Abstract
Objectives. We aimed to highlight sociodemographic differences in how patients access colonoscopy.
Methods. We invited all eligible patients (n = 2500) from 2 academy-affiliated colonoscopy centers in Alachua County, Florida (1 free standing, 1 hospital based), to participate in a precolonoscopy survey (September 2011–October 2013); patients agreeing to participate (n = 1841, response rate = 73.6%) received a $5.00 gift card.
Results. We found sociodemographic differences in referral pathway, costs, and reasons associated with obtaining the procedure. Patients with the ideal pathway (referred by their regular doctor for age-appropriate screening) were more likely to be Black (compared with other minorities), male, high income, employed, and older. Having the colonoscopy because of symptoms was associated with being female, younger, and having lower income. We found significant differences for 1 previously underestimated barrier, having a spouse to accompany the patient to the procedure.
Conclusions. Patients’ facilitators and barriers to colonoscopy differed by sociodemographics in our study, which implies that interventions based on a single facilitator will not be effective for all subgroups of a population.
Colorectal cancer (CRC), the second leading cause of US cancer deaths in 2013 (50 830),1 is not distributed equally. Nationally, it is estimated that incidence is 25% higher, and mortality from CRC 50% higher, in Black Americans than in Whites.2,3 Most CRC diagnoses follow evaluation by colonoscopy. Although consumers have a range of CRC screening tests, from least invasive (fecal occult blood test, fecal immunochemical test) to most invasive (sigmoidoscopy, colonoscopy),4 if polyps are indicated, a colonoscopy is required as follow-up. Thus, colonoscopy is both an entry point and a pivotal event in the process of preventing, detecting, and treating CRC. CRC can be prevented through the removal of precancerous polyps or detected at an early, easily treatable stage5; findings indicate6 that colonoscopy with polypectomy reduces mortality from CRC by 53%. Although rates of CRC screening have increased,3 there is need for improvement. More than one third of Americans are not in compliance with screening guidelines,7 with rates being lower in the southern United States.8
In 2008, Etzioni et al.9 presented a model of patient and provider-level factors that influence decision-making in colon cancer and that can lead to health disparities in disease recurrence and survival. The Etzioni model identifies key points of vulnerability in the treatment process where the potential to achieve high-quality, guideline-recommended care can be lost. The model captures patients after surgery, beginning with the decision to refer patients to a medical oncologist for adjuvant treatment; it is relevant because there is considerable evidence of inequities in who receives adjuvant treatment based on older age,10,11 comorbidities,12,13 low income,7 coverage with Medicaid rather than Medicare,13 Black race,14 female gender,15,16 and being unmarried.9
We propose that this model starts too late in the process; health disparities originate prior to colonoscopy and can increase at each decision point along a continuum. In an elaborated model (Figure 1), we suggest that CRC health disparities research should begin with an investigation of entry into the health care system and the subsequent pathways to colonoscopy. Referral patterns, costs, and patient demographics influence patient access to care, colonoscopy compliance, and postcolonoscopy decision-making.
FIGURE 1—
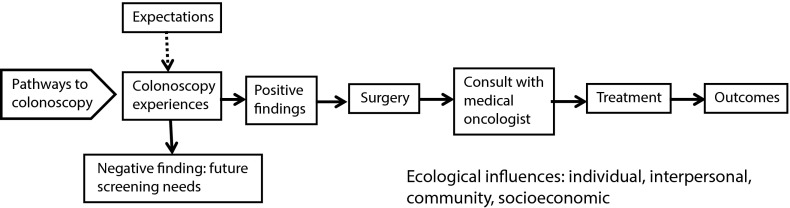
Pathways to colonoscopy, treatment, and outcomes.
PATHWAYS TO COLONOSCOPY
Colonoscopy is generally preceded by a referral from a primary care physician or specialist (with some supplemental support, such as Centers for Disease Control and Prevention’s Screen for Life Program17). Referral has been associated with these patient characteristics: younger age (within age-appropriate groups),18–20 higher education,20,21 higher income,19 White race,20 being married,18 having a comorbidity,18–20 and having a relative with CRC.19 Within the health care setting, referrals were found to be more frequent among internists than family practitioners,22 following a discussion with a physician,19,23 and when the patient had visited a doctor’s office within the past 12 months20 or 25 months.19
Once referred, patients must follow through by scheduling, preparing for, and keeping the colonoscopy appointment. Adherence has been found to be higher among Whites than Blacks in an urban diverse sample,24 among Medicare beneficiaries in Texas,25 and in a systematic review of studies of participants aged 65 years and older.26 However, using data from the 2010 National Health Survey, researchers27 found Blacks to have the highest adjusted level of colonoscopy (57.5%), followed by Whites (55.0%), American Indians/Alaskan natives (48.8%), and Asians (41.3%). Among at-risk individuals with first-degree family members affected by CRC, screening was significantly lower among Blacks than other groups.28 Other demographic characteristics associated with higher rates of completing screenings include male gender,19,26,29–32 not being of Mexican or Hispanic heritage,26,33 nonrural residence,34,35 higher income,22,27,36,37 having insurance,27,37,38 older age,19,27,32,38 and higher education.26,27 Particularly important factors are having a primary care physician as the referral source39 and having a usual source of care.22,26
Besides referral differences, other barriers to screening are also important to consider. CRC screening knowledge is lower among Blacks than Whites across national probability,40 urban,41 church-based,42 and previously unscreened43 samples, and this affects the screening decision.44 Opinions about colonoscopy are also mixed. Bass et al. noted that among never-screened Black men, there was “less trust of their doctors and the health care system and … an overall fear of going to the doctor”45(p121) Women in their study reported that, to agree to a colonoscopy, they needed a stronger relationship with their doctor.45 In their qualitative study of urban Blacks, Greiner et al.41 reported that fear of cancer and CRC screenings were prominent themes. Among other samples, barriers have included fear of the test,45,46 fear of cancer,45,46 and feelings of violation or embarrassment.46
CURRENT STUDY
We investigated demographic differences in pathways to the colonoscopy clinic. Consecutive eligible patients from 2 academic-affiliated colonoscopy centers (1 free standing, 1 hospital based) in Alachua County, Florida, were invited to enroll in a study investigating the colonoscopy experience. CRC incidence and mortality rates per 100 000 within both Florida and Alachua County are higher for Blacks (state incidence and mortality: 45.3 and 17.7; county incidence and mortality: 48.5 and 18.8) than for Whites (state incidence and mortality: 38.5 and 13.8; county incidence and mortality: 42.3 and 12.3).47 Also, percentages of the screening-eligible population who have had a sigmoidoscopy or colonoscopy within the past 5 years within Alachua County are higher for women (58.8%) than for men (49.4%) and for Whites (57.3%) than for Blacks (41.7%).47
We used precolonoscopy survey data to examine the following questions. (1) Is referral pattern associated with patient demographics? (2) Are out-of-pocket costs and perceptions of costs associated with patient demographics? (3) Are reasons for the colonoscopy related to patient demographics? Together, these 3 questions characterize the patient’s pathway to colonoscopy.
METHODS
We recruited participants from eligible patients scheduled for a colonoscopy (aged ≥ 18 years, able to read and write English, and cognitively able) from September 2011 through October 2013. The patient sample is described in Table 1. Of the 3237 eligible patients, we missed 737 (22.8%) because of scheduling or patient flow issues. Of the remaining 2500 patients, 1841 (73.6%) agreed to participate. Of those not participating, 396 of 659 (60.1%) completed an “opt-out” card. Top reasons given for not enrolling were physical condition (e.g., unwell, tired, hungry; 25%), time (e.g., feeling rushed; 16.8%), privacy concerns (14.2%), and not interested (12.7%). We subsequently excluded a small percentage of enrolled patients (n = 56; 3.0%) because of ineligibility, incomplete informed consent form, or at participant’s request. Patients received a $5.00 gift card for participating.
TABLE 1—
Patient-Reported Referral and Cost Factors Associated With Colonoscopy: Alachua County, FL, September 2011–October 2013
| Patient Characteristic | No. (%) | Patient’s First Colonoscopy (Yes = 42.4%) | P (χ2 or t) | Has Regular Doctor (Yes = 60.7%) | P (χ2 or t) | Paid Out of Pocket (Yes = 34.9%) | P (χ2 or t) | Procedure Was Financial Strain (Yes = 16.1%) | P (χ2 or t) | Procedure Was Worth the Cost (Yes = 93.1%) | P (χ2 or t) |
| Race, % | < .001 (χ2 = 25.7) | < .001 (χ2 = 15.5) | .012 (χ2 = 8.8) | .389 (χ2 = 1.9) | < .001 (χ2 = 35.3) | ||||||
| Black | 250 (15.4) | 52.6 | 72.8 | 27.2 | 16.2 | 86.8 | |||||
| White | 1247 (76.7) | 38.8 | 59.0 | 37.2 | 16.0 | 95.3 | |||||
| Other | 129 (8.0) | 55.9 | 60.8 | 33.3 | 20.8 | 85.1 | |||||
| Gender, % | .375 (χ2 = 0.8) | < .001 (χ2 = 12.2) | .13 (χ2 = 2.3) | .05 (χ2 = 3.8) | .57 (χ2 = 0.3) | ||||||
| Female | 1008 (61.8) | 41.5 | 57.7 | 33.6 | 17.7 | 93.0 | |||||
| Male | 623 (38.2) | 43.8 | 66.5 | 37.4 | 14.0 | 93.8 | |||||
| Income, % | < .001 (χ2 = 31.9) | < .001 (χ2 = 34.7) | < .001 (χ2 = 19.5) | < .001 (χ2 = 33.6) | < .008 (χ2 = 11.8) | ||||||
| < 20 000 | 380 (26.5) | 53.4 | 52.7 | 26.6 | 20.7 | 90.6 | |||||
| 20–49 000 | 490 (27.3) | 42.2 | 57.3 | 36.6 | 22.9 | 93.6 | |||||
| 50–79 000 | 286 (19.5) | 37.4 | 60.4 | 41.4 | 16.1 | 95.3 | |||||
| ≥ 80 000 | 392 (26.7) | 35.6 | 72.5 | 39.2 | 8.3 | 96.3 | |||||
| Employed (full- or part-time), % | < .001 (χ2 = 33.1) | .002 (χ2 = 9.4) | < .001 (χ2 = 41.6) | .349 (χ2 = 0.9) | .001 (χ2 = 10.8) | ||||||
| Yes | 898 (55.6) | 48.9 | 64.5 | 42.2 | 15.7 | 95.2 | |||||
| No | 716 (44.4) | 34.4 | 56.9 | 26.5 | 17.5 | 91.0 | |||||
| Lives with driver, % | .179 (χ2 = 1.8) | .014 (χ2 = 6.0) | .519 (χ2 = 0.4) | .952 (χ2 = 0.0) | .771 (χ2 = 0.8) | ||||||
| Yes | 1134 (69.9) | 41.3 | 62.8 | 35.6 | 16.1 | 93.3 | |||||
| No | 489 (30.1) | 44.9 | 56.3 | 33.9 | 16.3 | 93.7 | |||||
| Married or partnered, % | < .001 (χ2 = 13.9) | .106 (χ2 = 2.6) | .151 (χ2 = 2.1) | .534 (χ2 = 0.4) | .93 (χ2 = 0.0) | ||||||
| Yes | 1086 (67.3) | 39.2 | 62.5 | 36.5 | 16.8 | 93.3 | |||||
| No | 527 (32.7) | 49.1 | 58.2 | 32.8 | 15.5 | 93.4 | |||||
| Hispanic descent, % | .012 (χ2 = 6.3) | .034 (χ2 = 4.5) | .755 (χ2 = 0.1) | .486 (χ2 = 0.5) | .043 (χ2 = 4.1) | ||||||
| Yes | 88 (5.4) | 55.3 | 50.0 | 33.7 | 19.0 | 88.0 | |||||
| No | 1530 (94.6) | 41.5 | 61.6 | 35.4 | 16.2 | 93.6 | |||||
| Education, y (mean = 14.23 y) | .017 (t = −2.4) | .276 (t = 1.1) | < .001 (t = 3.8) | < .001 (t = −4.7) | < .001 (t = 4.3) | ||||||
| Answered “yes,” mean | 14.1 | 14.3 | 14.5 | 13.7 | 14.33 | ||||||
| Answered “no,” mean | 14.4 | 14.2 | 14.1 | 14.4 | 13.28 | ||||||
| Age, y (mean = 53.6 y) | < .001 (t = −13.0) | < .001 (t = 8.8) | < .001 (t = −7.1) | < .001 (t = −5.1) | .821 (t = 0.2) | ||||||
| Answered “yes,” mean | 48.8 | 55.9 | 50.4 | 49.8 | 53.48 | ||||||
| Answered “no,” mean | 57.1 | 50.0 | 55.3 | 54.4 | 53.18 |
Measures
A detailed, 6-page instrument provided information about the precolonoscopy experience, but we used only a subset of variables in the analyses presented here. Demographic variables included gender (1 = female, 2 = male), age (continuous), Hispanic or Latino (1 = yes, 2 = no), race (Black = 1, White = 2, all others = 3), employment (full- or part-time = 1, not employed or retired = 2), marital status (married or partnered = 1, single = 2), income (1 = ≤ $20 000, 2 = $20 000–$49 000, 3 = $50 000–$79 000, 4 = ≥ $80 000), education (continuous), and whether the patient lived with the person who drove to the colonoscopy center (1 = yes, 2 = no). Referral variables included whether it was the patient’s first colonoscopy (1 = yes, 2 = no) and who made the referral (1 = regular doctor, 2 = other). We included 3 cost variables: out-of-pocket costs (1 = yes, 2 = no), whether the colonoscopy was a financial strain (1 = yes, 2 = no), and whether the colonoscopy was worth the cost (1 = yes, 2 = no). Finally, patients were asked about why the colonoscopy was ordered (1 = yes, 2 = no): because it was routine for age, a family history of CRC, follow-up to a previous colonoscopy, follow-up to abnormal tests, having symptoms, patient asked for it, and a family member insisted. Patients could select all that applied.
Procedure and Data Analysis
After clinic registration, patients were approached by study staff. A brief description of the study was provided to eligible patients. Interested individuals received a detailed study description and a copy of the informed consent packet. All other eligible patients were asked to complete the opt-out card. Patients unable to complete the survey prior to their procedure were contacted later to complete via telephone.
We conducted analyses with SPSS version 21 (IBM, Armonk, NY). We performed the χ2 test and t test to compare demographic variables with referral and payment information and reasons for colonoscopies. We used multivariable logistic regression to determine the demographic variables independently associated with (1) referral and cost variables, (2) reasons for colonoscopy, and (3) a combined referral-source and reason-for-referral variable. For all logistic regression analyses, the comparison group was the one that was coded at the highest level (e.g., for a dichotomous variable, the comparison group was the group coded as 2). Odds ratios and 95% confidence intervals are reported. Bivariate analyses are reported in the tables and multivariate analyses are presented in the text.
RESULTS
The sample (Table 1) was predominantly White (76.7%), female (61.8%), employed (55.6%), living with the person who drove them to the clinic (69.9%), married or partnered (67.3%), and not of Hispanic descent (94.6%). Income was distributed across the 4 income groups; mean education was 14.23 years and mean age was 53.6 years.
Referral and Costs
Overall, 42.2% stated that this was their first colonoscopy and 60.7% reported that the procedure was ordered by their regular doctor (Table 1). Significant variables associated with first colonoscopy at the bivariate level included race, income, employment status, marital status, ethnicity, age, and education. In multivariable adjusted analyses using logistic regression with 95% confidence intervals, first-time colonoscopy was independently associated with being employed (odds ratio [OR] = 0.53; 95% confidence interval [CI] = 0.40, 0.71), lower income (OR = 0.42; 95% CI = 0.29, 0.60), being male (OR = 1.32; 95% CI = 1.03, 1.68), and younger age (OR = 1.05; 95% CI = 1.04, 1.06). Bivariate analyses indicated that being referred by the patient’s regular doctor was associated with race, gender, income, employment status, living with the driver, ethnicity, and age. After multivariate adjustment, being referred by the patient’s regular doctor was independently associated with being employed (OR = 0.54; 95% CI = 0.41, 0.71), being male (OR = 1.32; 95% CI = 1.04, 1.69), older age (OR = 0.96; 95% CI = 0.95, 0.97), and higher income. Patients in all of the 3 lower levels of income were significantly less likely to report having been referred by their regular doctor than were patients at the highest level of income (for income 1 [lowest income group], OR = 1.74; 95% CI = 1.21, 2.52; for income 2, OR = 1.99; 95% CI = 1.43, 2.76; for income 3, OR = 1.62; 95% CI = 1.14, 2.29).
Regarding costs, 34.9% had some level of self-pay, 16.1% reported that the colonoscopy was a financial strain, and 93.1% reported that the test was worth the cost. Significant bivariate associations with out-of-pocket costs were race, income, employment status, education, and age. Paying out-of-pocket costs was associated with being employed (OR = 0.66; 95% CI = 0.51, 0.87), higher income (OR for income 1 = 1.55; 95% CI = 1.07, 2.25), and younger age (OR = 1.03; 95% CI = 1.02, 1.04) after multivariate adjustment. In bivariate analyses, reporting that the colonoscopy was a financial strain was associated with income, education, and age. Logistic regression models showed that these same variables—lower income, lower education, and younger age—remained significant (for income 1, OR = 0.38; 95% CI = 0.22, 0.65; for income 2, OR = 0.31; 95% CI = 0.19, 0.50; for income 3, OR = 0.47; 95% CI = 0.28, 0.78; for education, OR = 1.08; 95% CI = 1.01, 1.16; for age, OR = 1.03; 95% CI = 1.02, 1.04); in addition, being married became significant (OR = 0.57; 95% CI = 0.40, 0.80) in the adjusted model. Finally, in bivariate analyses, reporting that the colonoscopy was worth the cost was associated with race, income, employment, and education. After adjustment, only White race (OR = 0.27; 95% CI = 0.14, 0.51) and higher education (OR = 0.86; 95% CI = 0.78, 0.96) remained statistically significant.
Reasons for the Colonoscopy
Stated reasons for the colonoscopy were as follows: routine based on age (49.4%), symptoms (39.7%), follow-up to a previous colonoscopy (29.3%), family history of CRC (21.1%), patient asked for test (19.3%), follow-up to abnormal tests (14.2%), and family insisted (5.1%). Given the low frequency of “family insisted,” we did not include it in further analyses.
In bivariate analyses (Table 2), having the colonoscopy because it was routine for their age was associated with gender, employment, living with the driver, marital status, age, and income. After multivariate adjustment, being employed (OR = 0.40; 95% CI = 0.30, 0.53), older age (OR = 0.93; 95% CI = 0.92, 0.94), and being in income 2 ($20 000–$49 000; OR = 1.63; 95% CI = 1.13, 2.34) versus income all other income levels remained significant. At the bivariate level, experiencing symptoms was associated with gender, income, not living with the driver, marital status, education, ethnicity, and age. After adjustment, having the colonoscopy because of symptoms was related to lower income (for income 1, OR = 0.58; 95% CI = 0.39, 0.85; for income 2, OR = 0.64; 95% CI = 0.44, 0.92; for income 3, OR = 0.68; 95% CI = 0.47, 0.99), female gender (OR = 0.68; 95% CI = 0.52, 0.88), and younger age (OR = 1.06; 95% CI = 1.05, 1.07).
TABLE 2—
Patient-Reported Reasons Patients Gave for Having a Colonoscopy: Alachua County, FL, September 2011–October 2013
| Patient Characteristic | Routine for Patient’s Age (Yes = 49.4%) | P (χ2 or t) | Having Symptoms (Yes = 39.7%) | P (χ2 or t) | Follow-Up Colonoscopy (Yes = 29.3%) | P (χ2 or t) | Family History (Yes = 21.1%) | P (χ2 or t) | Patient Asked For (Yes = 19.3%) | P (χ2 or t) | Follow-Up Abnormal (Yes = 14.2%) | P (χ2 or t) |
| Race, % | .841 (χ2 = 0.3) | .256 (χ2 = 2.7) | .301 (χ2 = 2.4) | .948 (χ2 = 0.1) | .232 (χ2 =2.9) | .191 (χ2 = 3.3) | ||||||
| Black | 50.7 | 38.5 | 25.1 | 19.9 | 18.4 | 17.8 | ||||||
| White | 49.0 | 39.3 | 29.9 | 20.8 | 18.9 | 13.3 | ||||||
| Other | 47.5 | 47.0 | 26.3 | 21.2 | 25.5 | 16.1 | ||||||
| Gender, % | < .001 (χ2 = 15.5) | < .001 (χ2 = 19.3) | .395 (χ2 = 0.7) | .003 (χ2 = 9.1) | .213 (χ2 = 1.5) | .264 (χ2 = 2.6) | ||||||
| Male | 55.6 | 32.6 | 30.2 | 16.6 | 20.7 | 12.6 | ||||||
| Female | 45.1 | 44.2 | 28.2 | 23.2 | 18.0 | 15.3 | ||||||
| Income, % | < .001 (χ2 = 23.1) | < .001 (χ2 = 31.7) | .249 (χ2 = 4.1) | .297 (χ2 = 3.7) | .54 (χ2 = 2.2) | .128 (χ2 = 5.7) | ||||||
| < 20 000 | 45.6 | 48.6 | 27.3 | 18.4 | 20.6 | 17.1 | ||||||
| 20–49 000 | 42.1 | 43.3 | 26.2 | 19.9 | 17.2 | 15.2 | ||||||
| 50–79 000 | 47.5 | 38.9 | 32.5 | 23.5 | 18.5 | 13.1 | ||||||
| ≥ 80 000 | 58.9 | 28.6 | 31.0 | 23.2 | 21.0 | 11.1 | ||||||
| Employed, % | .008 (χ2 = 7.1) | .918 (χ2 = 0.0) | < .001 (χ2 = 24.7) | .227 (χ2 = 1.5) | .577 (χ2 = 0.3) | < .001 (χ2 = 14.6) | ||||||
| Yes | 52.2 | 40.0 | 23.5 | 21.9 | 19.9 | 10.9 | ||||||
| No | 45.2 | 39.7 | 35.3 | 19.3 | 18.7 | 17.9 | ||||||
| Lives with driver, % | .018 (χ2 =5.6 ) | .003 (χ2 = 8.8) | .324 (χ2 = 1.0) | .689 (χ2 = 0.2) | .012 (χ2 = 6.3) | .207 (χ2 = 3.1) | ||||||
| Yes | 51.4 | 37.1 | 28.4 | 20.5 | 17.6 | 13.5 | ||||||
| No | 44.7 | 45.3 | 30.9 | 21.4 | 23.2 | 15.3 | ||||||
| Married or partnered, % | .002 (χ2 = 9.9) | < .001 (χ2 = 14.5) | .511 (χ2 = 0.4) | .698 (χ2 = 0.2) | .035 (χ2 = 4.4) | .55 (χ2 = 0.4) | ||||||
| Yes | 52.0 | 36.3 | 29.2 | 20.4 | 17.7 | 13.6 | ||||||
| No | 43.2 | 46.7 | 27.5 | 21.3 | 22.4 | 14.8 | ||||||
| Education, y | .282 (t = 1.1) | .001 (t = −3.2) | .191 (t = 1.3) | .71 (t = 0.4) | .239 (t = 1.2) | .001 (t = −3.5) | ||||||
| Answered “yes,” mean | 14.3 | 13.9 | 14.4 | 14.3 | 14.4 | 13.7 | ||||||
| Answered “no,” mean | 14.2 | 14.4 | 14.2 | 14.2 | 14.2 | 14.3 | ||||||
| Hispanic, % | .266 (χ2 = 1.2) | .014 (χ2 = 6.1) | .568 (χ2 = 0.3) | .378 (χ2 = 0.8) | .01 (χ2 = 6.7) | .008 (χ2 = 6.9) | ||||||
| Yes | 43.0 | 53.3 | 25.7 | 16.4 | 30.7 | 24.7 | ||||||
| No | 49.5 | 39.1 | 28.8 | 20.7 | 18.6 | 13.6 | ||||||
| Age, y | < .001 (t = 13.0) | < .001 (t = −14.1) | < .001 (t = 7.4) | .746 (t = 0.3) | .033 (t = 2.1) | .088 (t = −1.7) | ||||||
| Answered “yes,” mean | 57.8 | 47.6 | 57.4 | 53.5 | 55.0 | 51.9 | ||||||
| Answered “no,” mean | 49.3 | 56.9 | 51.8 | 53.2 | 53.1 | 53.6 |
Note. Patients could give multiple reasons for colonoscopy.
Follow-up to a previous colonoscopy was associated in both bivariate and logistic regression analyses with not being employed (OR = 1.34; 95% CI = 1.04, 1.75) and older age (OR = 0.97; 95% CI = 0.96, 0.98). Colonoscopy because of family history was associated in both analyses with being female (OR = 0.62; 95% CI = 0.46, 0.83). At the bivariate level, asking for the test was associated with not living with the driver, marital status, ethnicity, and age. Variables that remained independently associated in logistic regression analyses included being unmarried (OR for married or partnered = 1.62; 95% CI = 1.89, 2.20), being Hispanic (OR for ethnicity = 0.42; 95% CI = 0.24, 0.74), and older age (OR for age = 0.98; 95% CI = 0.97, 0.99). The final reason, follow-up to abnormal tests, was associated with employment, education, and ethnicity in bivariate analyses. Being unemployed (OR = 1.84; 95% CI = 1.29, 2.61) and Hispanic ethnicity (OR = 0.43; 95% CI = 0.23, 0.79) remained significant in logistic regression analyses.
A More Complete View of Pathways
Ideally, screening colonoscopy should be part of routine preventive care recommended by a patient’s regular doctor. Analyses indicated significant demographic differences among patients receiving colonoscopy according to whether or not they had symptoms and whether or not they were referred by their regular doctor. We created a new variable by which patients were classified as (1) referred by a regular doctor, no symptoms (n = 649); (2) referred by a regular doctor, with symptoms (n = 238); (3) not referred by a regular doctor, no symptoms (n = 207); and (4) not referred by a regular doctor, with symptoms (n = 324). Binary results for associations with demographic variables indicated that the groups were significantly different on all variables (Table 3). The largest differences occurred when we compared the highest and lowest incomes (26.4%) and Hispanics and non-Hispanics (16.4%). Using logistic regression, we then compared “ideal patients” (regular doctor, no symptoms) with all other patients. Significant associations for being in the regular doctor–no symptoms group included Black race compared with “other” (OR = 0.48; 95% CI = 0.26, 0.89); male gender (OR = 1.46; 95% CI = 1.12,1.90); highest income (group 4) compared with group 1 (OR = 1.68; 95% CI = 1.09, 2.61), group 2 (OR = 1.69; 95% CI = 1.16, 2.44), or group 3 (OR = 1.46; 95% CI = 1.01, 2.10); being employed (OR = 0.56; 95% CI = 0.41, 0.75); and being older (OR = 0.94, 95% CI = 0.92, 0.95).
TABLE 3—
Referral and Symptom Variables Associated With Getting a Colonoscopy, by Patient Demographic Characteristics: Alachua County, FL, September 2011–October 2013
| Patient Characteristic | Referred by Regular Doctor, No Symptoms, % (n = 649) | Referred by Regular Doctor, With Symptoms, % (n = 238) | Not Referred by Regular Doctor, No Symptoms, % (n = 207) | Not Referred by Regular Doctor, With Symptoms, % (n = 324) | P (χ2 or F) |
| Race | .001 (χ2 = 22.2) | ||||
| Black | 51.5 | 23.5 | 10.0 | 15.0 | |
| White | 45.8 | 15.1 | 15.0 | 24.1 | |
| Other | 38.9 | 24.1 | 13.0 | 24.1 | |
| Gender | < .001 (χ2 = 23.9) | ||||
| Female | 41.1 | 18.4 | 14.7 | 25.8 | |
| Male | 54.2 | 14.6 | 13.1 | 18.2 | |
| Income, $ | < .001 (χ2 = 51.7) | ||||
| < 20 000 | 33.7 | 21.6 | 16.7 | 28.0 | |
| 20 000–49 000 | 42.2 | 17.2 | 14.2 | 26.5 | |
| 50 000–79 000 | 46.8 | 16.3 | 14.7 | 22.2 | |
| ≥ 80 000 | 60.1 | 12.7 | 11.6 | 15.6 | |
| Employed | .007 (χ2 = 12.1) | ||||
| Yes | 48.4 | 17.2 | 11.3 | 23.1 | |
| No | 42.8 | 16.8 | 17.7 | 22.7 | |
| Lives with driver | .013 (χ2 = 10.8) | ||||
| Yes | 48.4 | 15.5 | 14.4 | 21.6 | |
| No | 39.4 | 19.7 | 15.0 | 25.9 | |
| Married or partnered | .005 (χ2 = 12.9) | ||||
| Yes | 49.0 | 15.3 | 14.4 | 21.2 | |
| No | 40.0 | 20.3 | 13.8 | 25.9 | |
| Hispanic | .023 (χ2 = 9.6) | ||||
| Yes | 30.4 | 18.8 | 14.5 | 36.2 | |
| No | 46.8 | 16.9 | 14.1 | 22.3 | |
| Education | 14.5 | 13.7 | 14.0 | 14.2 | < .001 (F = 8.4) |
| Age | 57.7 | 49.5 | 54.6 | 45.9 | < .001 (F = 72.6) |
Note. Percentages in the table are for the χ2; means for the F tests (age and education).
DISCUSSION
We examined 3 questions related to colonoscopy pathways: (1) Is referral pattern associated with patient demographics? (2) Are costs and perceptions of costs associated with patient demographics? (3) Are reasons for the colonoscopy related to patient demographics? The simple answer to each question is yes—in multivariate analyses, patient demographics were related to how and why patients appear for colonoscopies.
Review of Primary Findings
First-time colonoscopy patients were almost a decade younger and more likely to be male, of lower income, and employed than those with previous procedures. As average-risk individuals begin screening at age 50, this age difference is not surprising, and it is possible that age was a confounder between first colonoscopy and other demographics. However, among first-colonoscopy patients, males were actually older than females and patients in the highest income level were older than those in all other income groups. Employed patients were significantly younger than unemployed patients (including retired patients), so it is possible that this relationship was at least partially driven by age. Out-of-pocket costs were associated with the subgroup more likely to be covered by workplace insurance (employed, higher income, younger age) than by public programs. Other cost variables were associated with disadvantaged status. Reporting that the colonoscopy was a financial strain was more frequent among low-income, low-education, younger, married patients. Believing that colonoscopy was not worth the cost was associated with non-White race and lower education.
About half the patients (49.4%) were having their colonoscopies as part of routine screening care; overall, patients there for other reasons were more likely to be from at-risk groups (lower income, unemployed, Hispanic, unmarried, older, female). This pattern was particularly striking when we compared patients with no symptoms and referred by their regular doctor (the ideal screening situation) with all other patients. Patients following this “ideal pathway” were more likely to be Black (compared with other minorities), male, high income, employed, and older. On the surface, these findings appear to indicate some advantage to subgroups at higher risk for CRC—men and Blacks. However, it is critical to note that Blacks were only more advantaged than other minorities—not Whites—and, as noted, Blacks and men in the surrounding area were less likely to have had sigmoidoscopy or colonoscopy in the past 5 years.48 There remains a reservoir of inequality in terms of accessing care, receiving a referral, and following through on the referral. However, our findings suggest that Blacks and men may follow through when referred by their physicians; this reinforces previous findings regarding the importance of physician referral for CRC screening in general49,50 and referral by a trusted physician for Blacks.45
In many ways, colonoscopy is a “family procedure”; patients must be accompanied by an adult who stays on the premises (lenient clinics may allow continuous access via telephone). For most drivers, that entails a 2- to 3-hour wait plus travel time. Therefore, the driver must give up a half day that might have been used for work. Possibly, individuals who have lower resources (women, lower-income people) may put off having the colonoscopy (i.e., wait until they have symptoms) so that they do not inconvenience others, financially or otherwise. This reasoning is supported by our data—52.1% of women compared with 72.2% of men were driven by a spouse (χ2 = 67.4, P < .001). Likewise, living with the driver and income were highly related (χ 2 = 148.7, P < .001); 39.7% in the lowest level of income were driven by a spouse compared with 80.4% in the highest income level. The requirement to have someone on site during the procedure does not fit the daily reality of some subgroups. This requirement also has broader implications for care, as lower-income patients are significantly less likely to be accompanied by someone who could make medical decisions on their behalf should there be complications, critical follow-up instructions, or an abnormal finding such as cancer.
Limitations and Strengths
A limitation of the study is lack of geographic diversity; although the 2 clinics draw from a large rural–suburban area, both are academy affiliated. A second limitation is that patients were engaged immediately before the colonoscopy; therefore, we could not compare them with people who were never referred, never made an appointment, or did not show up for appointments. Additionally, although the sample was large and representative of the region, there was not enough variation to examine racial groups other than Whites and Blacks, and it is a disservice to refer to them as “other.” These limitations are counterbalanced by several strengths: a large sample size, high participation rate, characterization of nonresponders, and findings that are suggestive for potential interventions.
Implications
Previous studies have found higher rates of colonoscopies among males,20,27,30–33 people not of Mexican or Hispanic heritage,27,34 and people with nonrural residence,35,36 higher income,23,28,37,38 having insurance,28,38,39 older age,20,28,33,39 and higher education.27,28 This is most consistent with our profile of patients who were receiving a routine colonoscopy based on age—it does not reflect patients who were having the procedure for other reasons. Delay of colonoscopy until there are symptoms subverts the benefit of a highly effective screening program and highlights that it is not enough to simply count rates among groups; we need to account for factors that might reflect disparities in how and why people have the procedure. Studies need to explore below the surface of rates for more subtle group differences.
We also found 2 potential barriers that may signal sources of health disparities: the perceptions by minority and poorer patients that colonoscopy is not worth the cost and the finding that not having a driver is a potential barrier among certain subgroups of patients. Concerning the former, follow-up analyses showed that people who paid no costs were significantly more likely to think the colonoscopy was not worth it than were those who did pay. This finding is consistent with cognitive dissonance51; people tend to value what they pay for, especially when payment for something arduous is small. This suggests that a very small copay may make colonoscopy more desirable (and, if paid up front, might reduce rates of no-show). With community- or clinic-based interventions, it is important to address transportation for some subgroups, especially those with fewer social and economic resources. However, simply providing patient transportation does not solve the issue of having someone with decision-making authority nearby in an emergency.
Overall, these findings indicate that pathways to colonoscopy vary by demographic characteristics and that there is no single cause or solution for these differences. Differences originate across the ecological model—and it is the accumulation of factors that likely causes disparities in CRC screening, incidence, and mortality. Therefore, we must continue to look for causes and solutions across multiple levels and multiple settings. An effective intervention may be modular, with patients choosing the components that both motivate and remove perceived barriers. One role for a community health worker, patient advocate, or patient navigator might be to assess the components of an intervention needed by each individual patient (e.g., education, social support, costs, transportation). Such a modular intervention may be more difficult to implement and evaluate, but it more directly addresses the complexities of patient needs to support improved compliance with CRC screening.
Acknowledgments
The research reported in this article was supported by the Bankhead Coley Cancer Research Program (Award No. 1BG01-34188 to the first author).
We thank the many patients and caregivers who helped with this study, and the following colleagues: Lavonza “Quan” Holliman, MPH; Felix Lorenzo, MPH; Emmett Martin, MPH; Jane McGinley, BS; Jennifer Nguyen, MPH; Regina Reed, BS; Gregory Riherd, MPH; and Britany Telford, BS.
Note. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Bankhead Coley Cancer Research Program.
Human Participant Protection
This project was approved by the University of Florida institutional review board (01: 166-2011).
References
- 1.Cancer Facts & Figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics 2014. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Colorectal (colon) cancer. Available at: http://www.cdc.gov/cancer/colorectal/statistics/race.htm. Accessed December 19, 2012.
- 4.Tests to detect colorectal cancer and polyps. National Cancer Institute (NCI) Fact Sheet, last modified December 30, 2011. Available at: http://www.cancer.gov/cancertopics/factsheet/Detection/colorectal-screening. Accessed December 10, 2014.
- 5.American Society of Colon & Rectal Surgeons. Screening and surveillance for colorectal cancer. October 20102. Available at: http://www.fascrs.org/patients/treatments_and_screenings/assess_your_risk_for_colorectal_cancer/screening. Accessed December 10, 2014.
- 6.Zauber A, Winawer S, O’Brien M et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 8.Steele CB, Rim SH, Joseph DA, King JB, Steef LC. Colorectal cancer incidence and screening—United States, 2008 and 2010. MMWR Surveill Summ. 2013;62(suppl 3):53–60. [PubMed] [Google Scholar]
- 9.Etzioni DA, EI-Khoueiry AB, Beart RW., Jr Rates and predictors of chemotherapy use for stage Ill colon cancer. Cancer. 2008;113(12):3279–3289. doi: 10.1002/cncr.23958. doi:10.1002/cncr.23958. [DOI] [PubMed] [Google Scholar]
- 10.Ganapathi AM, Speicher PJ, Englum BR et al. Adjuvant chemotherapy for t1 node-positive colon cancers provides significant survival benefit. Dis Colon Rectum. 2014;57(12):1341–1348. doi: 10.1097/DCR.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley CJ, Given CW, Dahman B, Fitzgerald TL. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med. 2008;168(5):521–529. doi: 10.1001/archinternmed.2007.82. [DOI] [PubMed] [Google Scholar]
- 12.Sargent D, Sobrero A, Grothey A et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27(6):872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mano MS, Duhoux F. Colon cancer: update on adjuvant therapy. Clin Colorectal Cancer. 2008;7(3):178–183. doi: 10.3816/CCC.2008.n.023. [DOI] [PubMed] [Google Scholar]
- 14.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Lin JK, Chang SC et al. Is adjuvant chemotherapy beneficial to high risk stage II colon cancer? Analysis in a single institute. Int J Colorectal Dis. 2009;24(6):665–676. doi: 10.1007/s00384-009-0634-1. [DOI] [PubMed] [Google Scholar]
- 16.McGory ML, Zingmond DS, Sekeris E, Bastani R, Ko CY. A patient’s race/ethnicity does not explain the underuse of appropriate adjuvant therapy in colorectal cancer. Dis Colon Rectum. 2006;49(3):319–329. doi: 10.1007/s10350-005-0283-6. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Screen for Life: National Colorectal Cancer Action Campaign. Last update May 21, 2014. Available at: http://www.cdc.gov/cancer/colorectal/sfl. Accessed December 10, 2014.
- 18.Sewitch MJ, Fournier C, Dawes M et al. Do physician recommendations for colorectal cancer screening differ by patient age? Can J Gastronterol. 2007;21(7):435–438. doi: 10.1155/2007/938978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly JM, Xu Y, Levy BT. Patients whose physicians recommend colonoscopy and those who follow through. J Prim Care Community Health. 2013;4(2):83–94. doi: 10.1177/2150131912464887. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Ye J, Xu Z, Aladesanmi O. Provider recommendation for colorectal cancer screening: examining the role of patients’ socioeconomic status and health insurance. Cancer Epidemiol. 2009;33(3–4):207–211. doi: 10.1016/j.canep.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Higgins AY, Doubeni AR, Phillips KL et al. Self-reported colorectal cancer screening of Medicare beneficiaries in family medicine practices in the United States: a cross-sectional study. BMC Gastroenterol. 2012;12:23. doi: 10.1186/1471-230X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boguradzka A, Wiszniewski M, Kaminski MF et al. The effect of primary care physician counseling on participation rate and use of sedation in colonoscopy-based colorectal cancer screening program—a randomized controlled study. Scand J Gastroenterol. 2014;49(7):878–884. doi: 10.3109/00365521.2014.913191. [DOI] [PubMed] [Google Scholar]
- 24.Inadomi JM, Vijan S, Janz NK et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benarroch-Gampel J, Sheffield KM, Lin YL, Kuo YF, Goodwin JS, Riall TS. Colonoscopist and primary care physician supply and disparities in colorectal cancer screening. Health Serv Res. 2012;47(3 pt 1):1137–1157. doi: 10.1111/j.1475-6773.2011.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guessous I, Dash C, Lapin P et al. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50(1–2):3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murff H, Perterson N, Fowke J et al. Colonoscopy screening in African Americans and whites with affected first-degree relatives. Arch Intern Med. 2008;168(6):625–631. doi: 10.1001/archinte.168.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wernli K, Hubbard R, Johnson E, Chubak J, Kaminen A, Rutter C. A2-1: patterns of colorectal cancer testing in men and women newly eligible for screening. Clin Med Res. 2013;11(3):123. [Google Scholar]
- 30.Gancayco J, Soulos PR, Khiani V et al. Age-based and sex-based disparities in screening colonoscopy use among Medicare beneficiaries. J Clin Gastroenterol. 2013;47(7):630–636. doi: 10.1097/MCG.0b013e31828345c8. [DOI] [PubMed] [Google Scholar]
- 31.Yager SS, Chen L, Cheung WY. Sex-based disparities in colorectal cancer screening. Am J Clin Oncol. 2013 doi: 10.1097/COC.0b013e318282a830. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Denberg TD, Melhado TV, Coombes JM et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20(11):989–995. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda P, Johnson-Jennings M, Tarraf W et al. Using colorectal trends in the US to identify unmet primary care needs of vulnerable populations. Prev Med. 2012;55(2):131–136. doi: 10.1016/j.ypmed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole AM, Jackson JE, Doescher M. Urban–rural disparities in colorectal cancer screening: cross-sectional analysis of 1998–2005 data from the centers of Centers for Disease Control’s Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson AE, Henry KA, Samadder NJ, Merrill RM, Kinney AY. Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11(5):526–533. doi: 10.1016/j.cgh.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doubeni CA, Jambaulikar GD, Fouayz H et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population—a retrospective cohort study. PLoS One. 2012;7:e36392. doi: 10.1371/journal.pone.0036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver JS, Worley CB, Decoster J et al. Disparities in colorectal cancer screening behaviors: implications for African American men. Gastroenterol Nurs. 2012;35(2):93–98. doi: 10.1097/SGA.0b013e31824e2d7a. [DOI] [PubMed] [Google Scholar]
- 38.Halbert CH, Barg FK, Guerra CE et al. Cultural, economic, and psychological predictors of colonoscopy in a national sample. J Gen Intern Med. 2011;26(11):1311–1316. doi: 10.1007/s11606-011-1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singal AK, Lin YL, Kuo YF, Riall T, Goodwin JS. Primary care physicians and disparities in colorectal cancer screening in the elderly. Health Serv Res. 2013;48(1):95–113. doi: 10.1111/j.1475-6773.2012.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford JS, Coups EJ, Hay JL. Knowledge of colon cancer screening in a national probability sample in the United States. J Health Commun. 2006;11(suppl 1):19–35. doi: 10.1080/10810730600637533. [DOI] [PubMed] [Google Scholar]
- 41.Greiner K, Born W, Nallen N, Ahluwala J. Knowledge perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005;20(11):977–983. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng T, Hold C, Shipp M et al. Predictors of colorectal cancer knowledge and screening among church-attending African Americans and whites in the Deep South. J Community Health. 2009;34(2):90–97. doi: 10.1007/s10900-008-9128-2. [DOI] [PubMed] [Google Scholar]
- 43.Ruffin M, Creswell J, Jimbo M, Fetters M. Factors influencing choices for colorectal cancer screening among previously unscreened African and Caucasian Americans: findings from a triangulation mixed methods investigation. J Community Health. 2009;34(2):79–89. doi: 10.1007/s10900-008-9133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong CR, Bloomfield ER, Crookes DM, Jandorf L. Barriers and facilitators to adherence to screening colonoscopy among African-Americans: a mixed-methods analysis. J Cancer Educ. 2013;28(4):722–728. doi: 10.1007/s13187-013-0510-0. [DOI] [PubMed] [Google Scholar]
- 45.Bass S, Gordon T, Ruzek S et al. Perceptions of colorectal cancer screening in urban African American clinic patients: differences by gender and screening status. J Cancer Educ. 2011;26(1):121–128. doi: 10.1007/s13187-010-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bynum SA, Davis JL, Green BL, Katz RV. Unwillingness to participate in colorectal cancer screening: examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. Am J Health Promot. 2012;26(5):295–300. doi: 10.4278/ajhp.110113-QUAN-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jilcott Pitts SB, Lea CS, May CL et al. “Fault-line of an earthquake”: a qualitative examination of barriers and facilitators to colorectal cancer screening in rural, eastern North Carolina. J Rural Health. 2013;29(1):78–87. doi: 10.1111/j.1748-0361.2012.00424.x. [DOI] [PubMed] [Google Scholar]
- 48.Florida Dept of Health, Division of Public Health Statistics and Performance Management. Florida charts. Available at: http://www.floridacharts.com/charts/ChronicDiseases. Accessed January 30, 2014.
- 49.Lane DS, Messina CR, Cavanagh MF, Chenn JJ. A provider intervention to improve colorectal screening in county health centers. Med Care. 2008;46(9 suppl 1):S109–S116. doi: 10.1097/MLR.0b013e31817d3fcf. [DOI] [PubMed] [Google Scholar]
- 50.Taylor V, Lessler D, Mertens K et al. Colorectal cancer screening among African Americans: the importance of physician recommendation. J Natl Med Assoc. 2003;95(9):806–812. [PMC free article] [PubMed] [Google Scholar]
- 51.Aronson E. The effect of effort on the attractiveness of rewarded and unrewarded stimuli. J Abnorm Soc Psychol. 1961;63:375–380. doi: 10.1037/h0046890. [DOI] [PubMed] [Google Scholar]


