Background: Mycobacterium tuberculosis is a major cause of morbidity and mortality worldwide, and chemotherapeutic treatments are not always effective.
Results: Keratinocyte growth factor (KGF) administration results in an epithelium-dependent, GM-CSF-mediated induction of microbicidal programs in alveolar macrophages.
Conclusion: KGF induces GM-CSF and enhances clearance of M. tuberculosis from the lungs of mice.
Significance: KGF may be a useful adjunctive strategy for treatment of infection with M. tuberculosis.
Keywords: Epithelium, Growth Factor, Innate Immunity, Keratinocyte, Lung, Macrophage, Mycobacterium tuberculosis, Cell Activation, Rodent
Abstract
Augmentation of innate immune defenses is an appealing adjunctive strategy for treatment of pulmonary Mycobacterium tuberculosis infections, especially those caused by drug-resistant strains. The effect of intranasal administration of keratinocyte growth factor (KGF), an epithelial mitogen and differentiation factor, on M. tuberculosis infection in mice was tested in prophylaxis, treatment, and rescue scenarios. Infection of C57BL6 mice with M. tuberculosis resulted in inoculum size-dependent weight loss and mortality. A single dose of KGF given 1 day prior to infection with 105 M. tuberculosis bacilli prevented weight loss and enhanced pulmonary mycobacterial clearance (compared with saline-pretreated mice) for up to 28 days. Similar effects were seen when KGF was delivered intranasally every third day for 15 days, but weight loss and bacillary growth resumed when KGF was withdrawn. For mice with a well established M. tuberculosis infection, KGF given every 3 days beginning on day 15 postinoculation was associated with reversal of weight loss and an increase in M. tuberculosis clearance. In in vitro co-culture experiments, M. tuberculosis-infected macrophages exposed to conditioned medium from KGF-treated alveolar type II cell (MLE-15) monolayers exhibited enhanced GM-CSF-dependent killing through mechanisms that included promotion of phagolysosome fusion and induction of nitric oxide. Alveolar macrophages from KGF-treated mice also exhibited enhanced GM-CSF-dependent phagolysosomal fusion. These results provide evidence that administration of KGF promotes M. tuberculosis clearance through GM-CSF-dependent mechanisms and enhances host defense against M. tuberculosis infection.
Introduction
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB),2 infects one-third of the world's population and results in up to 1.2 million deaths per year (1). Strains resistant to all major anti-TB drugs have emerged, and novel approaches to therapy are needed. Inhaled M. tuberculosis bacteria impact the airway epithelial lining layer and are internalized into the phagosomes of alveolar macrophages (AMs) (2–4). Infected AMs secrete TNFα and chemokines that coordinate the recruitment of T lymphocytes into lung granulomas, which function to contain the infected cells and to facilitate the execution of microbicidal programs (5). T lymphocytes within the lesions secrete IFN-γ, GM-CSF and other cytokines that promote effective intracellular killing by AMs through both oxidative and non-oxidative mechanisms (6). Fusion of the macrophage phagosome with lysosomes is a tightly regulated event that exposes the bacterium to the acidic pH and digestive enzyme milieu within the lysosome and thereby promotes bacterial killing. M. tuberculosis can subvert host innate immune responses and survive within macrophages through a variety of adaptive mechanisms, including inhibition of phagolysosome fusion or acidification of the phagosome and scavenging of iron, leading to latency or progressive pulmonary infection (4). For the billions of individuals with latent TB, recrudescence years after the primary exposure is the most common source of new infections in the more than 9 million patients with active TB worldwide (1, 7). Treatment strategies focused on activating or reactivating the microbicidal activities of the AMs, including phagosome-lysosome fusion, are therefore intuitively appealing.
Keratinocyte growth factor (KGF) is a potent epithelial mitogen that is known to contribute to epithelial repair in several organs. KGF is expressed by mesenchymal cells and binds to FGFR2b receptors that are almost exclusively restricted to the epithelium (8). Previous studies indicate that KGF protects the lung from various insults, such as hypoxia, acid instillation, and intratracheal bleomycin (9–16) and enhances host survival following Pseudomonas aeruginosa-induced lung injury in vivo (17, 18). We have recently reported that exogenous KGF results in activation of AMs and enhanced clearance of Gram-negative bacteria from murine lungs at least in part by inducing the secretion of GM-CSF from the pulmonary epithelium and GM-CSF receptor-dependent activation of the STAT5 signaling pathway in AMs (19).
The objective of the current study was to determine whether KGF would promote pulmonary host defense against pulmonary M. tuberculosis infection in mice. We provide evidence that KGF enhances the clearance of M. tuberculosis through GM-CSF-induced activation of AM, nitric oxide-dependent killing, and an increase in phagosome-lysosome fusion.
EXPERIMENTAL PROCEDURES
Animals
Female C57BL/6 mice aged 6–8 weeks were purchased from Jackson Laboratories (Bar Harbor, ME) and housed under pathogen-free conditions in the animal facility of the University of Cincinnati College of Medicine. Mice were given ad libitum access to sterilized food and water. M. tuberculosis-infected mice were housed in a separate BSL3 animal facility. Animals were infected with M. tuberculosis by the intranasal route and treated with human recombinant KGF (Palifermin, a gift of Biovitrium UMEA) or saline. All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Culture and Isolation of M. tuberculosis
The virulent Erdman strain of M. tuberculosis was obtained from the ATCC (ATCC 35801). The GFP-expressing M. tuberculosis Erdman strain used for phagolysosome fusion experiments was a gift from Dr. Vojo Deretic (University of New Mexico). M. tuberculosis was cultivated on 7H11 agar plates and harvested in RPMI 1640 containing 10 mm HEPES. To minimize problems with clumping, suspensions were gently sonicated in a GenProbe bath sonicator. Inoculum size and mycobacterial burden were confirmed by serial dilution and determination of colony-forming units (cfu) on agar plates. To assess M. tuberculosis growth from the infected mice, the lungs were removed aseptically at specified time points, cut into small pieces, and homogenized. Viable M. tuberculosis in the lung tissue homogenates was quantified by serial dilution, plating in duplicate onto 7H11 Middlebrook agar in 6-well plates, incubation at 37 °C in a 5% CO2 incubator for 3 weeks, and counting of cfu (20). The plates were incubated for an additional 2 weeks to detect any slower growing M. tuberculosis colonies. The results are expressed as means ± S.E.
Cultured Cells
MLE-15 cells (gift of J. Whitsett, Cincinnati Children's Hospital Medical Center) are an immortalized cell line derived from the lung tumors of transgenic mice expressing the simian virus 40 (SV40) large T antigen under the transcriptional control of the human surfactant protein C (SP-C) promoter (21). MLE-15 cells retain many characteristics of alveolar type II cells, including epithelial cell morphology, microvilli, cytoplasmic multivesicular bodies, multilamellar inclusion bodies, and expression of SP-A, SP-B, and SP-C (22). MLE-15 cells were grown in RPMI 1640 (Gibco) containing 2% fetal bovine serum, 0.5% insulin-transferrin-sodium selenite (Sigma), 5 mg/liter transferrin, 10 mm HEPES, 10−8 m β-estradiol, and 10−8 m hydrocortisone. RAW 264.7 (RAW) cells (American Type Culture Collection, Manassas, VA) are a mouse macrophage cell line that was originally established from a tumor induced by the Abelson murine leukemia virus. RAW cells were maintained in DMEM (Invitrogen) supplemented with 10% heat-inactivated FBS (Invitrogen) and cultured at 37 °C in a 5% CO2 atmosphere. MLE-15 cells were plated at 25% confluence in DMEM (Gibco) in the lower well of transwell plates and were pretreated with KGF (100 ng/ml) or medium alone overnight. RAW cells were seeded at 25% confluence in the upper well of transwell dishes. On the third day, when the RAW cells were ∼50–60% confluent, they were incubated with M. tuberculosis at a multiplicity of infection of 10:1 for 2 h, washed, and co-incubated in transwell co-cultures with MLE-15 cells. After 5 days, the RAW cells were harvested, lysed, and plated onto 7H11 Middlebrook agar plates. M. tuberculosis cfu were quantified as outlined above.
ELISA
GM-CSF concentrations were measured in cell-free supernatants of culture samples or in lysates of freshly isolated AMs using a commercially available ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions and were expressed as pg/ml of the culture supernatant or lysate. In some experiments, NG-nitro-l-arginine methyl ester (l-NAME) was added to the medium to inhibit nitric-oxide synthase.
Isolation of Murine Alveolar Macrophages
Mice were sacrificed, and bronchoalveolar lavage (BAL) was performed by up to 10 cycles of instillation and aspiration of 1 ml of Hanks' balanced salt solution with 0.6 mm EDTA (23). The BAL fluid was centrifuged at 1500 × g for 5 min, and the cells were resuspended in lysis buffer containing 0.15 m NH4Cl with 0.01 m KHCO3, washed, and resuspended in 0.9% NaCl. Cells were quantified using a hemocytometer, and cell differentials were determined by examination of cytopreparation smears (Cytospin II, Shandon Southern Instruments, Inc., Sewickley, PA) after processing with the Hema 3 staining system (Fisher). Viability of AM was determined by trypan blue exclusion.
Infection of Mice with M. tuberculosis
Mice were infected i.n. with Erdman M. tuberculosis (2.5 × 101 to 1 × 106) suspended in 50 μl of Hanks' balanced salt solution in a class III biohazard safety cabinet, and maintained in the BSL3 animal facility at the University of Cincinnati. Mice were treated with intranasal saline (PBS) or KGF (5 mg/kg) as indicated and monitored for weight change and vital status or sacrificed for the determination of cfu in lung homogenates. The initial inoculum that was used to infect the mice was verified by plating onto 7H11 Middlebrook plates.
Assessment of Phagosome-Lysosome Fusion
To examine the co-localization of M. tuberculosis with phagosomes, AMs from KGF- or saline-treated mice were plated onto slides at 1 × 105 AMs/well in DMEM for 4 h. After incubation for 1 h at 37 °C in 5% CO2, GFP-M. tuberculosis Erdman was added at a multiplicity of infection of 10:1 for 2 h. For the neutralization of the GM-CSF-dependent phagolysosome fusion, anti-GM-CSF antibody (eBioscience, San Diego, CA) was added (100 μg/well) to AMs. After washing the monolayer, the AMs were fixed serially with 1 and 2% paraformaldehyde for 10 min each. The monolayers were permeabilized with 100% methanol for 5 min, washed three times with Dulbecco's PBS, and blocked overnight at 4 °C with PBS containing normal goat serum, 10% heat-inactivated FCS (Hyclone), and 5 mg/ml BSA. After washing, the monolayers were incubated with mouse anti-human LAMP-1 antibody (University of Iowa Hybridoma Facility, Iowa City, IA) for 1 h, followed by incubation with mouse IgG conjugated with Alexa Fluor 555 (1:5000 dilution; Molecular Probes). After washing, the slides were separated from the wells, overlaid with a coverslip and mounting medium, and sealed with nail polish. The slides were viewed using a confocal microscope (Olympus FV1000). On average, 200 cell-associated bacilli contained within ∼70 macrophages were counted from randomly selected confocal images in each test group. Results are expressed in percentage values of phagosome-lysosome fusion and shown as the mean ± S.E. of three independent experiments.
Statistical Analysis
All experiments were conducted in triplicate with a minimum of three experiments. The results were expressed as means ± S.E. or S.D. The statistical difference between the control and experimental data was determined by Student's t test or analysis of variance with paired comparisons. A p value of <0.05 was considered to be statistically significant.
RESULTS
Infection by M. tuberculosis Results in Inoculum Size-dependent Mortality in C57BL/6 Mice
Experiments were performed to determine the optimal M. tuberculosis inoculum size for subsequent experiments. A group of nine mice received Erdman M. tuberculosis i.n. at doses ranging from 102 to 109 bacteria and were observed daily for up to 35 weeks (Fig. 1). Mice that received 1 × 109 or 1 × 108 cfu of M. tuberculosis survived for up to 8 or 12 weeks, respectively, whereas those that received 1 × 104 to 1 × 102 M. tuberculosis survived beyond 32 weeks. We chose an inoculum of 105 organisms/mouse for most experiments, except where otherwise indicated. Collectively, these data indicate that infection with the M. tuberculosis (Erdman) strain results in a dose-dependent mortality in C57BL/6 mice.
FIGURE 1.
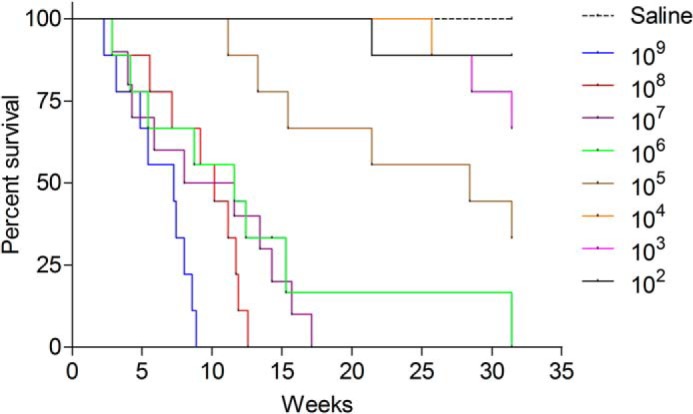
Inoculum size-dependent mortality of mice infected with M. tuberculosis. C57BL/6 mice were inoculated i.n. with 100 to 1 billion bacilli of the Erdman strain of M. tuberculosis, and vital status was monitored over a 32-week period (n = 10 mice/group).
KGF Protects Mice from Weight Loss and Enhances Clearance of M. tuberculosis from Mouse Lungs
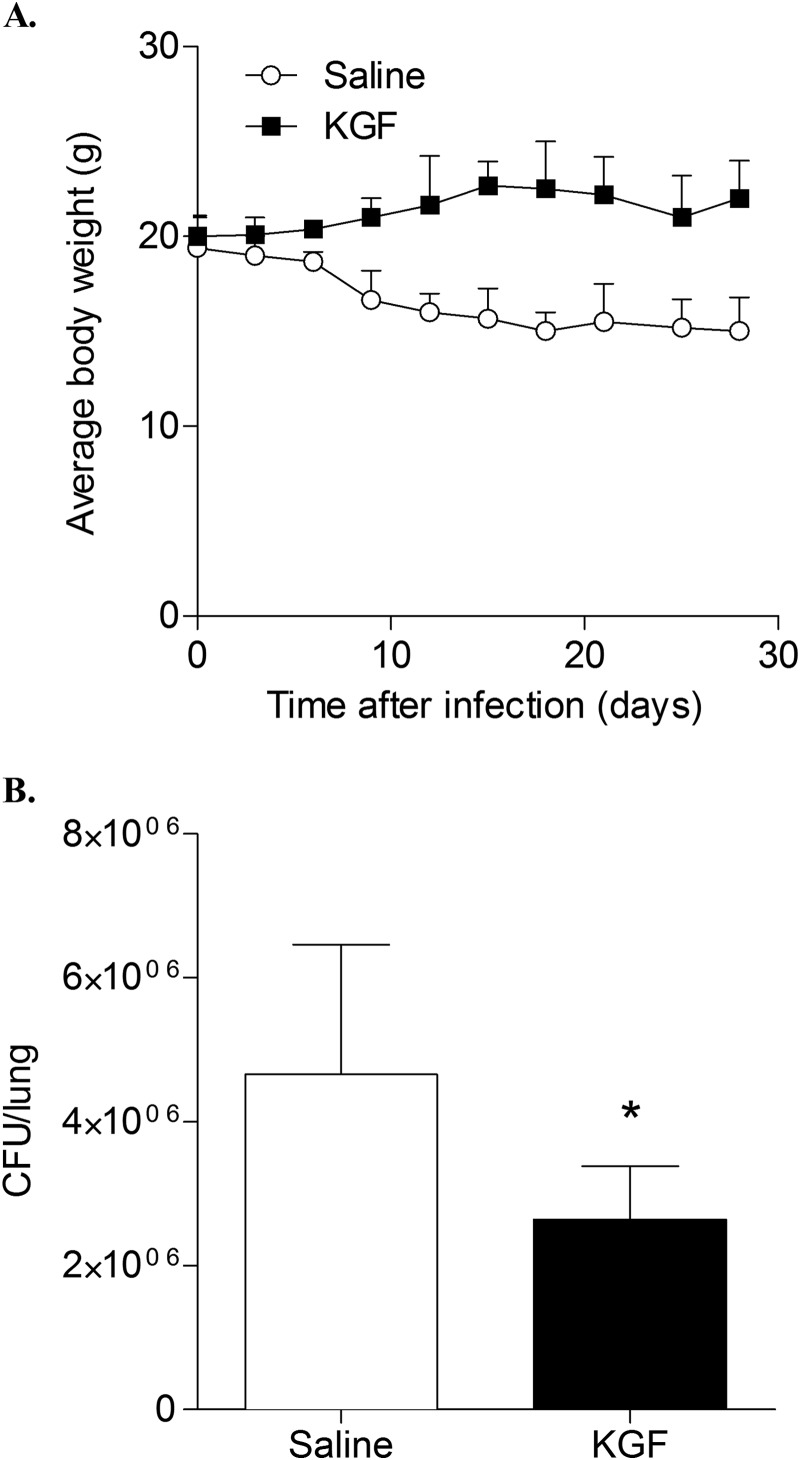
The effect of KGF pretreatment on body weight and pulmonary burden of M. tuberculosis bacilli was assessed. The dose and route of KGF administration were based on parameters shown to enhance pulmonary clearance of Gram-negative bacteria in a recent study from our group (19), which demonstrated a 2.5-fold increase in AMs recovered by BAL and type II cell proliferation without appreciable histological evidence of inflammation. Mice were treated with a single intranasal dose of KGF (5 mg/kg) or saline and then inoculated i.n. with 105 M. tuberculosis bacilli 24 h later. The mice that received saline lost more than 20% of their baseline body weight by day 20; in contrast, the mice that received KGF gained weight to about 20% above baseline by day 20 (n = 4 mice/group, p < 0.05) (Fig. 2A). At day 30, the lungs were harvested, and cfu in the lung homogenates were quantified (Fig. 2B). KGF pretreatment resulted in a 1.9 ± 0.25-fold reduction in the number of cfu in the lungs compared with the saline-treated controls (p < 0.05). There were no differences in mean weights of control mice treated with KGF or saline in the absence of infection (data not shown). Collectively, the data indicate that a single prophylactic dose of KGF prevents weight loss and enhances the clearance of M. tuberculosis from the lungs of mice.
FIGURE 2.
KGF enhances clearance of M. tuberculosis from mouse lungs. C57BL/6 mice were treated with intranasal KGF (5 mg/kg) or saline and then infected i.n. with 1 × 105 M. tuberculosis bacilli 24 h later. A, body weights were obtained at multiple time points. B, after 30 days, the lungs were harvested, and cfu of M. tuberculosis were quantified. The data are mean ± S.E. (error bars) (n = 5 mice/group). *, p < 0.05.
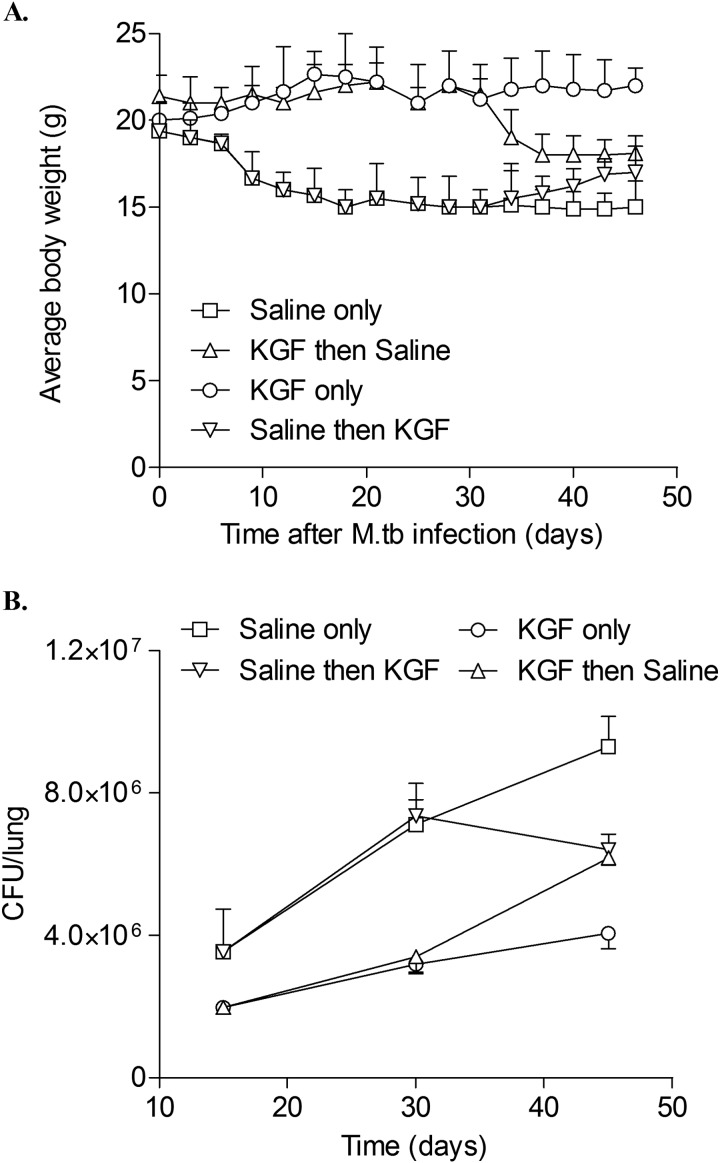
To determine whether serial treatment with KGF protects mice from weight loss and progressive mycobacterial infection, mice were inoculated i.n. with 105 M. tuberculosis bacilli and then treated with PBS or KGF i.n. every 3 days for 15 days (Fig. 3). At that point, saline-treated mice were divided into two groups: (a) continued saline administration every third day through day 45 (n = 4) (saline only) or (b) crossed over to receive KGF every third day through day 45 (n = 4) (saline then KGF). Similarly, mice that received KGF for the first 15 days were either given (a) KGF treatment every third day for 30 additional days through day 45 (KGF only) or (b) saline every third day from day 15 through 45 (KGF then saline).
FIGURE 3.
KGF treatment of M. tuberculosis-infected mice prevents weight loss and reduces M. tuberculosis proliferation in the lung. C57BL/6 mice were treated with KGF (5 mg/kg) or saline i.n. and then inoculated i.n. with 1 × 105 M. tuberculosis bacilli (Erdman) 24 h. later. Animals were divided into four groups: serial KGF or saline treatment every 3 days for 45 days or 15 days of KGF or saline treatment followed by crossover to the other group for days 30–45. Body weights (A) and M. tuberculosis cfu in homogenized lungs (B) were assessed for 45 days at the indicated time points. The data are expressed as mean ± S.E. (error bars) (n = 4 mice/group).
The saline-only mice lost ∼33% of their body weight (Fig. 3A). In contrast, the KGF-only mice gained weight, plateauing at about 10–11% above their baseline weight by day 15. The difference in body weight in the two groups was significant at day 20 (p < 0.001) and day 45 (p < 0.001). In the saline-only group, M. tuberculosis cfu in lung homogenates increased by ∼2.6-fold over the interval from 15 to 45 days, from 3.5 ± 0.6 × 106 bacteria at 15 days to 7.1 ± 0.6 × 106 bacteria at 30 days and 9.3 ± 0.4 × 106 bacteria at 45 days (Fig. 3B). The KGF-only mice had a lower burden of M. tuberculosis in the lung on day 15 compared with saline-treated mice and only a 1.6-fold log increase in the number of organisms over the following 30 days (from 2.0 ± 0.1 × 106 bacteria on day 15 to 3.2 ± 0.1 × 106 bacteria on day 30 and 4.1 ± 0.2 × 106 bacteria on day 45). We concluded that continuous treatment with KGF for 45 days protects mice from weight loss and reduces the growth of M. tuberculosis in the lungs.
Crossover experiments were performed to determine the effect of KGF initiation on established M. tuberculosis infection and the effect of withdrawing KGF on the subsequent course of infection. In “saline then KGF” mice, the weight fell upon saline treatment by 20% through day 15, but after serial KGF administration every 3 days commenced on day 16, weight plateaued and began to increase again (Fig. 3A). In the first 2 weeks after KGF administration commenced, the burden of organisms continued to increase, and it more than doubled from 3.5 ± 0.6 to 7.4 ± 0.2 × 106 cfu from day 15 to 30. By day 45 however, M. tuberculosis cfu in the lung had fallen to 6.4 ± 0.2 × 106 cfu (Fig. 3B). In “KGF then saline” mice, the weight was stable for approximately the first 30 days, despite KGF exposure for only the first 15 days, but at that point, it began to decrease. At 2.0 ± 0.01 × 106 bacteria on day 15, the bacterial burden in the KGF treatment animals was lower at the same time point than in the “saline alone” mice. After KGF withdrawal, M. tuberculosis growth was similar to that in “KGF only” mice through day 30 but then accelerated relative to that group over the interval from day 30 to 45, from 3.4 ± 0.2 × 106 to 6.2 ± 0.1 × 106 bacteria. We concluded that KGF can reduce M. tuberculosis growth in an established infection model and that withdrawal of KGF is associated with progression of M. tuberculosis infection.
KGF Enhances the Clearance of M. tuberculosis Inoculated at Doses That More Closely Mimic Inhalational Exposure
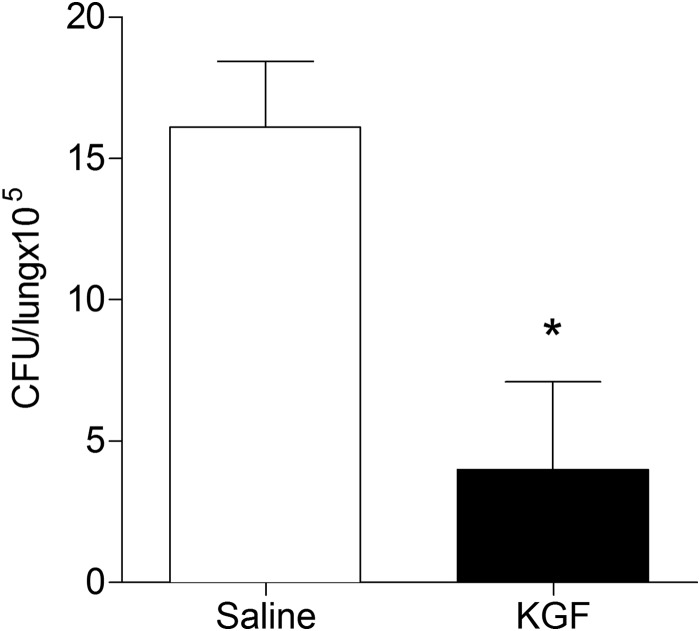
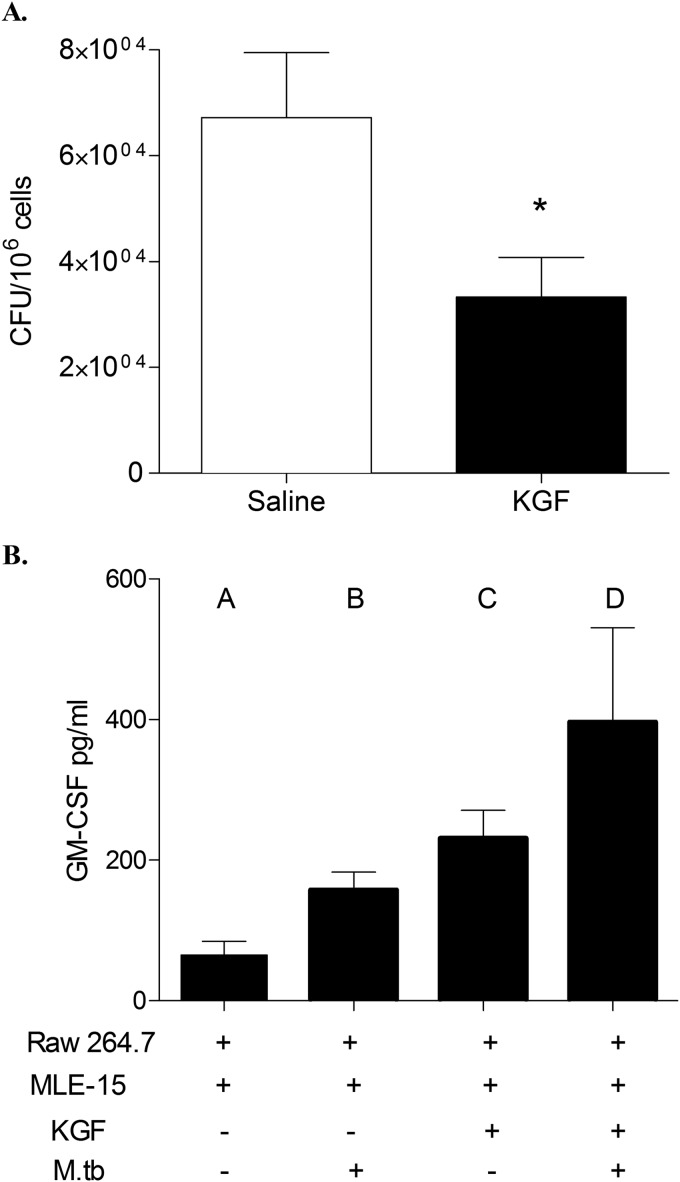
To more closely model natural infection with M. tuberculosis in humans, we measured the effect of KGF on the bacterial burden in the lungs after low dose M. tuberculosis inoculation. Mice were inoculated i.n. with 1 × 102 Erdman M. tuberculosis. After 30 weeks, mice were given intranasal saline or KGF (5 mg/kg) every third day for 2 weeks. Four weeks after commencement of the intervention, the lungs were harvested and homogenized, and cfu were quantified. We found that KGF enhanced the clearance of M. tuberculosis by >5-fold (n = 4, p < 0.001) (Fig. 4). These data indicate that KGF enhances the clearance of M. tuberculosis in an established infection model following a low dose inoculation that more closely mimics inhalational tuberculosis.
FIGURE 4.
KGF enhances the clearance of M. tuberculosis after low dose inoculation. Mice were inoculated i.n. with 1 × 102 Erdman M. tuberculosis. Beginning at 30 weeks postinfection, mice were given intranasal saline or KGF (5 mg/kg) on every third day for 2 weeks. The lungs were harvested and homogenized, and cfu were quantified. Data are means ± S.D. (error bars) of at least three independent experiments (n = 4 mice/group). *, p < 0.001.
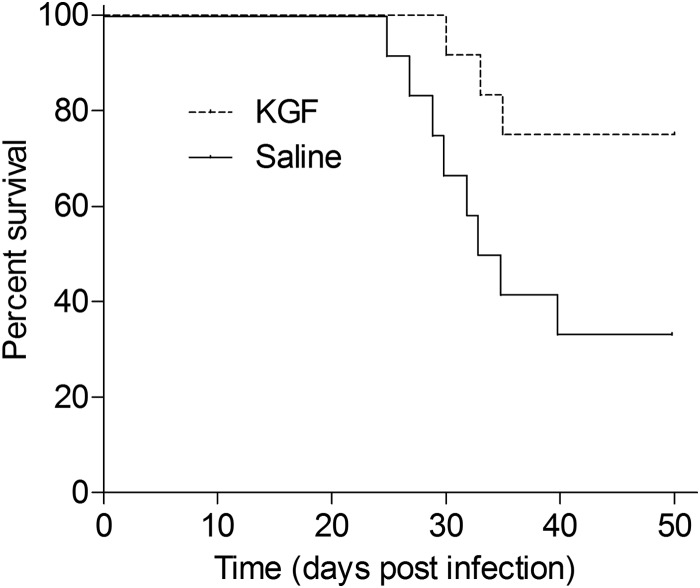
Finally, to determine whether KGF treatment enhances the survival of mice that harbor a heavy burden of M. tuberculosis (as occurs in advanced disease), mice were inoculated with 1 × 108 Erdman M. tuberculosis and then treated with intranasal KGF or saline every third day for 2 weeks. Vital status was monitored on a daily basis over a 50-day period starting from the day of initial infection. As shown in Fig. 5, 50-day survival was significantly greater in the KGF-treated group (75%) than in the saline-treated group (34%) (n = 9, p = 0.033). These data indicate that KGF protects mice from a lethal intranasal inoculum of M. tuberculosis.
FIGURE 5.
KGF treatment enhances the survival of mice that are infected with high dose M. tuberculosis. C57BL/6 mice were given intranasal saline or KGF (5 mg/kg), followed in 24 h by intranasal delivery of 1 × 108 Erdman M. tuberculosis. Treatment with intranasal KGF or saline was continued every third day for 2 weeks. Vital status was determined over a 50-day period (n = 9 mice/group; p < 0.05).
A Co-culture Model of Alveolar Epithelial Cells and Macrophages Demonstrates That GM-CSF-dependent Killing of M. tuberculosis Is Induced by KGF Administration
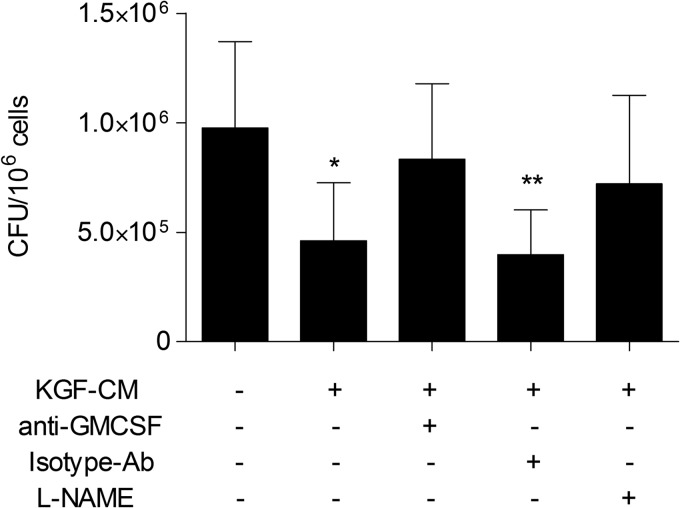
We have previously reported that KGF enhances pulmonary epithelial GM-CSF production, which in turn activates AMs and enhances bacterial clearance (19). To determine whether GM-CSF-dependent AM activation contributes to the enhanced M. tuberculosis clearance seen in KGF-treated mice, we conducted a series of in vitro co-culture experiments using macrophage (RAW) and alveolar pneumocyte (MLE-15) cell lines (Fig. 6). MLE-15 cells were plated in the lower well of transwell plates and pretreated with KGF (100 ng/ml) or medium alone overnight. RAW cells were infected with M. tuberculosis at a multiplicity of infection of 10:1, washed, and added to the upper well. After 5 days of co-culture, the RAW cells were harvested, lysed, and plated onto 7H11 Middlebrook agar plates, and M. tuberculosis cfu were quantified. We found that KGF treatment of MLE-15/RAW co-cultures reduced the growth of AM-associated M. tuberculosis ∼2.5-fold (p < 0.05) (Fig. 6A). The GM-CSF content of the medium supernatants under various culture conditions is shown in Fig. 6B. These data indicate that MLE-15 cells produce a small amount of GM-CSF under basal conditions, which is incrementally augmented by M. tuberculosis infection of AM, by the addition of KGF to the medium bathing MLE-15 cells, and by co-culture with uninfected AMs. Co-culture of M. tuberculosis-infected RAW cells with KGF-treated MLE-15 cell monolayers resulted in an ∼7-fold increase in the level of GM-CSF in culture medium, relative to the levels in the culture medium of unstimulated, uninfected cells (p < 0.05). Conditioned medium (CM) from KGF-treated MLE-15 cells was then used to determine whether GM-CSF was the factor responsible for the enhanced killing of M. tuberculosis (Fig. 7). Incubation of M. tuberculosis-infected RAW cells with CM reduced the growth of intracellular M. tuberculosis by about 2-fold. This effect of CM was reversed by a neutralizing antibody to GM-CSF but not by an isotype control antibody. In addition, the mycobactericidal effect of CM was blocked by the nitric oxide synthase inhibitor, l-NAME. Incubation of either anti-GM-CSF antibody or l-NAME with the RAW cells without CM did not have any effect on the M. tuberculosis growth (data not shown). These results indicate that KGF treatment of MLE-15 cells induces GM-CSF production that results in a decrease in M. tuberculosis burden in co-cultured RAW, at least in part through nitric oxide-dependent mechanisms. These data support an important role for GM-CSF and nitric oxide in the KGF-mediated anti-mycobacterial action of AMs.
FIGURE 6.
KGF treatment of MLE-15 cells results in a decrease in M. tuberculosis burden in co-cultured RAW cells and induces GM-CSF production. To determine the mechanism of KGF-enhanced M. tuberculosis clearance, MLE-15 cells plated in the lower well of transwell plates were pretreated with KGF (100 ng/ml) or medium only overnight. RAW cells infected with M. tuberculosis were then added to the upper well. A, after 5 days of co-culture, the RAW cells were harvested, lysed, and plated onto 7H11 Middlebrook agar plates, and cfu were quantified. Data are for n = 4 experiments; p < 0.05. B, the levels of GM-CSF in culture supernatants were measured by ELISA on culture day 5. Results are shown as the mean ± S.D. (error bars) and are representative of three independent experiments. p < 0.05 for comparison of columns A and C, A and D, and B and D.
FIGURE 7.
Conditioned medium from KGF-treated MLE15 cells reduces the growth of M. tuberculosis in RAW cells by GM-CSF- and nitric oxide-dependent mechanisms. M. tuberculosis-infected RAW cells were treated with conditioned medium from KGF-treated MLE15 cells (KGF-CM) in the presence or absence of neutralizing antibody to GM-CSF, isotype control antibody, or l-NAME (nitric-oxide synthase inhibitor). After 5 days, the RAW cells were lysed, plated on 7H11 Middlebrook agar, and cultured, and cfu were quantified. Results are shown as the mean ± S.D. (error bars) of quadruplicate samples and are representative of three independent experiments. **, p < 0.05; *, p < 0.001 versus untreated control cells.
Effect of KGF on Phagosome-Lysosome Fusion in M. tuberculosis-infected AMs
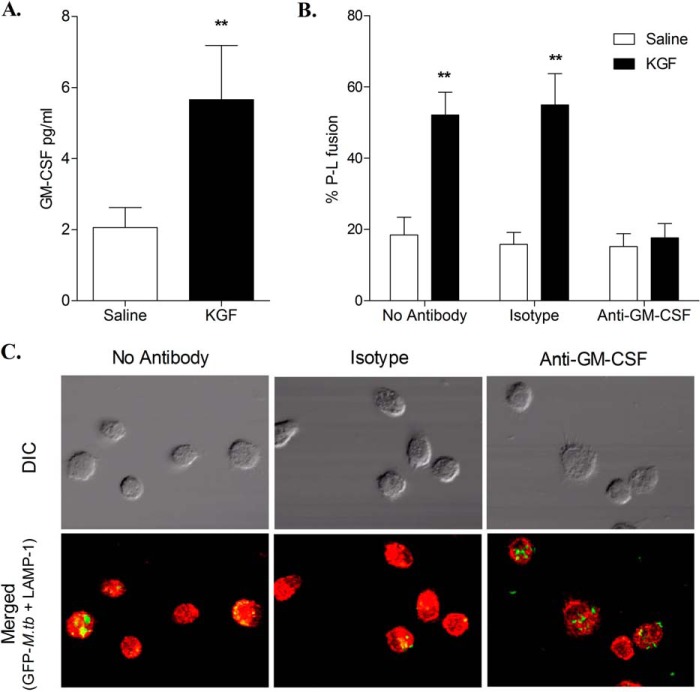
We also examined the effect of KGF on phagosome-lysosome fusion in M. tuberculosis-infected AMs (Fig. 8). AMs were isolated 24 h after treatment with KGF or saline and incubated with GFP-expressing M. tuberculosis. Following phagocytosis, green fluorescent M. tuberculosis bacilli were visible within phagosomes using confocal microscopy. The late endosomal/lysosomal compartment was visualized by staining with a red fluorescent LAMP-1 antibody. Co-localization of the red and green signals indicative of phagosome-lysosome fusion was quantified by counting yellow fluorescent signals. AMs isolated from KGF-treated mice showed increased co-localization of GFP M. tuberculosis with LAMP-1 (Fig. 8, A and B). The percentage of co-localized GFP M. tuberculosis was 28.9 ± 3.8% in KGF-treated mouse AMs compared with 10.9 ± 1.3% in saline-treated mouse AMs (p < 0.005) (Fig. 8C). We have previously found that lysates of AMs isolated from mice 1 h after in vivo KGF administration contained elevated GM-CSF levels, albeit lower than type II cell lysates from the same animals (19). In the prior report, we also found that type II cells but not AMs produced GM-CSF following in vitro exposure to KGF, a result that was not surprising given that the KGF receptor is known to be restricted to the epithelium. In order to assess the levels of GM-CSF that may be introduced into the phagolysosomal fusion assay, we harvested AMs by BAL 24 h after treating mice with saline or KGF and prepared lysates for GM-CSF quantitation by ELISA. GM-CSF levels associated with AMs from KGF-treated mice were ∼3-fold higher than the levels associated with AMs from saline-treated mice (p < 0.01) (Fig. 9A). To determine the role of GM-CSF in the enhanced phagolysosomal fusion induced by KGF, we isolated AMs 24 h following saline or KGF treatment and performed the phagolysosomal fusion assay as described previously with the exception that isolated AMs were incubated with no antibody, with isotype antibody, or with anti-GM-CSF antibody prior to the addition of GFP-expressing M. tuberculosis. Phagolysosomal fusion was enhanced ∼3-fold in AMs isolated from KGF-treated mice compared with those from saline-treated animals in the absence of the anti-GM-CSF antibody, whereas neutralization of GM-CSF abolished the KGF-induced enhancement of phagolysosomal fusion (Fig. 9, B and C). At least one prior report has demonstrated an increase in GM-CSF expression in human AMs 24 h following in vitro M. tuberculosis infection (24). To investigate the possibility that M. tuberculosis was inducing GM-CSF expression in our system, we performed experiments in which we infected cultured AMs isolated from KGF- or saline-treated animals with GFP-expressing M. tuberculosis and assessed GM-CSF expression 24 h later in medium by ELISA and in cells by RT-PCR. We did not detect GM-CSF protein in the assay medium from either group, and although we did detect a very low level of gene expression in AMs from KGF- and PBS-treated animals, there was no significant difference between the groups (not shown). Collectively, these results indicate that KGF promotes GM-CSF-dependent phagosome-lysosome fusion that, together with enhanced nitric-oxide production, promotes the killing of intracellular M. tuberculosis in AMs.
FIGURE 8.
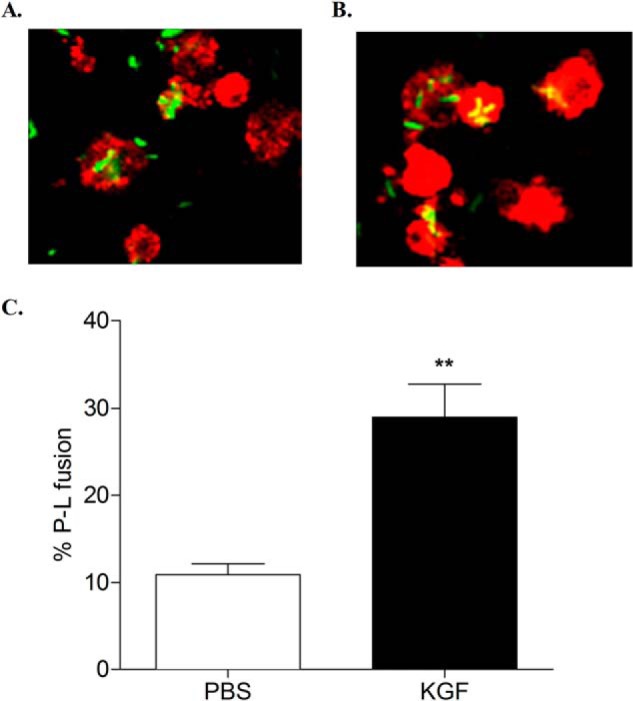
Effect of KGF on M. tuberculosis phagosome-lysosome (P-L) fusion in AMs. AMs were isolated from PBS- or KGF-pretreated mice, adhered for 4 h to overnight in a tissue culture chamber slide, and then infected with GFP-expressing M. tuberculosis. After 3 h, infected AMs were washed to remove unbound M. tuberculosis bacilli. AMs were then fixed, permeabilized, stained with LAMP-1 antibody, and processed for confocal microscopy. Shown are representative confocal images of M. tuberculosis-infected AMs isolated from mice that were pretreated with PBS (A) or KGF (B). Phagosome-lysosome fusion was expressed in percentage values and scored by counting the number of intracellular bacilli that fluoresced yellow, representing the co-localization of the GFP-M. tuberculosis (green) with the late endosomal/lysosomal marker LAMP-1 (red) (C). Results are shown as the mean ± S.D. (error bars) of triplicate samples and are representative of three independent experiments. **, p < 0.005 (two-tailed paired Student's t test).
FIGURE 9.
Neutralization of excess GM-CSF associated with AMs isolated from KGF-treated mice inhibits KGF-enhanced phagolysosomal fusion. AMs were isolated from mice 24 h after intranasal administration of PBS or KGF. GM-CSF levels associated with freshly isolated AMs were determined by ELISA of lysates prepared through two rapid freeze-thaw cycles of 2.5 × 105 BAL cells/100 μl of Hanks' balanced salt solution (A). ELISA results are shown as mean ± S.E. (error bars) for n = 4. **, p < 0.01 (Student's t test). In other experiments, isolated AMs were allowed to adhere for 4 h to overnight in a tissue culture chamber slide and then incubated with no antibody, isotype antibody, or anti-GM-CSF antibody for 1 h. The AM monolayer was infected with GFP-expressing M. tuberculosis for 3 h and then washed to remove unbound M. tuberculosis bacilli. AMs were fixed, permeabilized, and stained with the lysosomal marker LAMP-1. Phagolysosomal fusion was assessed using a confocal microscope by quantifying the percentage of organelles exhibiting yellow staining consistent with co-localization of the green and red fluorophores (B). Representative images (×1200 magnification) of AMs isolated from KGF-treated mice and assessed for phagolysosomal fusion in the presence of no antibody, of isotype antibody, or of anti-GM-CSF antibody are shown (C). Phagolysosomal fusion results are reported as mean ± S.E. of triplicate samples and are representative of two independent experiments. **, p < 0.01 (one-way analysis of variance). DIC, differential interference contrast.
DISCUSSION
We found that KGF administered prior to M. tuberculosis infection or serially postinfection protects mice from weight loss and reduces the burden of M. tuberculosis bacilli in lung tissue (Figs. 2 and 3). KGF was effective in enhancing the pulmonary clearance of M. tuberculosis after a low dose inoculum that more closely mimics inhalational exposure (Fig. 4) and in attenuating mortality from a lethal inoculum of M. tuberculosis (Fig. 5). We also found that serial KGF administration every 3 days protected against weight loss and reduced bacillary growth, either when begun prior to infection or in mice with well established infection, and that withdrawal of KGF from M. tuberculosis-infected mice resulted in a decline in weight and increased M. tuberculosis burden (Fig. 3). The mechanisms of enhanced clearance of M. tuberculosis were GM-CSF-dependent and included augmentation of both nitric oxide production and phagosome-lysosome fusion (Figs. 6–9). These findings suggest that administration of KGF (as adjunctive therapy) may be a plausible strategy for enhancing alveolar host defense during pulmonary M. tuberculosis infection.
Inhaled M. tuberculosis is readily phagocytosed by AMs and internalized into phagosomes (5). The bacteria are highly adapted to this intracellular niche and are able to subvert host defenses and proliferate intracellularly. Effective killing normally requires phagosome-lysosome fusion and a robust oxidative burst. These antimicrobial functions are regulated by a complex milieu of cytokines and chemokines that are produced by macrophages and lymphocytes in response to signals from the infected AMs (5). We have previously shown that KGF administration results in enhanced pulmonary clearance of Gram-negative bacteria through induction of GM-CSF and GM-CSF-mediated activation of phagocytic and oxidative mechanisms in AMs. (19) Given the central importance of GM-CSF in M. tuberculosis infection (25–30), we postulated that KGF would also augment host defense against this intracellular bacterium.
KGF administration enhanced M. tuberculosis clearance and blocked weight loss for a short period even after a single prophylactic dose (Fig. 2). These data suggest that KGF can prime alveolar defenses to resist infection. Serial KGF treatment attenuated weight loss but did not completely block the growth of M. tuberculosis. Interruption of KGF treatment resulted in resumption of weight loss and mycobacterial growth after an interval of about 15 days. KGF treatment begun in mice with established infection resulted in weight gain and a fall in the bacterial burden after a delay of about 2 weeks (Fig. 3B).
Administration of GM-CSF has been shown to enhance killing of M. tuberculosis in mice (31, 32). In addition, the absence of GM-CSF has been reported to increase the severity of M. tuberculosis infection in GM-CSF null animals (29). The use of GM-CSF as an adjuvant for the BCG vaccine has been shown to enhance protective immunity against TB (27, 33).
Our data suggest that GM-CSF at least partially mediates KGF-induced M. tuberculosis clearance. Using in vitro co-culture methods, we demonstrated that KGF treatment of MLE-15 cells induced GM-CSF and enhanced the killing of M. tuberculosis in co-cultured RAW cells (Fig. 6). Conditioned medium from KGF-treated MLE-15 cells also reduced the growth of M. tuberculosis in RAW cells in a manner that was blocked by anti-GM-CSF antibody (Fig. 7). We provide evidence that augmented oxidative killing via nitric oxide-dependent pathways (Fig. 7) and phagosome-lysosome fusion (Fig. 8) are important mechanisms by which KGF enhances clearance of M. tuberculosis, with the caveat that substantial differences in nitric oxide production exist between human and murine macrophages (34) and between primary cells and cell lines (35). Phagolysosome fusion was enhanced in AMs isolated from KGF-treated but not PBS-treated mice and was inhibited in AMs infected with M. tuberculosis ex vivo by the addition of anti-GM-CSF antibody (Fig. 9, B and C). The source of GM-CSF driving phagolysosome fusion in this assay was also explored. We found that excess levels of GM-CSF were associated with AMs isolated from mice following KGF treatment (Fig. 9A). We did not find evidence that GM-CSF produced by M. tuberculosis-infected AMs during the course of the assay mediated the enhanced phagolysosomal fusion. These data support our prior contention that type II cells are the principal source of alveolar GM-CSF following KGF administration (19).
Effects of KGF that are potentially distinct from those of GM-CSF, such as those on alveolar epithelial antimicrobial protein expression (36), collectin expression (37), barrier integrity, permeability, wound healing, and fluid transport (38), may be playing a role in KGF-enhanced survival and bacterial clearance following challenge with M. tuberculosis.
The Food and Drug Administration approved the use of KGF for chemotherapy-induced mucositis in December of 2004 (39). Clinical trials with KGF demonstrated reduced duration of oral lesions and salivary gland toxicity in patients with head and neck cancers treated with chemoradiation, diminished duration and severity of mucositis during bone marrow transplantation conditioning, and reduced incidence and severity of mucositis after chemotherapy for metastatic colorectal carcinoma (reviewed by Barasch et al. (40). Adverse effects were transient and mild to moderate in severity; the major side effects were skin flushing, temporary elevation of serum lipase and amylase, and hypersalivation. Phase I and II clinical trials have demonstrated that administration of KGF in doses up to 80 μg/kg/day is well tolerated with minimal side effects, and a recent randomized controlled trial indicates that even single dose KGF before each chemotherapeutic cycle reduces the incidence and severity of mucositis. A recent study supports the concept that parenteral KGF induces GM-CSF production and AM phagocytosis, enhancing bronchoalveolar lavage fluid antimicrobial activity in healthy volunteers (41). Food and Drug Administration approval, commercial availability, and favorable side effect profiles enhance our enthusiasm for exploring KGF as a potential therapeutic agent for TB, perhaps as an adjunctive agent to treat latent TB or active infections caused by drug-resistant strains, including multidrug-resistant or extremely drug-resistant strains.
We conclude that KGF enhances the clearance of M. tuberculosis in prophylactic treatment and rescue murine models by a mechanism that includes paracrine activation of AMs by epithelium-derived GM-CSF and enhancement of nitric oxide production and phagosome-lysosome fusion. Future experiments are needed to determine whether KGF acts synergistically with conventional chemotherapeutic agents to eradicate pulmonary infections due to M. tuberculosis.
Acknowledgment
KGF was a gift from Biovitrium.
This work was supported, in whole or in part, by National Institutes of Health, NCATS, Grant 8UL1TR000077-04 and by funding from NIH Grant T32 HL007752-21. This work was also supported by a Veterans Affairs Merit Award (to F. X. M.). Preliminary results from this work have been reported in poster form at the 2011 American Thoracic Society Meeting. Drs. McCormack and Pasula have applied for a patent entitled “Growth Factors for Treatment of Mycobacterial Infection” (PCT/US2013/038206).
- TB
- tuberculosis
- AM
- alveolar macrophage
- KGF
- keratinocyte growth factor
- BAL
- bronchoalveolar lavage
- CM
- conditioned medium.
REFERENCES
- 1. Zumla A., Raviglione M., Hafner R., von Reyn C. F. (2013) Tuberculosis. N. Engl. J. Med. 368, 745–755 [DOI] [PubMed] [Google Scholar]
- 2. Sundaramurthy V., Pieters J. (2007) Interactions of pathogenic mycobacteria with host macrophages. Microbes Infect. 9, 1671–1679 [DOI] [PubMed] [Google Scholar]
- 3. Valdivia-Arenas M., Amer A., Henning L., Wewers M., Schlesinger L. (2007) Lung infections and innate host defense. Drug Discov. Today Dis. Mech. 4, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guirado E., Schlesinger L. S., Kaplan G. (2013) Macrophages in tuberculosis: friend or foe. Semin. Immunopathol. 35, 563–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guirado E., Schlesinger L. S. (2013) Modeling the Mycobacterium tuberculosis granuloma: the critical battlefield in host immunity and disease. Front. Immunol. 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flynn J. L., Chan J. (2001) Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129 [DOI] [PubMed] [Google Scholar]
- 7. Flynn J. L., Chan J. (2003) Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr. Opin. Immunol. 15, 450–455 [DOI] [PubMed] [Google Scholar]
- 8. Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. (1989) Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 86, 802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yano T., Deterding R. R., Simonet W. S., Shannon J. M., Mason R. J. (1996) Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am. J. Respir. Cell Mol. Biol. 15, 433–442 [DOI] [PubMed] [Google Scholar]
- 10. Panos R. J., Bak P. M., Simonet W. S., Rubin J. S., Smith L. J. (1995) Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J. Clin. Invest. 96, 2026–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deterding R. R., Havill A. M., Yano T., Middleton S. C., Jacoby C. R., Shannon J. M., Simonet W. S., Mason R. J. (1997) Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc. Assoc. Am. Physicians 109, 254–268 [PubMed] [Google Scholar]
- 12. Chang Y., Edeen K., Lu X., De Leon M., Mason R. J. (2006) Keratinocyte growth factor induces lipogenesis in alveolar type II cells through a sterol regulatory element binding protein-1c-dependent pathway. Am. J. Respir. Cell Mol. Biol. 35, 268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mason R. J., Pan T., Edeen K. E., Nielsen L. D., Zhang F., Longphre M., Eckart M. R., Neben S. (2003) Keratinocyte growth factor and the transcription factors C/EBPα, C/EBPδ, and SREBP-1c regulate fatty acid synthesis in alveolar type II cells. J. Clin. Invest. 112, 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mason R. J., Gao B., Pan T., Jiang X., Eckart M., Neben S. (2002) Role of keratinocyte growth factor in regulating lipogenesis in alveolar type II cells: a gene-profiling approach. Chest 121, 77S. [DOI] [PubMed] [Google Scholar]
- 15. Fehrenbach H., Kasper M., Koslowski R., Pan T., Schuh D., Müller M., Mason R. J. (2000) Alveolar epithelial type II cell apoptosis in vivo during resolution of keratinocyte growth factor-induced hyperplasia in the rat. Histochem. Cell Biol. 114, 49–61 [DOI] [PubMed] [Google Scholar]
- 16. Charafeddine L., D'Angio C. T., Richards J. L., Stripp B. R., Finkelstein J. N., Orlowski C. C., LoMonaco M. B., Paxhia A., Ryan R. M. (1999) Hyperoxia increases keratinocyte growth factor mRNA expression in neonatal rabbit lung. Am. J. Physiol. 276, L105–L113 [DOI] [PubMed] [Google Scholar]
- 17. Viget N. B., Guery B. P., Ader F., Nevière R., Alfandari S., Creuzy C., Roussel-Delvallez M., Foucher C., Mason C. M., Beaucaire G., Pittet J. F. (2000) Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1199–L1209 [DOI] [PubMed] [Google Scholar]
- 18. Xu X., McCormick-Shannon K., Voelker D. R., Mason R. J. (1998) KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 18, 168–178 [DOI] [PubMed] [Google Scholar]
- 19. Wu H., Suzuki T., Carey B., Trapnell B. C., McCormack F. X. (2011) Keratinocyte growth factor augments pulmonary innate immunity through epithelium-driven, GM-CSF-dependent paracrine activation of alveolar macrophages. J. Biol. Chem. 286, 14932–14940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasula R., Britigan B. E., Turner J., Martin W. J., 2nd (2009) Airway delivery of silica increases susceptibility to mycobacterial infection in mice: potential role of repopulating macrophages. J. Immunol. 182, 7102–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeda K., Clark J. C., Bachurski C. J., Wikenheiser K. A., Cuppoletti J., Mohanti S., Morris R. E., Whitsett J. A. (1994) Immortalization of subpopulations of respiratory epithelial cells from transgenic mice bearing SV40 large T antigen. Am. J. Physiol. 267, L309–L317 [DOI] [PubMed] [Google Scholar]
- 22. Wikenheiser K. A., Vorbroker D. K., Rice W. R., Clark J. C., Bachurski C. J., Oie H. K., Whitsett J. A. (1993) Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 90, 11029–11033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pasula R., Downing J. F., Wright J. R., Kachel D. L., Davis T. E., Jr., Martin W. J., 2nd (1997) Surfactant protein A (SP-A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 17, 209–217 [DOI] [PubMed] [Google Scholar]
- 24. Silver R. F., Walrath J., Lee H., Jacobson B. A., Horton H., Bowman M. R., Nocka K., Sypek J. P. (2009) Human alveolar macrophage gene responses to Mycobacterium tuberculosis strains H37Ra and H37Rv. Am. J. Respir. Cell Mol. Biol. 40, 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mueller H., Detjen A. K., Schuck S. D., Gutschmidt A., Wahn U., Magdorf K., Kaufmann S. H., Jacobsen M. (2008) Mycobacterium tuberculosis-specific CD4+, IFNγ+, and TNFα+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43, 143–148 [DOI] [PubMed] [Google Scholar]
- 26. Higgins D. M., Sanchez-Campillo J., Rosas-Taraco A. G., Higgins J. R., Lee E. J., Orme I. M., Gonzalez-Juarrero M. (2008) Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J. Immunol. 180, 4892–4900 [DOI] [PubMed] [Google Scholar]
- 27. Ryan A. A., Wozniak T. M., Shklovskaya E., O'Donnell M. A., Fazekas de St Groth B., Britton W. J., Triccas J. A. (2007) Improved protection against disseminated tuberculosis by Mycobacterium bovis bacillus Calmette-Guerin secreting murine GM-CSF is associated with expansion and activation of APCs. J. Immunol. 179, 8418–8424 [DOI] [PubMed] [Google Scholar]
- 28. Szeliga J., Daniel D. S., Yang C. H., Sever-Chroneos Z., Jagannath C., Chroneos Z. C. (2008) Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis 88, 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez-Juarrero M., Hattle J. M., Izzo A., Junqueira-Kipnis A. P., Shim T. S., Trapnell B. C., Cooper A. M., Orme I. M. (2005) Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J. Leukoc. Biol. 77, 914–922 [DOI] [PubMed] [Google Scholar]
- 30. Sato K., Tomioka H., Shimizu T., Gonda T., Ota F., Sano C. (2002) Type II alveolar cells play roles in macrophage-mediated host innate resistance to pulmonary mycobacterial infections by producing proinflammatory cytokines. J. Infect. Dis. 185, 1139–1147 [DOI] [PubMed] [Google Scholar]
- 31. Francisco-Cruz A., Aguilar-Santelises M., Ramos-Espinosa O., Mata-Espinosa D., Marquina-Castillo B., Barrios-Payan J., Hernandez-Pando R. (2014) Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor. Med. Oncol. 31, 774. [DOI] [PubMed] [Google Scholar]
- 32. Francisco-Cruz A., Mata-Espinosa D., Estrada-Parra S., Xing Z., Hernández-Pando R. (2013) Immunotherapeutic effects of recombinant adenovirus encoding granulocyte-macrophage colony-stimulating factor in experimental pulmonary tuberculosis. Clin. Exp. Immunol. 171, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nambiar J. K., Ryan A. A., Kong C. U., Britton W. J., Triccas J. A. (2010) Modulation of pulmonary DC function by vaccine-encoded GM-CSF enhances protective immunity against Mycobacterium tuberculosis infection. Eur. J. Immunol. 40, 153–161 [DOI] [PubMed] [Google Scholar]
- 34. Gross T. J., Kremens K., Powers L. S., Brink B., Knutson T., Domann F. E., Philibert R. A., Milhem M. M., Monick M. M. (2014) Epigenetic silencing of the human NOS2 gene: rethinking the role of nitric oxide in human macrophage inflammatory responses. J. Immunol. 192, 2326–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sturrock A., Seedahmed E., Mir-Kasimov M., Boltax J., McManus M. L., Paine R., 3rd (2012) GM-CSF provides autocrine protection for murine alveolar epithelial cells from oxidant-induced mitochondrial injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L343–L351 [DOI] [PubMed] [Google Scholar]
- 36. Erdag G., Medalie D. A., Rakhorst H., Krueger G. G., Morgan J. R. (2004) FGF-7 expression enhances the performance of bioengineered skin. Mol. Ther. 10, 76–85 [DOI] [PubMed] [Google Scholar]
- 37. Yano T., Mason R. J., Pan T., Deterding R. R., Nielsen L. D., Shannon J. M. (2000) KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L1146–L1158 [DOI] [PubMed] [Google Scholar]
- 38. Blijlevens N., Sonis S. (2007) Palifermin (recombinant keratinocyte growth factor-1): a pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann. Oncol. 18, 817–826 [DOI] [PubMed] [Google Scholar]
- 39. Spielberger R., Stiff P., Bensinger W., Gentile T., Weisdorf D., Kewalramani T., Shea T., Yanovich S., Hansen K., Noga S., McCarty J., LeMaistre C. F., Sung E. C., Blazar B. R., Elhardt D., Chen M. G., Emmanouilides C. (2004) Palifermin for oral mucositis after intensive therapy for hematologic cancers. N. Engl. J. Med. 351, 2590–2598 [DOI] [PubMed] [Google Scholar]
- 40. Barasch A., Epstein J., Tilashalski K. (2009) Palifermin for management of treatment-induced oral mucositis in cancer patients. Biologics 3, 111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shyamsundar M., McAuley D. F., Ingram R. J., Gibson D. S., O'Kane D., McKeown S. T., Edwards A., Taggart C., Elborn J. S., Calfee C. S., Matthay M. A., O'Kane C. M. (2014) Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am. J. Respir. Crit. Care Med. 189, 1520–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]