Background: The mechanism by which centriole dimensions are regulated is not understood.
Results: The absence of centrobin leads to degradation of centrosomal protein 4.1-associated-protein (CPAP). High centrobin levels cause stabilization of CPAP and abnormal centrioles.
Conclusion: Centrobin plays a role in the stability and centriole elongation function of CPAP and limits the centriole length.
Significance: Identifying the regulatory mechanisms is crucial for understanding centriole biogenesis.
Keywords: Centriole, Centrosome, Confocal Microscopy, Proteasome, Ubiquitin
Abstract
Microtubule-based centrioles in the centrosome mediate accurate bipolar cell division, spindle orientation, and primary cilia formation. Cellular checkpoints ensure that the centrioles duplicate only once in every cell cycle and achieve precise dimensions, dysregulation of which results in genetic instability and neuro- and ciliopathies. The normal cellular level of centrosomal protein 4.1-associated protein (CPAP), achieved by its degradation at mitosis, is considered as one of the major mechanisms that limits centriole growth at a predetermined length. Here we show that CPAP levels and centriole elongation are regulated by centrobin. Exogenous expression of centrobin causes abnormal elongation of centrioles due to massive accumulation of CPAP in the cell. Conversely, CPAP was undetectable in centrobin-depleted cells, suggesting that it undergoes degradation in the absence of centrobin. Only the reintroduction of full-length centrobin, but not its mutant form that lacks the CPAP binding site, could restore cellular CPAP levels in centrobin-depleted cells, indicating that persistence of CPAP requires its interaction with centrobin. Interestingly, inhibition of the proteasome in centrobin-depleted cells restored the cellular and centriolar CPAP expression, suggesting its ubiquitination and proteasome-mediated degradation when centrobin is absent. Intriguingly, however, centrobin-overexpressing cells also showed proteasome-independent accumulation of ubiquitinated CPAP and abnormal, ubiquitin-positive, elongated centrioles. Overall, our results show that centrobin interacts with ubiquitinated CPAP and prevents its degradation for normal centriole elongation function. Therefore, it appears that loss of centrobin expression destabilizes CPAP and triggers its degradation to restrict the centriole length during biogenesis.
Introduction
Centrosomes are the major microtubule-organizing centers of the cell and are responsible for maintaining cell shape, motility, and polarity. They also have pivotal roles in organizing an accurate bipolar spindle during normal and asymmetric cell division, spindle orientation and positioning, biogenesis of the cilia and flagella, and immune response (1–6).
Every centrosome has two centrioles, which serve as the functional hub. The two centrioles, mother and daughter, that differ in age and morphology. They are duplicated only once in every cell cycle to generate two new centrioles that are then inherited by each daughter cell. Normal dimensions and morphology are achieved by the centrioles only after passage through one-and-a-half cell cycles. The mother centriole is more mature and longer than the daughter centriole and is identified by the presence of sub-distal and distal appendages (2, 7–12). Defects in centriolar duplication cause abnormal size, morphology, and number of centrioles, and these defects have been linked to genetic instability in tumorigenesis, neuropathies, and ciliopathies (13–20). In fact, almost all the mutations that have been identified in patients with microcephaly or Seckel syndrome are in the genes that encode for centriole proteins (21). Hence, understanding how the centriole biogenesis is regulated and identification of the players involved may contribute to the development of therapies for these clinical conditions.
In humans centriole assembly initiates at the S phase of cell cycle, and a new procentriole (daughter centriole) is generated from the proximal end of mother centriole (22–24). The duplication process involves three stages: initiation, elongation, and maturation. Initiation process includes the recruitment of essential centriolar proteins such as CEP152, PLK4, hSAS-6, STIL, CPAP2, CEP135, CP110, γ-tubulin, and centrobin to the proximal end of preexisting mother centrioles (25–36). Once this pro-centriolar protein complex is formed, centrioles are elongated to achieve a maximum length of ∼500 nm and width of ∼200 nm by the addition of α/β-tubulin heterodimers onto it (37). Exactly how these restricted dimensions are achieved in comparison with the dynamic and highly unstable cytoskeletal microtubules, which can vary in length, is not understood (38).
Recently, CPAP (also referred to as CENPJ) overexpression was reported to cause the centriolar microtubules to elongate beyond the predetermined length of 0.5 μm and led to accumulation of multipolar spindles (35, 39, 40). Depletion of cellular CPAP also resulted in loss of centrosome integrity and led to a higher number of cells with mono- and multipolar (35) as well as abnormal, misoriented spindles (4). Additionally, CPAP mutations have been linked to microcephaly and Seckel syndrome (41, 42). Disruption of CPAP gene expression in mice resulted in symptoms of microcephaly and Seckel syndrome as well as abnormal centrosome/centriole numbers and multi- and monopolar spindles (17). Hence, identifying the cellular mechanisms that regulate CPAP expression is critical and may also uncover the role of centriole duplication during brain development.
CPAP is ubiquitinated and degraded by the proteasome during mitosis (39, 43). Interestingly, the elongation phase of centriole duplication, which determines centriole length, is completed by the time cells reach mitosis (44). Although CEP120 and SPICE1 are known to promote centriole elongation and interact with CPAP (45, 46), they act downstream of CPAP in the centriole duplication process. Therefore, identifying the upstream targets and underlying signals that govern cellular CPAP levels will shed light on how the centriole length is restricted. In this regard, we recently reported that centrobin interacts with tubulin and CPAP, and these interactions are critical during the elongation stage of centriole duplication (47, 48).
Centrobin has a crucial role in centriole assembly, bipolar mitotic spindle assembly, microtubule polymerization, and asymmetric cell division (5, 36, 49–52). Although it is considered as a daughter centriole protein, centrobin was detected on mother centrioles of G2/M-arrested cells (47), suggesting that its levels are regulated on the centrioles at different stages of cell cycle. Our study, which reported that centrobin interacts with α-tubulin and is required for the stability and elongation of centrioles (47), demonstrated its pivotal structural and functional roles in centriole duplication. Most importantly, our recent report also shows that direct interaction between centrobin and CPAP is responsible for the recruitment and maintenance of CPAP on the centrioles, and prevention of this interaction inhibits the CPAP-mediated elongation of centrioles. However, the molecular mechanism by which centrobin contributes to the CPAP-mediated elongation of centrioles remains unknown.
In this study, for the first time we uncover a direct role for centrobin in limiting centriole length and the molecular mechanism associated with it. We show that centrobin expression levels regulate the cellular and centriolar levels of CPAP. Higher centrobin expression inhibits CPAP degradation and translates into increased ubiquitinated CPAP at the cellular and centriolar levels leading to abnormally elongated centrioles. On the other hand, reduced centrobin expression results in the destabilization and degradation of CPAP, an essential centriole elongation protein. Current observations in the context of our previous studies (47, 48) show that centrobin is required for maintaining CPAP levels and centriole elongation during normal cell cycle. However, once the right length of centrioles is achieved at mitosis (39), an unknown event, perhaps loss of centrobin, triggers CPAP degradation to limit centriole growth during biogenesis.
EXPERIMENTAL PROCEDURES
Cell Lines, Transfection, and Media
293T, HeLa, and U2OS osteosarcoma cells were grown in DMEM medium (Cellgro) supplemented with 10% fetal bovine serum, sodium pyruvate, minimum essential amino acids, and antibiotic-antimycotic solutions. The HeLa GFP-centrin-stable cells were kindly provided by Dr. Alexey Khodjakov, Wadsworth Center, New York State Department of Health (53). Plasmid transfections were done using the calcium phosphate method in the 293T cell line. Trans-IT2020 (Mirus) reagent was used to transfect plasmids in the U2OS cell line. siRNAs were transfected using Oligofectamine reagent (Invitrogen).
Antibodies and Reagents
Anti-centrobin monoclonal antibody has been described previously (47). The monoclonal anti-GFP antibody, used for immunoprecipitation, was purchased from Invitrogen™. The polyclonal antibody for CPAP was purchased from Proteintech. Antibodies against HA, actin, and tubulin were obtained from Sigma, and the anti-ubiquitin and myc tag (9E10) antibodies were from Santa Cruz Biotechnology. CP110 antibody was from Bethyl Laboratories. RNAi-mediated depletion of centrobin was achieved using either direct transfection of pretro-Super-shcentrobin or synthetic siRNAs from Thermo Fisher Scientific. The sequence of the targeting region has been described before (36). The Alexa Fluor 488/568/647-linked secondary antibodies as well as the α-tubulin-Alexa Fluor 488 conjugate were purchased from InvitrogenTM. Hydroxyurea, nocodazole, and MG-132 were purchased from Sigma.
Plasmids
The expression vectors carrying cDNAs for myc-centrobin (full-length centrobin) and centrobin mutant spanning residues 365–903 that do not bind to CPAP have been used and described before (47, 48). The GFP-C1-CPAP and pcDNA4-myc-CPAP expression constructs were kind gifts from Dr. Tang, Institute of Biomedical Sciences, Taiwan. The HA-ubiquitin expressing construct was kindly provided by Dr. Beichu Guo, Medical University of South Carolina.
Cell Culture, Transfection, and Drug Treatment
To study the stability effect of centrobin on CPAP, 293T or U2OS cells were transfected in 24 wells for 72 h with low concentrations of myc-centrobin and control plasmids. For proteasome inhibition, cells were treated with 20 μm MG-132 (Sigma) for 14 h. Because the MG-132 stock solution was prepared in DMSO, an equivalent amount of DMSO solution was added to cells as control treatment.
Immunofluorescence
For visualization of centrioles, cells grown on coverslips were left on ice for half an hour to depolymerize the cytoskeletal microtubules followed by treatment with an extraction buffer (20 mm Hepes, pH 7.4, 50 mm NaCl, 3 mm MgCl2, 300 mm sucrose, and 0.5% Triton X-100) to enhance centriole staining and fixed with ice-cold methanol at −20 °C. The cells were then incubated with appropriate primary antibodies diluted in PBS containing 0.02% Tween 20 and then incubated with Alexa Fluor 488/568/647-linked secondary antibodies. Coverslips were placed on slides in mounting media containing antifade and DAPI (Invitrogen), and imaging was conducted at room temperature. Images were acquired using the Olympus FV10i that has an automated scanning confocal system and is equipped with a 60× water immersion objective 1.345 n.a. Most images presented are maximum projections of Z-stacks. For mitotic spindle assay, cells were directly fixed with ice-cold methanol and stained using DAPI and α-tubulin and centrin antibodies. Images were acquired using the 60× oil objective with 1.4 n.a. of the Zeiss Axiovert Imager microscope.
Immunoprecipitation
In pulldown experiments, cells transfected with the indicated plasmids were washed in ice-cold PBS and then lysed using immunoprecipitation lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% sodium deoxycholate, 1% Triton X-100, and 100 mm PMSF) for 40 min on ice. The DNA was sheared using a 21-gauge needle, after which lysates were spun at 14,000 rpm for 20 min. Lysates were precleared with a 50% slurry of protein A/G beads (Pierce) followed by incubation with anti-GFP antibody overnight at 4 °C and then with protein A/G beads for 1 h at 4 °C to precipitate the immune complexes. After washing six times using lysis buffer, the bound proteins were fractionated on an SDS-PAGE gel, and Western blotting was performed to detect the bound proteins. For immunoprecipitation, 1 μg of anti-GFP antibody was used per ml of lysate.
Statistics
All experiments were performed independently at least three times. Experiments that involved enumeration have histograms presented where 50 cells were counted per group. Calculation of p value was done using Student's t test. The asterisk denotes that the results are significant.
RESULTS
Overexpression of Centrobin Results in Abnormal, Long Centriole-like Structures
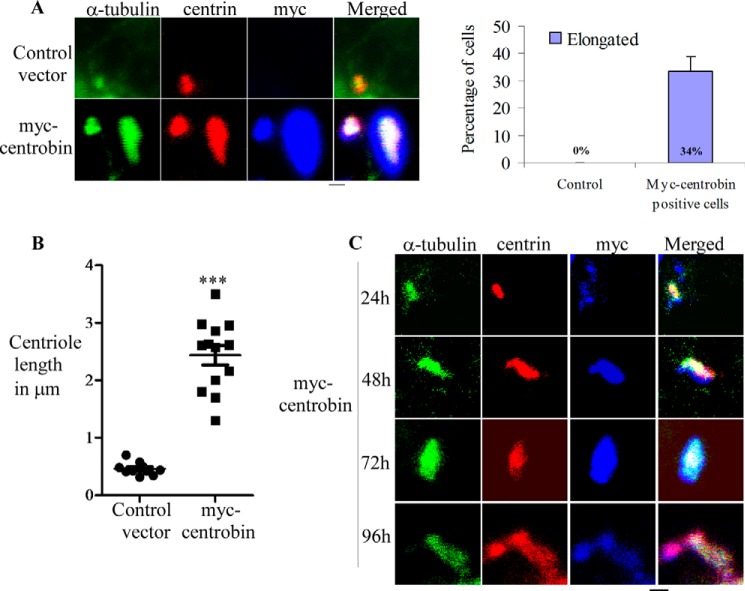
To understand the mechanism by which centrobin contributes to centriole elongation, U2OS cells were transfected with control or myc-tagged centrobin expression vector for 72 h, and the centriole length was determined in myc-positive cells. For better staining of the centrioles, cells were placed on ice to depolymerize the bulk of cytoskeletal microtubules and then extracted with a detergent-containing buffer as described under “Experimental Procedures.” Cells were then fixed with ice-cold methanol and stained using anti-α-tubulin, -myc, and -centrin antibodies for immunofluorescence microscopy. Confocal microscopy imaging revealed that in comparison with the centrioles of control cells, centrobin-overexpressing cells had abnormal, long centriolar structures (Fig. 1A, left panel). As shown previously by others (54, 55), tubulin and centrin staining along the length of these structures confirms that these are in fact microtubule-based, elongated centrioles.
FIGURE 1.
Overexpression of centrobin results in the abnormal elongation of centrioles. A, U2OS cells that were transfected with control or myc-centrobin expression vectors for 72 h were stained using the primary anti-myc, and -centrin antibodies followed by Alexa-568 and -647-linked secondary antibodies and α-tubulin-Alexa-488 direct conjugate antibody. The right panel depicts the percentage of myc-positive centrioles that showed abnormal elongation. Results represent three independent experiments with 50 cells examined/experiment. B, the centriole length was examined in control and myc-centrobin-expressing cells and has been plotted using the software Prism. p < 0.0001. C, same as A, except U2OS cells were stained at different time points after expression of myc-centrobin. Scale bar: 2 μm. Images were acquired using the Olympus FV10i confocal microscope and are maximum projections of Z-stacks.
Quantitation of elongated centrioles in myc-centrobin-positive cells from three independent experiments showed that 34% of the myc-positive centrioles were abnormally elongated beyond the predetermined 0.5 μm (Fig. 1A, right panel). At the 72-h time point, centriole length of myc-positive centrioles spanned between 1.6 and 3.4 μm, and the average length was found to be 2.8 μm as compared with control centrioles that spanned between 0.31 and 0.485 μm with an average length of 0.46 μm (Fig. 1B). A similar phenotype was observed in 293T cells upon overexpression of centrobin (data not shown), suggesting that the abnormal elongation of centrioles triggered by centrobin is not cell line-specific.
Next, we examined the timing of appearance of abnormal centrioles upon overexpression of centrobin. U2OS cells were transfected with myc-centrobin vector and imaged by confocal microscopy at 12, 24, 48, 72, and 96 h post-transfection. This time-course analysis of centrobin-overexpressing cells revealed that although the centriole size was not different from control cells at 12 h (data not shown) or 24 h post-transfection, the abnormal elongation was visible by 48 h, and the complexity of the microtubule-based structures increased profoundly at later time-points (Fig. 1C). As observed in Fig. 1C, centrioles were elongated to an average of 3 μm. The microtubule-based structures were found to be significantly longer and branched at the distal end by 96 h. These data confirm that increased cellular levels of centrobin lead to unregulated elongation and abnormal structure of the centrioles.
Centrobin Overexpression Results in Increased Cellular Levels of CPAP
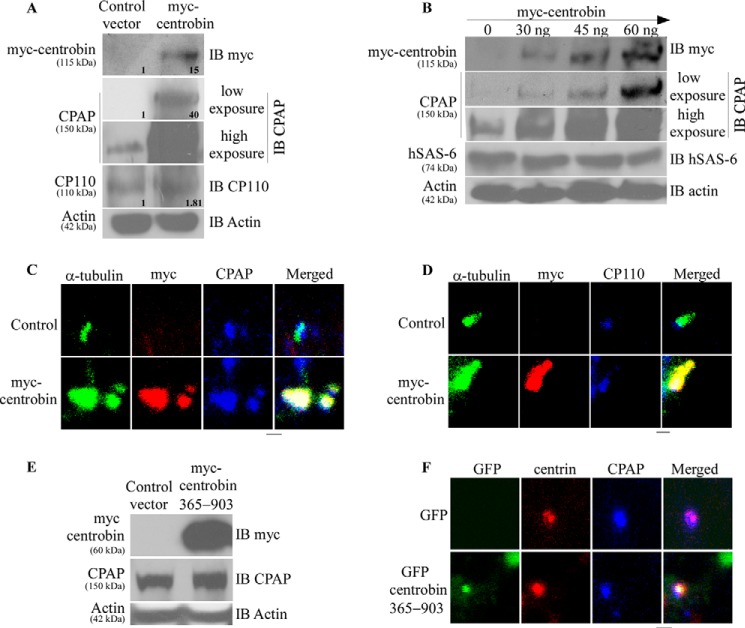
CPAP and CP110 play opposing roles in regulating centriole length. CPAP overexpression or CP110 depletion cause the centrioles to elongate abnormally (35, 39, 40). Therefore, to understand the underlying molecular mechanism by which centrobin-mediates centriole elongation, we examined the cellular expression levels of CPAP and CP110 in centrobin-overexpressing cells. 293T cells were transfected with control or myc-centrobin expression vectors, and cell lysates were prepared 72 h post-transfection and subjected to immunoblotting to detect the endogenous levels of CPAP, CP110, and tubulin. Fig. 2A shows that the centrobin-overexpressing, but not control cells, have a massive accumulation of the CPAP protein. On the other hand, the cellular level of CP110 in centrobin-overexpressing cells, albeit relatively higher than control, was not as profoundly different as CPAP levels, suggesting that centrobin-overexpression has a more robust effect on CPAP protein levels.
FIGURE 2.
Centrobin overexpression results in increased cellular CPAP but not CP110 and hSAS-6 levels. A, 293T cells transfected with control or myc-centrobin expression vectors were lysed after 72 h of transfection and immunoblotted (IB) using the anti-CPAP, -CP110, -myc, and -actin antibodies. Relative densitometric values are shown. Individual values were normalized against actin values, and the band intensities of control lane were considered as 1 for calculating relative values. B, 293T cells were transfected with control or indicated amounts of myc-centrobin vector followed by immunoblotting with the anti-myc, -CPAP, hSAS-6, and -actin antibodies. C, U2OS cells expressing control or myc-centrobin expression vectors were fixed with methanol and stained with anti-α-tubulin and, -myc, and -CPAP antibodies to mark the centrioles. D, same as B, except cells were stained with anti-CP110 antibody instead of CPAP. E, 293T cells were transfected with control or myc-centrobin-365–903 amino acid vectors and immunoblotted using the anti-myc, -CPAP, and -actin antibodies. F, U2OS cells were transfected with GFP and GFP-centrobin-365–903 vectors followed by immunostaining with anti-CPAP and -centrin antibodies. Images were acquired using the Olympus FV10i confocal microscope and are maximum projections of Z stacks. Scale bar: 2 μm.
To further confirm if expression levels of centrobin correlate with that of CPAP, 293T cells were transfected with varying amounts of myc-centrobin expression vector and examined for endogenous levels of CPAP. The cellular level of CPAP was directly proportional to amounts of centrobin expressed in the cell (Fig. 2B). Interestingly, corresponding levels of another essential centriole biogenesis protein, hSAS-6 (33), were not altered profoundly in centrobin-overexpressing cells. This suggests that cellular levels of centrobin, specifically, alter the cellular levels of CPAP.
Next, we examined whether the increased cellular levels of CPAP upon centrobin overexpression translate into their incorporation in the elongating centriole barrels. U2OS cells, which are commonly used for studies related to centriole biology due to mutations that uncouple the cell cycle and centriole duplication processes (56), were transfected with myc-centrobin expression vector for 72 h and stained for CPAP and CP110. The centriolar levels of CPAP mimicked the cellular levels, where centrobin-overexpression resulted in a profound increase in the staining intensity of CPAP as compared with control cells, specifically on the elongated centriole-like structures (Fig. 2C). CP110, which stains the distal ends of centrioles (27), was not significantly different in the control and centrobin-overexpressing cells (Fig. 2D). These results suggest that centrobin has a specific, stabilizing effect on the cellular levels of CPAP but not CP110. Because a constant dynamic exchange between the cytoplasmic and centriolar CPAP can occur (4), the abnormal centriole elongation of centrioles, when centrobin is expressed at higher levels, appears to be caused by stabilization of cellular levels of CPAP and accumulation of it on the centrioles.
Earlier, we showed that centrobin directly interacts with CPAP (48). To further evaluate if the observed stabilizing effect of centrobin on endogenous CPAP was indeed due to its binding ability, we tested the effect of a 365–903-amino acid fragment of centrobin that does not bind to CPAP but retains its tubulin binding ability (47, 48) (referred to as centrobin 365–903 in the rest of the manuscript) on the cellular and centriolar levels of CPAP. As observed in Fig. 2, E and F, overexpression of centrobin mutant, which does not bind to CPAP, did not alter the cellular and centriolar levels of CPAP, indicating that physical interaction with centrobin is necessary for the accumulation of CPAP.
Centrobin Depletion Results in the Degradation of Cellular CPAP
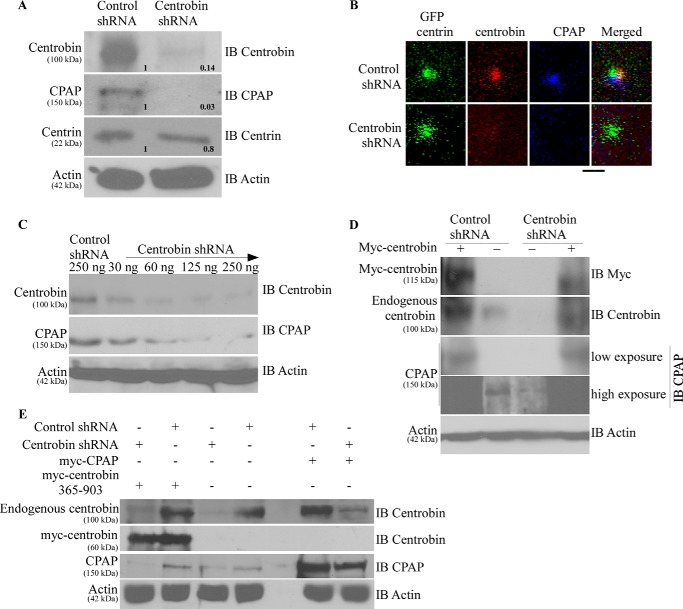
Recently, we reported that centrobin-CPAP interaction facilitates the maintenance of CPAP on centrioles and contributes to its elongation function (48). In fact, in this report we showed that depletion of centrobin causes the disappearance of CPAP from the centrioles and inhibition of CPAP-mediated centriole elongation. Here, we examined whether the cellular levels of CPAP are also affected upon centrobin depletion. 293T cells were transfected with either control or centrobin shRNA using the pRetro-Super expression vectors, and cell lysates were prepared after 72 h and subjected to SDS-PAGE and Western blotting to detect centrobin, CPAP, and another centriolar protein, centrin. Fig. 3A shows that endogenous CPAP was undetectable in centrobin-depleted cells, whereas the cellular level of centrin, a centriolar marker, was not affected considerably upon centrobin depletion (Fig. 3A).
FIGURE 3.
Centrobin depletion results in the degradation of cellular CPAP. A, 293T cells were transfected with control or centrobin shRNA for 72 h after which cells were lysed and probed with the anti-centrobin, -CPAP, -centrin, and -actin antibodies. Relative densitometric values are shown. Individual values were normalized against actin values, and the band intensities of control lane were considered as 1 for calculating relative values. IB, immunoblot. HeLa-GFP-centrin stable cells were transfected as described for A, and centrioles were imaged using the Olympus FV10i confocal microscope. Images are maximum projections of Z stacks. Scale bar: 2 μm. C. 293T cells were transfected with increasing amounts of centrobin shRNA- expressing vector for 72 h, and the lysates were immunoblotted using the indicated antibodies. D, to test the specificity of centrobin shRNA-induced effect, control or centrobin shRNA-transfected 293T cells after 48 h were re-transfected with myc-centrobin-expressing vector. Lysates were probed with antibodies against centrobin, CPAP, and actin. E, same experimental scheme as D, except control or centrobin shRNA cells were re-transfected with myc-centrobin-365–903 or myc-CPAP expressing vectors.
To assess if centrobin depletion has an effect on centriolar CPAP and if the effect is specific, HeLa cells that stably express GFP-centrin (53) were transfected with control or shRNA expression vectors for 72 h and stained for immunofluorescence microscopy. Although the GFP-positive centrioles in control shRNA-expressing cells showed the presence of centrobin and CPAP, centrobin depletion caused the disappearance of CPAP, but not centrin, from centrioles (Fig. 3B). This indicates that centrobin depletion-associated effect on CPAP level is specific.
To further assess the effect of centrobin deficiency on cellular levels of CPAP protein, 293T cells were transfected with varying amounts of centrobin shRNA expression vector for 72 h, and the cell lysates were subjected to immunoblotting to detect CPAP levels. In agreement with the observations shown in Fig. 2D, where increasing amounts of myc-centrobin expression led to a synergistic stabilizing effect on CPAP, progressive loss of endogenous cellular CPAP was observed with increasing depletion of centrobin (Fig. 3C). These observations indicate that maintenance of cellular and centriolar levels of CPAP requires the presence of centrobin.
Reintroduction of Full-length Centrobin, but Not CPAP, Restored the Cellular Levels of CPAP
Next, we examined whether the loss of cellular CPAP in centrobin-depleted cells is due to the loss of centrobin by reintroducing centrobin expression. Because the shRNA used for depleting centrobin targets the non-coding 3′-UTR region, this reagent does not affect the expression of exogenously introduced centrobin cDNA. Centrobin was depleted in 293T cells for 36 h as described above and re-transfected with the myc-centrobin expression vector. After 72 h, cell lysates were probed for endogenous and exogenous centrobin and endogenous CPAP by immunoblotting. Fig. 3D demonstrates that centrobin knockdown resulted in the loss of endogenous CPAP, similar to Fig. 3, A and C, but reintroduction of centrobin in these cells restored the cellular levels of CPAP. This observation, in association with the results of Fig. 2, confirms that the loss of CPAP seen in centrobin-deficient cells is specific to centrobin levels, and the persistence of cellular and centriolar CPAP is determined by centrobin levels.
In a recent report (48), we showed that the U2OS cells overexpressing GFP-CPAP were unable to elongate centrioles in the absence of centrobin due to lack of centrobin-mediated recruitment of CPAP to the centrioles. Hence, we examined whether the lack of centriolar expression of CPAP in centrobin-depleted cells is associated with the lack of interaction of centrobin with CPAP. 293T cells were transfected with control or centrobin shRNA vector for 36 h, re-transfected with centrobin mutant that does not bind to CPAP (centrobin-365–903) or full-length CPAP itself (Fig. 3E), expression vectors for an additional 36 h, and the cell lysates were subjected to immunoblotting to detect centrobin and CPAP. Fig. 3E demonstrates that although robust expression of the exogenously delivered myc-CPAP was seen in control cells, relatively lower levels of CPAP were detected in the centrobin shRNA-expressing cells. This confirms that centrobin at least partially regulates the stability and persistence of CPAP in cells. In addition, expression of centrobin-365–903 did not restore the CPAP expression in centrobin-depleted cells (Fig. 3E). These results confirm that protein instability due to lack of interaction with centrobin is the mechanism responsible for disappearance of CPAP in centrobin-deficient cells.
Inhibition of the Proteasome Pathway Restores Cellular CPAP Levels in Centrobin-depleted Cells
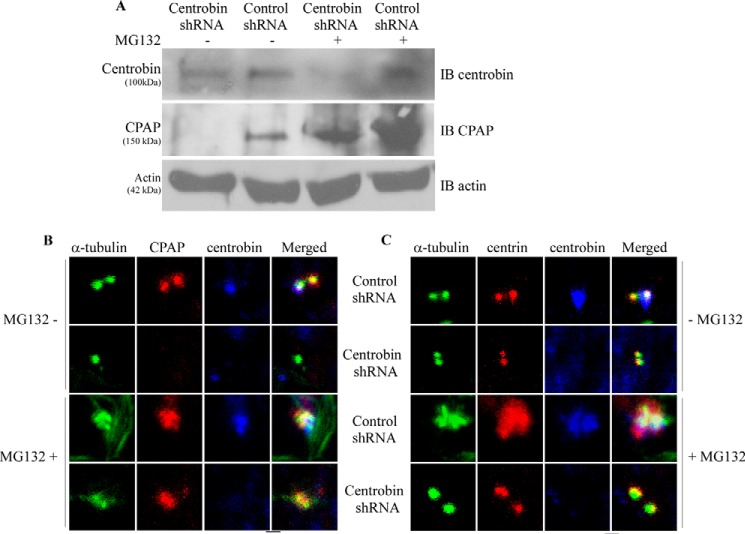
CPAP is degraded by the proteasome in late mitotic/early G1 cells (39, 43). However, what triggers CPAP degradation and termination of centriole elongation at this phase is not known. Considering our observations that CPAP stability is dependent on the cellular levels of centrobin, we sought to understand the mechanism by which CPAP expression is lost in centrobin-depleted cells. 293T cells were transfected with control or centrobin shRNA for 72 h and treated with a proteasome inhibitor, MG-132, for an additional 14 h, and the cell lysates were subjected to immunoblotting for detection of CPAP. Our results show that in comparison with untreated cells, a significant accumulation of CPAP occurs in MG-132-treated control shRNA cells (Fig. 4A), reiterating earlier observations that CPAP is proteasomally degraded (39, 43). Importantly, although CPAP was significantly down-regulated in untreated centrobin-depleted cells, MG-132 treatment did restore the CPAP expression in them, albeit not equivalent to the levels of MG-132-treated control cells.
FIGURE 4.
Proteasome inhibition restores CPAP expression in centrobin-depleted cells. A, 293T cells were transfected with control or centrobin shRNA expression vectors for 72 h after which cells were treated with either DMSO vehicle control or MG-132 (20 μm) for 14 h. Cells were then lysed and immunoblotted (IB) with the indicated antibodies. B, U2OS cells were transfected with control or centrobin siRNAs for 72 h followed by treatment with DMSO or MG-132 for 14 h. Cells were then fixed with methanol followed by staining with anti-α-tubulin, -CPAP, and -centrobin antibodies to visualize the centrioles. C, same as B, except cells were stained with centrin instead of CPAP. Images were acquired using the Olympus FV10i confocal microscope and are maximum projections of Z stacks. Scale bar: 2 μm.
Inhibition of the Proteasome Restores CPAP on the Centrioles
Our previous report shows that CPAP is not recruited to the centrioles in the absence of centrobin (48). Therefore, we determined whether inhibition of proteasome activity also led to recruitment and restoration of CPAP to the centrioles in centrobin-depleted cells. U2OS cells were treated with either control or centrobin siRNAs for 72 h and MG-132 for another 14 h and stained using anti-α-tubulin, -centrobin, and -CPAP antibodies for immunofluorescence microscopy. As observed in Fig. 4B, control and centrobin-depleted cells that are treated with MG-132 had normal sized centrioles. However, although CPAP was undetectable in centrobin-depleted cells that are not treated with MG-132, proteasome inhibition restored CPAP recruitment to the centrioles in these cells. As anticipated, inhibition of proteasome activity in control cells also resulted in higher expression of CPAP on the centrioles. These observations that proteasome inhibition restores centriolar levels of CPAP in centrobin-depleted cells confirm that CPAP is targeted for proteasome-mediated degradation in the absence of centrobin.
Proteasome Inhibition Is Not Sufficient to Restore the Centriole Elongation Activity of CPAP in Centrobin-deficient Cells
Previous studies have shown that overexpression of CPAP causes abnormal centriole elongation (35, 39, 40) and centrobin is required for this elongation to proceed (47, 48). Our previous and current observations indicate that centrobin promotes this centriole elongation activity by regulating the stability of cellular and centriolar CPAP. It has also been shown that de novo abnormal elongation of centrioles can occur upon inhibition of the proteasome activity (57). Because inhibition of the proteasome degradation pathway restored CPAP expression to the centrioles in centrobin-depleted cells (Fig. 4B), we examined whether prevention of CPAP degradation by inhibiting proteasome activity alone is sufficient to cause abnormal centriole elongation in the absence of centrobin. U2OS cells were transfected with control or centrobin siRNA, treated with MG-132, and stained with antibodies to detect α-tubulin, centrobin, and centrin to assess the overall centriole size. As reported before (57), we observed abnormally elongated centrioles in the MG-132-treated control cells (Fig. 4C). Centrioles in centrobin-deficient cells, in spite of treatment with MG-132, did not show abnormal elongation of centrioles as compared with control cells, perhaps due to relatively lower amounts of cellular and centriolar CPAP as observed in Fig. 4, A and B. This reiterates that the final length of centrioles is determined by the amount of both centrobin and CPAP present in the cell.
Interaction with Centrobin Prevents the Proteasome-mediated Degradation of Ubiquitinated CPAP
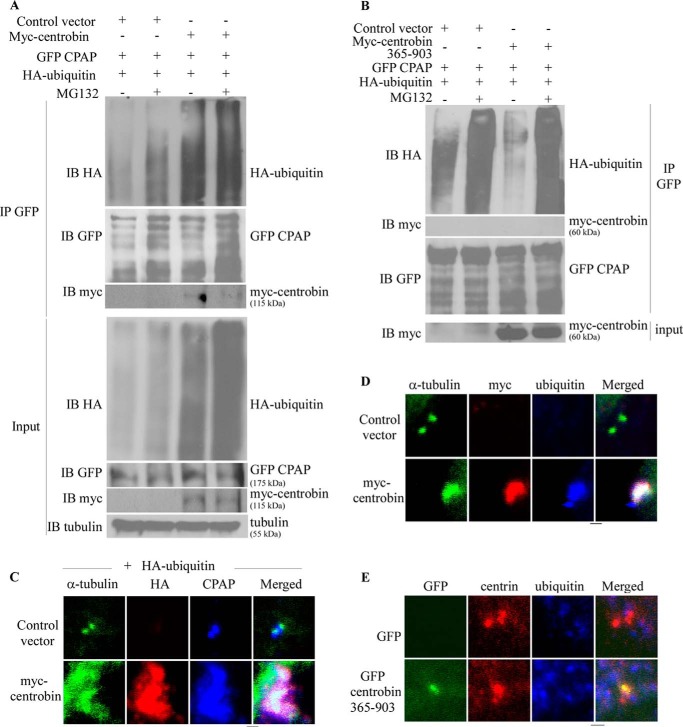
Because ubiquitination precedes proteasomal degradation of proteins, we examined the ubiquitination status of CPAP in centrobin overexpressing cells. Myc-centrobin, GFP-CPAP, and HA-ubiquitin co-transfected 293T cells, and the appropriate control cells were treated with DMSO or MG-132. The cell lysates were then subjected to immunoprecipitation using anti-GFP antibody to pull down GFP-CPAP and immunoblotted (IB) with anti-HA, -GFP, and -myc antibodies to detect ubiquitin, CPAP, and centrobin, respectively. As anticipated, MG-132 treatment resulted in an increase in the amount of ubiquitinated proteins in control cells (Fig. 5A, Input panel). Interestingly, when the anti-GFP-(CPAP) immunoprecipitates were probed using anti-HA (ubiquitin) antibody, centrobin-overexpressing cells showed much higher levels of ubiquitinated proteins even without MG-132 treatment. The smear, but not ladder, pattern (Fig. 5A, upper panel) upon probing the immunoprecipitates suggests that other ubiquitinated proteins may be co-precipitated along with CPAP. Therefore, the same blot-membrane was re-probed using anti-GFP antibody to detect ubiquitinated CPAP. As observed in the middle IB panel of Fig. 5A, CPAP detected using anti-GFP antibody showed a ladder pattern indicating that it is the ubiquitinated form of CPAP. The control cells that were treated with MG-132 showed higher levels of ubiquitinated CPAP compared with untreated cells, indicating that inhibition of proteasome prevents the degradation of ubiquitinated CPAP. Importantly, centrobin overexpressing cells also showed profoundly higher levels of ubiquitinated CPAP compared with control cells even in the absence of MG-132. In fact, the immunoprecipitates prepared from GFP-CPAP-expressing cells using anti-GFP antibody showed the presence of myc-centrobin (Fig. 5A) confirming our earlier observation that centrobin and CPAP interact (48). Importantly, expression of centrobin-365–903 did not cause the accumulation of ubiquitinated CPAP in non-proteasome inhibitor-treated cells (Fig. 5B), suggesting that CPAP is accumulated only through its interaction with centrobin. Overall, these results indicate that centrobin interacts with an ubiquitinated form of CPAP, and this ubiquitinated form of CPAP does not get targeted for proteasome-mediated degradation in the presence of centrobin.
FIGURE 5.
Centrobin overexpression results in the accumulation of ubiquitinated CPAP at the cellular and centriole level. A, 293T cells were transfected with control or myc-centrobin expression vectors along with GFP-CPAP and HA-ubiquitin plasmids and were treated with or without MG-132 (20 μm) for the last 14 h. Lysates were prepared using the radioimmune precipitation assay buffer and treated with anti-GFP antibody. Immunoprecipitates (IP) were separated by SDS-PAGE and immunoblotted using the anti-HA, -myc, -CPAP, and -GFP antibodies. Input: 5% of lysate. B, the assay was performed as described for panel A using centrobin-365–903 expression vector. C, U2OS cells were transfected with control or myc-centrobin along with the HA-ubiquitin expression vectors. Cells were fixed and stained using the anti-α-tubulin, -HA, and -CPAP antibodies. D, U2OS cells were transfected with control or myc-centrobin expressing vector and immunostained with anti-α-tubulin, -ubiquitin, and -CPAP antibodies. E, U2OS cells were transfected with GFP or GFP-centrobin-365–903 and stained using the anti-α-tubulin, -ubiquitin, and -CPAP antibodies. Images were acquired using the Olympus FV10i confocal microscope and are maximum projections of Z stacks. Scale bar: 2 μm.
Persistence of Ubiquitinated CPAP in Centrobin-overexpressing Cells Leads to the Elongation of Centrioles
The elongation phase of centriole duplication is completed when the cell reaches mitosis (39). It has been shown that CPAP is degraded through proteasome targeting, and persistence of CPAP can lead to long, abnormal centrioles (44). Our results show that centrobin-overexpressing cells have abnormal, elongated centrioles (Fig. 1) as well as high levels of ubiquitinated CPAP. This raises the possibility that ubiquitinated CPAP may also accumulate on the centrioles of centrobin-overexpressing cells due to persistent interaction with centrobin (4), resulting in uninterrupted elongation function.
Because centrobin overexpression caused accumulation of ubiquitinated CPAP in the cell and the localization of ubiquitinated proteins on the centrioles has never been reported before, we examined whether ubiquitinated proteins are accumulated on centrioles in the context of centrobin overexpression. U2OS cells were transfected with the myc-centrobin expression vector and HA-ubiquitin vector. Cells were co-stained with anti-HA and -CPAP antibodies along with anti-tubulin antibody to mark the centrioles for immunofluorescence microscopy. Fig. 5C shows that although the anti-HA antibody did not stain the centrioles, centrobin-overexpressing cells showed high levels of HA staining on the centrioles indicating centriolar accumulation of ubiquitinated proteins. Importantly staining using ubiquitin-specific antibody also showed high amounts of ubiquitinated proteins on the elongated centrioles of full-length centrobin (Fig. 5D) but not on centrioles of cells expressing centrobin-365–903 (Fig. 5E). Although the identity of ubiquitinated proteins on the centrioles of centrobin-overexpressing cells is unknown, our results, in association with the known role of CPAP in centriole elongation (39), suggest that centrobin is responsible for the stabilization and accumulation of ubiquitinated CPAP on centrioles and their elongation.
Centrobin Depletion and Overexpression Affects the Bipolar Spindle Morphology
Bipolar spindle assembly ensures the correct segregation of chromosomes during cell division. Perturbing the centrosome integrity due to short centrioles or abnormal sized centrioles has been shown to affect the bipolar spindle assembly process (35, 51), which can contribute to chromosome instability (15, 58). Hence, we evaluated the physiological effects of CPAP loss as a consequence of centrobin depletion as well as excess CPAP due to overexpression of centrobin in a mitotic bipolar spindle assembly assay. U2OS cells were transfected with either centrobin shRNA or myc-centrobin expression vector along with appropriate control vectors for 96 h. The spindle morphology in mitotic cells from these cultures was analyzed by staining with anti-α-tubulin and -centrin antibodies and DAPI (Fig. 6). As reported earlier (51), depletion of centrobin caused an accumulation of cells with abnormal spindles that were aberrant, unfocused, mini, or collapsed. Importantly, a higher number of centrobin- overexpressing cells also demonstrated these types of abnormal spindles compared with control vector-transfected cells, confirming that a normal expression level of centrobin is necessary for maintaining centrosome integrity, which is critical for normal bipolar spindle assembly during cell division.
FIGURE 6.
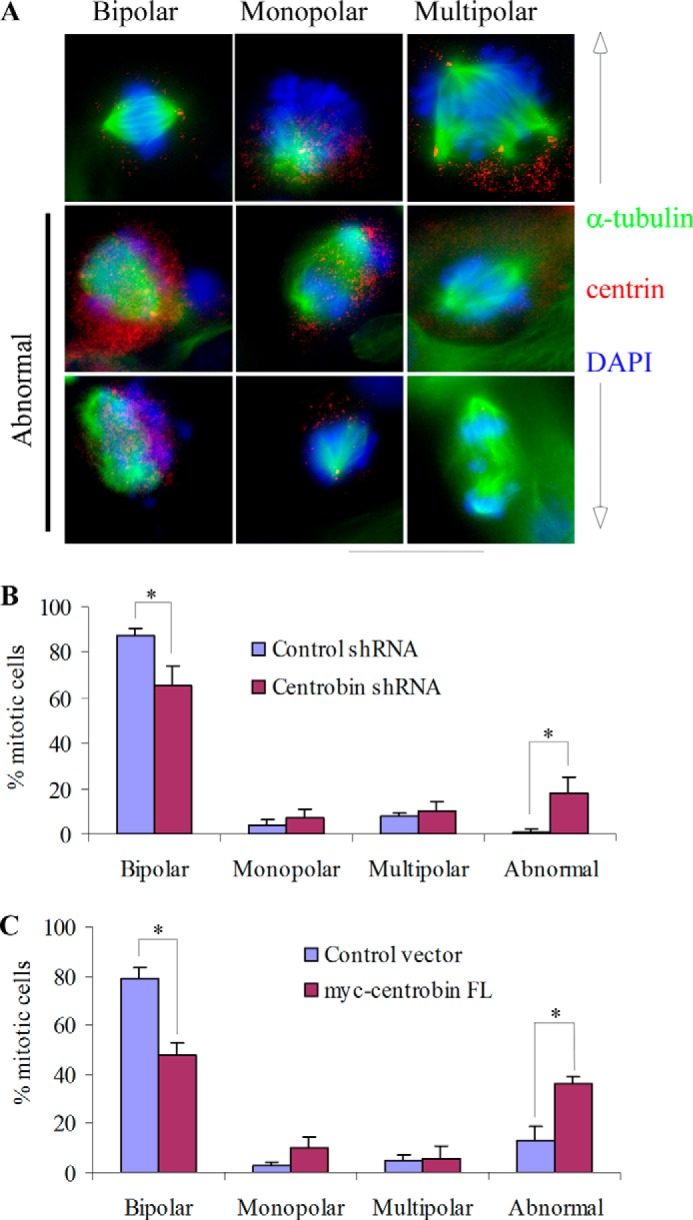
Centrobin depletion and overexpression results in spindles with abnormal morphology. U2OS cells were transfected with control or centrobin shRNA and control or myc-centrobin vectors for 72 h followed by staining with DAPI (blue) to mark the nucleus and α-tubulin (green) and centrin (red) primary antibodies to stain the spindle and centrioles. Representative images for the major categories of spindles observed have been shown in the top row of panel A, and examples of visibly abnormal spindle-bearing cells are shown in middle and lower rows of this panel. Quantification of mitotic cells in centrobin-depleted cells and centrobin-overexpressing cells are shown in panels B and C, respectively. Images were acquired using the Zeiss Axiovert imaging microscope with the 60× oil immersion objective. Scale bar: 20 μm. *, p value <0.001.
DISCUSSION
Here we have identified the molecular mechanism by which centrobin contributes to the assembly of centrioles. We found that centrobin is critical for stabilizing the cellular and centriolar levels of CPAP. Although depletion of centrobin leads to cellular CPAP degradation, overexpression of centrobin causes the accumulation of CPAP and abnormal, long centrioles. In association with our previous report that centrobin and CPAP interact directly (48), this study demonstrates that loss of centrobin-CPAP interaction results in targeting of ubiquitinated CPAP for proteasome-mediated degradation, a potential mechanism for restricting the centriole length to ∼500 nm.
Although several centriolar proteins such as STIL, SAS-6, CEP120, SPICE1, and CEP135 can interact with CPAP and positively regulate the centriole duplication and elongation process (30, 32, 45, 46), this is the first study that uncovers the mechanism by which CPAP levels are regulated in the cell to restrict the centriole length. Earlier we showed that centrobin binds to CPAP directly, and this interaction is essential for maintaining CPAP levels on centrioles (48). Here, we show that centrobin expression levels determine the cellular levels of CPAP. Although excess centrobin causes the persistence of ubiquitinated CPAP and uncontrolled elongation of centrioles due to prolonged interaction with centrobin, lack of centrobin leads to degradation of ubiquitinated CPAP by the proteasome and inhibition of centriole elongation.
It has been shown that CPAP is ubiquitinated by the APC-Cdh1 complex, which is active during mitosis (39). Once a protein is ubiquitinated, it is often targeted to the proteasome and degraded. This is achieved by the action of specific deubiquitinases, which recycle the ubiquitin back to the cytoplasm (59), making the protein vulnerable to the action of proteases. Therefore, ubiquitination of CPAP at mitosis and targeting it for proteasomal degradation is one of the mechanisms by which centriolar CPAP levels are regulated to halt centriole elongation at the predetermined dimensions of ∼500-nm length (39). In fact, this previous study also showed that expression of a degradation-resistant mutant form of CPAP, which lacks the ubiquitination site, could lead to unregulated elongation of centrioles. However, the ubiquitination pattern of CPAP and its centriole localization were not reported.
Our previous report showed that the centriolar recruitment and maintenance of CPAP is dependent on its interaction with centrobin (48). However, how centrobin-mediated stabilization of CPAP on the centrioles is achieved was not known. Although disassociation of the centrobin-CPAP interaction, due to inability of CPAP to target to the centrioles, was thought to be responsible, our current study clearly shows that the cellular levels of CPAP, determined by its interaction with centrobin, may be critical for a dynamic exchange of these proteins between cytoplasm and centrioles. In this regard our observation that inhibition of proteasome activity using MG-132 is sufficient to restore the cellular and centriolar CPAP indicates that centrobin stabilizes cellular levels of CPAP and prevents its proteasome-dependent degradation. This notion has been further substantiated by profound accumulation of CPAP in the cell and centrioles upon centrobin overexpression.
We also observed that overexpression of centrobin causes massive accumulation of ubiquitinated CPAP in the cell and unregulated elongation of centrioles. An intriguing question is why the ubiquitinated CPAP is not targeted for degradation in the presence of high levels of centrobin. The most obvious reason is that the interaction of ubiquitinated CPAP with centrobin prevents it from being targeted for proteasomal degradation. Therefore, ubiquitinated CPAP levels are expected to be proportionate to centrobin levels, and the absence of centrobin results in proteasomal degradation of ubiquitinated CPAP. Our observations shown here suggest this as the potential mechanism. However, alternatively, it is possible that centrobin has a direct or indirect role in inhibiting the proteasome, which prevents the degradation of ubiquitinated proteins, CPAP in particular, possibly by affecting the deubiquitinase function. For example, the activity of deubiquitinase USP33 has been linked to the regulation of centriole distal end capping protein, CP110 (60). It is possible that deubiquitination of CPAP, before protease degradation in the proteasome, may be dependent on USP33 or similar enzymes, and high levels of centrobin may have a regulatory effect on them, which needs to be investigated in the future. Yet another possibility is that certain forms of ubiquitination, as shown for the NF-κB signaling pathway (61, 62), are actually required to perpetuate signaling activities, and CPAP may be ubiquitinated differently in the presence and absence of centrobin. Both the above-mentioned possibilities will be tested in the future.
Previous reports have shown that CPAP is degraded at the G2/M stage. At this stage, the microtubule assembly on the centrioles is completed, and the centrioles have reached their predetermined dimensions (39). Hence, it is believed that degradation of CPAP is a trigger to stop further elongation of the centrioles. Importantly, both centrobin and CPAP directly bind to tubulin and have microtubule-stabilizing property (49, 52, 63), suggesting that these proteins contribute to the actual polymerization of microtubules. Future studies will address how the tubulin binding property of centrobin and CPAP coordinates and contributes toward centriole assembly and regulation of centriole length during biogenesis. In addition, because the cellular levels of centrobin remain unchanged throughout the cell cycle (39), what triggers CPAP degradation in the presence of centrobin for restricting the centriole length is another important question. Centrobin, which is considered a predominantly daughter centriolar protein (36), has also been detected on the mother centrioles in G2/M-arrested cells (47), suggesting the levels of this protein are regulated on centrioles at different stages of cell cycle. Furthermore, in G2/M-arrested cells, centrobin undergoes PLK1-mediated phosphorylation (52) indicating its altered function. It is possible that in mitotic cells centrobin gets phosphorylated causing termination of centrobin-CPAP interaction at least at centriolar level, resulting in CPAP degradation and cessation of centriole elongation. This notion is currently being investigated.
Centrobin-CPAP interaction appears to be crucial for bipolar mitotic spindle formation. Our results show that abnormal levels of centrobin are associated with loss of centrosome integrity, defective mitotic spindles with abnormal spindle poles and orientation. In fact this is in agreement with previous reports that showed defective mitotic spindle formation and partial loss of centrosome integrity upon centrobin depletion of centrobin or CPAP (35, 51) as well as upon overexpression of CPAP (35). The normal functioning of mitotic spindles is critical for the fidelity of chromosome segregation and tumor suppression. Therefore, our observations highlight the significance of centrobin-CPAP interaction during cell division.
Overall, this study shows that centrobin regulates the CPAP levels and contributes to centriole assembly during elongation as depicted in the model (Fig. 7). Loss of centrobin-CPAP interaction at mitosis by an unknown mechanism targets CPAP for degradation and restricts the centriole length at a predetermined length. However, excess centrobin facilitates prolonged interaction with CPAP and accumulation of ubiquitinated proteins, potentially including CPAP, on centrioles, thereby causing the centrioles to elongate abnormally. On the other hand, depletion of centrobin results in the degradation of CPAP, causing inhibition of the centriole duplication process. Although our observations clearly show that ubiquitinated CPAP is accumulated on the centrioles and abnormally elongated centrioles are generated in the presence of excessive centrobin, additional studies are needed to realize whether ubiquitinated CPAP is needed for, or if it is functional in, promoting centriole elongation.
FIGURE 7.
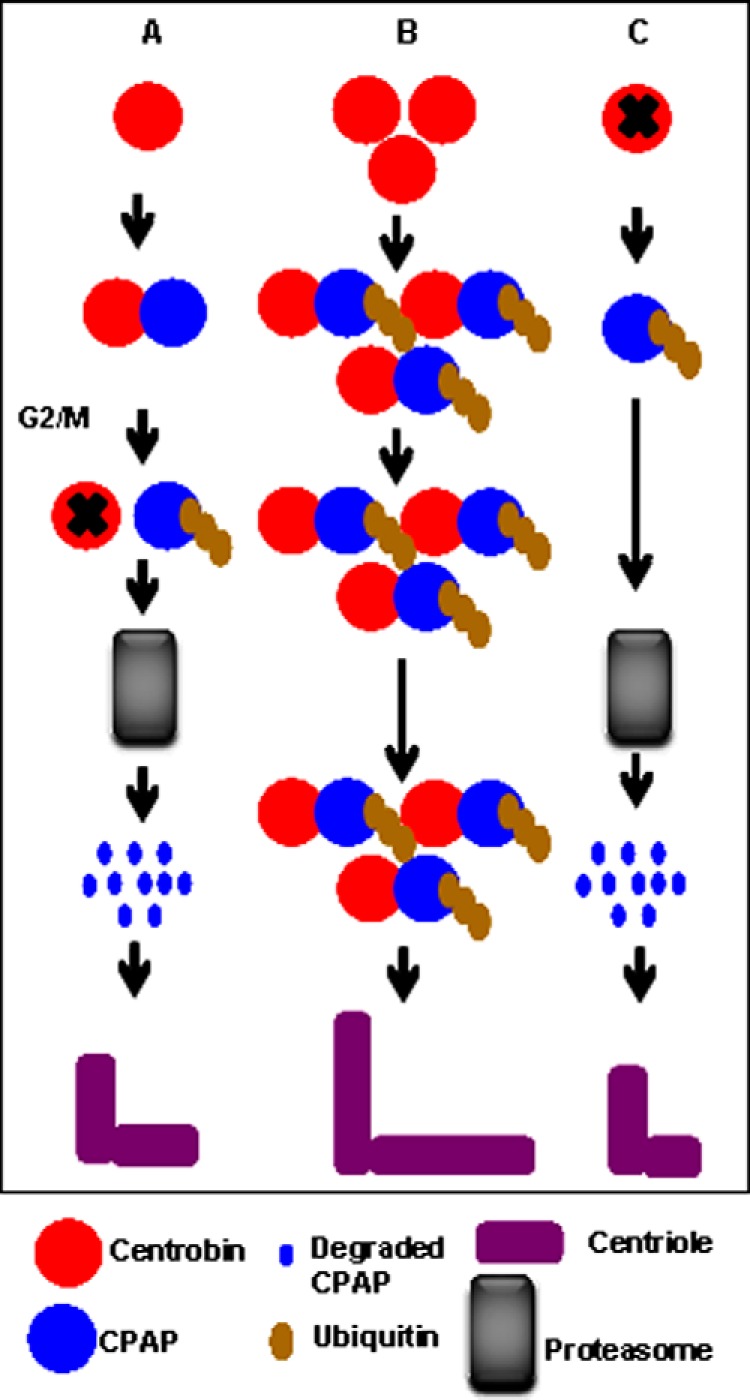
Schematic depiction of the differential effects of forced and depleted centrobin expression on CPAP stability. A, under normal circumstances interaction between centrobin and CPAP maybe terminated at the G2/M phase by an unknown mechanism, leading to the ubiquitination and proteasomal degradation of CPAP and regulation of centriole length. B, high levels of constitutive expression of centrobin cause inhibition of proteasomal degradation of CPAP, resulting in accumulation and continued interaction between centrobin and ubiquitinated CPAP. This culminates in uncontrolled elongation and abnormal structure of centrioles. C, the absence of centrobin causes the destabilization of cellular CPAP due to rapid ubiquitination and proteasome-mediated degradation, potentially leading to inhibition of centriole elongation.
In summary, we report the identification of centrobin as an upstream regulator of CPAP, a centrosomal protein with crucial role in centriole assembly, spindle orientation, brain development, and ciliogenesis (4, 17, 27, 35, 41, 64). Of clinical importance, patients with microcephaly and Seckel syndrome were found to have mutations in this gene (41, 42). Furthermore, mice with disrupted CPAP expression develop symptoms of microcephaly and Seckel syndrome (17). Therefore, our observations that centrobin regulates CPAP levels and restricts the length of centrioles are highly significant and contribute to the understanding of centriole biogenesis.
Acknowledgments
We thank Dr. T. K. Tang and Dr. Beichu Guo for invaluable help in providing reagents. We also thank Dr. Alexey Khodjakov, Wadsworth Center, NY for kindly sharing the HeLa-GFP-centrin stable cells and the Cell and Molecular Imaging Shared Resource facility at the Medical University of South Carolina. Imaging facilities for this research were supported, in part, by National Institutes of Health Cancer Center Support Grant P30 CA138313 to the Hollings Cancer Center, Medical University of South Carolina.
This work was supported by internal funds from the Departments of Surgery and Microbiology and Immunology, MUSC (to C. V.).
- CPAP
- centrosomal protein 4.1-associated-protein.
REFERENCES
- 1. Doxsey S. (2001) Re-evaluating centrosome function. Nat. Rev. Mol. Cell Biol. 2, 688–698 [DOI] [PubMed] [Google Scholar]
- 2. Nigg E. A., Raff J. W. (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 [DOI] [PubMed] [Google Scholar]
- 3. Lüders J., Stearns T. (2007) Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161–167 [DOI] [PubMed] [Google Scholar]
- 4. Kitagawa D., Kohlmaier G., Keller D., Strnad P., Balestra F. R., Flückiger I., Gönczy P. (2011) Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. J. Cell Sci. 124, 3884–3893 [DOI] [PubMed] [Google Scholar]
- 5. Januschke J., Reina J., Llamazares S., Bertran T., Rossi F., Roig J., Gonzalez C. (2013) Centrobin controls mother-daughter centriole asymmetry in Drosophila neuroblasts. Nat. Cell Biol. 15, 241–248 [DOI] [PubMed] [Google Scholar]
- 6. Stinchcombe J. C., Salio M., Cerundolo V., Pende D., Arico M., Griffiths G. M. (2011) Centriole polarisation to the immunological synapse directs secretion from cytolytic cells of both the innate and adaptive immune systems. BMC Biol. 9, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doxsey S., Zimmerman W., Mikule K. (2005) Centrosome control of the cell cycle. Trends Cell Biol. 15, 303–311 [DOI] [PubMed] [Google Scholar]
- 8. Robbins E., Jentzsch G., Micali A. (1968) The centriole cycle in synchronized HeLa cells. J. Cell Biol. 36, 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azimzadeh J., Bornens M. (2007) Structure and duplication of the centrosome. J. Cell Sci. 120, 2139–2142 [DOI] [PubMed] [Google Scholar]
- 10. Hinchcliffe E. H., Li C., Thompson E. A., Maller J. L., Sluder G. (1999) Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 283, 851–854 [DOI] [PubMed] [Google Scholar]
- 11. Meraldi P., Nigg E. A. (2002) The centrosome cycle. FEBS Lett. 521, 9–13 [DOI] [PubMed] [Google Scholar]
- 12. Chrétien D., Buendia B., Fuller S. D., Karsenti E. (1997) Reconstruction of the centrosome cycle from cryoelectron micrographs. J. Struct. Biol. 120, 117–133 [DOI] [PubMed] [Google Scholar]
- 13. Lingle W. L., Lutz W. H., Ingle J. N., Maihle N. J., Salisbury J. L. (1998) Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. U.S.A. 95, 2950–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pihan G. A., Purohit A., Wallace J., Malhotra R., Liotta L., Doxsey S. J. (2001) Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 61, 2212–2219 [PubMed] [Google Scholar]
- 15. Ganem N. J., Godinho S. A., Pellman D. (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X., Tsai J. W., Imai J. H., Lian W. N., Vallee R. B., Shi S. H. (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461, 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McIntyre R. E., Lakshminarasimhan Chavali P., Ismail O., Carragher D. M., Sanchez-Andrade G., Forment J. V., Fu B., Del Castillo Velasco-Herrera M., Edwards A., van der Weyden L., Yang F., Sanger Mouse Genetics Project, Ramirez-Solis R., Estabel J., Gallagher F. A., Logan D.W., Arends M. J., Tsang S. H., Mahajan V. B., Scudamore C. L., White J. K., Jackson S. P., Gergely F., Adams D. J. (2012) Disruption of mouse Cenpj, a regulator of centriole biogenesis, phenocopies Seckel syndrome. PLoS Genet. 8, e1003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singla V., Romaguera-Ros M., Garcia-Verdugo J. M., Reiter J. F. (2010) Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell 18, 410–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bettencourt-Dias M., Hildebrandt F., Pellman D., Woods G., Godinho S. A. (2011) Centrosomes and cilia in human disease. Trends Genet. 27, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pihan G. A., Purohit A., Wallace J., Knecht H., Woda B., Quesenberry P., Doxsey S. J. (1998) Centrosome defects and genetic instability in malignant tumors. Cancer Res. 58, 3974–3985 [PubMed] [Google Scholar]
- 21. Thornton G. K., Woods C. G. (2009) Primary microcephaly: do all roads lead to Rome? Trends Genet. 25, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strnad P., Gönczy P. (2008) Mechanisms of procentriole formation. Trends Cell Biol. 18, 389–396 [DOI] [PubMed] [Google Scholar]
- 23. Hinchcliffe E. H., Miller F. J., Cham M., Khodjakov A., Sluder G. (2001) Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291, 1547–1550 [DOI] [PubMed] [Google Scholar]
- 24. Hinchcliffe E. H., Sluder G. (2001) “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 15, 1167–1181 [DOI] [PubMed] [Google Scholar]
- 25. Blachon S., Gopalakrishnan J., Omori Y., Polyanovsky A., Church A., Nicastro D., Malicki J., Avidor-Reiss T. (2008) Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics 180, 2081–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bettencourt-Dias M., Rodrigues-Martins A., Carpenter L., Riparbelli M., Lehmann L., Gatt M. K., Carmo N., Balloux F., Callaini G., Glover D. M. (2005) SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 15, 2199–2207 [DOI] [PubMed] [Google Scholar]
- 27. Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y. D., Nigg E. A. (2007) Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 [DOI] [PubMed] [Google Scholar]
- 28. Habedanck R., Stierhof Y. D., Wilkinson C. J., Nigg E. A. (2005) The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7, 1140–1146 [DOI] [PubMed] [Google Scholar]
- 29. Chen Z., Indjeian V. B., McManus M., Wang L., Dynlacht B. D. (2002) CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 3, 339–350 [DOI] [PubMed] [Google Scholar]
- 30. Lin Y. C., Chang C. W., Hsu W. B., Tang C. J., Lin Y. N., Chou E. J., Wu C. T., Tang T. K. (2013) Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. EMBO J. 32, 1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vulprecht J., David A., Tibelius A., Castiel A., Konotop G., Liu F., Bestvater F., Raab M. S., Zentgraf H., Izraeli S., Krämer A. (2012) STIL is required for centriole duplication in human cells. J. Cell Sci. 125, 1353–1362 [DOI] [PubMed] [Google Scholar]
- 32. Tang C. J., Lin S. Y., Hsu W. B., Lin Y. N., Wu C. T., Lin Y. C., Chang C. W., Wu K. S., Tang T. K. (2011) The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO J. 30, 4790–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leidel S., Delattre M., Cerutti L., Baumer K., Gönczy P. (2005) SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7, 115–125 [DOI] [PubMed] [Google Scholar]
- 34. Stearns T., Evans L., Kirschner M. (1991) γ-Tubulin is a highly conserved component of the centrosome. Cell 65, 825–836 [DOI] [PubMed] [Google Scholar]
- 35. Kohlmaier G., Loncarek J., Meng X., McEwen B. F., Mogensen M. M., Spektor A., Dynlacht B. D., Khodjakov A., Gönczy P. (2009) Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 19, 1012–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zou C., Li J., Bai Y., Gunning W. T., Wazer D. E., Band V., Gao Q. (2005) Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 171, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vorobjev I. A., Chentsov Y. S. (1980) The ultrastructure of centriole in mammalian tissue culture cells. Cell Biol. Int. Rep. 4, 1037–1044 [DOI] [PubMed] [Google Scholar]
- 38. Kochanski R. S., Borisy G. G. (1990) Mode of centriole duplication and distribution. J. Cell Biol. 110, 1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang C. J., Fu R. H., Wu K. S., Hsu W. B., Tang T. K. (2009) CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 11, 825–831 [DOI] [PubMed] [Google Scholar]
- 40. Schmidt T. I., Kleylein-Sohn J., Westendorf J., Le Clech M., Lavoie S. B., Stierhof Y. D., Nigg E. A. (2009) Control of centriole length by CPAP and CP110. Curr. Biol. 19, 1005–1011 [DOI] [PubMed] [Google Scholar]
- 41. Al-Dosari M. S., Shaheen R., Colak D., Alkuraya F. S. (2010) Novel CENPJ mutation causes Seckel syndrome. J. Med. Genet. 47, 411–414 [DOI] [PubMed] [Google Scholar]
- 42. Gul A., Hassan M. J., Hussain S., Raza S. I., Chishti M. S., Ahmad W. (2006) A novel deletion mutation in CENPJ gene in a Pakistani family with autosomal recessive primary microcephaly. J. Hum. Genet. 51, 760–764 [DOI] [PubMed] [Google Scholar]
- 43. Kim M. K., Dudognon C., Smith S. (2012) Tankyrase 1 regulates centrosome function by controlling CPAP stability. EMBO Rep. 13, 724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsou M. F., Wang W. J., George K. A., Uryu K., Stearns T., Jallepalli P. V. (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev. Cell 17, 344–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Comartin D., Gupta G. D., Fussner E., Coyaud É., Hasegan M., Archinti M., Cheung S. W., Pinchev D., Lawo S., Raught B., Bazett-Jones D. P., Lüders J., Pelletier L. (2013) CEP120 and SPICE1 cooperate with CPAP in centriole elongation. Curr. Biol. 23, 1360–1366 [DOI] [PubMed] [Google Scholar]
- 46. Lin Y. N., Wu C. T., Lin Y. C., Hsu W. B., Tang C. J., Chang C. W., Tang T. K. (2013) CEP120 interacts with CPAP and positively regulates centriole elongation. J. Cell Biol. 202, 211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gudi R., Zou C., Li J., Gao Q. (2011) Centrobin-tubulin interaction is required for centriole elongation and stability. J. Cell Biol. 193, 711–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gudi R., Zou C., Dhar J., Gao Q., Vasu C. (2014) Centrobin-Centrosomal Protein 4.1-associated Protein (CPAP) Interaction Promotes CPAP Localization to the Centrioles during Centriole Duplication. J. Biol. Chem. 289, 15166–15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeong Y., Lee J., Kim K., Yoo J. C., Rhee K. (2007) Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J. Cell Sci. 120, 2106–2116 [DOI] [PubMed] [Google Scholar]
- 50. Balestra F. R., Strnad P., Flückiger I., Gönczy P. (2013) Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev. Cell 25, 555–571 [DOI] [PubMed] [Google Scholar]
- 51. Jeffery J. M., Urquhart A. J., Subramaniam V. N., Parton R. G., Khanna K. K. (2010) Centrobin regulates the assembly of functional mitotic spindles. Oncogene 29, 2649–2658 [DOI] [PubMed] [Google Scholar]
- 52. Lee J., Jeong Y., Jeong S., Rhee K. (2010) Centrobin/NIP2 is a microtubule stabilizer whose activity is enhanced by PLK1 phosphorylation during mitosis. J. Biol. Chem. 285, 25476–25484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piel M., Meyer P., Khodjakov A., Rieder C. L., Bornens M. (2000) The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Middendorp S., Küntziger T., Abraham Y., Holmes S., Bordes N., Paintrand M., Paoletti A., Bornens M. (2000) A role for centrin 3 in centrosome reproduction. J. Cell Biol. 148, 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bornens M. (2002) Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34 [DOI] [PubMed] [Google Scholar]
- 56. Stucke V. M., Silljé H. H., Arnaud L., Nigg E. A. (2002) Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Korzeniewski N., Cuevas R., Duensing A., Duensing S. (2010) Daughter centriole elongation is controlled by proteolysis. Mol. Biol. Cell 21, 3942–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Godinho S. A., Pellman D. (2014) Causes and consequences of centrosome abnormalities in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Komander D., Clague M. J., Urbé S. (2009) Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563 [DOI] [PubMed] [Google Scholar]
- 60. Li J., D'Angiolella V., Seeley E. S., Kim S., Kobayashi T., Fu W., Campos E. I., Pagano M., Dynlacht B. D. (2013) USP33 regulates centrosome biogenesis via deubiquitination of the centriolar protein CP110. Nature 495, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iwai K. (2012) Diverse ubiquitin signaling in NF-κB activation. Trends Cell Biol. 22, 355–364 [DOI] [PubMed] [Google Scholar]
- 62. Schmukle A. C., Walczak H. (2012) No one can whistle a symphony alone: how different ubiquitin linkages cooperate to orchestrate NF-κB activity. J. Cell Sci. 125, 549–559 [DOI] [PubMed] [Google Scholar]
- 63. Hung L. Y., Chen H. L., Chang C. W., Li B. R., Tang T. K. (2004) Identification of a novel microtubule-destabilizing motif in CPAP that binds to tubulin heterodimers and inhibits microtubule assembly. Mol. Biol. Cell 15, 2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu K. S., Tang T. K. (2012) CPAP is required for cilia formation in neuronal cells. Biology Open 1, 559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]