Abstract
Many recent breakthroughs in our understanding of termite biology have been facilitated by “omics” research. Omic science seeks to collectively catalog, quantify, and characterize pools of biological molecules that translate into structure, function, and life processes of an organism. Biological molecules in this context include genomic DNA, messenger RNA, proteins, and other biochemicals. Other permutations of omics that apply to termites include sociogenomics, which seeks to define social life in molecular terms (e.g., behavior, sociality, physiology, symbiosis, etc.) and digestomics, which seeks to define the collective pool of host and symbiont genes that collaborate to achieve high-efficiency lignocellulose digestion in the termite gut. This review covers a wide spectrum of termite omic studies from the past 15 years. Topics covered include a summary of terminology, the various kinds of omic efforts that have been undertaken, what has been revealed, and to a degree, what the results mean. Although recent omic efforts have contributed to a better understanding of many facets of termite and symbiont biology, and have created important new resources for many species, significant knowledge gaps still remain. Crossing these gaps can best be done by applying new omic resources within multi-dimensional (i.e., functional, translational, and applied) research programs.
Keywords: holobiome, digestome, sociogenomics, symbiosis, metabolomics, DNA methylation, sociobiology, socioevolution
Introduction
Overview and Terminology
In a broad sense, the underlying goals of omic1 science are to catalog, quantify, and characterize pools of biological molecules that translate into structure, function, and life processes of an organism or environment. The types of biological molecules receiving focus in omics2 include genomic DNA, messenger RNA (mRNA), protein, and metabolites (Figure 1). DNA, mRNA, and protein are respectively the foci of genomics, transcriptomics, methylomics, and proteomics. Genomics, methylomics, and transcriptomics rely on nucleic acid sequencing, whereas proteomics utilizes peptide sequencing procedures. By contrast, metabolomics is rooted more in analytical chemistry and focuses on biochemicals, metabolites, or pathways. Another relevant omic approach is the cataloging of bacterial and protist symbionts using high-throughput 16S and 18S rRNA sequencing.
FIGURE 1.
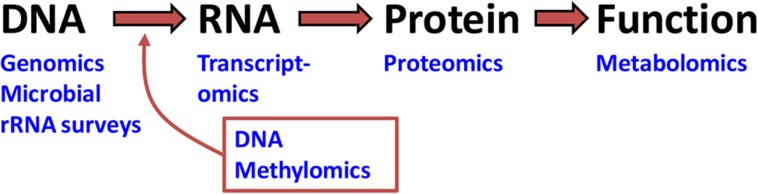
The continuum of biological organization and function addressed by omic research. The three bio-molecules listed (DNA, RNA, and protein) constitute the Central Dogma of Biology. Omic approaches that target these molecules can at best infer function. Proving function requires metabolomics and other functional or translational approaches not covered in this review (Scharf, 2015).
Termite omic research has focused on the host termite, individual gut microbial symbionts or entire populations of gut microbes. In the latter case, these “meta” analyses focusing broadly on collective microbiota occurring in the gut microenvironment have been popular, particularly with microbiologists specializing in termite intestinal microbiology. Although it presents significant bioinformatic challenges, a more inclusive approach that considers host and symbionts together as a single functional unit is the best approach for appreciating the full functional capacity of termites. A fundamental advantage of omic research over more traditional organismal research is that it enables direct mechanistic insights into termite and symbiont physiology and biochemistry. The use of omic technologies has led to new insights into behavior, social structure, digestion, and host-symbiont/symbiont–symbiont interactions, and many other aspects of termite biology. However, also as addressed throughout this review, omic science has limits for being able to define biological function.
Termite Symbiosis and the Holobiont Concept
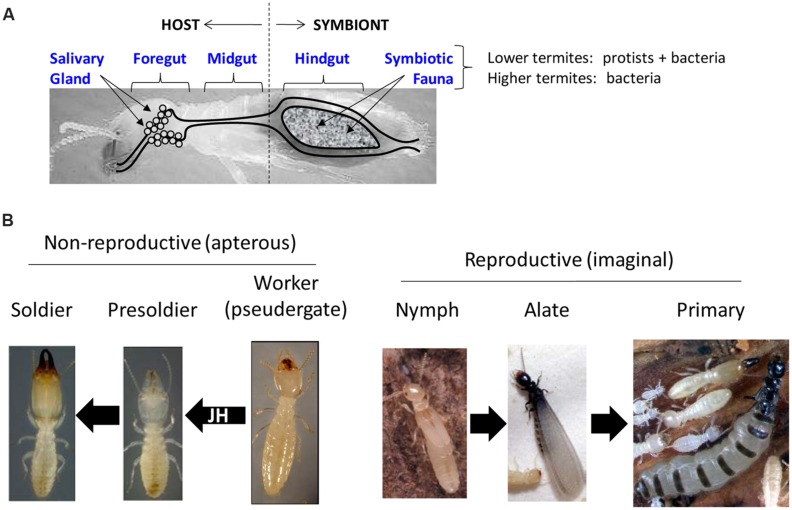
Termites are perhaps best know1n for their symbiotic associations with gut microbes (König et al., 2013; Brune, 2014) that are often linked to digestive processes, although lignocellulose digestion is not mediated entirely by gut microbes (Watanabe and Tokuda, 2010; Figure 2A). The more ancestral lower termites have tri-partite symbioses that include host, bacteria and protozoa; whereas in higher termites, symbiosis has been reduced to a two-way association between host and bacteria (but some higher termites also maintain ecto-symbiotic associations with fungi; Brune, 2014). The host component of termite symbiotic systems adds substantially to the digestive process both in terms of contributing enzymes and maintaining a favorable gut microenvironment for symbiosis and digestion to occur (Watanabe et al., 1998; Tartar et al., 2009; Scharf et al., 2011; Sethi et al., 2013a; Tokuda et al., 2014). Because of the high degree of interplay that occurs between the termite host and gut symbionts, a key idea moving forward will be to consider termites from the perspective of the “holobiont” (a single functional unit in which host and symbionts are physiologically tightly connected). Omic research has enabled a multifaceted systemic understanding of gut digestomes that is central to understanding the termite holobiome from an applied perspective (Scharf, 2015).
FIGURE 2.
Fundamental ideas behind digestomic and sociogenomic research in termites. (A) Key components associated with termite digestomes and digestomic research. Different gut regions have been studied in an attempt to dissect host and symbiont contributions to digestion. An important distinction between lower and higher termites is the presence of protist and bacterial symbiota in lower termites, and only bacteria in higher termites. (B) Caste and phenotype-associated transitions addressed through sociogenomic research. Left: non-reproductive or “apterous” (wingless) phenotypes of lower termites. Presoldiers and soldiers differentiate from workers in response to elevated juvenile hormone (JH) titers. Right: nymphs give rise to alates that become primary reproductives; a process akin to typical hemimetabolous insect development.
Sociogenomics and Digestomics
The term sociogenomics was coined to describe the use of omic approaches for defining social life in molecular terms, which began with studies on the honey bee, Apis mellifera (Robinson et al., 2005). A parallel idea cited as rationale for many omic studies in social insects, including termites, is that solitary genes and traits were likely co-opted for new functions as solitary ancestors transitioned to social lifestyles (West-Eberhard, 2003; Nelson et al., 2007). Understanding such traits is essential for understanding termite social evolution (Miura and Scharf, 2011; Figure 2B). Another term used specifically in relation to digestive research is digestomics, which was coined to describe the collective pool of host and symbiont genes that collaborate to achieve high-efficiency lignocellulose digestion in the termite gut (Scharf and Tartar, 2008; Tartar et al., 2009; Figure 2A). Such terminology is useful because of the large number of symbionts that occupy termite guts and collaborate with the host in lignocellulose digestion. A related term is termitosphere, which is the full complement of gut and ectosymbiotic (nest) microbes present in termites, termite colonies, and their surrounding nest structures (Roose-Amsaleg et al., 2004; Bastien et al., 2013). Whether in relation to social, solitary or symbiont genes, proteins or other biomolecules, sociogenomic and digestomic research in termites has created an explosion of new sequence data.
Omic Studies in Termites: What has been Done?
Based on a recent literature survey (Table 1), at the time of writing this article around 70 papers had been published describing omic efforts in termite systems. These studies include all the themes introduced above, as well as microbial 16S and 18S surveys.
Table 1.
A comprehensive literature summary of termite omic research, organized by approaches taken.
| Omic approach taken | Termite group | Termite species | Host or symbiont | Tissue or fraction | Approach | Method | Major finding | Reference |
|---|---|---|---|---|---|---|---|---|
| Transcriptome | Lower | Coptotermes formosanus | Host | Whole-body polyphenic library | Hypothesis-driven (virgin vs. egg-laying queens) | Sanger sequencing + microarray | 7663 ESTs sequenced that aligned into 4726 contigs; microarray analysis revealed 94 differentially expressed genes between virgin and reproductive queens | Husseneder et al. (2012) |
| Combined | Whole workers | Hypothesis-driven | Sanger sequencing | 1511 total unigenes (362 contigs + 1149 singletons) | Hussain et al. (2013) | |||
| Coptotermes gestroi | Host | Head tissue | Descriptive | Sanger sequencing | 3003 high quality ESTs were obtained that aligned into 695 unigenes (245 contigs and 450 singlets) | Do et al. (2014) | ||
| Cryptotermes secundus | Combined | Whole workers and neotenic reproductives | Hypothesis-driven | Representational difference analysis, Sanger sequencing | 187 differentially expressed library clones were identified that aligned into 35 unigene contigs | Weil et al. (2007) | ||
| Hodotermopsis sjostedti | Host | Head tissue of differentiated workers and soldiers | Hypothesis-driven | Differential display, Sanger sequencing | 11 candidate bands were identified, including the SOL1 gene, which was detected mostly in soldier heads | Miura et al. (1999) | ||
| Host | Mandibular tissue of workers, presoldiers, and soldiers | Hypothesis-driven (JHA induced gene expression) | Fluorescent differential display (FDD), Sanger sequencing | 81 candidate bands identified by FDD that aligned into 12 unigenes upregulated in mandibular tissue during soldier differentiation | Koshikawa et al. (2005) | |||
| Host | Whole worker termites without guts | Hypothesis-driven (JHA up and downregulated genes) | FDD, Sanger sequencing | 28 candidate bands identified by FDD; 18 aligned into ca. 5 unigenes | Cornette et al. (2006) | |||
| Host | Worker brain and subesophageal ganglion | Hypothesis-driven (JHA induced gene expression) | FDD, Sanger sequencing | No differences in expression patterns detected between pseudergates and soldiers; five genes up-regulated in brain and/or SOG during differentiation | Ishikawa et al. (2010) | |||
| Host | Whole worker termites without guts | Hypothesis-driven (JHA up and downregulated genes) | Subtractive libraries, filter arrays, and Sanger sequencing | 87 and 64 JHA up- and downregulated clones identified | Cornette et al. (2013) | |||
| Mastotermes darwiniensis | Host | Worker head, thorax and front legs | Hypothesis-driven (genes upregulated by Metarhizium anisopliae infection) | Subtractive cDNA library, Sanger sequencing | The number of differentially expressed clones was not specified | Thompson et al. (2003) | ||
| Reticulitermes flavipes | Combined | Whole worker termites | Hypothesis-driven | Sanger sequencing | 19 total unigenes (13 contigs + 6 singletons) | Gao et al. (2012) | ||
| Multi-species: C. secundus and Cryptotermes cynocephalus | Combined | Whole workers and neotenic reproductives | Hypothesis-driven | Representational difference analysis, Sanger sequencing | 16 differentially expressed genes were identified in C. cynocephalus with significant homology to genes identified previously in C. secundus | Weil et al. (2009) | ||
| Lower and higher | Multi-species: H. sjostedti, Reticulitermes speratus, and Nasutitermes takasagoensis | Host | Whole-body minus gut | Hypothesis-driven | 454 pyrosequencing | >1.2 million quality-filtered reads yielding >400 million bases for each of the three species. Caste transcriptomes compared by GO and orthology searches. Putative JH and caste differentiation genes annotated | Hayashi et al. (2013) | |
| Higher | Macrotermes gilvus | Symbiont | Fungal ectosymbiont (Termitomyces) | Descriptive | Subtractive cDNA library, Sanger sequencing | 1,382 and 325 EST contigs were obtained for non-subtracted (lignocellulose fed) and subtracted (lignocellulose minus lab diet) cDNA libraries | Johjima et al. (2006) | |
| N. takasagoensis | Host | Soldier frontal gland | Descriptive | 454 pyrosequencing | 50,290 sequence reads were assembled into 1111 contigs (774 unigenes) | Hojo et al. (2012) | ||
| Odontotermes formosanus | Host | Head tissue | Descriptive | Illumina sequencing | 116,885 unigene sequences; 30,646 with significant identity | Huang et al. (2012) | ||
| Species unknown | Symbiont | Fungal ectosymbiont of higher termite (Termitomyces albuminosus ) | Descriptive | 454 pyrosequencing | 6494 candidate genes (3301 contigs + 3193 singletons) | Yang et al. (2012) | ||
| Proteome | Lower | R. flavipes (santonensis) | Symbiont | Gut symbiota (bacteria, protist) | Descriptive | LC-MS/MS (ion trap) and 2-D PAGE | Tubulins proved to be the most suitable protein family with which to identify flagellate populations from hindgut samples | Bauwens et al. (2013) |
| Higher | Nasutitermes corniger | Symbiont | Bacterial gut symbiota | Descriptive | LC-MS | 886 proteins identified, 197 with known enzymatic function; very few cellulases identified | Burnum et al. (2011) | |
| Higher and lower | Multi-species: 12 species (10 lower, 2 higher) | Host | Labial glands of workers and soldiers | Descriptive | N-terminal peptide sequencing (Edman degradation) | Endogenous (host) endoglucanase cellulases were identified in worker labial glands of all species | Sillam-Dussès et al. (2012) | |
| Metatranscriptome, metagenome, and 16S pyrosequencing | Higher | Multi-species: Amitermes wheeleri, N. corniger | Symbiont | Bacterial gut symbiota | Hypothesis-driven (differences between wood and dung feeders) | 454 pyrosequencing | Firmicutes and Spirochaetes dominated in A. wheeleri, while Spirochaetes and Fibrobacteres dominated in N. corniger | He et al. (2013) |
| Metatranscriptome and proteome | Lower | R. flavipes | Combined | Worker termite gut and protist microbiota | Hypothesis-driven (comparison of cellulose vs. wood vs. lignin feeding) | 454 pyrosequencing + LC-MS proteomics | 347,798 sequence reads aligned into 97,254 singlets + 9553 differentially expressed contigs; proteome and transcriptome results showed congruence | Sethi et al. (2013a) |
| Metatranscriptome and proteome | Lower | R. speratus | Symbiont | Protist gut symbiota (hindgut lumen) | Descriptive | Sanger sequencing + proteomics | 910 total clones sequenced; 580 candidate genes identified | Todaka et al. (2007) |
| Metatranscriptome | Lower | C. formosanus | Combined | Whole workers, nymphs, soldiers, and alates | Descriptive | Sanger sequencing (normalized polyphenic library) | 25,939 candidate genes (16 691 contigs and 9248 singletons) | Zhang et al. (2012) |
| Symbiont | Protist gut symbiota | Descriptive | 454 pyrosequencing | 75,122 candidate genes (2891 contigs + 72,231 singletons) | Xie et al. (2012) | |||
| H. sjostedti | Combined | Worker termite gut (salivary gland, foregut, midgut, and hindgut) and protist microbiota | Descriptive | Sanger sequencing | Different compositions of expressed genes were identified across gut regions | Yuki et al. (2008) | ||
| Multi-species: R. speratus, H. sjostedti, Neotermes koshunensis, M. darwiniensis, Cryptocercus punctulatus | Symbiont | Protist gut symbiota (hindgut lumen) | Hypothesis-driven | Sanger sequencing | 910, 920, 1056, 1021, and 868 clones sequenced from each taxon (n = 4775) that aligned into 3780 unigene contigs; 77 full-length GHF7 cellulases were identified | Todaka et al. (2010) | ||
| R. flavipes | Combined | Whole-body polyphenic library (non-normalized) | Descriptive | Sanger sequencing (random clones) | 88 random clones were sequenced that aligned into 49 unigene contigs | Wu-Scharf et al. (2003) | ||
| Combined | Whole-body polyphenic library (non-normalized) | Hypothesis-driven (worker vs. soldier) | Filter arrays, Sanger sequencing | 105 differentially expressed clones were identified that aligned into 34 unigene contigs | Scharf et al. (2003) | |||
| Combined | Whole-body polyphenic library (non-normalized) | Hypothesis-driven (worker vs. immature reproductive) | Filter arrays, Sanger sequencing | 68 differentially expressed clones were identified that aligned into 25 unigene contigs | Scharf et al. (2005) | |||
| Combined | Worker termite gut and protist microbiota | Hypothesis-driven | Sanger sequencing | 6555 total transcripts (3044 host, 3511 protist symbiont) | Tartar et al. (2009) | |||
| Combined | Whole-body polyphenic library (soldier, worker, alate, early and late larvae) | Hypothesis-driven (comparisons among castes) | Sanger sequencing (random clones) | 15,259 random clones sequenced representing 6991 total genes | Steller et al. (2010) | |||
| Combined | Worker termite gut and protist microbiota | Hypothesis-driven (comparison of JH, soldier head extract, live soldiers, and reproductives) | Microarray | 543 total gut genes differentially expressed after 24-h exposures (151 host + 392 protist symbiont) | Sen et al. (2013) | |||
| Combined | Worker termite gut and protist microbiota | Hypothesis-driven (comparison of wood and cellulose/paper feeding) | Microarray | 544 total gut genes differentially expressed after 7-days feeding periods (236 host + 301 protist symbiont) | Raychoudhury et al. (2013) | |||
| Zootermopsis angusticollis | Symbiont | Bacterial gut symbiota (Treponema spirochetes) | Hypothesis-driven (comparison of two Treponema in co-culture) | Illumina sequencing | Total database size = 3,855,671 reads; 45% of reads were 16S and 23S rRNAs; >97% of all non-rRNA genes were unique | Rosenthal et al. (2011) | ||
| Metagenome and proteome | Higher | N. corniger | Symbiont | Bacterial gut symbiota (P3 luminal contents) | Descriptive | Sanger sequencing + 454 pyrosequencing + LC-MS proteomics | 12 bacterial phyla and 216 phylotypes identified; >71 Mb of DNA sequenced; ∼700 glycoside hydrolase domains corresponding to 45 different carbohydrate active enzymes were identified (including putative cellulases and hemicellulases) | Warnecke et al. (2007) |
| Metagenome and 16S survey | Higher | Odontotermes yunnanensis | Symbiont | Bacterial gut symbiota | Descriptive | 454 pyrosequencing | 548,807 total sequence reads; no evidence of lignases; 205 total cellulase and hemicellulase genes annotated | Liu et al. (2013) |
| Metagenome | LOWER | C. gestroi | Symbiont | Bacterial gut symbiota | Descriptive | Illumina de novo genome sequencing | 316 candidate cellulase ORFs, 259 candidate hemicellulase ORFs, and 12 candidate pectinase ORFs | Do et al. (2014) |
| R. flavipes (santonensis) | Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (beta glucosidase) + Sanger sequencing | 9 beta glucosidase positive clones were identified from GH1, GH3, and GH4 | Mattéotti et al. (2011b) | ||
| Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (beta glucosidase) + Sanger sequencing | 1 beta glucosidase positive clone was identified (GH1) | Mattéotti et al. (2011a) | |||
| Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (xylosidase) + Sanger sequencing | 1 putative endo-1,4-beta-xylanase was identified from GH11 | Mattéotti et al. (2012) | |||
| Higher | Globitermes sulphureus | Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (beta glucosidase) + Sanger sequencing | 1 beta glucosidase positive clones was identified and functionally expressed | Wang et al. (2012) | |
| Macrotermes annandalei | Symbiont | Bacterial gut symbiota | Descriptive | 454 pyrosequencing (bacterial fosmid libraries grown under selective conditions) | 13 positive clones identified encoding 1 xylanase and 12 beta-glucosidases | Liu et al. (2011) | ||
| Microcerotermes sp. | Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (cellulase and xylanase) + Sanger sequencing | Fourteen independent active clones (2 cellulases and 12 xylanases) were obtained by functional screening (GHF 5,8,10,11) | Nimchua et al. (2012) | ||
| Pseudacanthotermes militaris | Symbiont | Gut and fungal comb bacteria | Descriptive | 454 pyrosequencing | 1.46 Mbp of metagenome sequence | Bastien et al. (2013) | ||
| Trinervitermes trinervoides | Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (esterase)+ Sanger sequencing | 68 fosmid clones were identified with esterase activity, of which the 14 most active were sub cloned and sequenced | Rashamuse et al. (2012) | ||
| Symbiont | Bacterial gut symbiota | Descriptive | Functional screening (feruloyl “FAE” esterase) + Sanger sequencing | Seven FAE-positive fosmid clones were identified | Rashamuse et al. (2014) | |||
| Metabolome | Lower | H. sjostedti | Combined | Worker termite gut | Descriptive | Isotope-ratio mass spectrometry (IR-MS) | Localized the majority of glucose release from 13C-cellulose to the foregut region | Tokuda et al. (2014) |
| Z. angusticollis | Combined | Worker termite gut | Descriptive | TMAH thermocemical lysis coupled with GC-MS | Results transformed the view of lignin degradation in the termite gut | Geib et al. (2008) | ||
| C. formosanus | Combined | Worker termite gut | Descriptive | TMAH thermocemical lysis coupled with CP-MAS-NMR spectroscopy, and Py-GC/MS | During gut passage the native lignin macromolecular assembly undergoes structural modification but with conservation of the abundant β-O-4′ interunit lignin linkage and retention of the original aromatic properties | Ke et al. (2011) | ||
| C. formosanus | Combined | Worker termite gut | Descriptive | TMAH thermocemical lysis coupled with GC-MS | Results suggest that the plant cell wall deconstruction process in C. formosanus consists of stepwise unlocking reactions that affect the lignin matrix and lignin–carbohydrate associations | Ke et al. (2013) | ||
| Higher And lower | Multi-species: eight species (seven lower, one higher) | Host | Labial glands of workers and soldiers | Descriptive | HPLC MALDI-TOF and GC-TOF-MS | Hydroquinone and other glucose and benzene-linked compounds identified in labial gland secretions of workers and soldiers | Sillam-Dussès et al. (2012) | |
| Genome | Lower | C. formosanus | Symbiont | Bacteroidales endosymbiont (phylotype CfPt1-2) of the cellulolytic protist Pseudotrichonympha grassii | Descriptive | Combination of Sanger and 454 pyrosequencing | 1,114,206 bp chromosome containing 758 putative protein-coding sequences, 38 transfer RNA genes, and 4 rRNA genes | Hongoh et al. (2008a) |
| M. darwiniensis | Symbiont | Blattabacterium bacterial endosymbiont | Descriptive | Illumina sequencing | 594 candidate genes identified (544 protein-coding + 40 RNA-coding) | Sabree et al. (2012) | ||
| R. flavipes | Symbiont | Bacterial gut symbiont (Opitutaceae bacterium strain TAV1; Verrucomicrobia) | Descriptive | Combination of Illumina + 454 pyrosequencing | Genome contains 6,051 genes with 5,987 CDS; 64 structural RNAs were identified with the presence of one rRNA operon | Isanapong et al. (2012) | ||
| Reticulitermes lucifugus | Symbiont | Bacterial gut symbiont (Sebadella termitidis strain NCTC 11300T; Phylum Fusobacteria) | Descriptive | Combined Sanger, Illumina, and 454 pyrosequencing | 4,486,650 bp long genome containing 4,264 predicted genes (4,210 protein-coding, 54 RNAs) | Harmon-Smith et al. (2010) | ||
| R. speratus | Symbiont | Endomicrobia “TG-1” endosymbiont (phylotype Rs-D17) of the cellulolytic protist Trichonympha agilis | Descriptive | Combination of Sanger and 454 pyrosequencing | 1,125,857 bp chromosome encoding 761 putative protein-coding genes | Hongoh et al. (2008b) | ||
| Genome, transcriptome, and DNA methylome | Lower | Zootermopsis nevadensis | Host | Worker, soldier, reproductive, larvae | Descriptive | Illumina + 454 pyrosequencing | 562 Mb genome sequenced with 98x coverage; 96 miRNA, and 17,737 protein coding genes were identified | Terrapon et al. (2014) |
| Genome, fungal symbiont genome, and gut microbial metagenome | Higher | Macrotermes natalensis (and Termitomyces sp. symbiont) | Combined | Genomic DNA of M. natalensis queen and Termitomyces homokaryon, metagenomic DNA of major worker, minor soldier, and queen gut | Descriptive | Illumina sequencing | First sequencing of a tripartite symbiotic system; 1.3 Gb host genome, 84 Mb fungal symbiont genome; 816 Mb gut prokaryotic metagenomes; major emphasis on cellulose digestion; greatly reduced gut microbiome in queens relative to major workers and minor soldiers | Poulsen et al. (2014) |
| DNA methylome | Lower | Coptotermes lacteus | Host | Workers, soldiers and nymphs | Descriptive | Methylation-targeted amplification fragment length polymorphism (AFLP) | Found evidence for DNA methylation, but no differences in methylation levels among castes | Lo et al. (2012) |
| Lower and higher | Multi-species: H. sjostedti, R. speratus and N. takasagoensis | Host | Whole-body minus gut | Hypothesis-driven | 454 Pyrosequencing | >1.2 million filtered reads yielding >400 million bases for each of the three species. DNA methyltransferases putatively responsible for DNA methylation were represented in all three species | Hayashi et al. (2013) | |
| Lower | Multi-species: R. flavipes, C. formosanus | Host | Whole-body polyphenic libraries | Descriptive | Sanger sequencing | Signatures of high DNA methylation levels exist in R. flavipes and C. formosanus. Results suggest the presence of DNA methylation in R. flavipes and C. formosanus potentially at high levels or widely targeted across the lengths of genes, relative to other insect taxa | Glastad et al. (2013) | |
| 18S sequencing | Lower | Multi-species: Reticulitermes, Zootermopsis, Cryptocercus | Symbiont | Protist gut symbiota | Descriptive | 454 pyrosequencing | Protist diversity estimated by 18S SSU sequencing is much higher than when estimated by protist morphology | Tai and Keeling (2013) |
| Lower | Z. angusticollis | Symbiont | Protist gut symbiota (single cell) | Descriptive | Sanger sequencing | Seven protists identified by rRNA sequence | Tai et al. (2013) | |
| 18S and bacterial 16S sequencing | Lower | Multi-species: 24 lower termites and three Cryptocercus cockroaches | Symbiont | Protist and bacterial gut symbiota | Hypothesis-driven | 454 pyrosequencing | Although microbial communities are vertically inherited and codiversification with the host termite has had a prominent role in structuring symbiont communities, dispersal appears to have a larger role in community composition | Tai et al. (2015) |
| 16S sequencing | Lower | C. formosanus | Symbiont (positions 27-1492) | Cuticular bacteria | Hypothesis-driven | Sanger sequencing | 25 total ribotypes detected (20 and 14 from simple and extended families) | Husseneder et al. (2010b) |
| Symbiont (positions 27-1492) | Bacterial gut symbiota (whole gut) | Hypothesis-driven | Sanger sequencing | 1,876 total 16S reads that sorted into 213 bacteria ribotypes and 13 phyla | Husseneder et al. (2010a) | |||
| Multi-species: R. flavipes (santonensis), H. sjostedti, Z. nevadensis, M. darwiniensis, Kalotermes flavicollis, Neotermes castaneus, C. secundus | Symbiont (positions 27-1492) | Bacterial endosymbionts of protist gut symbionts | Descriptive | Sanger sequencing | Each protist morphotype harbored “Endomicrobia” from unique phylogenetic lineages | Stingl et al. (2005) | ||
| Multi-species: R. flavipes, C. formosanus, Z. angusticollis | Symbiont (positions 63-1492) | Spirochaete gut symbiota (whole gut) | Descriptive | Sanger sequencing | >21 new species of Treponema identified in each of the three species studied | Lilburn et al. (1999) | ||
| R. flavipes | Symbiont (entire SSU region) | Bacterial gut symbiota (hindgut lumen) | Descriptive | Sanger sequencing + ARDRA analysis | Six phyla and 261 species-level phylotypes estimated | Fisher et al. (2007) | ||
| Symbiont (V5–V6 region) | Bacterial gut symbiota (hindgut lumen) | Hypothesis-driven | 454 pyrosequencing | 475,980 total 16S reads that sort into eight major bacterial phyla and 4761 species-level phylotypes (5% divergence level) | Boucias et al. (2013) | |||
| R. flavipes (santonensis) | Symbiont positions (27-1492) | Bacterial gut symbiota (midgut, protozoa, hindgut fluid and wall) | Descriptive | Sanger sequencing + T-RFLP analysis | 392 clones sequenced; seven major phyla and >200 species-level bacterial ribotypes identified | Yang et al. (2005) | ||
| R. speratus | Symbiont (positions 563-1114) | Bacterial gut symbiota (whole gut) | Descriptive | Sanger sequencing | 1344 clones sequenced; 11 phyla and 268 species-level phylotypes identified | Hongoh et al. (2003) | ||
| Z. angusticollis | Symbiont (positions 27-1492) | Bacterial gut symbiota | Hypothesis-driven (effects of antibiotic rifampicin) | Sanger sequencing | Six and 17 species-level OTUs were identified for rifampin and control treatments (n = 85–87 clones) | Rosengaus et al. (2011) | ||
| Higher | Cornitermes cumulans | Symbiont (positions 21 or 27-907) | Whole gut | Descriptive | Sanger sequencing | >8 Phyla identified (species-level estimates not provided) | Grieco et al. (2013) | |
| Cubitermes niokoloensis | Symbiont (positions 338-518) | Bacterial symbionts from gut regions, soil and mound | Hypothesis-driven | Sanger sequencing + DGGE | 212 total clones sequenced; 101 different species-level phylotypes identified | Fall et al. (2007) | ||
| Multi-species: Odontotermes somaliensis, Odontotermes sp., Microtermes sp. | Symbiont (positions 27-1492) | Bacterial gut symbiota | Hypothesis-driven | Sanger sequencing | 100, 100 and 96 clones sequenced from each taxon; 151 different phylotypes identified | Makonde et al. (2013) | ||
| Multi-species: | Symbiont (positions 341-806) | Bacterial gut symbiota | Descriptive | 454 pyrosequencing | Performed 16S sequencing on nine fungus-growing termite species from one geographic region of Ivory Coast; Identified 16 phyla and 42 genera total, with 11 genera occurring in all nine species | Otani et al. (2014) | ||
| N. corniger | Symbiont (V1–V2 and V8 regions) | Bacterial gut symbiota (P3 lumen) | Hypothesis-driven | 454 pyrosequencing | 2269 species-level OTUs of which 1617 and 652 were from the V1–V2 and V8 regions, respectively | Engelbrektson et al. (2010) | ||
| Symbiont (V3–V4 region) | Bacterial gut symbiota (six whole gut regions) | Descriptive | 454 pyrosequencing | 3,200-26,000 16S reads per gut region (crop, midgut and paunch P1–P4) that sort into seven major bacterial phyla | Köhler et al. (2012) | |||
| N. takasagoensis | Symbiont (positions 27-1390) | Bacterial gut symbiota (whole gut) | Hypothesis-driven | Sanger sequencing + T-RFLP analysis | 388 total clones sequenced; 10 major phyla identified; 31–43 species-level phylotypes | Miyata et al. (2007) | ||
| T. trinervoides | Symbiont (positions 1170-1492) | Bacterial gut symbiota | Hypothesis-driven (difference between grass and sugarcane feeding field colonies) | 454 pyrosequencing | 2274 and 2943 species-level OTUs sampled from sugarcane and grass feeding colonies (1% divergence level); nine major phyla sampled; Firmicutes and Bacteroidetes most common | Sanyika et al. (2012) |
Taxonomic Distribution
In total, 82 termite species have been investigated using various omic approaches, with greater representation by lower than higher termites (72 vs. 28%). Among lower termites the top genera studied are important pest groups (Reticulitermes and Coptotermes), followed by non-pests from Hodotermopsis, Mastotermes, and Cryptotermes. Among higher termite genera, Nasutitermes dominate, followed by Odontotermes, Trinervitermes, and several other minor groups. Two termite genome sequences have now been published from the lower termite Zootermopsis angusticollis and the higher termite Macrotermes natalensis (see below).
Host vs. Symbiont Investigation
Of the various omic studies to date considering symbiosis and symbiotic partnerships in termite systems, the majority have taken an exclusive symbiont-oriented approach (>60%), whereas a minority have considered the host termite separately (<20%). The remainder have considered host and symbiont together (∼20%). In the latter category of host and symbiont combined, some studies have been a case of “accidental metatranscriptomics” (because protist symbionts have polyadenylated transcripts that are represented in cDNA libraries along with host transcripts; e.g., Scharf et al., 2003, 2005; Steller et al., 2010), but others have been deliberate metatranscriptomic studies (e.g., Tartar et al., 2009; Raychoudhury et al., 2013; Sen et al., 2013). The greater emphasis on gut symbiota compared to the host termite is likely because of the stereotypically well-recognized presence of gut microbes in termites.
Experimental Approaches and Types of Sequencing
In terms of experimental approaches taken, there has been an approximately equal split between descriptive and hypothesis-driven studies. Regarding the types of sequencing performed, transcriptomics and metatranscriptomics have been the dominant approaches (25 and 21% of studies), followed by microbial surveys for cataloging purposes (23%). The transcriptomic approaches used can be further divided into different methodologies such as cDNA library sequencing (Sanger, pyrosequencing or Illumina RNA-seq) and microarrays. Other efforts have targeted symbiont metagenomes (15%), symbiont or termite genomes (9%), proteomes (3%), and DNA methylomes (3%).
Omic Studies in Termites: What has been Revealed?
Genomics
Host Termite Genomes
At present only two termite genome sequences are available (Table 1); one from the lower termite Zootermopsis nevadensis (Terrapon et al., 2014) and one from the higher termite M. natalensis (Poulsen et al., 2014). Z. nevadensis was selected for sequencing based on its small genome size of 562 Mb relative to other termites, most of which are over 1000 Mb (Koshikawa et al., 2008). The Z. nevadensis sequencing approach involved shotgun genome sequencing of genomic DNA from symbiont-free soldier heads (n = 50 and 150 heads for 2 and 20 kb libraries, respectively). The transcriptomes of castes and various phenotypes were also sequenced for both gene prediction and comparative transcriptomic purposes. Transcriptome data were also used to search for DNA methylation machinery and methylation/epigenetic differences among castes and developmental stages.
The Z. nevadensis genome provided the first hints into how termites differ at the genome level from their eusocial counterparts in the order Hymenoptera, which evolved sociality independently. For making socio-evolutionary comparisons, emphasis was placed on gene family expansions, male fertility, chemoreception, immunity, polyphenism/division of labor, and potential epigenetic caste regulation. An expansion of genes related to male fertility and upregulated gene expression in male reproductives are consistent with differences in mating biology between termites and Hymenoptera. Regarding chemoreception, divergent numbers of genes and gene families relative to Hymenoptera were identified, as were variations in chemoreception gene expression among castes. Regarding caste polyphenism and division of labor, caste-associated gene expression profiles were readily identifiable. Key caste-regulatory and reproduction-associated genes identified through preceding work (e.g., hexamerins, vitellogenins, and CYP genes) were further defined and verified as gene families at the genomic level. Interestingly, there are 76 cytochrome P450 genes in the Z. nevadensis genome; which is nearly 2x as many as encoded by the honey bee genome (Honey Bee Genome Sequencing Consortium, 2006). Lastly, DNA methylation signatures and patterns of alternative splicing provided some evidence to suggest epigenetic caste regulation (see later).
The M. natalensis sequencing considered not only the host genome, but also the entire tri-partite system of this higher fungus-growing termite. This included the 1.3 Gb host genome, the 84 Mb genome of the Termitomyces sp. fungal symbiont and 816 Mb of prokaryotic gut metagenome from major workers, minor soldiers, and queens. Emphasis was placed mostly on cellulose digestion, which revealed a rich complement of glycosyl hydrolases from host, fungi, and gut microbes that likely collaborate in lignocellulose digestion. Another major finding was that gut microbiota composition is reduced by over 50% in queens relative to workers and soldiers, suggesting that queen gut microbiota undergo substantial compositional changes during colony founding, which points toward the local environment or other external factors as sources of microbiota as incipient colonies grow and age. Moving forward, the Z. nevadensis and M. natalensis genomes will be important resources for termitologists, and will also provide important scaffolds for assembly of additional termite genomes that will facilitate study of genes related to many evolutionary and biological processes.
Individual Symbiont Genomes
Five individual symbiont genomes have been sequenced (Table 1), with several others published or in progress since the writing of this article. No protist genomes have yet been sequenced. Two bacterial endosymbionts of hindgut protists from Coptotermes formosanus and Reticulitermes speratus (phylum Elusimicrobia or “TG1”) were the first symbiont genomes sequenced; they were obtained from isolated individual cells after whole-genome amplification (Hongoh et al., 2008a,b). No lignocellulase genes were identified; however, both genomes encoded capabilities to fix nitrogen, recycle host nitrogen wastes for amino acid and cofactor biosynthesis, and import glucose and xylose as energy and carbon sources. The next symbiont genomes were from gut bacteria in the phyla Verrucomicrobia and Fusobacteria, from the termites Reticulitermes flavipes and R. lucifugus (Harmon-Smith et al., 2010; Isanapong et al., 2012). These genomes were from culturable isolates and were found to encode genes related to cellulose degradation and nitrogen fixation. Another example is the genome of an obligate fat body endosymbiont Blattabacterium from the basal termite Mastotermes darwiniensis (Sabree et al., 2012). This bacterium displays a reduction in genome size and loss of genes required for amino acid production relative to free-living gut bacteria, which is consistent with its ability to recycle nitrogenous wastes and its role as a co-evolved endosymbiotic partner of the host termite.
Symbiont Metagenomes
At the time of writing this article, at least 12 prokaryotic metagenomes had been partially sequenced (Table 1). Most metagenome publications have reported on lignocellulase identification from genome sequences of gut bacteria that selectively grew on lignocellulose media (Liu et al., 2011; Mattéotti et al., 2011a,b, 2012; Nimchua et al., 2012; Rashamuse et al., 2012, 2014; Wang et al., 2012). Another study used targeted xylanase screening from gut and ectosymbiotic fungi-associated bacteria of the higher termite Pseudacanthotermes militaris (Bastien et al., 2013). Other studies took broader approaches to sequence from gut bacterial communities of higher termites. By combining metagenome sequencing with 16S surveys and metatranscriptomics, these studies revealed new information on bacterial cellulase diversity from termites with different symbiosis strategies (i.e., with and without fungal ectosymbionts; Warnecke et al., 2007; Liu et al., 2013) and from different feeding guilds (dung vs. wood; He et al., 2013). While these studies provided a wealth of new high-impact information on bacterial symbionts, they did not consider how symbionts from the gut and/or nest termitosphere collaborate with or complement the host termite.
Transcriptomics
Host Transcriptome
Around 15 transcriptomic studies to date have focused on physiological processes or tissues in the host termite (Table 1). Early studies looked for caste-biased gene expression, but the approaches employed had low resolving power and typically revealed only small numbers of differentially expressed genes. These studies mainly used subtractive hybridizations or cDNA “macro” arrays (reviewed by Miura and Scharf, 2011). Also, these early studies in lower termites often fell into the category of “accidental metatranscriptomics” as described earlier. The majority of focus in termite transcriptomic work has been on differences among castes or during caste differentiation (reviewed by Miura and Scharf, 2011). Mainly, newer studies are considered here.
Because of the importance of juvenile hormone (JH) to soldier caste differentiation and the reliability of JH treatment for inducing soldier caste differentiation, continuing focus has been placed on this transition in hypothesis-driven studies that combine JH assays with transcriptomics (e.g., Cornette et al., 2013; Sen et al., 2013). Caste-regulatory primer pheromones and the social environment have also been studied in the same context (Tarver et al., 2010; Sen et al., 2013). Other studies have included tissue-directed subtractive hybridizations, random/de novo cDNA library sequencing and/or cDNA oligonucleotide microarrays to reveal caste-biased gene expression (Weil et al., 2009; Ishikawa et al., 2010; Leonardo et al., 2011; Hojo et al., 2012; Huang et al., 2012; Husseneder et al., 2012; Terrapon et al., 2014). The over-arching themes emerging from this work include caste and morphogenesis-associated gene expression, endocrine signaling, vitellogenesis, reproduction-related processes, and regulatory mechanisms that maintain juvenile worker states in lower termites.
The immune response is another aspect of host termite physiology investigated through transcriptomics. Four studies have revealed responses to immune challenges by both stereotypical and unprecedented immune-responsive genes (Thompson et al., 2003; Yuki et al., 2008; Gao et al., 2012; Hussain et al., 2013). Finally, an emerging theme has been to investigate pathogen-xenobiotic interactions at the transcriptome level (Husseneder and Simms, 2014; Sen et al., 2015).
Symbiont-Host Metatranscriptomes
In addition to host-targeted studies noted above, other studies have considered symbiont or host-symbiont metatranscriptome composition (Table 1). Early examples in this category showed worker-biased expression of protist cellulases (Scharf et al., 2003) and differential expression of symbiont cellulases between dispersing and non-dispersing adult reproductives (Scharf et al., 2005). Subsequent studies focused on metatranscriptome composition of bacteria, protist and/or fungal symbionts, mostly for the purpose of identifying digestive cellulases (reviewed by Scharf and Tartar, 2008). Recent work has probed deeper into gut metatranscriptomes by taking advantage of both traditional and next-generation sequencing technology (Todaka et al., 2010; Rosenthal et al., 2011; Xie et al., 2012; Zhang et al., 2012; He et al., 2013). Other work has sought to partition host and symbiont digestive contributions and identify candidate enzymes expressed specifically in response to wood (i.e., complex lignocellulose), cellulose and lignin feeding (Tartar et al., 2009; Raychoudhury et al., 2013; Sethi et al., 2013a).
One microarray study investigated gut metatranscriptome changes in responses to JH, primer pheromones and socio-environmental conditions, suggesting interesting linkages between gut symbiota and caste differentiation (Sen et al., 2013). Another microarray study investigated host and symbiont gene expression in response to pathogen and nicotinoid-insecticide challenges, providing new insights into immunological roles played by bacterial and protist gut symbionts in defending against invading fungal and bacterial pathogens (Sen et al., 2015), building on the ideas of extended disease resistance as conferred by fecal nest bacteria (Chouvenc et al., 2013) and gut microbiota (Rosengaus et al., 2014).
Proteomics
Proteomics (Table 1) is important to validate transcriptome studies, particularly for determining if a gene’s presence and/or its transcription and translation are proportional. For example, proteomic studies in a higher termite were unable to identify most of the bacterial cellulase proteins predicted by metagenome sequencing (Warnecke et al., 2007; Burnum et al., 2011). Alternatively, proteomic studies in lower termites were able to identify both protist cellulases and other host lignocellulases initially identified via metatranscriptome sequencing (Todaka et al., 2007; Sethi et al., 2013a). Another study investigated proteins present in labial gland secretions of 12 lower and higher termite species, identifying endogenous GHF9 cellulases as dominant components of worker labial gland secretions in most species investigated (Sillam-Dussès et al., 2012). Another study used proteomics to catalog gut microbial communities, but with limited resolution (Bauwens et al., 2013). Clearly, more proteomic efforts are needed to resolve issues related to: (1) congruency between nucleic acid and protein sequencing approaches, and (2) to verify open reading frames predicted by metagenome and transcriptome sequencing.
DNA Methylomes
Four studies to date have looked at methylation signatures across termite castes with somewhat differing results. A seminal study used a methylation-targeted amplification fragment length polymorphism (AFLP) approach in Coptotermes lacteus to look for methylation signature differences among castes (Lo et al., 2012). Evidence of methylation was found, but no significant caste-associated methylation patterns were identified.
A subsequent study was done in silico using database sequences from R. flavipes and C. formosanus (Glastad et al., 2013). In this study and the two described below, transcriptome data were mined to determine the specific distribution of CpG dinucleotides (i.e., 5′–3′ cytosine followed by guanine), in order to predict DNA methylation levels in silico. Evidence of DNA methylation machinery and methylation signatures was found at high levels among expressed genes. Results also suggested that DNA methylation in R. flavipes is targeted to genes with ubiquitous (rather than differential) expression among castes and morphs. A third study examined host transcriptomes of three termite species that included two lower (Hodotermopsis sjostedti, R. speratus) and one higher termite (Nasutitermes takasagoensis; Hayashi et al., 2013). Pyrosequencing was done in combination with 69 caste and phenotypic libraries from the three termite species. Sequence analysis revealed that DNA methyltransferases potentially responsible for DNA methylation were present in each species, and verified the presence of methylation signatures. However, only limited evidence of caste-associated methylation profiles was detectable across the three species.
Finally, DNA methylation was assessed in Z. nevadensis as part of genome and transcriptome sequencing efforts (Terrapon et al., 2014). Transcriptome data were used to determine the specific distribution of CpG dinucleotides, in order to make in silico predictions of DNA methylation levels and explore for epigenetic differences among castes. In addition to verifying the presence of genes that encode for DNA methylation machinery (i.e., DNA methyltransferases 1 and 3), results showed greater methylation of genes rather than intergenic DNA, and a greater presence in introns than exons. This evidence, along with findings that alternatively spliced genes have greater degrees of methylation, suggests intronic methylation may impact alternative splicing.
While it is clear that DNA methylation exists in termites, so-far inconclusive results have been obtained to suggest epigenetic caste regulation. As concluded previously in relation to genetic caste determination (Vargo and Husseneder, 2009), the field of epigenetic caste regulation is in its infancy and epigenetic phenomena may or may not be relevant in natural colonies. More importantly, in silico methylation studies can only suggest that methylation may exist and which genes might be differentially methylated. Functional/translational research will be required to verify whether or not such genes truly are methylated, as well as the functions of those genes.
Metabolomics
Metabolomic studies are useful for assessing in situ processes, both as an exploratory approach and for functional/translational studies to verify nucleotide sequences. Soldier defensive secretions previously received much attention in this respect (Prestwich, 1984; Nelson et al., 2001). A more recent study investigated chemical components of labial gland secretions in soldier and worker termites from 7 lower and 1 higher termite (Sillam-Dussès et al., 2012). This study confirmed hydroquinone and other glucose and benzene-linked compounds as common labial gland secretions among most species.
Other metabolomic studies have focused on lignocellulose digestion. One main question addressed has been: does lignin digestion or modification occur during passage through the termite gut? Several studies over the past 25 years have addressed this question (reviewed by Ni and Tokuda, 2013) but recent metabolomic studies have been particularly informative (Geib et al., 2008; Ke et al., 2011, 2013). In general, findings are consistent regarding modification of lignin during passage through the gut, but evidence of actual lignin depolymerization has been more elusive. One possible reason for this could relate to insufficient detection procedures. Another possibility is that lignin-ether bonds, broken during depolymerization, only remain in this state for a short time and thus appear as intact lignin in frass. The induction of numerous antioxidant and detoxification enzymes by lignin feeding, as well as increased saccharification in the presence of lignin-associated phenoloxidases, supports the latter possibility (Sethi et al., 2013a). Despite convincing evidence of lignin modification during passage through the termite gut, and related omic studies revealing lignin-associated changes in host oxidative enzymatic machinery, the topic of lignin digestion/modification in termite guts remains contentious (Brune, 2014).
Another aspect of termite metabolomic research considers cellulose digestion and relative contributions of host and symbiont to this process. A recent metabolomic study investigated in situ digestion of 13C-labeled crystalline cellulose by H. sjostedti (Tokuda et al., 2014). Novel insights obtained related to both cellulose digestion and nitrogen metabolism. The results not only confirmed preceding work showing that endogenous cellulose digestion by the host is substantial, but also suggested other novel possibilities; for example (i) a significant digestive contribution by hindgut bacteria is phosphorolysis of cello-oligosaccharides to glucose-1-phosphate, and (ii) essential amino acid acquisition occurs via lysis of hindgut microbes obtained through proctodeal trophallaxis. The rapid buildup of glucose observed in the foregut agrees well with prior studies showing that host foregut cellulases can produce high levels of glucose directly from wood lignocellulose (Scharf et al., 2011; Sethi et al., 2013a,b). Additionally, higher glucose levels observed in the hindgut than other regions agrees with estimates that glucose release from lignocellulose is about 1/3 host and 2/3 symbiont (Scharf et al., 2011). However, since this study only focused on metabolite identification in gut tissue, it could not account for nutrients/metabolites transported out of the foregut and catabolized in other areas of the body.
Symbiont 16S and 18S Surveys
Bacterial 16S rRNA sequence surveys have been used extensively for cataloging bacteria and archaea (Wang and Qian, 2009), whereas 18S small subunit (SSU) rRNA surveys are just beginning to gain attention for cataloging protist symbionts (Tai and Keeling, 2013). Over 20 bacterial 16S surveys have been published to date using both cloning-dependent and -independent, high- and low-throughput approaches (Table 1). Highly variable species-level compositions have been obtained across the different termite species investigated, but, in general, six major bacterial phyla are represented across higher and lower termites: Bacteroidetes, Firmicutes, Spirochaetes, Proteobacteria, Fibrobacteres, and Elusimicrobia (Brune, 2014). Surveys conducted in parallel with higher-termite metagenome studies have been very informative for matching functional and taxonomic diversity (Warnecke et al., 2007; He et al., 2013); however, a study comparing multiple colonies through pyrosequencing of 16S amplicons found that bacterial compositions were different among colonies and likely influenced by local environment (Boucias et al., 2013). Additionally, 16S surveys revealed that lignocellulosic diet shifts have no short-term impacts on termite and cockroach microbiota composition (Sanyika et al., 2012; Boucias et al., 2013; Schauer et al., 2014). Another 16S survey of fungus-growing termites suggested a core microbiota of 42 genera that was shared among all nine termite species tested (Otani et al., 2014). This core microbiota was very different from other higher and lower termites, leading the authors to conclude the 42 common genera represent a core microbiota of fungus-growing termites. Conversely, since the termites were sampled from a limited geographic area it is possible that the core genera represent common microbes acquired from the local environment.
In comparison to prokaryotic 16S surveys, comparatively few protist 18S SSU surveys have been conducted (Table 1). These studies, conducted using a combination of cloning-dependent and independent approaches, have been transformative. Two studies provided new evidence to suggest greater protist symbiont diversity than originally indicated by traditional morphological identification (James et al., 2013; Tai et al., 2013). Two other studies used high-throughput 16S and 18S SSU sequencing to compare 24 lower termites with three wood-feeding cockroaches (Tai and Keeling, 2013; Tai et al., 2015). Like their predecessors, these studies found protist diversity to be higher than when estimated by morphology, and also that protist symbiont taxa tend to be highly endemic to a host genus, which is different than relationships between termite hosts and bacterial symbiota. These findings illustrate the significant opportunities that exist for development of high-throughput techniques for assessing protist symbiont communities and studying protist-bacterial symbiont relationships.
Needs and Opportunities
Termite omic research in the last 10–15 years has led to a new era of understanding for termite and symbiont biology. Omics has also enabled the development of new unparalleled resources (i.e., transcriptome, genome, proteome, metabolome, symbiont meta-omic, and symbiont rDNA) useful for moving ahead with targeted functional work. The stage is now set for making significant headway in many aspects of termite research, including, but not limited to digestion, symbiosis, caste differentiation, and social evolution. However, key needs and opportunities remain in specific areas that seem particularly relevant for filling in knowledge gaps and potentially leading to transformative, paradigm-shifting outcomes.
Having the Z. nevadensis and M. natalensis genomes available not only facilitates further study of genes related to a range of evolutionary and biological processes, but these resources also provide important scaffolds for assembly of additional lower and higher termite genomes. Once multiple termite genomes are available, this would certainly better inform our view of termite social evolution. On the topic of host-symbiont “hologenomes,” sequencing more host genomes and symbiont metagenomes from the same termites concurrently (as recently done for M. natalensis), would provide unprecedented insights into the scope of interactions and synergies occurring in termite holobiomes. Such efforts could further reveal important differences between clades of higher and lower termites, leading to new evolutionary insights. Such datasets would also provide unmatched resources for advancing integrative sociogenomic, digestomic, termitosphere, and other research topics.
On the topic of proteomics, more studies are needed in species that have had genomes, transcriptomes, metagenomes, or metatranscriptomes sequenced. Combining proteomics with nucleic acid sequencing will better resolve gene prediction models and better test for congruency between transcription and translation profiles. On the topic of metabolomics, termite digestion remains an area much in need of metabolomic research focusing on how complex lignocellulose is broken down in termite guts and converted to energy. Also, tracking metabolites as they leave the gut and are utilized in the termite body would be very informative for testing hypotheses on the relative importance of nutrient flow into symbiont metabolic pathways.
On the topic of DNA methylomics, while it is now clear that DNA methylation happens in termites, so-far inconclusive results have been obtained regarding the role of DNA methylation in caste regulation. In silico methylation studies as performed can only suggest that methylation may exist and which genes are potentially differentially methylated. Functional and translational research is needed to understand the roles of such genes.
Substantial opportunities and needs still remain for 16S and 18S rRNA-based symbiont cataloging. Protist 18S SSU cataloging capabilities in particular have recently been developed, and can continue to improve provided that several conditions are met, such as: (1) appropriate primers can be developed, (2) statistically sound sampling regimes can be developed at biologically relevant scales, (3) single-cell microbiology and other data sources can be integrated, and (4) appropriate analytical tools developed (Tai and Keeling, 2013). This line of research has already begun to transform the view of protist diversity and co-evolution with host termites but more studies are needed in different termite species with established omic resources.
Finally, regarding prokaryotic 16S surveys, much has already been done, but an important gap in knowledge is the extent to which environment influences bacterial microbiota composition. This is important information for understanding differences in behavior and physiology across the geographic range for a termite species, as well as potentially for limiting the extent to which generalizations can be made about the relative importance of individual microbes or core microbiota in gut communities.
Conclusion
This review has covered many aspects related to outcomes, findings and trends resulting from termite omic research. To date, omic research in diverse termite species has provided key insights into caste differentiation, digestion, pathogen defense and microbiomes, and most recently has provided two termite genome sequences. Termite omics has also created important tools and resources for conducting targeted, functional, translational, and applied research. However, these resources have only received limited attention to date for asking hypothesis-driven questions to elucidate the functional and evolutionary significance for pools of identified genes, proteins, and microbes. In recent years sequencing has rapidly moved into the realm of super high-throughput, with accompanying assembly and analyses requiring proportional super-computing power and bioinformatics expertise, but only limited resolution of biology or function. Transitioning from research that produces lists of genes, proteins and microbes, to research that determines their functional significance, is where the most important challenges lie for the next phases of termite science.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Apologies are extended to investigators whose research could not be cited because of space limitations. The author thanks Priya Rajarapu, Brittany Peterson, and Andres Sandoval for manuscript review, Vera Tai for sharing prepublication data, as well as his collaborators and all members of his laboratory, past and present, for their contributions and input.
Funding. Work conducted in the author’s laboratory was supported by the following funding sources: USDA-CSREES-NRI grant no. 2007-35607-17777, USDA-NIFA-AFRI grant nos. 2009-05245 and 2010-65106-30727, Consortium for Plant Biotechnology Research-DOE grant no. DE-FG36-02GO12026, DOE-SBIR grant nos. DE-FG02-08ER85063 and DE-85538 S08-II, NSF grant no. 1233484CBET, and the O.W. Rollins/Orkin Endowment at Purdue University. M.E.S. is an inventor on the following patents: US Patent No. 7,968,525, US Patent No. 8,445,240, US Provisional Patent No. 61/602,149, and US Provisional Patent No. 61/902,472.
The singular term “omic" is used as an adjective in this review.
The plural term “omics" is used as a noun.
REFERENCES
- Bastien G., Arnal G., Bozonnet S., Laguerre S., Ferreira F., Fauré R., et al. (2013). Mining for hemicellulases in the fungus-growing termite Pseudacanthotermes militaris using functional metagenomics. Biotechnol. Biofuels. 6:78 10.1186/1754-6834-6-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwens J., Millet C., Tarayre C., Brasseur C., Destain J., Vandenbol M., et al. (2013). Symbiont diversity in Reticulitermes santonensis: investigation strategy through proteomics. Environ. Entomol. 42 882–887 10.1603/EN13112 [DOI] [PubMed] [Google Scholar]
- Boucias D. G., Cai Y., Sun Y., Lietze V. U., Sen R., Raychoudhury R., et al. (2013). The hindgut lumen prokaryotic microbiota of the termite Reticulitermes flavipes and its responses to dietary lignocellulose composition. Mol. Ecol. 22 1836–1853 10.1111/mec.12230 [DOI] [PubMed] [Google Scholar]
- Brune A. (2014). Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12 168–180 10.1038/nrmicro3182 [DOI] [PubMed] [Google Scholar]
- Burnum K. E., Callister S. J., Nicora C. D., Purvine S. O., Hugenholtz P., Warnecke F., et al. (2011). Proteome insights into the symbiotic relationship between a captive colony of Nasutitermes corniger and its hindgut microbiome. ISME J. 5 161–164 10.1038/ismej.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouvenc T., Efstathion C. A., Elliott M. L., Su N. Y. (2013). Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc. Biol. Sci. 280:20131885 10.1098/rspb.2013.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornette R., Koshikawa S., Hojo M., Matsumoto T., Miura T. (2006). Caste-specific cytochrome P450 in the damp-wood termite Hodotermopsis sjostedti. Insect Mol. Biol. 15 235–244 10.1111/j.1365-2583.2006.00632.x [DOI] [PubMed] [Google Scholar]
- Cornette R., Hayashi Y., Koshikawa S., Miura T. (2013). Differential gene expression in response to juvenile hormone analog treatment in the damp-wood termite Hodotermopsis sjostedti. J. Insect Physiol. 59 509–518 10.1016/j.jinsphys.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Do T. H., Nguyen T. T., Nguyen T. N., Le Q. G., Nguyen C., Kimura K., et al. (2014). Mining biomass-degrading genes through Illumina-based de novo sequencing and metagenomic analysis of free-living bacteria in the gut of the lower termite Coptotermes gestroi harvested in Vietnam. J. Biosci. Bioeng. 6 665–671 10.1016/j.jbiosc.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Engelbrektson A., Kunin V., Wrighton K. C., Zvenigorodsky N., Chen F., Ochman H., et al. (2010). Experimental factors affecting PCR-based estimates of microbial species richness and evenness. ISME J. 4 642–647 10.1038/ismej.2009.153 [DOI] [PubMed] [Google Scholar]
- Fall S., Hamelin J., Ndiaye F., Assigbetse K., Aragno M., Chotte J. L., et al. (2007). Differences between bacterial communities in the gut of a soil-feeding termite (Cubitermes niokoloensis) and its mounds. Appl. Environ. Microbiol. 73 5199–5208 10.1128/AEM.02616-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Miller D., Brewster C., Husseneder C., Dickerman A. (2007). Diversity of gut bacteria of Reticulitermes flavipes as examined by 16S rRNA gene sequencing and amplified rDNA restriction analysis. Curr. Microbiol. 55 254–259 10.1007/s00284-007-0136-8 [DOI] [PubMed] [Google Scholar]
- Gao Q., Tancredi S. E., Thompson G. J. (2012). Identification of mycosis-related genes in the eastern subterranean termite by suppression subtractive hybridization. Arch. Insect Biochem. Physiol. 80 63–76 10.1002/arch.21026 [DOI] [PubMed] [Google Scholar]
- Geib S. M., Filley T. R., Hatcher P. G., Hoover K., Carlson J. E., Jimenez-Gasco Mdel M., et al. (2008). Lignin degradation in wood-feeding insects. Proc. Natl. Acad. Sci. U.S.A. 105 12932–12937 10.1073/pnas.0805257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glastad K. M., Hunt B. G., Goodisman M. A. D. (2013). Evidence of a conserved functional role for DNA methylation in termites. Insect Mol. Biol. 22 143–154 10.1111/imb.12010 [DOI] [PubMed] [Google Scholar]
- Grieco M. A., Cavalcante J. J., Cardoso A. M., Vieira R. P., Machado E. A., Clementino M. M., et al. (2013). Microbial community diversity in the gut of the South American termite Cornitermes cumulans. Microb. Ecol. 65 197–204 10.1007/s00248-012-0119-6 [DOI] [PubMed] [Google Scholar]
- Harmon-Smith M., Celia L., Chertkov O., Lapidus A., Copeland A., Glavina Del Rio T., et al. (2010). Complete genome sequence of Sebaldella termitidis type strain (NCTC 11300). Stand. Genomic Sci. 2 220–227 10.4056/sigs.811799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Shigenobu S., Watanabe D., Toga K., Saiki R., Shimada K., et al. (2013). Construction and characterization of normalized cDNA libraries by 454 pyrosequencing and estimation of DNA methylation levels in three distantly related termite species. PLoS ONE 8:e76678 10.1371/journal.pone.0076678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Ivanova N., Kirton E., Allgaier M., Bergin C., Scheffrahn R. H., et al. (2013). Comparative metagenomic and metatranscriptomic analysis of hindgut paunch microbiota in wood- and dung-feeding higher termites. PLoS ONE 8:e61126 10.1371/journal.pone.0061126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M., Maekawa K., Saitoh S., Shigenobu S., Miura T., Hayashi Y., et al. (2012). Exploration and characterization of genes involved in the synthesis of diterpene defence secretion in nasute termite soldiers. Insect Mol. Biol. 21 545–557 10.1111/j.1365-2583.2012.01162.x [DOI] [PubMed] [Google Scholar]
- Honey Bee Genome Sequencing Consortium. (2006). Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443 931–949 10.1038/nature05260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongoh Y., Ohkuma M., Kudo T. (2003). Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus. FEMS Microbiol. Ecol. 44 231–242 10.1016/S0168-6496(03)00026-6 [DOI] [PubMed] [Google Scholar]
- Hongoh Y., Sharma V. K., Prakash T., Noda S., Toh H., Taylor T. D., et al. (2008a). Genome of an endosymbiont coupling N2 fixation to cellulolysis within protist cells in termite gut. Science 322 1108–1109 10.1126/science.1165578 [DOI] [PubMed] [Google Scholar]
- Hongoh Y., Sharma V. K., Prakash T., Noda S., Taylor T. D., Kudo T., et al. (2008b). Complete genome of the uncultured Termite Group 1 bacteria in a single host protist cell. Proc. Natl. Acad. Sci. U.S.A. 105 5555–5560 10.1073/pnas.0801389105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Sun P., Zhou X., Lei C. (2012). Characterization of head transcriptome and analysis of gene expression involved in caste differentiation and aggression in Odontotermes formosanus. PLoS ONE 7:e50383 10.1371/journal.pone.0050383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Li Y. F., Cheng Y., Liu Y., Chen C. C., Wen S. Y. (2013). Immune-related transcriptome of Coptotermes formosanus Shiraki workers: the defense mechanism. PLoS ONE 8:e69543 10.1371/journal.pone.0069543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseneder C., Ho H. Y., Blackwell M. (2010a). Comparison of the bacterial symbiont composition of the Formosan subterranean termite from its native and introduced range. Open Microbiol. J. 4 53–66 10.2174/1874285801004010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseneder C., Simms D. M., Aluko G. K., Delatte J. (2010b). Colony breeding system influences cuticular bacterial load of Formosan subterranean termite workers. Environ. Entomol. 39 1715–1723 10.1603/EN09238 [DOI] [PubMed] [Google Scholar]
- Husseneder C., McGregor C., Lang R. P., Collier R., Delatte J. (2012). Transcriptome profiling of female alates and egg-laying queens of the Formosan subterranean termite. Comp. Biochem. Physiol. D. 7 14–27. [DOI] [PubMed] [Google Scholar]
- Husseneder C., Simms D. M. (2014). Effects of caste on the expression of genes associated with septic injury and xenobiotic exposure in the Formosan subterranean termite. PLoS ONE 9:e105582 10.1371/journal.pone.0105582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isanapong J., Goodwin L., Bruce D., Chen A., Detter C., Han J., et al. (2012). High-quality draft genome sequence of the Opitutaceae bacterium strain TAV1 a symbiont of the wood-feeding termite Reticulitermes flavipes. J. Bacteriol. 194 2744–2745 10.1128/JB.00264-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Okada Y., Ishikawa A., Miyakawa H., Koshikawa S., Miura T. (2010). Gene expression changes during caste-specific neuronal development in the damp-wood termite Hodotermopsis sjostedti. BMC Genomics 11:314 10.1186/1471-2164-11-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James E. R., Tai V., Scheffrahn R. H., Keeling P. J. (2013). Trichonympha burlesquei n. sp. from Reticulitermes virginicus and evidence against a cosmopolitan distribution of Trichonympha agilis in many termite hosts. Int. J. Syst. Evol. Microbiol. 63 3873–3876 10.1099/ijs.0.054874-0 [DOI] [PubMed] [Google Scholar]
- Johjima T., Taprab Y., Noparatnaraporn N., Kudo T., Ohkuma M. (2006). Large-scale identification of transcripts expressed in a symbiotic fungus (Termitomyces) during plant biomass degradation. Appl. Microbiol. Biotechnol. 73 195–203 10.1007/s00253-006-0570-8 [DOI] [PubMed] [Google Scholar]
- Ke J., Laskar D. D., Chen S. (2013). Tetramethylammonium hydroxide (TMAH) thermochemolysis for probing in situ softwood lignin modification in each gut segment of the termite. J. Agric. Food Chem. 61 1299–1308 10.1021/jf3048548 [DOI] [PubMed] [Google Scholar]
- Ke J., Laskar D. D., Singh D., Chen S. (2011). In situ lignocellulosic unlocking mechanism for carbohydrate hydrolysis in termites: crucial lignin modification. Biotechnol. Biofuels 4:17 10.1186/1754-6834-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler T., Dietrich C., Scheffrahn R. H., Brune A. (2012). High-resolution analysis of gut environment and bacterial microbiota reveals functional compartmentation of the gut in wood-feeding higher termites (Nasutitermes spp.). Appl. Environ. Microbiol. 78 4691–4701 10.1128/AEM.00683-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., Li L., Fröhlich J. (2013). The cellulolytic system of the termite gut. Appl. Microbiol. Biotechnol. 97 7943–7962 10.1007/s00253-013-5119-z [DOI] [PubMed] [Google Scholar]
- Koshikawa S., Cornette R., Hojo M., Maekawa K., Matsumoto T., Miura T. (2005). Screening of genes expressed in developing mandibles during soldier differentiation in the termite Hodotermopsis sjostedti. FEBS Lett. 579 1365–1370 10.1016/j.febslet.2005.01.031 [DOI] [PubMed] [Google Scholar]
- Koshikawa S., Miyazaki S., Cornette R., Matsumoto T., Miura T. (2008). Genome size of termites (Insecta, Dictyoptera, Isoptera) and wood roaches (Insecta, Dictyoptera, Cryptocercidae). Naturwissenschaften 95 859–867 10.1007/s00114-008-0395-7 [DOI] [PubMed] [Google Scholar]
- Leonardo F. C., da Cunha A. F., da Silva M. J., Carazzolle M. F., Costa-Leonardo A. M., Costa F. F., et al. (2011). Analysis of the workers head transcriptome of the Asian subterranean termite, Coptotermes gestroi. Bull. Entomol. Res. 101 383–391 10.1017/S0007485310000556 [DOI] [PubMed] [Google Scholar]
- Lilburn T. G., Schmidt T. M., Breznak J. A. (1999). Phylogenetic diversity of termite gut spirochaetes. Environ. Microbiol. 1 331–345 10.1046/j.1462-2920.1999.00043.x [DOI] [PubMed] [Google Scholar]
- Liu N., Yan X., Zhang M., Xie L., Wang Q., Huang Y., et al. (2011). Microbiome of fungus-growing termites: a new reservoir for lignocellulase genes. Appl. Environ. Microbiol. 77 48–56 10.1128/AEM.01521-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhang L., Zhou H., Zhang M., Yan X., Wang Q., et al. (2013). Metagenomic insights into metabolic capacities of the gut microbiota in a fungus-cultivating termite (Odontotermes yunnanensis). PLoS ONE 8:e69184 10.1371/journal.pone.0069184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo N., Li B., Ujvari B. (2012). DNA methylation in the termite Coptotermes lacteus. Insect Soc. 59 257–261 10.1007/s00040-011-0213-7 [DOI] [Google Scholar]
- Makonde H. M., Boga H. I., Osiemo Z., Mwirichia R., Mackenzie L. M., Göker M., et al. (2013). 16S-rRNA-based analysis of bacterial diversity in the gut of fungus-cultivating termites (Microtermes and Odontotermes species). Antonie Van Leeuwenhoek 104 869–883 10.1007/s10482-013-0001-7 [DOI] [PubMed] [Google Scholar]
- Mattéotti C., Bauwens J., Brasseur C., Tarayre C., Thonart P., Destain J., et al. (2012). Identification and characterization of a new xylanase from Gram-positive bacteria isolated from termite gut (Reticulitermes santonensis). Protein Expr. Purif. 83 117–127 10.1016/j.pep.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Mattéotti C., Haubruge E., Thonart P., Francis F., De Pauw E., Portetelle D., et al. (2011a). Characterization of a new β-glucosidase/β-xylosidase from the gut microbiota of the termite (Reticulitermes santonensis). FEMS Microbiol. Lett. 314 147–157 10.1111/j.1574-6968.2010.02161.x [DOI] [PubMed] [Google Scholar]
- Mattéotti C., Thonart P., Francis F., Haubruge E., Destain J., Brasseur C., et al. (2011b). New glucosidase activities identified by functional screening of a genomic DNA library from the gut microbiota of the termite Reticulitermes santonensis. Microbiol. Res. 166 629–642 10.1016/j.micres.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Miura T., Kamikouchi A., Sawata M., Takeuchi H., Natori S., Kubo T., et al. (1999). Soldier caste-specific gene expression in the mandibular glands of Hodotermopsis japonica. Proc. Natl. Acad. Sci. U.S.A. 96 13874–13879 10.1073/pnas.96.24.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Scharf M. E. (2011). “Molecular mechanisms underlying caste differentiation in termites,” in Biology of Termites: A Modern Synthesis, eds Bigness D. E., Roisin Y., Lo N. (Dordrecht: Springer; ), 211–253. [Google Scholar]
- Miyata R., Noda N., Tamaki H., Kinjyo K., Aoyagi H., Uchiyama H., et al. (2007). Influence of feed components on symbiotic bacterial community structure in the gut of the wood-feeding higher termite Nasutitermes takasagoensis. Biosci. Biotechnol. Biochem. 71 1244–1251 10.1271/bbb.60672 [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Ihle K. E., Fondrk M. K., Page R. E., Amdam G. V. (2007). The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5:e62 10.1371/journal.pbio.0050062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. J., Cool L. G., Forschler B. T., Haverty M. I. (2001). Correspondence of soldier defense secretion mixtures with cuticular hydrocarbon phenotypes for chemotaxonomy of the termite genus Reticulitermes in North America. J. Chem. Ecol. 27 1449–1479 10.1023/A:1010325511844 [DOI] [PubMed] [Google Scholar]
- Ni J., Tokuda G. (2013). Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnol. Adv. 31 838–850 10.1016/j.biotechadv.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Nimchua T., Thongaram T., Uengwetwanit T., Pongpattanakitshote S., Eurwilaichitr L. (2012). Metagenomic analysis of novel lignocellulose-degrading enzymes from higher termite guts inhabiting microbes. J. Microbiol. Biotechnol. 22 462–469 10.4014/jmb.1108.08037 [DOI] [PubMed] [Google Scholar]
- Otani S., Mikaelyan A., Nobre T., Hansen L. H., Koné N. A., Sørensen S. J., et al. (2014). Identifying the core microbial community in the gut of fungus-growing termites. Mol. Ecol. 23 4631–4644 10.1111/mec.12874 [DOI] [PubMed] [Google Scholar]
- Poulsen M., Hu H., Li C., Chen Z., Xu L., Otani S., et al. (2014). Complementary symbiont contributions to plant decomposition in a fungus-farming termite. Proc. Natl. Acad. Sci. U.S.A. 111 14500–14505 10.1073/pnas.1319718111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich G. D. (1984). Defense mechanisms of termites. Ann. Rev. Entomol. 29 201–232 10.1146/annurev.en.29.010184.001221 [DOI] [Google Scholar]
- Rashamuse K., Mabizela-Mokoena N., Sanyika T. W., Mabvakure B., Brady D. (2012). Accessing carboxylesterase diversity from termite hindgut symbionts through metagenomics. J. Mol. Microbiol. Biotechnol. 22 277–286 10.1159/000342447 [DOI] [PubMed] [Google Scholar]
- Rashamuse K., Ronneburg T., Sanyika W., Mathiba K., Mmutlane E., Brady D. (2014). Metagenomic mining of feruloyl esterases from termite enteric flora. Appl. Microbiol. Biotechnol. 98 727–737 10.1007/s00253-013-4909-7 [DOI] [PubMed] [Google Scholar]
- Raychoudhury R., Sen R., Cai Y., Sun Y., Lietze V. U., Boucias D. G., et al. (2013). Comparative metatranscriptomic signatures of wood and paper feeding in the gut of the termite Reticulitermes flavipes. Insect Mol. Biol. 22 155–171 10.1111/imb.12011 [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Grozinger C. M., Whitfield C. W. (2005). Sociogenomics: social life in molecular terms. Nat. Rev. Genet. 6 257–270 10.1038/nrg1575 [DOI] [PubMed] [Google Scholar]
- Roose-Amsaleg C., Brygoo Y., Harry M. (2004). Ascomycete diversity in soil-feeding termite nests and soils from a tropical rainforest. Environ. Microbiol. 6 462–469 10.1111/j.1462-2920.2004.00579.x [DOI] [PubMed] [Google Scholar]
- Rosengaus R. B., Schultheis K. F., Yalonetskaya A., Bulmer M. S., DuComb W. S., Benson R. W., et al. (2014). Symbiont-derived β-13-glucanases in a social insect: mutualism beyond nutrition. Front. Microbiol. 5:607 10.3389/fmicb.2014.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengaus R. B., Zecher C. N., Schultheis K. F., Brucker R. M., Bordenstein S. R. (2011). Disruption of the termite gut microbiota and its prolonged consequences for fitness. Appl. Environ. Microbiol. 77 4303–4312 10.1128/AEM.01886-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. Z., Matson E. G., Eldar A., Leadbetter J. R. (2011). RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture. ISME J. 5 1133–1142 10.1038/ismej.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabree Z. L., Huang C. Y., Arakawa G., Tokuda G., Lo N., Watanabe H., et al. (2012). Genome shrinkage and loss of nutrient-providing potential in the obligate symbiont of the primitive termite Mastotermes darwiniensis. Appl. Environ. Microbiol. 78 204–210 10.1128/AEM.06540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyika T. W., Rashamuse K. J., Hennesy F., Brady D. (2012). Luminal hindgut bacterial diversities of the grass and sugarcane feeding termite Trinervitermes trinervoides. African J. Microbiol. Res. 6 2639–2648. [Google Scholar]
- Scharf M. E. (2015). Termites as targets and models for biotechnology. Ann. Rev. Entomol. 60 77–102 10.1146/annurev-ento-010814-020902 [DOI] [PubMed] [Google Scholar]
- Scharf M. E., Karl Z. J., Sethi A., Boucias D. G. (2011). Multiple levels of synergistic collaboration in termite lignocellulose digestion. PLoS ONE 6:e21709 10.1371/journal.pone.0021709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf M. E., Scharf W. D., Pittendrigh B. R., Bennett G. W. (2003). Caste- and development-associated gene expression in a lower termite. Genome Biol. 4:R62 10.1186/gb-2003-4-10-r62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf M. E., Tartar A. (2008). Termite digestomes as sources for novel lignocellulases. Biofuels Bioprod. Bioref. 2 540–552 10.1002/bbb.107 [DOI] [Google Scholar]
- Scharf M. E., Wu-Scharf D., Zhou X., Pittendrigh B. R., Bennett G. W. (2005). Gene expression profiles among immature and adult reproductive castes of the termite Reticulitermes flavipes. Insect Mol. Biol. 14 31–44 10.1111/j.1365-2583.2004.00527.x [DOI] [PubMed] [Google Scholar]
- Schauer C., Thompson C., Brune A. (2014). Pyrotag sequencing of the gut microbiota of the cockroach Shelfordella lateralis reveals a highly dynamic core but only limited effects of diet on community structure. PLoS ONE 9:e85861 10.1371/journal.pone.0085861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Raychoudhury R., Cai Y., Sun Y., Lietze V. U., Boucias D. G., et al. (2013). Differential impacts of juvenile hormone, soldier head extract and alternate caste phenotypes on host and symbiont transcriptome composition in the gut of the termite Reticulitermes flavipes. BMC Genomics 14:491 10.1186/1471-2164-14-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Raychoudhury R., Cai Y., Sun Y., Lietze V. U., Boucias D. G., et al. (2015). Metatranscriptomic signatures of nicotinoid-pathogen synergy in the termite gut. PLoS ONE (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A., Slack J. M., Kovaleva E. S., Buchman G. W., Scharf M. E. (2013a). Lignin-associated metagene expression in a lignocellulose-digesting termite. Insect Biochem. Mol. Biol. 43 91–101 10.1016/j.ibmb.2012.10.001 [DOI] [PubMed] [Google Scholar]
- Sethi A., Kovaleva E. S., Slack J. M., Brown S., Buchman G. W., Scharf M. E. (2013b). A GHF7 cellulase from the protist symbiont community of Reticulitermes flavipes enables more efficient lignocellulose processing by host enzymes. Arch. Insect Biochem. Physiol. 84 175–193 10.1002/arch.21135 [DOI] [PubMed] [Google Scholar]
- Sillam-Dussès D., Krasulová J., Vrkoslav V., Pytelková J., Cvačka J., Kutalová K., et al. (2012). Comparative study of the labial gland secretion in termites. PLoS ONE 7:e46431 10.1371/journal.pone.0046431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller M. M., Kambhampati S., Caragea D. (2010). Comparative analysis of expressed sequence tags from three castes and two life stages of the termite Reticulitermes flavipes. BMC Genomics 11:463 10.1186/1471-2164-11-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl U., Radek R., Yang H., Brune A. (2005). “Endomicrobia”: cytoplasmic symbionts of termite gut protozoa form a separate phylum of prokaryotes. Appl. Environ. Microbiol. 71 1473–1479 10.1128/AEM.71.3.1473-1479.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai V., James E. R., Nalepa C. A., Scheffrahn R. H., Perlman S. J., Keeling P. J. (2015). The role of host phylogeny varies in shaping microbial diversity in the hindguts of lower termites. Appl. Environ. Microbiol. 81 1059–1070 10.1128/AEM.02945-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai V., James E. R., Perlman S. J., Keeling P. J. (2013). Single-Cell DNA barcoding using sequences from the small subunit rRNA and internal transcribed spacer region identifies new species of Trichonympha and Trichomitopsis from the hindgut of the termite Zootermopsis angusticollis. PLoS ONE 8:e58728 10.1371/journal.pone.0058728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai V., Keeling P. J. (2013). Termite hindguts and the ecology of microbial communities in the sequencing age. J. Eukaryot. Microbiol. 60 421–428 10.1111/jeu.12048 [DOI] [PubMed] [Google Scholar]
- Tartar A., Wheeler M. M., Zhou X., Coy M. R., Boucias D. G., Scharf M. E. (2009). Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termite R. flavipes. Biotechnol. Biofuels 2:25 10.1186/1754-6834-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarver M. R., Zhou X., Scharf M. E. (2010). Socio-environmental and endocrine influences on developmental and caste-regulatory gene expression in the eusocial termite Reticulitermes flavipes. BMC Mol. Biol. 11:28 10.1186/1471-2199-11-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrapon N., Li C., Robertson H. M., Ji L., Meng X., Booth W., et al. (2014). Molecular traces of alternative social organization in a termite genome. Nat. Commun. 5:3636 10.1038/ncomms4636 [DOI] [PubMed] [Google Scholar]
- Thompson G. J., Crozier Y. C., Crozier R. H. (2003). Isolation and characterization of a termite transferrin gene up-regulated on infection. Insect Mol. Biol. 12 1–7 10.1046/j.1365-2583.2003.00381.x [DOI] [PubMed] [Google Scholar]
- Todaka N., Inoue T., Saita K., Ohkuma M., Nalepa C. A., Lenz M., et al. (2010). Phylogenetic analysis of cellulolytic enzyme genes from representative lineages of termites and a related cockroach. PLoS ONE 5:e8636 10.1371/journal.pone.0008636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaka N., Moriya S., Saita K., Hondo T., Kiuchi I., Takasu H., et al. (2007). Environmental cDNA analysis of the genes involved in lignocellulose digestion in the symbiotic protist community of Reticulitermes speratus. FEMS Microbiol. Ecol. 59 592–599 10.1111/j.1574-6941.2006.00237.x [DOI] [PubMed] [Google Scholar]
- Tokuda G., Tsuboi Y., Kihara K., Saitou S., Moriya S., Lo N., et al. (2014). Metabolomic profiling of 13C-labelled cellulose digestion in a lower termite: insights into gut symbiont function. Proc. Biol. Sci. 281 1789 10.1098/rspb.2014.0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo E. L., Husseneder C. (2009). Biology of subterranean termites: insights from molecular studies of Reticulitermes, and Coptotermes. Annu. Rev. Entomol. 54 379–403 10.1146/annurev.ento.54.110807.090443 [DOI] [PubMed] [Google Scholar]
- Wang Q., Qian C., Zhang X. Z., Liu N., Yan X., Zhou Z. (2012). Characterization of a novel thermostable β-glucosidase from a metagenomic library of termite gut. Enzyme Microb. Technol. 51 319–324 10.1016/j.enzmictec.2012.07.015 [DOI] [PubMed] [Google Scholar]
- Wang Y., Qian P. Y. (2009). Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 4:e7401 10.1371/journal.pone.0007401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke F., Luginbühl P., Ivanova N., Ghassemian M., Richardson T. H., Stege J. T., et al. (2007). Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 450 560–565 10.1038/nature06269 [DOI] [PubMed] [Google Scholar]
- Watanabe H., Noda H., Tokuda G., Lo N. (1998). A cellulase gene of termite origin. Nature 394 330–331 10.1038/28527 [DOI] [PubMed] [Google Scholar]
- Watanabe H., Tokuda G. (2010). Cellulolytic systems in insects. Annu. Rev. Entomol. 55 609–632 10.1146/annurev-ento-112408-085319 [DOI] [PubMed] [Google Scholar]
- Weil T., Korb J., Rehli M. (2009). Comparison of queen-specific gene expression in related lower termite species. Mol. Biol. Evol. 26 1841–1850 10.1093/molbev/msp095 [DOI] [PubMed] [Google Scholar]
- Weil T., Rehli M., Korb J. (2007). Molecular basis for the reproductive division of labour in a lower termite. BMC Genomics 8:198 10.1186/1471-2164-8-198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard M. J. (2003). Developmental Plasticity and Evolution. Oxford: Oxford University Press. [Google Scholar]
- Wu-Scharf D., Scharf M. E., Pittendrigh B. R., Bennett G. W. (2003). Expressed sequence tags from a polyphenic Reticulitermes flavipes cDNA library. Sociobiology 41 479–490. [Google Scholar]
- Xie L., Zhang L., Zhong Y., Liu N., Long Y., Wang S., et al. (2012). Profiling the metatranscriptome of the protistan community in Coptotermes formosanus with emphasis on the lignocellulolytic system. Genomics 99 246–255 10.1016/j.ygeno.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Yang H., Schmitt-Wagner D., Stingl U., Brune A. (2005). Niche heterogeneity determines bacterial community structure in the termite gut (Reticulitermes santonensis). Environ. Microbiol. 7 916–932 10.1111/j.1462-2920.2005.00760.x [DOI] [PubMed] [Google Scholar]
- Yang F., Xu B., Li J., Huang Z. (2012). Transcriptome analysis of Termitomyces albuminosus reveals the biodegradation of lignocellulose. Wei Sheng Wu Xue Bao 52 466–477. [PubMed] [Google Scholar]
- Yuki M., Moriya S., Inoue T., Kudo T. (2008). Transcriptome analysis of the digestive organs of Hodotermopsis sjostedti, a lower termite that hosts mutualistic microorganisms in its hindgut. Zoolog. Sci. 25 401–406 10.2108/zsj.25.401 [DOI] [PubMed] [Google Scholar]
- Zhang D., Lax A. R., Henrissat B., Coutinho P., Katiya N., Nierman W. C., et al. (2012). Carbohydrate-active enzymes revealed in Coptotermes formosanus transcriptome. Insect Mol. Biol. 21 235–245 10.1111/j.1365-2583.2011.01130.x [DOI] [PubMed] [Google Scholar]