Background: Factor XIIa (FXIIa) binds to the cell surface; however, the underlying mechanism remains underexplored.
Results: Heparan sulfate (HS) enhances FXIIa binding capacity and consequently migration of human lung fibroblasts (HLF) isolated from fibrotic lungs.
Conclusion: HS is responsible for local accumulation of FXIIa on the cell surface.
Significance: Enhanced association of FXIIa with HLF derived from diseased lungs suggests its role in fibrogenesis.
Keywords: Cell Biology, Glycosaminoglycan, Heparan Sulfate, Proteoglycan, Pulmonary Fibrosis, Factor XIIa
Abstract
Hageman factor (FXIIa) initiates the intrinsic coagulation pathway and triggers the kallikrein-kinin and the complement systems. In addition, it functions as a growth factor by expressing promitogenic activities toward several cell types. FXIIa binds to the cell surface via a number of structurally unrelated surface receptors; however, the underlying mechanisms are not yet fully understood. Here, we demonstrate that FXIIa utilizes cell membrane-bound glycosaminoglycans to interact with the cell surface of human lung fibroblasts (HLF). The combination of enzymatic, inhibitory, and overexpression approaches identified a heparan sulfate (HS) component of proteoglycans as an important determinant of the FXIIa binding capacity of HLF. Moreover, cell-free assays and competition experiments revealed preferential binding of FXIIa to HS and heparin over dextran sulfate, dermatan sulfate, and chondroitin sulfate A and C. Finally, we demonstrate that fibroblasts isolated from the lungs of the patients suffering from idiopathic pulmonary fibrosis (IPF) exhibit enhanced FXIIa binding capacity. Increased sulfation of HS resulting from elevated HS 6-O-sulfotransferase-1 expression in IPF HLF accounted, in part, for this phenomenon. Application of RNA interference technology and inhibitors of intracellular sulfation revealed the cooperative action of cell surface-associated HS and urokinase-type plasminogen activator receptor in the accumulation of FXIIa on the cell surface of IPF HLF. Moreover, FXIIa stimulated IPF HLF migration, which was abrogated by pretreatment of cells with heparinase I. Collectively, our study uncovers a novel role of HS-type glycosaminoglycans in a local accumulation of FXIIa on the cell membrane. The enhanced association of FXIIa with IPF HLF suggests its contribution to fibrogenesis.
Introduction
Coagulation factor XII (FXII2; Hageman factor) is the zymogen of serine protease factor XIIa (FXIIa), which expresses procoagulant and proinflammatory activities. FXII is predominantly synthesized in the liver and is composed of fibronectin type I and type II domains, two epidermal growth factor-like domains, a kringle region, a proline-rich domain, and a catalytic domain (1). Together with high molecular weight kininogen and plasma kallikrein, FXII forms the plasma contact system (2). Negatively charged surfaces promote FXII autoactivation by inducing a conformational change in the zymogen resulting in its enhanced susceptibility to proteolytic cleavage and the formation of FXIIa. FXIIa triggers the intrinsic coagulation pathway via activation of factor XI. It initiates the classical complement pathway and converts prekallikrein to kallikrein, which in turn reciprocally activates FXII and liberates bradykinin from high molecular weight kininogen (3).
Both FXII and FXIIa bind to the plethora of negatively charged artificial and physiologically relevant substances, including extracellular matrix components, glycosaminoglycans (GAGs), polyphosphate, extracellular RNA, or misfolded proteins (1, 3). The importance of heparin, a highly sulfated GAG, in the modulation of plasma contact system activity was stressed by Hojima et al. (4), who reported that autoactivation and reciprocal activation of FXII critically depend on a heparin negative charge. Interestingly, heparin-initiated FXII activation promoted bradykinin, but not FXIa, formation, suggesting that heparin, via its ability to modulate FXIIa generation, stimulates the kallikrein-kinin system, whereas the intrinsic coagulation cascade remains unaffected (5). Heparin was also found to protect FXIIa from inhibition by C1 esterase inhibitor (6), supporting the notion that surface-bound FXIIa may effectively hydrolyze its physiologic substrates.
Although binding to and activation of FXII on negatively charged surfaces are well characterized, much less is known about FXII interaction with the cell surface. Association of FXII with neutrophils (7), platelets, and endothelial cells (8–10) has been reported, pointing toward the urokinase-type plasminogen activator receptor (u-PAR), gC1qR, and cytokeratin 1 on endothelial cells (11) and GPIbα on platelets (12) as FXII docking sites on the cell membrane. Although all aforementioned receptors are structurally unrelated, with no common FXII binding sites being characterized, they are identified as glycoproteins. GPIbα, for example, contains a considerable amount of O-glycans rich in sialic acid residues, and u-PAR is subjected to extensive N-linked glycosylation (13). Putative N-glycosylation sites have also been described in gC1qR (2). In view of these findings and taking into account the important role of GAGs in the modulation of FXIIa activity, we speculated that GAG may also contribute to the binding of FXIIa to the cell surface. To verify this assumption, we used a combined strategy of enzymatic digestion, metabolic inhibition, and recombinant overexpression and found that FXIIa utilizes the heparan sulfate (HS) component of proteoglycans (PGs) to bind to the surface of the cells. Our data reveal a novel role of cell surface-associated HS in a local accumulation of FXIIa on the cell membrane.
EXPERIMENTAL PROCEDURES
Reagents
FXIIa was from American Diagnostica (Pfungstadt, Germany). Monoclonal antibody 10E4, recognizing an epitope of the intact HS chains, was purchased from U.S. Biological (Salem, MA; catalog no. H1890). Neuraminidase (sialidase) from Vibro cholerae and N-glycosidase F (recombinant, expressed in Escherichia coli) were from Roche Applied Science. Heparinase I, heparinase II, chondroitinase ABC, chondroitin sulfate (CS)-A, CS-C, CS-A/C (3:1), tosylphenylalanyl chloromethyl ketone-treated trypsin from bovine pancreas, papain, proteinase K, tunicamycin, dextran sulfate 5000, glucose, and p-nitrophenyl-β-d-xylopyranoside (β-d-xyloside) were purchased from Sigma-Aldrich. Heparinase III, low molecular weight heparin, heparan sulfate, dermatan sulfate, and heparin/GAG binding plates were from Iduron (Manchester, UK).
Cell Isolation and Culture
Human lung fibroblasts (HLF) were isolated and cultured as described previously (14). Human fetal lung fibroblasts (HLF-1, ATCC® CCL-153TM) were obtained from the American Type Culture Collection (Manassas, VA). Wild-type Chinese hamster ovary (CHO) cells, CHO-A745 and CHO-D677 cells, were a kind gift of Dr. Jeffrey D. Esko (University of California, San Diego, La Jolla, CA). HLF-1 and CHO cells were cultured in Dulbecco's modified Eagle's medium/F-12 (Life Technologies, Inc.) supplemented with 10% fetal calf serum (FCS) and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere with 5% CO2.
RNA Isolation and Real-time Quantitative RT-PCR
RNA isolation and real-time quantitative RT-PCR (RT-qPCR) were performed as described previously (15). The following oligonucleotide primers were used: human HS 6-O-sulfotransferase (HS6ST)-1 (NM_004807.2) forward, 5′-GTCTCGTAGCAGGGTGAT-3′; human HS6ST-1 reverse, 5′-GACCGAGCTCACCAACTG-3′; human HS6ST-2 (NM_001077188.1) forward, 5′-GAGGATGGTGATGTAGTGGAA-3′; human HS6ST-2 reverse, 5′-CTCTTCTCCAGGTTCTCCAC-3′; human u-PAR (NM_002659.3) forward, 5′-GACCCTGAGCTATCGGACTG-3′; human u-PAR reverse, 5′-GCTTCGGGAATAGGTGACAG-3′; human syndecan-1 (SDC-1; NM_001006946.1) forward, 5′-TGACAACTTCTCCGGCTCAG-3′; human SDC-1 reverse, 5′-GAGACGTGGGAATAGCCGTC-3′; human β-actin (NM_001101.3) forward, 5′-ATTGCCGACAGGATGCAGGAA-3′; human β-actin reverse, 5′-GCTGATCCACATCTGCTGGAA-3′. β-Actin served as a reference gene. Cycling conditions were 95 °C for 6 min, followed by 40 cycles of 95 °C for 20 s, 58 °C for 30 s, and 73 °C for 30 s. Due to the non-selective dsDNA binding of the SYBR Green dye, melting curve analysis and gel electrophoresis were performed to confirm the exclusive amplification of the expected PCR product. All changes in the target gene mRNA levels are presented as ΔCq, which was calculated by subtracting the Cq value of the target gene from the Cq value of the reference gene. The higher values of ΔCq correspond to higher relative expression of the gene of interest.
Western Blotting
Cells were lysed in ice-cold lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 μm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 mm PMSF, 1 μg/ml Complete protease inhibitor mixture (Roche Applied Science)). Protein lysates were separated on a 10% SDS-polyacrylamide gel under reducing conditions, followed by electrotransfer to a PVDF membrane. After blocking, the membrane was probed with a mouse anti-His tag antibody (Millipore, Schwalbach, Germany; catalog no. 70796). Afterward, the membrane was incubated with peroxidase-labeled secondary antibody (Dako, Gostrup, Denmark). Final detection of proteins was performed using an ECL Plus kit (Amersham Biosciences). To determine the amounts of protein loaded on the gel, the blot was stripped and reprobed using mouse anti-β-actin (Sigma-Aldrich; catalog no. A2228) antibody.
Labeling of FXIIa
One mg of FXIIa was labeled using the EZ-Link® sulfo-NHS-biotinylation kit (Thermo Scientific, Erlangen, Germany) according to the manufacturer's instruction. Alternatively, FXIIa was labeled with Alexa Fluor® 546 dye (Life Technologies) using the APEXTM antibody labeling kit (Life Technologies) according to the instructions provided by the manufacturer.
Immunocytochemistry
For immunocytochemical analysis, CHO cells either untreated or treated with 0.0016 IU of heparinase I overnight were fixed with 4% paraformaldehyde for 10 min, blocked with 1% bovine serum albumin (BSA) in PBS for 1 h at room temperature, and incubated overnight at 4 °C with a mouse anti-HS antibody. Afterward, the slides were incubated with a fluorescein-conjugated secondary antibody (Dianova, Hamburg, Germany) and mounted with Vectashield mounting medium (Vector, Burlingame, CA). Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) staining. Controls were performed by substituting the primary antibody with a species-matched isotype control. The images were captured by a Leica DMR microscope (Leica, Heidelberg, Germany) with a ×63/1.25–0.75 numerical aperture oil objective. All images illustrated are representative of at least four other areas per section, seen on at least three independent sections. To monitor binding of FXIIa to HLF, cells were fixed and blocked as detailed above and incubated with Alexa Fluor® 546-labeled FXIIa overnight at 4 °C. Slides were analyzed by confocal laser-scanning microscopy using a ×63/1.4 numerical aperture plan apochromat oil objective (LSM 780, Carl Zeiss).
FXIIa Binding to HLF
Fibroblasts or CHO cells were seeded in 96-well plates, cultured overnight, and then washed several times with HEPES-Tyrode's buffer (135 mm NaCl, 2.7 mm KCl, 11.9 mm NaHCO3, 0.36 mm NaH2PO4, 14.7 mm HEPES, 50 μm ZnCl2, 1 mm MgCl2, 2 mm CaCl2, 3.5 mg/ml BSA, 3.5 mg/ml glucose, pH 7.4). Cells were incubated for 1 h at 37 °C with 2.75 μg/ml FXIIa in the absence or presence of heparin, HS, dermatan sulfate (DS), dextran sulfate (DxS), glucose (100 μg/ml each), CS-A, or CS-C (both 200 μg/ml) in HEPES-Tyrode's buffer. In some experiments, cells were preincubated for 60 min at 37 °C with various concentrations of sialidase or N-glycosidase F; for 3 min at 37 °C with various concentrations of trypsin, papain, or proteinase K; for 2, 3, or 4 days with various concentrations of β-d-xyloside or tunicamycin; for 2 days with 10 mm Na3ClO3 and/or 30 mm Na2SO4; for 2 h with 0.004 units of chondroitinase ABC; or overnight with various concentrations of heparinase I or heparinase III. Afterward, the cells were washed with HEPES-Tyrode's buffer and then incubated with goat anti-FXII antibody (Zytomed Systems, Berlin, Germany; catalog no. 206-0056). FXII binding to the cells was detected using horseradish peroxidase-conjugated anti-goat antibody (Dako) and the peroxidase-specific substrate 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB; Thermo Fisher Scientific). Bound FXIIa was determined by measuring the absorbance at 450 nm using the microtiter plate reader (Molecular Devices, Biberach, Germany).
Cytotoxicity Assay
Cell death was assessed by measuring the activity of cytoplasmic lactate dehydrogenase released into the cell culture medium using a cytotoxicity detection kit (Roche Applied Science) according to the manufacturer's instructions.
Antisense Oligonucleotides
Commercially available siRNA sequences directed against human u-PAR, HS6ST-1, and SDC-1 and a universal negative-control siRNA (all from Ambion, Austin, TX) were employed. Cells were treated with siRNAs (100 nm each) using the siLentFect lipid reagent (Bio-Rad) according to the manufacturer's instructions. The siRNA-mediated down-regulation of the target genes was assessed 24, 48, or 72 h after transfection by RT-qPCR or Western blotting. Twenty-four h after transfection, cells were seeded onto 96-well plates for a binding assay.
Syndecan-1 Overexpression
Probe specific for mouse SDC-1 ectodomain 775-bp cDNA (232–1007 bp, X15487) was prepared by RT-PCR from mouse primary macrophages using sense primer (5′-TGCAGGATCCTGGGCAGCATGAGACGCGCGGCGCTCTGG-3′) and antisense primer (5′-CCGCTCGAGTCAGTGGTGATGGTGATGATGTCCCAGCACTTCCTTCCTGTCCAAAAGGCT-3′) containing His6 tag sequence and cloned into BamHI/SacII sites of vector pcDNA3.1(+) (Life Technologies). To reach high transfection efficiency, plasmids were transfected into HLF-1 using Lipofectamine® 2000 (Life Technologies) according to the manufacturer's instructions. Expression of His-tagged SDC-1 was confirmed by Western blotting 24 h after transfection. Eight h after transfection, cells were seeded onto 96-well plates for a binding assay.
Filter Binding Assay
To assess FXIIa binding to GAGs a filter-binding assay was performed, where a nylon membrane was soaked with buffer A (10 mm Tris-Cl (pH 7.5), 50 mm NaCl, 1 mm EDTA, and 1× Denhardt's solution (0.02% (m/v) Ficoll, 0.02% (m/v) polyvinylpyrrolidone, and 0.02% (m/v) BSA)) and fixed into a slot-blot apparatus. One hundred μl containing 10, 20, or 30 μg of DxS or DS; 2.5, 5, or 10 μg of heparin or HS; or 10, 20, or 40 μg of CS-A, CS-C, or CS-A/C were aspirated for 30 min through the filter and then cross-linked for 30 min by exposure to ultraviolet light (254 nm). After extensive washing with buffer A, the membrane was incubated at room temperature for 2 h with 10 μg/ml FXIIa in TBS containing 0.1% (v/v) Tween 20 (TBS-T), followed by incubation with goat antibody raised against FXII (Zytomed Systems) at 4 °C overnight. After extensive washing with TBS-T, the membrane was incubated with peroxidase-labeled secondary antibody (Dako), and FXIIa-GAG complexes were detected by enhanced chemiluminescence (ECL Plus kit, Amersham Biosciences). To demonstrate the efficiency of the GAG immobilization, the membrane was stained for 15 min with 0.1% toluidine blue (Serva, Heidelberg, Germany) in 10% (v/v) acetic acid and 40% (v/v) methanol.
Solid-phase Binding Assay
Heparin/GAG binding plates were coated with heparin, HS, DS, DxS, or glucose (25 μg/ml each) or with CS-A or CS-C (250 μg/ml each) according to the instructions provided by the manufacturer. The efficacy of GAG immobilization was controlled by the digoxigenin glycan detection kit (Roche Applied Science). The plates were washed three times with SAB buffer (100 mm NaCl, 50 mm sodium acetate, 0.2% Tween 20) and blocked with 1% BSA in PBS for 1 h at 37 °C. After washing the plates with SAB buffer, different concentrations of FXIIa/biot-FXIIa in SAB buffer were added into the wells, and HS, DS, DxS, CS-A, CS-C, or glucose was allowed to bind to FXIIa/biot-FXIIa for 2 h at 37 °C. After extensive washing with SAB buffer, bound FXIIa was detected using either goat anti-FXII antibody (Zytomed Systems) followed by peroxidase-labeled secondary antibody or peroxidase-labeled streptavidin (Dako). Final detection was performed using peroxidase-specific substrate, TMB. For competition experiments, the plates were coated with HS, heparin, or DS (25 μg/ml each), and serial dilutions of heparin, HS, DS, DxS, CS-A, CS-C, glucose, or unlabeled FXIIa were added simultaneously with FXIIa/biot-FXIIa into the wells. The uncoated plate was used as a control.
Microscale Thermophoresis
FXIIa interaction with HS, DxS, or CS-A was determined by changes in the thermophoretic movement by means of the microscalethermophoresis binding assay (NanoTemper Technologies, Munich, Germany) as described previously (16). Briefly, FXIIa was labeled with the fluorophore NT-647 (Monolith NT Protein Labeling Kit, NanoTemper Technologies) in the presence of a small peptide-based inhibitor, Phe-Pro-Arg-chloromethylketone. An 11-fold titration series of HS, DxS, or CS-A (5 mm to 2.44 μm) diluted 1:1 with PBS containing 0.05% Tween 20 was prepared. The concentration of NT-647-labeled factor XIIa was kept constant at 250 nm. To enable binding, FXIIa was incubated with GAG for 30 min in the dark. The reaction was then aspirated into hydrophobic coated glass capillaries and sealed with wax. At a laser voltage of 30%, the thermophoretic movement of labeled proteins was monitored with a laser on for 30 s and off for 5 s. Fluorescence was measured before laser heating (FInitial) and after 30 s of laser-on time (FHot). Normalized fluorescence, FNorm = FHot/FInitial, represents the concentration ratio of labeled molecules. To obtain the relative change in fluorescence per mill, FNorm was plotted directly and multiplied by a factor of 10. KD was calculated with NanoTemper Software (NanoTemper Technologies) from at least three independent thermophoresis measurements.
Cell Surface Expression of Heparan Sulfate
The presence of cell surface HS was detected using a 10E4 antibody. Donor or IPF HLF were seeded in 96-well plates (2 × 104 cells/well) and cultured overnight. On the following day, cells were washed with FCS-free medium and incubated with 0.001 IU of either heparinase I, II, or III in medium without FCS overnight. After the medium was removed, cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. Nonspecific binding sites were blocked by incubation with 0.1% BSA in PBS for 1 h. Next, cells were washed with PBS and incubated with 50 ng of 10E4 antibody at 4 °C overnight. Following the washing with PBS, cells were incubated with horseradish peroxidase-conjugated anti-mouse antibody (Dako). Final detection was performed using peroxidase-specific substrate, TMB.
Gap Closure Assay
HLF migratory potential was assessed using silicone cell culture inserts (Ibidi, Martinsried, Germany) attached to a 12-well plate. First, 5 × 104 cells were seeded into each silicone insert compartment and allowed to attach. Next, silicone cell culture inserts were removed, the gap area was photographed, and cells were treated with 0.0025 IU of heparinase I for 2 h in medium without FCS, followed by the addition of 0.7 μg of FXIIa. After 21 h, images of the gap area were taken, and the cells that had migrated into the gap area were counted using ImageJ (National Institutes of Health, Bethesda, MD).
Statistics
Comparison of multiple groups was performed by analysis of variance followed by Tukey's post hoc test. For comparison of two groups, Student's t test was used. p < 0.05 was considered statistically significant. Data are presented as mean ± S.D.
RESULTS
PGs Mediate FXIIa Binding to the HLF Surface
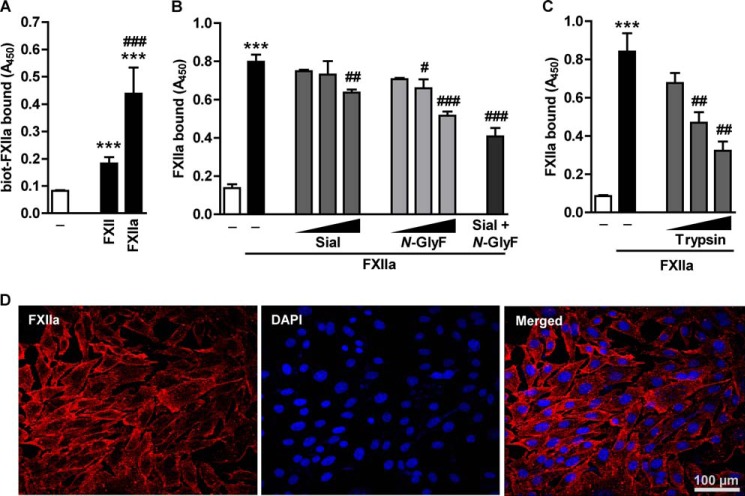
Because FXII receptors do not share a common protein sequence motif that could serve as docking sites on the cell surface, we set out to evaluate whether their carbohydrate moieties may play a role in the FXII binding to the cell membrane. Employing a cell-based ELISA, we found that HLF robustly accommodated FXIIa on the cell surface (Fig. 1A). Pretreatment of HLF with sialidase, which hydrolyzes terminal sialic acid residues, or N-glycosidase F, which entirely cleaves off N-linked GAG chains, reduced FXIIa binding in a dose-dependent manner. This effect was potentiated when these two enzymes were applied simultaneously (Fig. 1B). In order to elucidate the role of the protein component in the association of FXIIa with HLF, the cells were preincubated with trypsin, papain, or proteinase K. Whereas pretreatment of HLF with trypsin diminished binding of FXIIa by 60% (Fig. 1C), minute amounts of papain and protease K caused cell loss, precluding the assessment of FXIIa interaction with the cell surface (data not shown). Together, these results indicate that glycoproteins/proteoglycans may be involved in the binding of FXIIa to the cell surface. We also monitored the binding of Alexa Fluor® 546-labeled FXIIa to HLF by an immunofluorescence analysis. A prominent pericellular staining, predominantly localized to cell-cell contacts and lamellopodia-like structures, was observed (Fig. 1D). Collectively, all of these experiments prompted the question of whether PG might contribute to the binding of FXIIa to the cell surface.
FIGURE 1.
Glycosidases and proteases affect FXIIa binding to the HLF surface. A, HLF were treated with 1 μg/ml biotin-labeled FXII/FXIIa for 1 h at 37 °C. Following the incubation with peroxidase-labeled streptavidin, binding of FXII/FXIIa to the cell surface was analyzed. Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus control; ###, p < 0.001 versus FXII. B, cells were treated with 1, 0.1, or 0.01 units/ml sialidase (Sial), N-Glycosidase F (N-GlyF), or a combination of both enzymes (0.1 units/ml each) for 60 min at 37 °C to remove surface GAG. C, to digest surface proteins, cells were incubated with 0.00025, 0.0001, or 0.00005% (w/v) trypsin for 3 min at 37 °C. B and C, after enzyme removal, cells were incubated with 2.75 μg/ml FXIIa and subjected to cell-based ELISA with anti-FXII antibodies to determine the degree of FXIIa accumulation on the cell surface. Data represent mean ± S.D. (error bars) (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus FXIIa only. D, representative immunofluorescence pictures demonstrating binding of Alexa Fluor® 546-labeled FXIIa to HLF. Magnification was ×630.
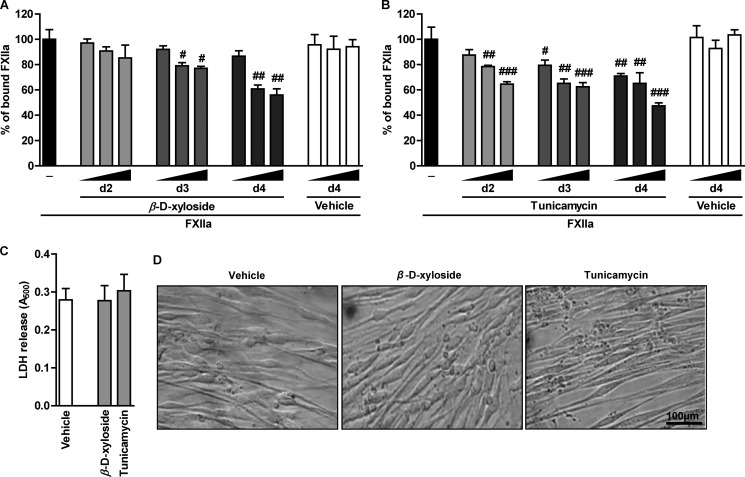
To examine the role of PG GAG side chains in the interaction of FXIIa with HLF, the cells were treated with β-d-xyloside, an inhibitor of O-linked GAG attachment to the core protein, and thereafter the association of FXIIa with the cell membrane was studied. As illustrated in Fig. 2A, FXIIa binding was gradually decreased during incubation with increasing concentrations of β-d-xyloside. Moreover, prolonged incubation with β-d-xyloside resulted in pronounced reduction of FXIIa binding capacity (to 56% of control cells treated with the vehicle). In parallel, the impact of protein N-glycosylation on the attachment of FXIIa to HLF was investigated. Treatment of cells with tunicamycin, an inhibitor of N-linked oligosaccharide biosynthesis, resulted in a time- and concentration-dependent reduction of FXIIa binding to 50% of control cells grown in the presence of the vehicle (Fig. 2B). To exclude the possibility that the changes in FXIIa binding capacity of HLF result from cell damage caused by 4-day-long incubation of cells with 10 μm β-d-xyloside or 200 ng/ml tunicamycin, a lactate dehydrogenase cytotoxicity assay was performed. This experimental procedure revealed that β-d-xyloside and tunicamycin exhibited no cytotoxic effects on the cells (Fig. 2C). Furthermore, microscopic inspection of the cells treated for 4 days with β-d-xyloside or tunicamycin did not display any changes in the cell shape (Fig. 2D). In summary, these data suggest that PG-associated GAGs may contribute to the FXIIa binding to HLF.
FIGURE 2.
Interference with GAG biosynthesis decreases accumulation of FXIIa on the cell surface. A and B, HLF were treated with 2.5, 5, or 10 μm β-d-xyloside (A) or 50, 100, or 200 ng/ml tunicamycin (B) for 2, 3, or 4 days to prevent initiation of GAG synthesis or to inhibit N-linked glycosylation, respectively. Following the FXIIa treatment, accumulation of FXIIa on the cell surface was assessed by cell-based ELISA, and resulting values are given as a percentage of FXIIa binding to β-d-xyloside- or tunicamycin-untreated cells (set as 100%) (n = 4); #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus FXIIa only. C and D, cell damage caused by 4-day-long incubation of HLF with 10 μm β-d-xyloside or 200 ng/ml tunicamycin as assessed by release of lactate dehydrogenase (LDH) into cell culture media (C) or by microscopic inspection of the cells (D). The data are representative of three independent experiments. Magnification was ×100. Data represent mean ± S.D. (error bars).
HS Provides Binding Sites for FXIIa on HLF
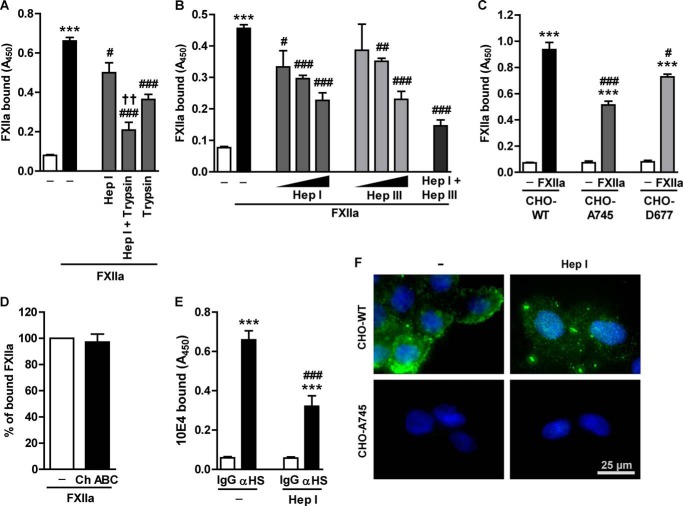
Previously, Badellino and Walsh (17) provided evidence of a FXIIa interaction with immobilized heparin, a GAG structurally related to HS. Therefore, we wondered whether membrane-bound HS-PG might be involved in FXIIa accumulation on the fibroblast surface. Enzymatic digestion of surface HS by heparinase I or protein removal by trypsin treatment reduced FXIIa binding by 24 and 45%, respectively (Fig. 3A). This effect was potentiated when heparinase I and trypsin were applied simultaneously. Next, we investigated the role of differently sulfated HS structures in FXIIa binding by using heparinase I and heparinase III. N- and 6-O-sulfated glucosamine linked to 2-O-sulfated iduronic acid is a preferred dissacharide recognized by heparinase I, whereas heparinase III cleaves glycosidic linkage between N-acetylated or N-sulfated glucosamine and glucuronic acid (18). This distinct substrate specificity dictates degradation of high- or low-sulfated HS regions by heparinase I or heparinase III, respectively. As shown in Fig. 3B, treatment of cells with heparinase I or heparinase III decreased FXIIa binding in a dose-dependent manner. Interestingly, at a lower enzyme concentration, the heparinase III effect was less pronounced than that of heparinase I, implying that highly sulfated HS domains account for the majority of FXIIa binding to HLF. Eventually, however, a higher dose of heparinase III reduced FXIIa binding to the same level as did heparinase I, implying that the progressive removal of the otherwise intact highly sulfated domains accounts for this phenomenon. Combined action of heparinase I and heparinase III diminished FXIIa binding capacity below the level observed when these two enzymes were applied separately. To ultimately prove the involvement of HS in the FXIIa binding, we employed cells that do not express GAG entirely (CHO-A745 cell line) or specifically lack HS (CHO-D677 cell line) at the cell surface (19). As shown in Fig. 3C, mutant cell lines showed a significantly lower FXIIa binding capacity. Accumulation of FXIIa on CHO-A745 was reduced by 45% and on CHO-D677 by 22% when compared with wild-type cells. Although CHO-D677 cells are HS-deficient, this cell line expresses increased amounts of CS at the cell surface (17). Because the reduction of FXIIa binding to CHO-D677 was less pronounced than to CHO-A745, we assumed that elevated CS levels compensate for the lack of HS and assist in the interaction of FXIIa with the cell surface. To verify whether CS moieties participate in the FXIIa binding to HLF, the cells were treated with chondroitinase ABC in order to remove CS chains. As seen in Fig. 3D, FXIIa binding was not affected by chondroitinase ABC treatment, demonstrating that CS is not involved in the FXIIa sequestration to HLF. Collectively, these data indicate that HS-PG plays an important role in the interaction of FXIIa with the cell surface.
FIGURE 3.
HS mediates interaction of FXIIa with HLF. A, to simultaneously remove HS and surface proteins, HLF were co-incubated with 0.0016 IU of heparinase I (Hep I) and 0.0001% (w/v) trypsin. B, HLF were incubated with 0.0032, 0.0016, or 0.0008 IU of heparinase I; with 0.001, 0.0004, or 0.0002 IU of heparinase III (Hep III); or with both enzymes (0.0016 IU of heparinase I and 0.001 IU of heparinase III) to remove HS from the cell surface. A and B, after incubation with anti-FXII antibodies, FXIIa binding capacity of HLF was analyzed by cell-based ELISA. Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus FXIIa only; ††, p < 0.01 versus Hep I-treated cells. C, wild-type (CHO-WT), GAG-deficient (CHO-A745), or HS-deficient (CHO-D677) CHO cells were treated with FXIIa. Surface accumulation of FXIIa was examined as described above. Data represent mean ± S.D. (error bars) (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ###, p < 0.001 versus CHO-WT cells incubated with FXIIa. D, impact of CS degradation on FXIIa binding. Cells were treated with 0.004 units of chondroitinase ABC (Ch ABC) for 2 h, followed by incubation with FXIIa and measurement of FXIIa binding capacity as described above. Date are given as a percentage of FXIIa binding to chondroitinase ABC-untreated cells (set as 100%) (n = 3). E, effect of heparinase I enzymatic activity on HS surface expression. Cell-based ELISA was performed on the intact HLF and on the cells treated with 0.0016 IU of heparinase I and subsequently incubated with 10E4 antibodies directed against native HS-type GAG (αHS). Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus IgG; ###, p < 0.001 versus anti-HS-type GAG only. F, representative fluorescence microscopy images of CHO-WT and CHO-A745 cells stained with antibodies directed against native heparin sulfate chains (green). Cells were either treated with 0.0016 IU of heparinase I or left untreated (CTRL). Magnification was ×400.
Heparinase I activity toward cell surface HS was controlled by cell-based ELISA performed with 10E4 antibody, which recognizes native HS-type GAG. Heparinase I decreased 10E4 antibody binding to HLF as compared with control (Fig. 3E). Likewise, HS immunoreactivity was reduced upon heparinase I treatment of CHO-WT cells and was not visible on CHO-A745 cells (Fig. 3F).
FXIIa Binds to Immobilized HS
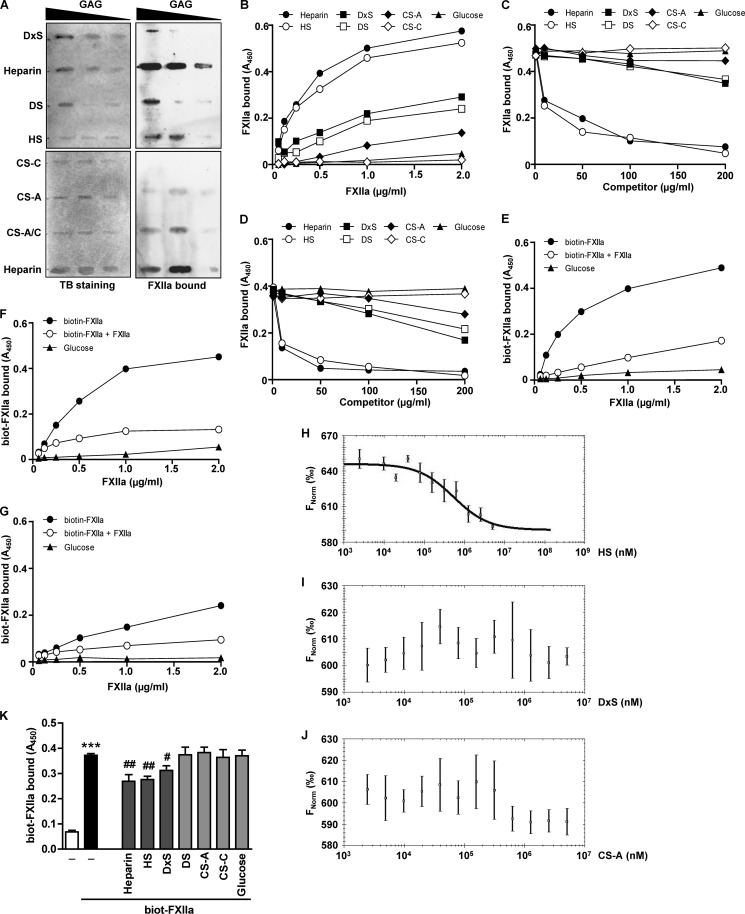
To analyze the binding of FXIIa to immobilized GAGs, a slot-blot filter-binding assay was performed. Increasing concentrations of DxS, DS, HS, CS-C, CS-A, and CS-A/C were spotted on the membrane and subsequently probed with FXIIa. Heparin was employed as a positive control (17). Although we observed association of FXIIa with heparin, HS, DS, DxS, and CS-A (Fig. 4A, right), a direct comparison of FXIIa interaction with diverse GAGs was not possible due to different binding affinities of GAGs to the nylon membrane (Fig. 4A, left panel). To further characterize the interaction of FXIIa with various GAGs, a solid-phase binding assay was employed. Here, a microtiter plate was coated with GAGs, and increasing concentrations of FXIIa were applied. The efficacy of the coating procedure was monitored by a digoxigenin-based enzyme-linked immunosorbent assay (data not shown). While the most efficient binding of FXIIa to heparin and HS was observed, the interaction of FXIIa with DxS, DS, and CS-A was less pronounced. FXIIa did not bind CS-C and glucose applied as a control (Fig. 4B).
FIGURE 4.
FXIIa binds to immobilized and cell surface-associated HS. A, binding of FXIIa to DxS or DS (30, 20 or 10 μg of each), heparin or HS (10, 5, or 2.5 μg of each), and CS-A, CS-C, or CS-A/C (40, 20, or 10 μg of each) immobilized on a nylon membrane. Toluidine blue (TB) staining and immunodetection with anti-FXII antibodies were used to visualize immobilized GAGs and bound FXIIa, respectively. The membranes shown are representative of six independent experiments. B–D, FXIIa binding to GAGs immobilized onto the surface of microtiter plates. B, heparin, HS, DxS, DS, or glucose (25 μg/ml each) and CS-A or CS-C (250 μg/ml each) were surface-immobilized and incubated with the indicated amounts of FXIIa. C and D, surface-immobilized HS (C) or DS (D) was incubated with 2 μg/ml FXIIa in the presence of the indicated amounts of heparin, HS, DxS, DS, CS-A, CS-C, or glucose used as competitors. E–G, surface-immobilized HS (E), heparin (F), or DS (G) was incubated with increasing amounts of biotinylated FXIIa in the presence or absence of a 100-fold molar excess of unlabeled FXIIa acting as a competitor. Following the washing steps, bound FXIIa was quantified using anti-FXII antibody (B–D) or directly with peroxidase-coupled streptavidin (E–G). B–G, data are representative of three independent experiments performed in triplicates. H–J, binding of FXIIa to HS (H), DxS (I), or CS-A (J) as assessed by microscale thermophoresis. KD values were calculated from three independent thermophoresis measurements. K, effect of solubilized GAG on the FXIIa binding to HLF. Biotinylated FXIIa was incubated with HLF in the absence or presence of 100 μg/ml heparin, HS, DxS, DS, or glucose or 200 μg/ml CS-A or CS-C. After extensive washing steps to remove unbound FXIIa and competitors, the amount of cell surface bound FXIIa was quantified using peroxidase-coupled streptavidin. Data represent mean ± S.D. (error bars) (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ##, p < 0.01 versus FXIIa only.
To further analyze the binding of FXIIa to GAGs, we performed competition experiments with HS- or DS-coated microwell plates, which were then incubated with FXIIa in the presence of serial dilutions of heparin, HS, DxS, DS, CS-A, CS-C, or glucose. Free heparin and HS effectively blocked binding of FXIIa to immobilized HS (Fig. 4C) or DS (Fig. 4D). DxS, DS, and CS-A at the highest concentration used (100–200 μg/ml) moderately competed with immobilized HS or DS for FXIIa binding, whereas CS-C and glucose were ineffective (Fig. 4, C and D). Next, we followed the interaction between immobilized HS and biotinylated FXIIa in the presence of unlabeled FXIIa acting as a competitor. Unlabeled FXIIa efficiently blocked binding of biotin-labeled FXIIa to HS (Fig. 4E). Similar results were obtained when immobilized heparin instead of HS was used (Fig. 4F). Although biotinylated FXIIa did not bind efficiently to DS, this interaction was also abrogated by unlabeled FXIIa (Fig. 4G). To confirm FXIIa binding to HS, we performed microscale thermophoresis. As depicted in Fig. 4H, FXIIa bound to HS with a KD of 270 ± 38 μm. Conversely, no binding of FXIIa to DxS (Fig. 4I) or CS-A (Fig. 4J) was observed. The measurements of FXIIa interaction with C1 inhibitor served as a positive control (data not shown).
Finally, we tested whether free GAGs compete with FXIIa binding to HLF. To this end, HLF were incubated with FXIIa in the absence or presence of heparin, HS, DxS, DS, CS-A, CS-C, or glucose. As shown in Fig. 4K, heparin and HS inhibited the association of FXIIa with HLF by 29 and 26%, respectively. When compared with heparin and HS, DxS showed a moderate effect (15%), whereas DS, CS-A, CS-C, and glucose failed to interfere with the binding of FXIIa to the cells (Fig. 4K).
Overexpression of HS-type PG Modulates FXIIa Binding to Cell Surface
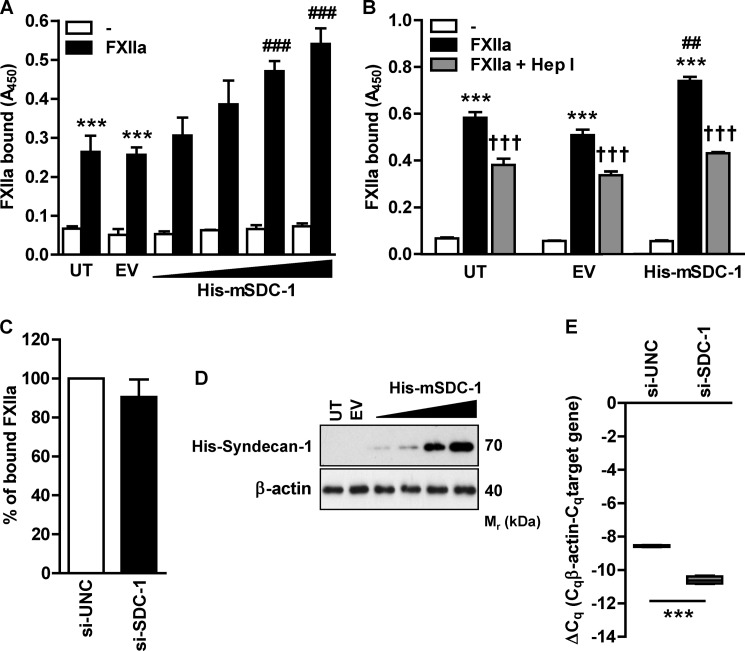
We then explored whether transfection of HLF-1 with a vector carrying SDC-1, a prototype transmembrane HS-PG, might influence FXIIa binding capacity of the cells (20). Gradual SDC-1 overexpression progressively enhanced FXIIa accumulation, ultimately doubling the binding capacity of untransfected cells (Fig. 5A). Next, we verified whether the HS component of SDC-1 is responsible for increased FXIIa binding capacity of HLF-1. Digestion of sulfate-rich HS domains by heparinase I abolished the favorable effect of SDC-1 overexpression and reduced FXIIa binding to the level observed in untransfected cells (Fig. 5B). Collectively, these results demonstrate that HS-type PGs mediate FXIIa accumulation on the cell surface. To investigate whether decreased SDC-1 expression will reduce FXIIa binding, we employed siRNA directed against SDC-1. Interestingly, FXIIa binding capacity of HLF was not affected upon SDC-1 knockdown (Fig. 5C). This implies that endogenous SDC-1 provides a minority of FXIIa binding sites on HLF and that other HS-PGs are responsible for accumulation of FXIIa on this cell type. Fig. 5D demonstrates the efficacy of SDC-1 overexpression in HLF-1, and Fig. 5E shows the efficacy of SDC-1 knockdown.
FIGURE 5.
Overexpression of HS-PG increases FXIIa binding capacity of HLF. A, HLF-1 cells were either left untransfected (UT) or transfected with empty vector (EV) or with increasing concentrations of SDC-1-encoding vector (His-mSDC-1), incubated with FXIIa on the following day, and subjected to cell-based ELISA with anti-FXII antibodies to determine the degree of FXIIa accumulation on the cell surface. Data represent mean ± S.D. (error bars) (n = 4); ***, p < 0.001 versus control; ###, p < 0.001 versus FXIIa-treated cells transfected with empty vector. B, prior to the incubation with FXIIa, SDC-1-overexpressing cells were treated with 0.0016 IU of heparinase I (Hep I). The amount of FXIIa on the cell surface was measured as described above. Data represent mean ± S.D. (n = 3); ***, p < 0.001 versus control; ##, p < 0.01 versus FXIIa-treated cells transfected with empty vector; †††, p < 0.001 versus cells treated with FXIIa only. C, SDC-1 or universal control siRNA (si-SDC-1 or si-UNC, respectively)-transfected cells were incubated with FXIIa, followed by measurement of FXIIa binding capacity using a cell-based ELISA. Date are given as a percentage of FXIIa binding to siUNC-treated cells (set as 100%). (n = 3). D, evaluation of SDC-1 overexpression in HLF-1 cells. Cells were either left untransfected or transfected with empty vector or various doses of SDC-1-encoding vector (His-mSDC-1). Expression of SDC-1 was detected with anti-His antibody, and β-actin served as a loading control. Representative blots are shown (n = 3). E, evaluation of SDC-1 knockdown. Cells were transfected with 100 nm si-SDC-1 or si-UNC. After 72 h, the efficacy of SDC-1 knockdown was assessed by RT-qPCR. RT-qPCR data are presented as ΔCq. β-Actin served as a reference gene (n = 3).
Altered HS Sulfation Enhances FXIIa Binding Capacity of IPF HLF
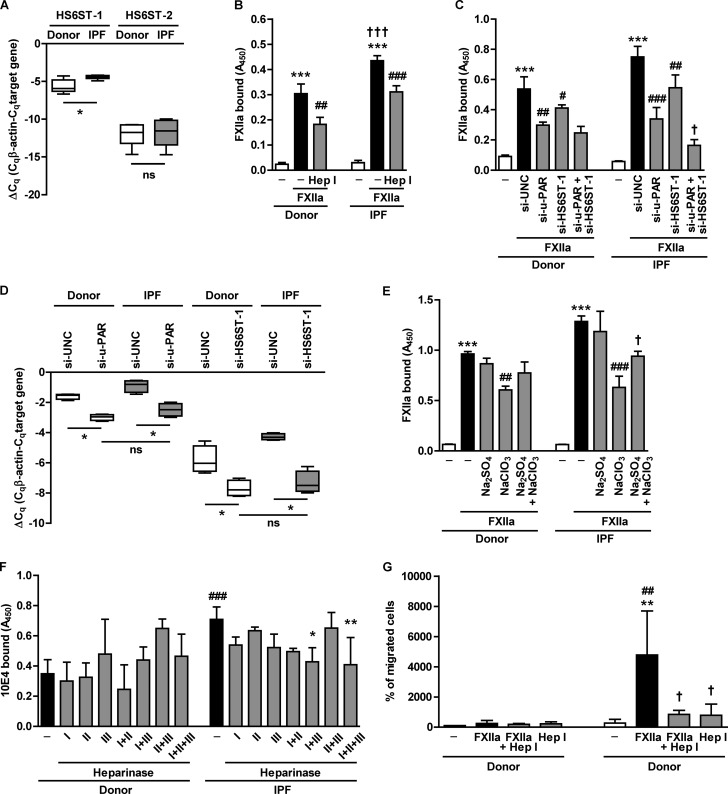
In a recent paper, Lu et al. (21) reported elevated levels of HS 6-O-sulfation in the lungs of patients suffering from idiopathic pulmonary fibrosis (IPF). This phenomenon was associated with the increased expression of HS 6-O-sulfotransferase (HS6ST)-1 and -2 (21), enzymes catalyzing sulfation at the 6-O-position of N-acetylglucosamine residues. Thus, we investigated whether increased HS 6-O-sulfation may have an impact on FXIIa binding capacity of IPF HLF. In agreement with previously published data (21), analysis of HS6ST-1 and HS6ST-2 expression revealed increased HS6ST-1 mRNA levels in fibroblasts isolated from IPF lungs as compared with fibroblasts isolated from donor lungs (Fig. 6A). Moreover, IPF HLF bound FXIIa more efficiently than donor HLF (Fig. 6B). Pretreatment of cells with heparinase I reduced FXIIa association with HLF, irrespective of whether the cells were derived from donor or IPF lungs (Fig. 6B). Bearing in mind that heparinase I is unable to entirely remove surface HS (22), we assumed that residual HS chains attached to the core proteins after heparinase I treatment can still act as functional FXIIa binding sites and confer similar FXIIa binding capacity of both cell types. To ultimately prove the role of increased HS6ST-1 expression in the FXIIa binding capacity of IPF HLF, HS6ST-1 was knocked down using siRNA technology. Depletion of HS6ST-1 reduced the accumulation of FXIIa on both the donor and IPF fibroblast cell surface (Fig. 6C). This effect was potentiated when u-PAR, the receptor involved in the binding of FXIIa to HLF,3 was knocked down. Remarkably, simultaneous depletion of u-PAR and HS6ST-1 had an additive effect on FXIIa binding to IPF, but not to donor, HLF (Fig. 6C), implying that enhanced HS 6-O-sulfation, not related to u-PAR, plays a role in the attachment of FXIIa to IPF HLF. The efficiency of u-PAR and HS6ST-1 knockdown in donor and IPF HLF is demonstrated in Fig. 6D. To further analyze the role of HS sulfation in the accumulation of FXIIa on IPF HLF, we performed experiments in the presence of sodium chlorate, an inhibitor of intracellular sulfation. Pretreatment of the cells with NaClO3 inhibited the attachment of FXIIa to donor HLF by 37% and to IPF HLF by 51% when compared with the respective control (Fig. 6E). The sodium chlorate effect in IPF HLF was attenuated when the culture medium was supplemented with sodium sulfate used to overcome sodium chlorate inhibition. In order to investigate the sulfation pattern of donor and IPF HLF, we assessed the binding of a 10E4 antibody to the cells pretreated with heparinases from Flavobacterium heparinum. Under control conditions, the binding of the 10E4 antibody to IPF HLF was 2-fold higher than to donor HLF (Fig. 6F). Because the 10E4 antibody epitope contains N- or O-sulfate groups (23), these data point to an increased amount of these groups associated with HS on the cell surface of fibroblasts derived from diseased lung. When the cells were subjected to enzymatic HS digestion, we observed that combined heparinase I and III or I, II, and III treatment reduced the amount of 10E4 epitopes on IPF HLF by 40 and 42%, respectively (Fig. 6F). As mentioned above, heparinase I cleaves within highly sulfated HS domains, whereas heparinase III displays specificity toward non-sulfated HS regions, thus indicating that combined treatment resulted in the progressive removal of the major portion of cell surface HS. Moreover, because the effect of heparinase treatment was observed only in cells isolated from diseased lungs, we postulate that HS chains on IPF HLF are characterized by a higher sulfation when compared with donor HLF. Collectively, these results point to an important role of HS sulfation in the elevated FXIIa binding capacity of IPF HLF.
FIGURE 6.
Sulfate groups mediate increased FXIIa accumulation on the surface of IPF HLF. A, HS6ST-1 and HS6ST-2 mRNA expression in fibroblasts isolated from donor (n = 6) or IPF (n = 6) lungs. RT-qPCR data are presented as ΔCq. β-Actin served as a reference gene. *, p < 0.05; n.s., non-significant. B, donor and IPF HLF were treated with 0.001 IU of heparinase I (Hep I), followed by incubation with FXIIa. FXIIa binding capacity was analyzed by cell-based ELISA with anti-FXII antibodies. Data represent mean ± S.D. (error bars) (n = 4); ***, p < 0.001 versus control; ##, p < 0.01; ###, p < 0.001 versus HLF treated with FXIIa only; †††, p < 0.001 versus FXIIa-treated donor HLF. C, donor and IPF HLF were transfected with universal control siRNA (si-UNC) or siRNA directed against u-PAR (si-u-PAR) or HS6ST-1 (si-HS6ST-1) (100 nm each) and incubated with FXIIa. Binding of FXIIa on the cell surface was estimated as described above. Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus control; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus FXIIa-treated cells transfected with si-UNC; †, p < 0.05 versus IPF HLF transfected with si-u-PAR. D, efficacy of u-PAR and HS6ST-1 knockdown in donor and IPF HLF. RT-qPCR data are presented as ΔCq. β-Actin served as a reference gene (n = 3); *, p < 0.05; ns, not significant. E, binding of FXIIa to donor and IPF HLF treated for 48 h with 10 mm NaClO3, 30 mm Na2SO4, or a combination of these reagents. Surface accumulation of FXIIa was measured by cell-based ELISA. Data represent mean ± S.D. (n = 4); ***, p < 0.001 versus control; ##, p < 0.01; ###, p < 0.001 versus FXIIa; †, p < 0.05 versus NaClO3- and FXIIa-treated cells. F, effect of heparinase enzymatic activity on HS surface expression on donor and IPF HLF. Cells were treated with 0.001 IU of either heparinase I, II, or III or with combinations of these enzymes. The expression of cell surface HS was assessed by cell-based ELISA using the 10E4 antibody. Data represent mean ± S.D. (n = 3); *, p < 0.05; **, p < 0.01 versus heparinase-untreated HLF; ###, p < 0.001 versus heparinase-untreated donor HLF. G, migratory potential of donor and IPF HLF as assessed by the gap closure assay. Cells were incubated with 0.0025 IU of heparinase I, followed by treatment with 0.7 μg of FXIIa. Cell migration is expressed as a percentage of migrated cells in various experimental groups relative to the number of migrating untreated donor HLF (set as 100%). Data represent mean ± S.D. (n = 3); **, p < 0.01 versus untreated IPF HLF; ##, p < 0.01 versus FXIIa-treated donor HLF; †, p < 0.05 versus FXIIa-treated IPF HLF.
HS Mediates FXIIa-induced IPF HLF Migration
Finally, our efforts concentrated on elucidating the functional consequence of increased FXIIa binding to IPF HLF. We profiled the expression of several profibrotic genes upon stimulation of donor or IPF HLF with FXIIa and observed that expression of collagen 1, collagen 4, matrix metalloproteinase-9, matrix metalloproteinase-2, or vimentin remained unaffected by the treatment (data not shown). We then endeavored to determine whether FXIIa may modify HLF cellular properties and observed no FXIIa effect on the donor HLF migration (Fig. 6G). However, FXIIa had a striking effect on the IPF HLF by inducing pronounced cell migration that was 18-fold higher than in the non-stimulated cells. Moreover, heparinase I treatment nearly abolished the effect of FXIIa and reduced fibroblast migration to a value just 3-fold higher then the control cells, indicating that the promigratory effect of FXIIa on IPF HLF depends on the presence of cell surface HS chains.
DISCUSSION
In the present work, we demonstrate that HS-type PGs contribute to FXIIa binding capacity of HLF. HS is a linear biopolymer containing numerous negatively charged sulfated groups (24). The repetitive nature of HS chain structure is used by numerous proteins to bind to the cell surface. These interactions usually occur between positively charged amino acids of the respective protein and the negatively charged sulfate groups of HS (25). Thus, changes in the sulfation pattern of HS may result in altered affinity for growth factors, adhesion molecules, or cytokines. Our study demonstrates the direct binding of FXIIa to immobilized HS and heparin and, to a lesser extent, to other GAG species. Interestingly, heparin interacted with FXIIa slightly stronger than with HS. Compared with heparin, HS is characterized by lower sulfation of disaccharide residues and, thereby, lower negative net charge. Because the strength of FXIIa binding depends on the negative charge density of the surface (3), the reduced number of sulfated residues in HS may explain the slightly diminished FXIIa binding capacity of HS and, in addition, points toward the role of sulfation in the association of GAGs with FXIIa.
At present, we can only speculate about the region of FXIIa involved in the interaction with HS chains. In general, the protein HS-binding platforms are organized into large, positively charged surfaces composed of several basic amino acids that do not display a common sequence pattern (24). Although several structural domains of FXII/FXIIa have been described to bind negatively charged surfaces, the precise location of the FXII site involved in the interaction with GAG chains has not yet been identified (1, 17). Analysis of heparin binding to a number of other coagulation factors may provide mechanistic and structural insights as to how FXIIa associates with HS-type GAGs (25). For example, heparin binding to thrombin is mediated by a large electropositive area on the protein surface composed of several basic amino acids (26). Similarly, the number of basic amino acids on the surface of activated protein C provides a binding site for heparin and HS (27). Moreover, structural studies on factor IX or factor X indicate that these proteins interact with heparin via positively charged surfaces homologous to the heparin binding site described in thrombin (28, 29). Interestingly, the study of FXII architecture reveals the presence of an electropositive path on the protein surface formed by the fibronectin type II domain and the epidermal growth factor-like region on the protein N terminus (30). Because similar structures were found to mediate the binding of various coagulation factors to heparin, it is tempting to speculate that this electropositive area may mediate FXIIa interaction with HS-type GAGs. However, whether this positively charged region of FXIIa is essential for the binding to HS-type GAGs remains the objective of further studies.
Although the role of GAGs in the modulation of FXII activity is well characterized, studies describing the involvement of GAGs in the binding of FXIIa to the cell surface are lacking, and available reports rather highlight the role of GAG chains in association with other coagulation factors and their inhibitors with the cell membrane. Ho et al. (31) reported that microvascular endothelial cells utilize HS to bind, and subsequently degrade, factor Xa-tissue factor pathway inhibitor complexes. Furthermore, cell surface HS chains were found to bind thrombin to the endothelial cells (32). HS was also shown to bind antithrombin, a physiological inhibitor of thrombin, to the microvascular endothelial cells (33, 34). Finally, several studies demonstrated the importance of HS for the attachment of HK to the cell surface (35, 36). Interestingly, the degree of HS involvement in this process varied depending on the cell type used. Surface HS was shown to account for the majority of HK binding to endothelial cells, whereas accumulation of HK on fibroblasts and epithelial cells predominantly depended on chondroitin sulfate (37). With regard to FXII/FXIIa, several studies demonstrated its binding to the cell surface. Whereas FXII was shown to interact with neutrophils (7), endothelial cells and platelets were found to bind both FXII and FXIIa (8–10, 12). Moreover, FXIIa was shown to compete with HK for the binding to endothelial cells and platelets, suggesting that these two proteins share binding sites on the cells (10, 11). The present study develops this idea and demonstrates that FXIIa utilizes HS-PG to dock on the cell surface of HLF. Furthermore, it provides evidence of a collaborative action of u-PAR and HS-PG in the binding of FXIIa to the cell surface of fibroblasts isolated from the lungs of patients suffering from IPF. Idiopathic pulmonary fibrosis is a devastating disease that is characterized by excessive proliferation and invasive phenotype of fibroblasts in the lungs of affected patients (38–40). Here, we demonstrate that FXIIa induces IPF, but not donor, HLF migration in an HS-dependent manner. The role of HS-decorated PG in modulation of cell motility was stressed before (e.g. in breast cancer cells, where elevated syndecan-1 and -4 expression was associated with changes in cell adhesion and migration) (41, 42). Moreover, our unpublished results4 demonstrating potent mitogenic activities of FXIIa toward HLF further support a role of FXIIa in fibrogenesis. The ability of FXII/FXIIa to stimulate proliferation of alveolar type II cells, aortic smooth muscle cells, hepatocytes, and endothelial cells has been described previously (43, 44). Furthermore, FXII/FXIIa-induced endothelial cell proliferation was shown to be physiologically relevant because it had a profound impact on angiogenesis (45). Thus, HS-dependent sequestration of FXII/FXIIa to the cell surface may have important (but not related to its procoagulant activities) consequences in vivo.
Interestingly, the association of FXIIa with IPF HLF was dependent on the degree of HS sulfation, because the knockdown of HS6ST-1 and the addition of sodium chlorate to the culture medium markedly diminished FXIIa binding capacity of this cell type. The expression of HS6ST-1 was found to be dysregulated in the lungs of IPF patients. In particular, HS6ST-1-positive fibroblast foci were detected in the IPF lungs, and increased HS6ST-1 mRNA levels were found in primary IPF lung fibroblasts (21). The amount of O-sulfate groups, especially 6-O-sulfates, in HS determines its ability to interact with proteins. For example, 6-O-sulfation of HS was found to regulate TGF-β signaling in fibroblasts and to promote the interaction of urokinase with u-PAR on the cell surface (46). In addition, 6-O-sulfation mediated the binding of the platelet-derived growth factor, hepatocyte growth factor, and lipoprotein lipase to the HS, whereas the binding of the epidermal growth factor, fibroblast growth factor (FGF)-2, and FGF receptor to HS was dependent on both 6-O- and 2-O-sulfation, and the interaction with antithrombin required the presence of 6-O- and 3-O-sulfate groups (47, 48). Moreover, the expression of SULF1, an enzyme catalyzing the removal of 6-O-sulfate groups from HS, was found to be down-regulated in several cancer cell types, resulting in increased HS sulfation and subsequently enhanced epidermal growth factor signaling (41). Here, we demonstrate an increased 10E4 antibody binding to the IPF HLF when compared with donor HLF, an effect that was reduced following treatment with heparinase I and III. The specificity of heparinase I directs its activity toward a linkage between N- and 6-O-sulfated glucosamine and 2-O-sulfated iduronic acid, whereas heparinase III cleaves between non-sulfated or N-sulfated glucosamine and iduronic acid or glucoronic acid (18). Because immunoreactivity of IPF, but not donor, HLF was reduced after combined treatment with heparinase I and III, we concluded that IPF HLF contain more N-sulfated and 6-O- and 2-O-sulfated HS chains at the cell surface. This interpretation is in line with the results of our FXIIa binding experiments with sodium chlorate and HS6ST-1 knockdown, which showed greater susceptibility of IPF HLF to the inhibition of HS sulfation. Thus, we propose that elevated HS sulfation observed in IPF lungs, although not directly required, could help to bring FXIIa into close proximity of its signaling receptors and help to increase FXIIa local concentration. The question of whether HS chains may regulate FXII activation on the fibroblast cell surface still requires further investigation.
In summary, our results demonstrate an important role of HS-decorated PG in the binding of FXIIa to the cell surface and in particular to IPF HLF characterized by increased expression of u-PAR and HS6ST-1. Thus, enhanced sequestration of FXIIa to IPF HLF may dictate its new, until now undiscovered, role in the pathogenesis of pulmonary fibrosis.
Acknowledgments
We thank B. Taborski, Y. Horn, and H. Thiele for excellent technical assistance.
This study was supported by Deutsche Forschungsgemeinschaft Grant WY119/1-1 (to M. W.) and SFB 815, Project A5 (to L. S.), the Excellence Cluster “Cardiopulmonary System” (to M. W. and L. S.), and the University Medical Center Giessen and Marburg (to M. W.).
L. Wujak and M. Wygrecka, unpublished observation.
E. Jablonska and M. Wygrecka, unpublished results.
- FXII
- factor XII
- FXIIa
- activated FXII
- GAG
- glycosaminoglycan
- HLF
- human lung fibroblast(s)
- HS
- heparan sulfate
- IPF
- idiopathic pulmonary fibrosis
- u-PAR
- urokinase-type plasminogen activator receptor
- PG
- proteoglycan
- HS6ST
- HS 6-O-sulfotransferase
- CS
- chondroitin sulfate
- β-d-xyloside
- p-nitrophenyl-β-d-xylopyranoside
- RT-qPCR
- real-time quantitative RT-PCR
- SDC-1
- syndecan-1
- DS
- dermatan sulfate
- DxS
- dextran sulfate
- TMB
- 3,3′,5,5′-tetramethylbenzidine dihydrochloride.
REFERENCES
- 1. Stavrou E., Schmaier A. H. (2010) Factor XII: what does it contribute to our understanding of the physiology and pathophysiology of hemostasis & thrombosis. Thromb. Res. 125, 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghebrehiwet B., Lim B. L., Peerschke E. I., Willis A. C., Reid K. B. (1994) Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular “heads” of C1q. J. Exp. Med. 179, 1809–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Renné T., Schmaier A. H., Nickel K. F., Blombäck M., Maas C. (2012) In vivo roles of factor XII. Blood 120, 4296–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hojima Y., Cochrane C. G., Wiggins R. C., Austen K. F., Stevens R. L. (1984) In vitro activation of the contact (Hageman factor) system of plasma by heparin and chondroitin sulfate E. Blood 63, 1453–1459 [PubMed] [Google Scholar]
- 5. Oschatz C., Maas C., Lecher B., Jansen T., Björkqvist J., Tradler T., Sedlmeier R., Burfeind P., Cichon S., Hammerschmidt S., Müller-Esterl W., Wuillemin W. A., Nilsson G., Renné T. (2011) Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity 34, 258–268 [DOI] [PubMed] [Google Scholar]
- 6. Pixley R. A., Schmaier A., Colman R. W. (1987) Effect of negatively charged activating compounds on inactivation of factor XIIa by Cl inhibitor. Arch. Biochem. Biophys. 256, 490–498 [DOI] [PubMed] [Google Scholar]
- 7. Henderson L. M., Figueroa C. D., Müller-Esterl W., Bhoola K. D. (1994) Assembly of contact-phase factors on the surface of the human neutrophil membrane. Blood 84, 474–482 [PubMed] [Google Scholar]
- 8. Schousboe I. (2003) Binding of activated Factor XII to endothelial cells affects its inactivation by the C1-esterase inhibitor. Eur. J. Biochem. 270, 111–118 [DOI] [PubMed] [Google Scholar]
- 9. Schousboe I. (2001) Rapid and cooperative binding of factor XII to human umbilical vein endothelial cells. Eur. J. Biochem. 268, 3958–3963 [DOI] [PubMed] [Google Scholar]
- 10. Reddigari S. R., Shibayama Y., Brunnée T., Kaplan A. P. (1993) Human Hageman factor (factor XII) and high molecular weight kininogen compete for the same binding site on human umbilical vein endothelial cells. J. Biol. Chem. 268, 11982–11987 [PubMed] [Google Scholar]
- 11. Mahdi F., Madar Z. S., Figueroa C. D., Schmaier A. H. (2002) Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1 on endothelial cell membranes. Blood 99, 3585–3596 [DOI] [PubMed] [Google Scholar]
- 12. Bradford H. N., Pixley R. A., Colman R. W. (2000) Human factor XII binding to the glycoprotein Ib-IX-V complex inhibits thrombin-induced platelet aggregation. J. Biol. Chem. 275, 22756–22763 [DOI] [PubMed] [Google Scholar]
- 13. Ploug M., Rahbek-Nielsen H., Nielsen P. F., Roepstorff P., Dano K. (1998) Glycosylation profile of a recombinant urokinase-type plasminogen activator receptor expressed in Chinese hamster ovary cells. J. Biol. Chem. 273, 13933–13943 [DOI] [PubMed] [Google Scholar]
- 14. Jablonska E., Markart P., Zakrzewicz D., Preissner K. T., Wygrecka M. (2010) Transforming growth factor-β1 induces expression of human coagulation factor XII via Smad3 and JNK signaling pathways in human lung fibroblasts. J. Biol. Chem. 285, 11638–11651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wygrecka M., Marsh L. M., Morty R. E., Henneke I., Guenther A., Lohmeyer J., Markart P., Preissner K. T. (2009) Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood 113, 5588–5598 [DOI] [PubMed] [Google Scholar]
- 16. Moreth K., Frey H., Hubo M., Zeng-Brouwers J., Nastase M. V., Hsieh L. T., Haceni R., Pfeilschifter J., Iozzo R. V., Schaefer L. (2014) Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 35, 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badellino K. O., Walsh P. N. (2001) Localization of a heparin binding site in the catalytic domain of factor XIa. Biochemistry 40, 7569–7580 [DOI] [PubMed] [Google Scholar]
- 18. Wei Z., Lyon M., Gallagher J. T. (2005) Distinct substrate specificities of bacterial heparinases against N-unsubstituted glucosamine residues in heparan sulfate. J. Biol. Chem. 280, 15742–15748 [DOI] [PubMed] [Google Scholar]
- 19. Rostand K. S., Esko J. D. (1997) Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mali M., Elenius K., Miettinen H. M., Jalkanen M. (1993) Inhibition of basic fibroblast growth factor-induced growth promotion by overexpression of syndecan-1. J. Biol. Chem. 268, 24215–24222 [PubMed] [Google Scholar]
- 21. Lu J., Auduong L., White E. S., Yue X. (2014) Up-regulation of heparan sulfate 6-O-sulfation in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 50, 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ben-Zaken O., Tzaban S., Tal Y., Horonchik L., Esko J. D., Vlodavsky I., Taraboulos A. (2003) Cellular heparan sulfate participates in the metabolism of prions. J. Biol. Chem. 278, 40041–40049 [DOI] [PubMed] [Google Scholar]
- 23. Ai X., Do A. T., Lozynska O., Kusche-Gullberg M., Lindahl U., Emerson C. P., Jr. (2003) QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 162, 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarrazin S., Lamanna W. C., Esko J. D. (2011) Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu D., Esko J. D. (2014) Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 83, 129–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carter W. J., Cama E., Huntington J. A. (2005) Crystal structure of thrombin bound to heparin. J. Biol. Chem. 280, 2745–2749 [DOI] [PubMed] [Google Scholar]
- 27. Friedrich U., Blom A. M., Dahlbäck B., Villoutreix B. O. (2001) Structural and energetic characteristics of the heparin-binding site in antithrombotic protein C. J. Biol. Chem. 276, 24122–24128 [DOI] [PubMed] [Google Scholar]
- 28. Johnson D. J., Langdown J., Huntington J. A. (2010) Molecular basis of factor IXa recognition by heparin-activated antithrombin revealed by a 1.7-Å structure of the ternary complex. Proc. Natl. Acad. Sci. U.S.A. 107, 645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pol-Fachin L., Verli H. (2014) Structural glycobiology of heparin dynamics on the exosite 2 of coagulation cascade proteases: implications for glycosaminoglycans antithrombotic activity. Glycobiology 24, 97–105 [DOI] [PubMed] [Google Scholar]
- 30. Beringer D. X., Kroon-Batenburg L. M. (2013) The structure of the FnI-EGF-like tandem domain of coagulation factor XII solved using SIRAS. Acta Crystallogr Sect. F Struct. Biol. Cryst. Commun. 69, 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ho G., Broze G. J., Jr., Schwartz A. L. (1997) Role of heparan sulfate proteoglycans in the uptake and degradation of tissue factor pathway inhibitor-coagulation factor Xa complexes. J. Biol. Chem. 272, 16838–16844 [DOI] [PubMed] [Google Scholar]
- 32. Shimada K., Ozawa T. (1985) Evidence that cell surface heparan sulfate is involved in the high affinity thrombin binding to cultured porcine aortic endothelial cells. J. Clin. Invest. 75, 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kojima T., Leone C. W., Marchildon G. A., Marcum J. A., Rosenberg R. D. (1992) Isolation and characterization of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J. Biol. Chem. 267, 4859–4869 [PubMed] [Google Scholar]
- 34. de Agostini A. I., Watkins S. C., Slayter H. S., Youssoufian H., Rosenberg R. D. (1990) Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J. Cell Biol. 111, 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Renné T., Schuh K., Müller-Esterl W. (2005) Local bradykinin formation is controlled by glycosaminoglycans. J. Immunol. 175, 3377–3385 [DOI] [PubMed] [Google Scholar]
- 36. Renné T., Dedio J., David G., Müller-Esterl W. (2000) High molecular weight kininogen utilizes heparan sulfate proteoglycans for accumulation on endothelial cells. J. Biol. Chem. 275, 33688–33696 [DOI] [PubMed] [Google Scholar]
- 37. Renné T., Müller-Esterl W. (2001) Cell surface-associated chondroitin sulfate proteoglycans bind contact phase factor H-kininogen. FEBS Lett. 500, 36–40 [DOI] [PubMed] [Google Scholar]
- 38. Coward W. R., Saini G., Jenkins G. (2010) The pathogenesis of idiopathic pulmonary fibrosis. Ther. Adv. Respir. Dis. 4, 367–388 [DOI] [PubMed] [Google Scholar]
- 39. Tager A. M., LaCamera P., Shea B. S., Campanella G. S., Selman M., Zhao Z., Polosukhin V., Wain J., Karimi-Shah B. A., Kim N. D., Hart W. K., Pardo A., Blackwell T. S., Xu Y., Chun J., Luster A. D. (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 [DOI] [PubMed] [Google Scholar]
- 40. Sakai N., Tager A. M. (2013) Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim. Biophys. Acta. 1832, 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skandalis S. S., Afratis N., Smirlaki G., Nikitovic D., Theocharis A. D., Tzanakakis G. N., Karamanos N. K. (2014) Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers: focus on the role and impact of proteoglycans. Matrix Biol. 35, 182–193 [DOI] [PubMed] [Google Scholar]
- 42. Malavaki C. J., Roussidis A. E., Gialeli C., Kletsas D., Tsegenidis T., Theocharis A. D., Tzanakakis G. N., Karamanos N. K. (2013) Imatinib as a key inhibitor of the platelet-derived growth factor receptor mediated expression of cell surface heparan sulfate proteoglycans and functional properties of breast cancer cells. FEBS J. 280, 2477–2489 [DOI] [PubMed] [Google Scholar]
- 43. Gordon E. M., Venkatesan N., Salazar R., Tang H., Schmeidler-Sapiro K., Buckley S., Warburton D., Hall F. L. (1996) Factor XII-induced mitogenesis is mediated via a distinct signal transduction pathway that activates a mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 93, 2174–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LaRusch G. A., Mahdi F., Shariat-Madar Z., Adams G., Sitrin R. G., Zhang W. M., McCrae K. R., Schmaier A. H. (2010) Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood 115, 5111–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmeidler-Sapiro K. T., Ratnoff O. D., Gordon E. M. (1991) Mitogenic effects of coagulation factor XII and factor XIIa on HepG2 cells. Proc. Natl. Acad. Sci. U.S.A. 88, 4382–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pucci M., Fibbi G., Magnelli L., Del Rosso M. (2001) Regulation of urokinase/urokinase receptor interaction by heparin-like glycosaminoglycans. J. Biol. Chem. 276, 4756–4765 [DOI] [PubMed] [Google Scholar]
- 47. Afratis N., Gialeli C., Nikitovic D., Tsegenidis T., Karousou E., Theocharis A. D., Pavão M. S., Tzanakakis G. N., Karamanos N. K. (2012) Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 279, 1177–1197 [DOI] [PubMed] [Google Scholar]
- 48. Thacker B. E., Xu D., Lawrence R., Esko J. D. (2014) Heparan sulfate 3-O-sulfation: a rare modification in search of a function. Matrix Biol. 35, 60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]