cardiac CCK gene transcription.
Plasma Pro-CCK Is a Prognostic Marker in Patients with Stable Heart Failure
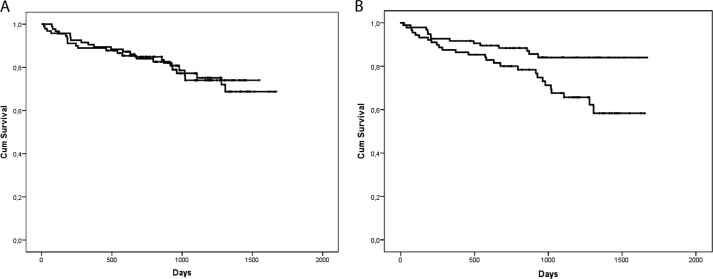
To investigate whether the N-terminally truncated pro-CCK plays a role in human pathophysiology, we examined patients with stable heart failure. Baseline clinical characteristics according to the pro-CCK concentrations are listed in Table 1. Ischemic heart disease was the most prevalent cause of heart failure (57%). The median CCK and pro-CCK concentrations in plasma were 1.2 and 17.8 pmol/liters, respectively. Plasma pro-CCK was only weakly associated with pro-BNP (r = 0.27, p < 0.001). During a median follow-up period of 2.6 years, 42 (22.6%) of the patients died. When analyzed as a continuous variable, plasma CCK concentrations did not predict overall mortality (Fig. 5A). However, the concentrations of pro-CCK above the median were associated with a significantly increased risk of mortality (hazard ratio for death of patients with pro-CCK at >17.8 pmol/liter = 2.2 (1.2–4.2), p = 0.016) (Fig. 5B). Adjustment for clinically relevant variables in chronic heart failure (age, gender, left ventricular ejection fraction, New York Heart Association class, and creatinine clearance) did not modify this association (hazard ratio per ln pro-CCK = 3.8 (1.1–12.9), p = 0.034).
TABLE 1.
Patient characteristics
BMI, body mass index; DM, diabetes mellitus; IFG, impaired fasting glucose; LVEF, left ventricular ejection fraction; HbA1c, hemoglobin A1c.
| Pro-CCK |
p value | |||
|---|---|---|---|---|
| Total (n = 186) | <17.75 pm (n = 93) | ≥17.75 pm (n = 93) | ||
| Demographics | ||||
| Age (years)a | 69.3 ± 10.4 | 66.2 ± 10.5 | 72.3 ± 9.4 | 0.122 |
| Females/males (n) | 54/132 | 22/71 | 32/61 | 0.106 |
| BMI (kg/m2)a | 27.1 ± 5.0 | 28.3 ± 5.1 | 25.8 ± 4.6 | 0.495 |
| Cardiovascular disease (n) | ||||
| Nonischemic heart disease | 78 | 41 | 37 | 0.506 |
| Ischemic heart disease | 103 | 49 | 54 | |
| Missing | 5 | 3 | 2 | |
| Diabetic status (n) | ||||
| Nondiabetic | 119 | 55 | 64 | 0.532 |
| DM history | 38 | 21 | 17 | |
| DM newly diagnosed | 8 | 6 | 2 | |
| IFG | 14 | 7 | 7 | |
| Not classified | 7 | 4 | 3 | |
| Heart failure measurementsa | ||||
| LVEF (%) | 30.3 ± 8.3 | 30.0 ± 8.2 | 30.5 ± 8.4 | 0.600 |
| Heart rate (beats/min) | 74.6 ± 15.2 | 75.8 ± 13.8 | 73.3 ± 16.5 | 0.022 |
| Diastolic blood pressure (mm Hg) | 78.5 ± 14.4 | 80.5 ± 14.4 | 76.4 ± 14.5 | 0.526 |
| Systolic blood pressure (mm Hg) | 138.6 ± 25.2 | 138.3 ± 23.3 | 138.9 ± 27.7 | 0.272 |
| 6-min walk test (m) | 367.9 ± 143.9 | 382.8 ± 135.7 | 346.3 ± 83.1 | 0.392 |
| Metabolic status | ||||
| Fasting insulin (pmol/liter)b | 82.8 | 84.4 | 81.4 | 0.636 |
| HbA1c (%)a | 6.2 ± 1.3 | 6.28 ± 1.4 | 6.17 ± 1.1 | 0.116 |
| Cholesterol (mm)a | 5.2 ± 1.3 | 5.1 ± 1.1 | 5.2 ± 1.5 | 0.074 |
| Creatinine (μm)a | 100 ± 32.1 | 95.2 ± 28.0 | 105.3 ± 36.0 | 0.333 |
| Fasting blood glucose (mm)a | 5.7 ± 2.3 | 5.8 ± 2.5 | 5.6 ± 2.1 | 0.238 |
a Values are expressed as mean ± S.D.
b Values are expressed as the median.
FIGURE 5.
Kaplan-Meier curves for 186 elderly patients with stable heart failure. The patients were divided into higher and lower groups based on the median concentrations of plasma CCK (A) or plasma pro-CCK (B). Cum, cumulative.
DISCUSSION
CCK is a classic intestinal hormone and a transmitter in the central and peripheral nervous systems (18). Intestinal CCK is synthesized in duodenal and jejunal I-cells in α-amidated and O-sulfated forms (CCK-58, CCK-33, CCK-22, and CCK-8) and released to blood in response to food ingestion. Circulating CCK peptides stimulate smooth muscle contraction in the gallbladder, pancreatic enzyme secretion, and long-term growth of the pancreas (19). Neural CCK (mainly sulfated CCK-8) acts as a potent neurotransmitter in the central and peripheral nervous systems. The CCK gene is also expressed in small amounts in a few other organs. For instance, CCK peptides are expressed in pituitary corticotrophs, thyroid C-cells, the adrenal medulla, and neuroendocrine cells in the bronchial mucosa (20–23). In pituitary corticotrophs, most of the stored peptides are processing intermediates, where a C-terminal dibasic cleavage and subsequent carboxyamidation of glycine do not occur (24). This pituitary pattern therefore resembles the pattern of cardiac pro-CCK, albeit with a marked difference in organ size, tissue concentration, and hence total CCK gene expression at the protein/peptide level.
The identification of the triple-sulfated and truncated pro-CCK fragment 25–94 leaves unexplained the complementary N-terminal fragment 1–24. We have developed an immunoassay specific for the N terminus of this fragment, but so far, it has not been possible to detect immunoreactivity corresponding to this peptide in cardiac extracts. Thus, the N-terminal fragment is either degraded or heavily modified during the post-translational processing to an extent to which epitopes deduced from CCK cDNA are not available.
Our identification of cardiac-specific, truncated, and triple-sulfated pro-CCK enlarges our understanding of the heart as an endocrine organ. A previous report showed that CCK mRNA is expressed in developing mouse hearts (5). Surprisingly, the authors of that study commented only on intestinal and cerebral (but not cardiac) CCK gene expression, and they did not examine the latter further. Cardiac CCK expression is not regulated by hypoxia, but is markedly regulated by isoprenaline, which suggests that the expression represents adaption to increased sympathetic activity. In addition, our findings add to the information about the biosynthetic processing apparatus in cardiomyocytes, where only the endoprotease corin has been directly implicated in the biosynthesis of hormonal precursors (25).
In addition to endocrine I-cells in the small intestinal mucosa and neurons in the central and peripheral nervous systems, this study shows that pro-CCK is also synthesized in abundant amounts in cardiac myocytes. Apparently, there is no interference between the pro-CCK products from the different cell types, partly because of the blood-brain barrier that prohibits pro-CCK fragments in the peripheral circulation from interfering with the synaptic transmission in cerebral neurons and partly because of the cell-specific processing pattern in the heart. Thus, the known CCK-A and CCK-B receptors cannot bind the cardiac pro-CCK product. Future work should elucidate the exact mechanisms and physiological targets of cardiac pro-CCK expression. Nevertheless, the clinical data presented here are highly encouraging. Notably, clinical studies on fasting heart failure patients are rare. This might at least partially explain the fact that little is known about intestinal endocrine regulation of the heart in patients with heart failure. The basal CCK concentrations in our patients confirmed that the patients were indeed fasting, whereas increased pro-CCK concentrations reflected the risk of mortality. More studies are needed to determine whether the N-terminally truncated pro-CCK adds significant information to the existing battery of biomarkers in heart failure.
Acknowledgments
We are grateful to Julie Smith for providing tissue from neonatal piglets. The skillful technical assistance of Dijana Terzic, Lone Olsen, Allan Kastrup, and Alice Lieth is gratefully acknowledged.
This work was supported by grants from the Novo Nordisk Foundation, the Lundbeck Foundation, and The Danish Biotechnology Center for Cellular Communication.
- CCK
- cholecystokinin
- ANP
- atrial natriuretic peptide
- BNP
- brain natriuretic peptide.
REFERENCES
- 1. Goetze J. P. (2012) B-type natriuretic peptide: from posttranslational processing to clinical measurement. Clin. Chem. 58, 83–91 [DOI] [PubMed] [Google Scholar]
- 2. Deftos L. J., Burton D. W., Brandt D. W. (1993) Parathyroid hormone-like protein is a secretory product of atrial myocytes. J. Clin. Invest. 92, 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ito H., Hirata Y., Adachi S., Tanaka M., Tsujino M., Koike A., Nogami A., Murumo F., Hiroe M. (1993) Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J. Clin. Invest. 92, 398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dschietzig T., Richter C., Bartsch C., Laule M., Armbruster F. P., Baumann G., Stangl K. (2001) The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 15, 2187–2195 [DOI] [PubMed] [Google Scholar]
- 5. Lay J. M., Gillespie P. J., Samuelson L. C. (1999) Murine prenatal expression of cholecystokinin in neural crest, enteric neurons, and enteroendocrine cells. Dev. Dyn. 216, 190–200 [DOI] [PubMed] [Google Scholar]
- 6. Christoffersen C., Goetze J. P., Bartels E. D., Larsen M. O., Ribel U., Rehfeld J. F., Rolin B., Nielsen L. B. (2002) Chamber-dependent expression of brain natriuretic peptide and its mRNA in normal and diabetic pig heart. Hypertension 40, 54–60 [DOI] [PubMed] [Google Scholar]
- 7. Christoffersen C., Bollano E., Lindegaard M. L. S., Bartels E. D., Goetze J. P., Andersen C. B., Nielsen L. B. (2003) Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144, 3483–3490 [DOI] [PubMed] [Google Scholar]
- 8. Goetze J. P., Gore A., Møller C. H., Steinbrüchel D. A., Rehfeld J. F., Nielsen L. B. (2004) Acute myocardial hypoxia increases BNP gene expression. FASEB J. 18, 1928–1930 [DOI] [PubMed] [Google Scholar]
- 9. Rehfeld J. F. (1978) Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassay for porcine triacontatriapeptide cholecystokinin. J. Biol. Chem. 253, 4016–4021 [PubMed] [Google Scholar]
- 10. Cantor P., Rehfeld J. F. (1989) Cholecystokinin in pig plasma: release of components devoid of a bioactive COOH-terminus. Am. J. Physiol. 256, G53–G61 [DOI] [PubMed] [Google Scholar]
- 11. Hilsted L., Rehfeld J. F. (1986) Measurement of precursors for α-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal. Biochem. 152, 119–126 [DOI] [PubMed] [Google Scholar]
- 12. Rehfeld J. F. (1998) Accurate measurement of cholecystokinin in plasma. Clin. Chem. 44, 991–1001 [PubMed] [Google Scholar]
- 13. Zimmer H. G. (1997) Catecholamine-induced cardiac hypertrophy: significance of proto-oncogene expression. J. Mol. Med. 75, 849–859 [DOI] [PubMed] [Google Scholar]
- 14. Smith J., Christoffersen C., Nørgaard L. M., Olsen L. H., Vejlstrup N. G., Andersen C. B., Goetze J. P. (2013) Cardiac natriuretic peptide gene expression and plasma concentrations during the first 72 hours of life in piglets. Endocrinology 154, 1864–1872 [DOI] [PubMed] [Google Scholar]
- 15. Yasuoka C., Ihara Y., Ikeda S., Miyahara Y., Kondo T., Kohno S. (2004) Antiapoptotic activity of Akt is down-regulated by Ca2+ in myocardiac H9c2 cells. J. Biol. Chem. 279, 51182–51192 [DOI] [PubMed] [Google Scholar]
- 16. Weidemann A., Klanke B., Wagner M., Volk T., Willam C., Wiesener M. S., Eckardt K. U., Warnecke C. (2008) Hypoxia, via stabilization of the hypoxia-inducible factor HIF-1α, is a direct and sufficient stimulus for brain-type natriuretic peptide induction. Biochem. J. 409, 233–242 [DOI] [PubMed] [Google Scholar]
- 17. Kistorp C., Faber J., Galatius S., Gustafsson F., Frystyk J., Flyvbjerg A., Hildebrandt P. (2005) Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 112, 1756–1762 [DOI] [PubMed] [Google Scholar]
- 18. Larsson L. I., Rehfeld J. F. (1979) Localization and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res. 165, 201–218 [DOI] [PubMed] [Google Scholar]
- 19. Rehfeld J. F., Sun G., Christensen T., Hillingsø J. G. (2001) The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J. Clin. Endocrinol. Metab. 86, 251–258 [DOI] [PubMed] [Google Scholar]
- 20. Rehfeld J. F., Friis-Hansen L., Goetze J. P., Hansen T. V. O. (2007) The biology of cholecystokinin and gastrin peptides. Curr. Top. Med. Chem. 7, 1154–1165 [DOI] [PubMed] [Google Scholar]
- 21. Rehfeld J. F. (1987) Preprocholecystokinin processing in the normal human anterior pituitary. Proc. Natl. Acad. Sci. U.S.A. 84, 3019–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bardram L., Hilsted L., Rehfeld J. F. (1989) Cholecystokinin, gastrin and their precursors in pheochromocytomas. Acta Endocrinol. 120, 479–484 [DOI] [PubMed] [Google Scholar]
- 23. Geijer T., Folkesson R., Rehfeld J. F., Monstein H. J. (1990) Expression of the cholecystokinin gene in a human (small-cell) lung carcinoma cell line. FEBS Lett. 270, 30–32 [DOI] [PubMed] [Google Scholar]
- 24. Rehfeld J. F. (1986) Accumulation of nonamidated progastrin and procholecystokinin products in porcine pituitary corticotrophs: Evidence of posttranslational control of cell differentiation. J. Biol. Chem. 261, 5841–5847 [PubMed] [Google Scholar]
- 25. Wu Q., Xu-Cai Y. O., Chen S., Wang W. (2009) Corin: new insights into the natriuretic peptide system. Kidney Int. 75, 142–146 [DOI] [PMC free article] [PubMed] [Google Scholar]