Background: The roles of Rictor/mTORC2 in folliculogenesis and follicle survival are unknown.
Results: Loss of Rictor in oocytes causes excessive follicular atresia and the mutant mice demonstrate progressive POF phenotype.
Conclusion: Rictor/mTORC2 plays key roles in folliculogenesis, follicle survival, and female fertility.
Significance: This study establishes a novel function of mTORC2 in folliculogenesis and a potential link between mTORC2 and POF.
Keywords: Apoptosis, Gene Knockout, Mammalian Target of Rapamycin (mTOR), Oocyte, Ovary, Folliculogenesis, Follicle Survival, mTORC2, Oogenesis, Premature Ovarian Failure, Rictor
Abstract
Molecular basis of ovarian folliculogenesis and etiopathogenesis of premature ovarian failure (POF), a common cause of infertility in women, are not fully understood. Mechanistic target of rapamycin complex 2 (mTORC2) is emerging as a central regulator of cell metabolism, proliferation, and survival. However, its role in folliculogenesis and POF has not been reported. Here, we showed that the signaling activity of mTORC2 is inhibited in a 4-vinylcyclohexene diepoxide (VCD)-induced POF mouse model. Notably, mice with oocyte-specific ablation of Rictor, a key component of mTORC2, demonstrated POF phenotypes, including massive follicular death, excessive loss of functional ovarian follicles, abnormal gonadal hormone secretion, and consequently, secondary subfertility in conditional knock-out (cKO) mice. Furthermore, reduced levels of Ser-473-phosphorylated Akt and Ser-253-phosphorylated Foxo3a and elevated pro-apoptotic proteins, Bad, Bax, and cleaved poly ADP-ribose polymerase (PARP), were observed in cKO mice, replicating the signaling alterations in 4-VCD-treated ovaries. These results indicate a critical role of the Rictor/mTORC2/Akt/Foxo3a pro-survival signaling axis in folliculogenesis. Interestingly, loss of maternal Rictor did not cause obvious developmental defects in embryos or placentas from cKO mice, suggesting that maternal Rictor is dispensable for preimplantation embryonic development. Our results collectively indicate key roles of Rictor/mTORC2 in folliculogenesis, follicle survival, and female fertility and support the utility of oocyte-specific Rictor knock-out mice as a novel model for POF.
Introduction
The reproductive lifespan of women and most other mammals is ultimately dependent on the size of the ovarian primordial follicle pool, which is generally established during embryonic development or after birth (1, 2). To produce mature oocytes for fertilization, primordial follicles are recruited from the reservoir of dormant follicles into the growing follicle pool through a process termed follicular activation, and subsequently undergo a series of development steps. During this entire process, a large number of follicles undergo apoptotic death (atresia) if not selected for further growth (3–5). Menopause or ovarian failure occurs when the primordial follicle pool is exhausted. Premature ovarian failure (POF),4 also known as premature menopause, is characterized by cessation of ovarian function, amenorrhea, hypoestrogenism, and elevated gonadotropin levels in women under 40 years of age, affecting 1–2 and 0.1% of women under the ages of 40 and 30, respectively (6). A disastrous consequence of POF is loss of fertility, which is due to the absence of follicles in most cases, and in others, the inability of the remaining follicles to respond to hormonal stimulation (7). Nevertheless, the detailed etiology of POF remains to be established, and the factors and mechanisms involved in regulating the processes of primordial follicle activation, follicle growth, and follicle survival or atresia are not completely understood at present.
Mechanistic target of rapamycin (mTOR) is a highly conserved Ser/Thr protein kinase that forms two distinct functional complexes termed mTOR complex 1 (mTORC1) and mTORC2 (8, 9). mTORC1, essentially comprising mTOR, mLST8, and rapamycin-sensitive adaptor protein of mTOR (Raptor), regulates cell growth, proliferation and metabolism (10, 11). Activation of mTORC1 promotes the phosphorylation of two downstream targets, p70 ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E binding protein-1 (4E-BP1), stimulating ribosome biogenesis and protein synthesis (12, 13). However, mTORC2 is insensitive to rapamycin. The essential core of mTORC2 comprises mTOR, mSIN1, mLST8, and the rapamycin-insensitive subunit, Rictor (8, 9). mTORC2 regulates the activity of Akt and PKC and controls cell survival and cytoskeletal organization (14, 15). In contrast to mTORC1, for which many upstream signals and cellular functions have been defined, relatively little is known about mTORC2 biology.
PI3K signaling is significant in folliculogenesis processes, including follicle activation, development, and survival (16–19). As a downstream target of PI3K, mTORC1 is involved in the regulation of folliculogenesis. Stimulation of mTORC1 leads to overactivation of the entire pool of primordial follicles, causing POF and infertility (20–22). However, the roles of Rictor/mTORC2 in folliculogenesis are currently unknown. In this study, we demonstrated that Rictor activity and downstream mTORC2 signaling are suppressed in ovaries treated with the ovotoxicant, 4-vinylcyclohexene diepoxide (VCD), an occupational chemical shown to cause POF in human and animal models by accelerating the apoptotic process of atresia (23–27). Additionally, induction of oocyte-specific ablation of Rictor with the Cre-loxp system using Zp3-Cre and Rictorloxp/loxp mice resulted in POF phenotypes. Our findings support a critical role of Rictor/mTORC2 in follicle survival and oogenesis.
EXPERIMENTAL PROCEDURES
Mice, Husbandry, and Genotyping
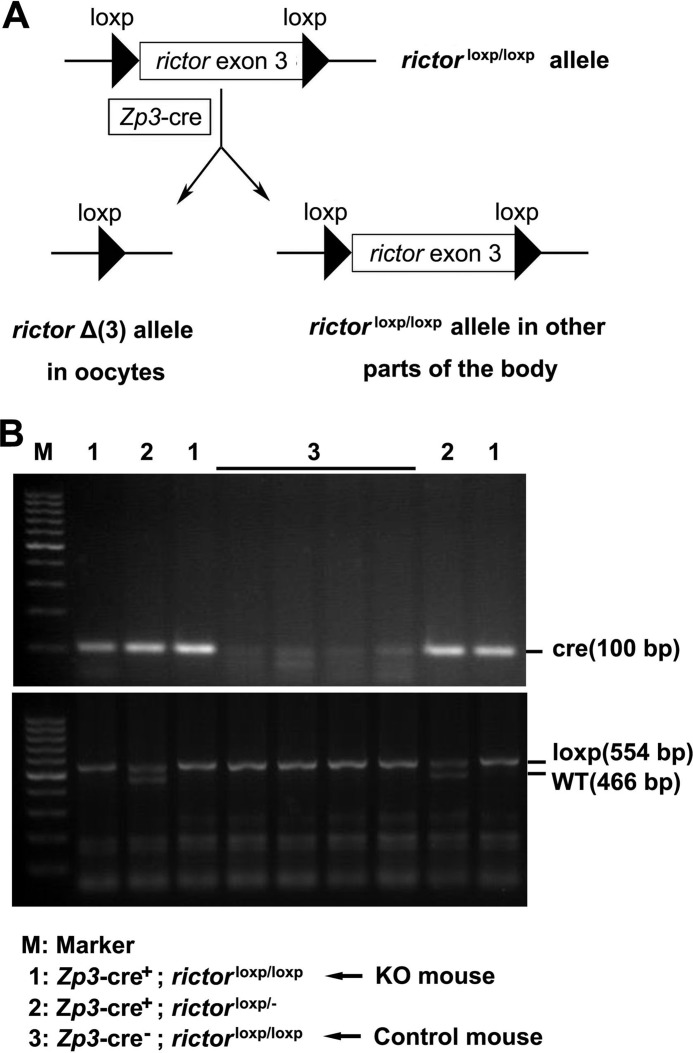
Zp3-Cre mice (Jax number 003650) were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). Rictor loxp mice were kindly provided by Professor Mark A. Magnuson (Vanderbilt University). For oocyte-specific knock-out experiments, males (but not females) carrying the Cre transgene were used for breeding to prevent universal knock-out of target genes in the whole body (28). Male Zp3-Cre mice were mated with female homozygous Rictor loxp (Rictorloxp/loxp) mice to yield males heterozygous for loxp Rictor and Zp3-Cre, which were then bred with female mice homozygous for loxp Rictor to obtain female mice homozygous for loxp Rictor and heterozygous for Zp3-Cre. These mice displayed oocyte-specific deletion of Rictor (Zp3-Cre+, Rictorloxp/loxp) and were therefore designated conditional knock-out (cKO) mice. Female mice homozygous for loxp Rictor without Zp3-Cre (Zp3-Cre−, Rictorloxp/loxp) from the same litter were taken as control mice. Genotyping involved the use of the following primers: Zp3-F, 5′-GCGGTCTGGCAGTAAAAACTATC-3′, Zp3-R, 5′-GTGAAACAGCATTGCTGTCACTT-3′; Rictor-F, 5′-GAAGTTATTCAGATGGCCCAGC-3′; Rictor-R, 5′-ACTGAATATGTTCATGGTTGTG-3′. DNA extraction, PCR amplification, and agarose electrophoresis were performed according to the instructions of The Jackson Laboratory. All animal experiments were approved by the Southern Medical University Committee on the Use and Care of Animals and performed in accordance with the Committee's guidelines and regulations.
Histological Analysis
Ovaries and uteri of mice were removed, weighed, fixed in 4% paraformaldehyde, and processed using paraffin wax and standard methods. For follicle counting, five sections of each ovary (5 μm taken 200 μm apart) were used for H&E staining and follicle classification performed as described previously (29). For immunohistochemistry analysis, the following primary antibodies were used: anti-Rictor (1:50), anti-phospho-Rictor (p-Rictor, Thr-1135) (1:50), and anti-p-S6 (Ser-235/Ser-236) (1:100) from Cell Signaling Technology; anti-p-Akt (Ser-473) (1:50) from Santa Cruz Biotechnology; and anti-p-Foxo3a (Ser-253) (1:50), anti-Bad (1:100), anti-Bax (1:100), and anti-placental lactogen 1 (PL-1, 1:50) from Epitomics. Horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and diaminobenzidine substrate (ZSGB Biotechnology, Beijing, China) were used for visualization. Sections were finally counterstained with hematoxylin. Cell apoptosis of follicles was evaluated on 5-μm sections by TUNEL assay using a commercial kit (Promega).
Western Blot Analysis
After euthanasia, ovaries were immediately removed, subsequently triturated and lysed on ice, and finally boiled in SDS loading buffer. Protein extracts were subjected to 6–12% SDS-PAGE and subsequent processes following the standard protocol. Apart from the primary antibodies specified for immunohistochemistry analysis, those used for Western blot were as follows: anti-p-S6K (Thr-389), anti-S6K, anti-cleaved PARP, and anti-p-NDRG1 (Thr-346) (Cell Signaling Technology); anti-Akt and anti-α-Tubulin (Santa Cruz Biotechnology); and anti-Foxo3a (Proteintech) (Chicago, IL). An ECL kit (PerkinElmer) was used for visualization.
Serum Hormone Measurement
After anesthesia, mid-line dermatotomy of the chest was performed, and blood was drawn via cardiocentesis. After 10 min of centrifugation at 3,000 × g, serum was collected and stored at −20 °C until use. Plasma FSH, luteinizing hormone (LH), estrogen hormone (E2), and progesterone levels were measured using ELISA (Nanjing Jiancheng Bioengineering, Nanjing, China).
Fertility Assay and Embryonic Development Assessment
To evaluate the fertility of female mice, 4- or 6-month-old control or cKO mice were mated with wild-type male C57 mice with proven fertility at a proportion of 1:1. Successful conception was defined by the presence of vaginal plug and subsequent visibly growing abdomen, and pregnant female mice were separated and monitored. The number of pups from each litter was counted, each pup was weighed, and physical status was examined. The duration from the day of mating to the day of delivery was defined as a childbirth cycle. Female mice that did not conceive within 2 months of mating were defined as infertile. For embryonic development assessment, female control or cKO mice with vaginal plug were designated E0.5 and killed at E5.0, E10, and E15. Embryos and placenta were removed, counted, and weighed. E15 placentas were fixed in 4% paraformaldehyde, processed using paraffin wax, and sectioned for histological analysis.
VCD Treatment
Female wild-type C57 mice (4 weeks old) were administered daily intraperitoneal injections of either sesame oil (2.5 ml/kg, vehicle control) or VCD (Sigma) dissolved in sesame oil (80 mg/kg) for 15 days, as described previously (25). At 12 h following the final dose, mice were killed. One ovary of each mouse was triturated, lysed, and boiled in SDS loading buffer for Western blot analysis. The other ovary was fixed in 4% paraformaldehyde and processed using paraffin wax and standard methods. Histological analysis was performed as described above.
Statistical Analysis
All experiments were carried out in duplicate. Data were expressed as mean values ± S.E., and differences between groups were analyzed using Student's t test (SPSS 13.0) if data violated normal distribution via nonparametric Mann-Whitney test. p < 0.05 was considered statistically significant. For Western blot analysis, one representative set of data is shown.
RESULTS
4-VCD Destroys Early-stage Follicles and Suppresses the Rictor/mTORC2 Signaling Pathway
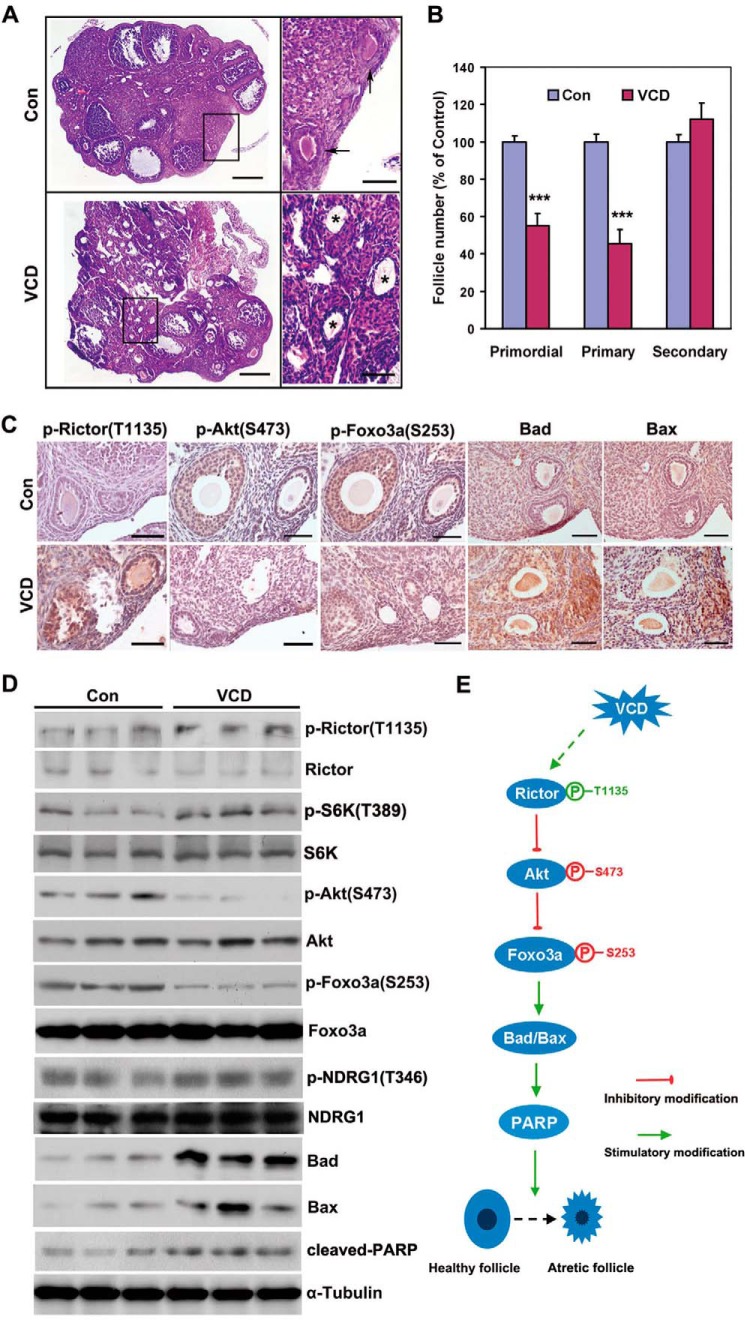
To assess the role of mTORC2 in POF, the activity of Rictor, a central component of the mTORC2 signaling pathway, was finely monitored in 4-VCD-treated ovaries. As expected, 15 days of VCD treatment reduced the number of primordial follicles by 45% and primary follicles by 55% that of control mice, respectively, with a weak toxic effect on follicles of higher grade (Fig. 1, A and B). Interestingly, the level of phospho-Rictor (p-Rictor, Thr-1135), an inhibitory modifier of Rictor, was up-regulated in VCD-treated ovaries (Fig. 1C). Rictor is a core component of mTORC2 that promotes cell survival through phosphorylating and activating Akt in various contexts, in turn, phosphorylating and inhibiting Foxo3a, a pro-apoptotic transcription factor (9, 15). As expected, expression of p-Akt (Ser-473) and p-Foxo3a (Ser-253) was reduced in VCD-treated ovaries (Fig. 1C). Simultaneously, the levels of Bad, Bax, and cleaved PARP, three typical pro-apoptotic proteins, were elevated (Fig. 1, C and D). Serum- and glucocorticoid-regulated kinase 1 (SGK1), another mTORC2 substrate, was not affected because the phosphorylation of N-Myc downstream-regulated 1 (NDRG1) at Thr-346, a readout for SGK1 activity, was comparable between the control and VCD-treated mice (Fig. 1D). In addition, we observed an increase in Thr-389-phosphorylated S6K1 (Fig. 1D). These results provide in vivo evidence supporting the previous finding that S6K1 mediates direct phosphorylation and inhibition Rictor at Thr-1135, acting as a novel mTORC1-dependent inhibitory feedback loop on mTORC2 (30–32). Thus, phosphorylation and inactivation of Rictor appear to be significantly involved in VCD-induced follicular apoptosis (Fig. 1E).
FIGURE 1.
4-VCD destroys small follicles and inhibits Rictor/mTORC2 signaling. A, representative H&E images of ovaries from 4-week-old mice treated with VCD or vehicle (Con) for 15 days, respectively. Arrows indicate primordial or primary follicles in the control group, whereas massive atretic (apoptotic) follicles were observed in the VCD group (asterisk). Scale bar = 500 μm for the left panel, 50 μm for the right panel. B, the percentage of primordial and primary follicles in the control and VCD groups. Follicles at each stage were classified, counted, and calculated in proportion to the total number of follicles in each group. Bars indicate mean ± S.E. ***, p < 0.001. C, immunohistochemical analysis showed changes in Rictor/mTORC2 signaling after VCD treatment. Scale bar = 50 μm. D, Western blot analysis of Rictor/mTORC2 signal activity before and after VCD treatment. Protein samples were extracted from the whole ovary. Three individual mice of each group are shown. NDRG1, N-Myc downstream-regulated 1. E, schematic of the mechanism underlying VCD-induced ovarian toxicity in which Rictor plays a central role.
Oocyte-specific Deletion of Rictor Replicates the Effect of VCD on Oocytes
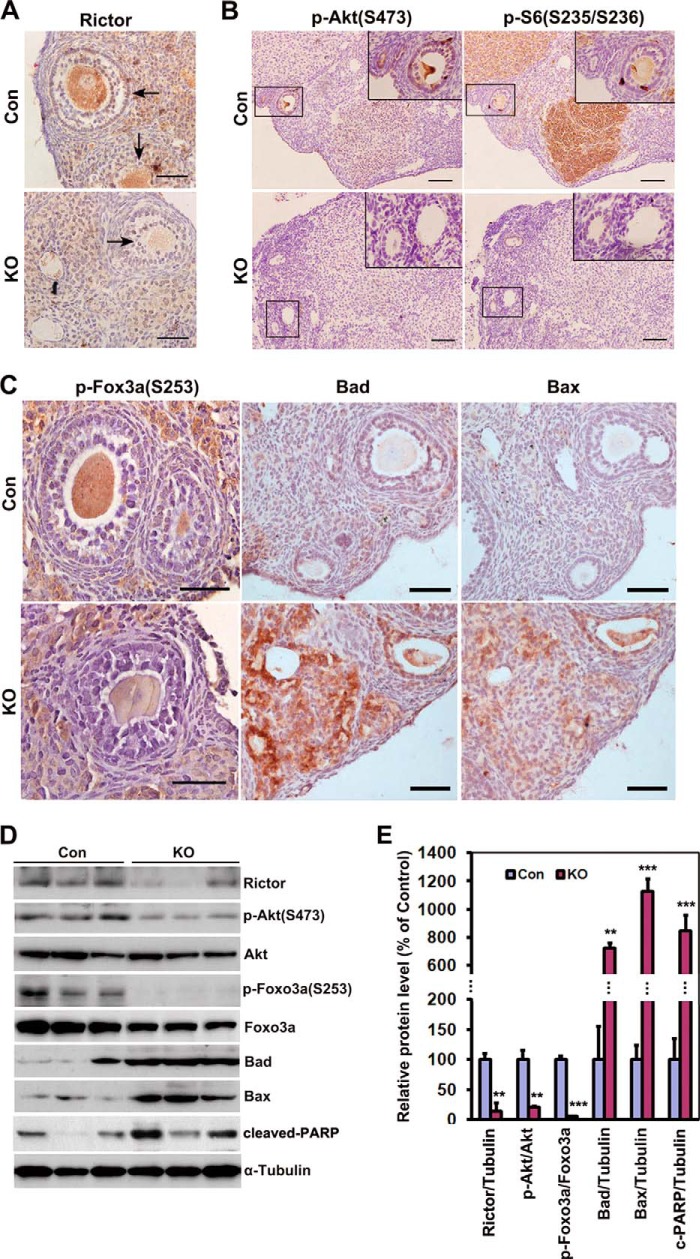
To identify the role of Rictor/mTORC2 in follicle survival, we generated mice with oocyte-specific deletion of Rictor using the Cre-loxp system (Fig. 2). As expected, Rictor expression was observed in ovarian follicles of control littermates, but not those of cKO mice (Fig. 3A), suggesting complete Cre-mediated recombination of the floxed Rictor gene. A decrease in p-Akt (Ser-473) was evident, attributable to the loss of Rictor (Fig. 3B). Interestingly, we observed specific reduction of p-S6 (Ser-235/Ser-236), a downstream signal/target of mTORC1, particularly in the corpus luteum (Fig. 3B). This result was in line with the previous finding that mTORC1 is activated by mTORC2 via inhibition of its negative regulator, tuberous sclerosis complex 1/2 (TSC1/2), through Akt (9, 10).
FIGURE 2.
Generation of mice with oocyte-specific deletion of Rictor. A, schematic of deletion of Rictor exon 3 by Zp3-cre-mediated recombination. B, genotyping the offspring after mating transgenic Cre and loxp mice. Genotyping was performed as described under “Experimental Procedures.”
FIGURE 3.
Elimination of Rictor in oocyte replicates the toxicological effects of VCD on Rictor/mTORC2 signaling. A, immunohistochemical analysis showed successful deletion of Rictor in oocytes of cKO mice. No Rictor expression was observed in oocytes of cKO mice. Con, control. B, reduced Ser-473-phosphorylated Akt and Ser-235/Ser-236-phosphorylated S6 in ovaries of cKO mice, particularly in corpus luteum. The inset represents an enlargement of the area in the box. C, decreased level of Ser-253-phosphorylated Foxo3a simultaneously observed with enhancement of Bad, Bax, and cleaved PARP in oocytes of cKO mice. D, Western blot analysis for comparison of Rictor/mTORC2 signaling in the ovaries of control and cKO mice. Protein samples were extracted from the whole ovary. Three individual mice of each group are shown. E, quantification of the results of D. Ovaries were from 6-month old control and cKO mice. Bars indicate mean ± S.E. **, p < 0.01, ***, p < 0.001. Scale bar = 50 μm for A and C, 200 μm for B.
Next, we monitored the expression of downstream signals/targets of Rictor/mTORC2. Loss of Rictor led to decreased p-Foxo3a (Ser-253) and increased Bad, Bax, and cleaved PARP levels (Fig. 3, C and D). These changes in signaling molecules were confirmed via Western blot analysis (Fig. 3, D and E). Thus, oocyte-specific ablation of Rictor duplicates the effects of VCD on the underlying signaling pathway controlling follicular survival in the ovary. Our results collectively reveal a central role of Rictor in the signaling axis of mTORC2/Akt/Foxo3a/pro-apoptotic proteins for regulation of follicular survival.
Loss of Rictor in Oocytes Causes Progressively Extensive Cell Apoptosis and Follicle Loss
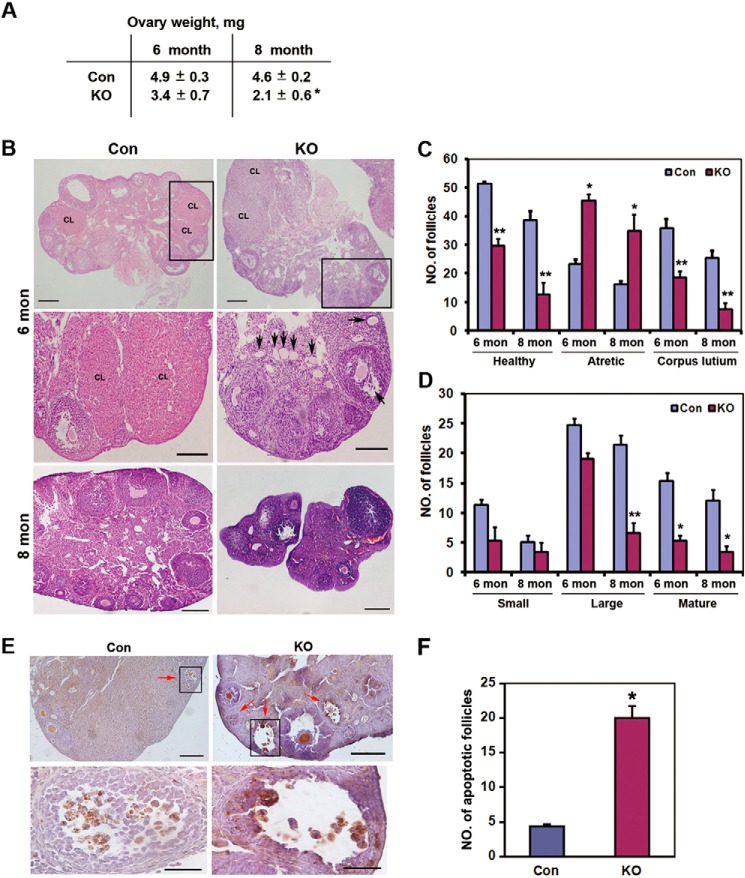
Next, we focused on the follicle number in cKO mice. cKO mice developed normally but had smaller ovaries than control mice, with a ovary weight decreased to 45% of the control mice at the age of 8 months (Fig. 4A). Consistent with enhanced expression of pro-apoptotic proteins, significantly fewer healthy follicles and less corpus luteum and more atretic follicles were morphologically observed in the ovaries of cKO mice. Notably, follicles were rarely found in cKO mice at the age of 8 months (Fig. 4B). To precisely assess follicle loss caused by Rictor deletion, the number of follicles at each stage of five sections from each ovary was classified and counted. When compared with control mice, we observed 40% loss of healthy follicles and 50% decrease in corpus luteum in 6-month-old cKO mice. In 8-month-old cKO mice, a 67% loss of healthy follicles and a 71% decrease in corpus luteum were observed (Fig. 4C), suggesting a progressive follicle loss after Rictor deletion in oocytes. Corpus luteum develops from follicular granulosa cells after ovulation (33). Less corpus luteum indicates lower levels of ovulation or availability of a smaller dominant follicle reserve for ovulation, which may be attributed to excessive follicle loss or arrest of development from small to mature follicles. In this regard, it is reasonable to believe that excessive follicle loss is one of the critical causes because more atretic follicles were detected in cKO mice (Fig. 4C). To further determine at which follicular stage follicle loss occurs, the number of follicles at each stage was analyzed. As shown in Fig. 4D, although cKO mice had fewer small follicles (including primordial and primary follicles) than control mice, this difference was not statistically significant. It has been shown that ZP3-Cre-mediated gene deletion occurs at the primary and/or later follicular stages (34), and thus deprivation of Rictor in oocytes did not cause significant primordial follicle loss. Indeed, massive follicle loss in cKO mice occurred at large and mature follicles (Fig. 4D). Consistently, acceleration of cell apoptosis was observed in large and mature follicles in 8-month-old cKO mice (Fig. 4, E and F), in line with the result of elevated pro-apoptotic protein levels in cKO mice. These results indicate that deletion of Rictor in oocytes enhanced cell apoptosis and follicle loss, and thus decreased follicular reserve available for ovulation.
FIGURE 4.
Histological comparison of ovarian morphology between control and cKO mice. A, comparison of ovary weight between control (Con) and cKO mice at the indicated age. Values present mean ± S.E. *, p < 0.05. B, overall view of morphological differences between a cross-section of control and cKO ovaries at the indicated age. Arrows indicate atretic follicles rich in cKO mice. CL, corpus luteum. Scale bar = 500 μm for the upper panel, 100 μm for the middle panel, and 200 μm for the lower panel. C, comparison of the total number of classified follicles between control and cKO mice at the indicated age. Data represent the average total number of indicated follicles counted from five sections (200 μm apart) of each ovary. n = 8 for 6-month group, n = 3 for 8-month group. Bars indicate mean ± S.E. *, p < 0.05; **, p < 0.01. D, comparison of the number of follicles at each stage in healthy follicles. Small follicles include primordial and primary follicles. Large follicles are designated secondary and preantral follicles, whereas mature follicles represent antral follicles. n = 8 for 6-month group, n = 3 for 8-month group. Bars indicate mean ± S.E. *, p < 0.05. E, cell apoptosis assay in 8-month-old control and cKO mice. Red arrows indicate follicle with apoptotic cells. Scale bar = 200 μm for the left panel, and 50 μm for the magnification panel. F, quantification of apoptotic follicles in E. Bars indicate mean ± S.E. *, p < 0.05.
Depletion of Rictor in Oocytes Causes Subfertility in cKO Mice
In general, diminished follicle reserve routinely results in reduced female fertility. To further evaluate the fertility of cKO mice, 4-, 6-, and 8-month-old female control and cKO mice were mated with wild-type C57BL/6J males with proven fertility. As expected, progressive loss of fertility was observed in cKO mice. All control mice successfully conceived and delivered their pups, whereas one cKO mouse (11%) from both the 4-month-old and the 6-month-old groups did not conceive within the 2-month observation period. Of note, all 8-month-old cKO mice were sterile (Table 1). Interestingly, one mouse (11%) from the 4-month-old group and four (44%) from the 6-month-old group died of dystocia (Table 1), characterized by aberrantly long gestation duration and delivery of dead pups. Consequently, the cKO group had a significantly lower number of live pups than the control group (Table 1). Even for the cKO mice displaying successful parturition, the frequency of delivery was decreased (Table 1). In summary, cKO mice exhibited a typical age-dependent decline in reproductive fitness and became sterile at the age of 8 months.
TABLE 1.
Fertility assessment of age-matched control and cKO mice
Control and cKO mice at indicated age were crossed with wild-type C57 males with proven fertility for 2 months, respectively. The number of pups per litter, status of pups (alive or dead), and time from the day of mating to the day of delivery (gestation duration) were assessed and recorded. Values present mean ± S.E. —, not tested.
| 4 month, n = 9 |
6 month, n = 9 |
8 month, n = 3 |
||||
|---|---|---|---|---|---|---|
| Con | cKO | Con | cKO | Con | cKO | |
| Non-pregnant, n | 0 | 1 | 0 | 1 | 0 | 3 |
| Died of dystocia, n | 0 | 1 | 0 | 4 | 0 | 0 |
| Gestation duration, days | 21.2 ± 0.9 | 25.5 ± 2.6 | 20.8 ± 0.5 | 30 ± 5.3 | 22.3 ± 0.6 | — |
| Total no. of live pups | 149 | 78 | 136 | 41 | 37 | 0 |
| Average no. of pups/litter | 8.3 ± 0.4 | 4.3 ± 0.7 | 7.5 ± 0.3 | 2.3 ± 0.7 | 6.2 ± 0.6 | 0 |
Abnormal Hormone Secretion in cKO Mice
As a reproductive organ, the ovary is largely regulated by gonadal hormones (FSH and LH), and in turn, produces other gonadal hormones (E2 and P4). Accordingly, we assessed the hormone parameters in cKO mice. Both FSH and LH levels in cKO mice were higher, although not to a significant extent, when compared with those in control mice (Fig. 5, A and B). Considering the existence of follicular development arrest and deficient ovulation, these changes may represent certain positive feedback responses because FSH is responsible for follicular development and LH is responsible for ovulation. In contrast, both estradiol and progesterone secretion were significantly decreased in cKO mice (Fig. 5C), especially progesterone with a reduction of almost 50% that of the control level (Fig. 5D). As an important secretory source for estradiol and the exclusive source for progesterone (33), less functional corpus luteum in cKO mice consequently produced insufficient hormones, partly leading to subfertility in cKO mice.
FIGURE 5.
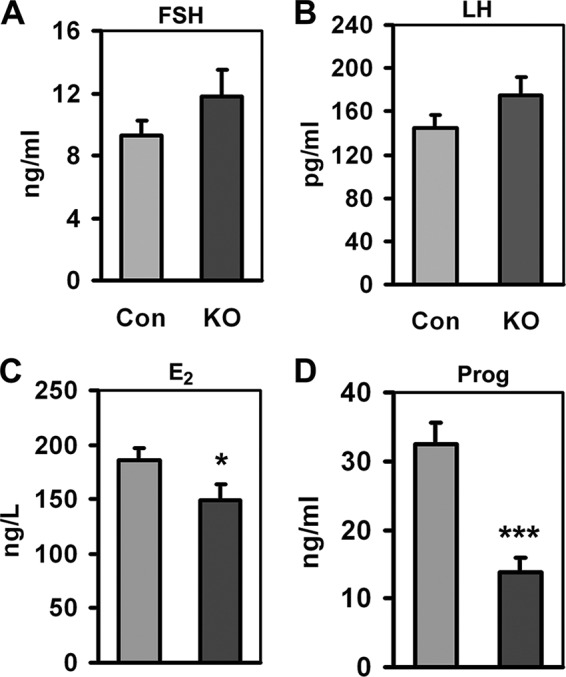
Gonadal hormone parameters of control (Con) and cKO mice. A, FSH. B, LH. C, estrogen. D, progesterone (Prog). Samples were from serum, n = 10–14 for each tested item. Bars indicate mean ± S.E. *, p < 0.05. ***, p < 0.001.
Maternal Rictor Is Dispensable for Embryonic Development
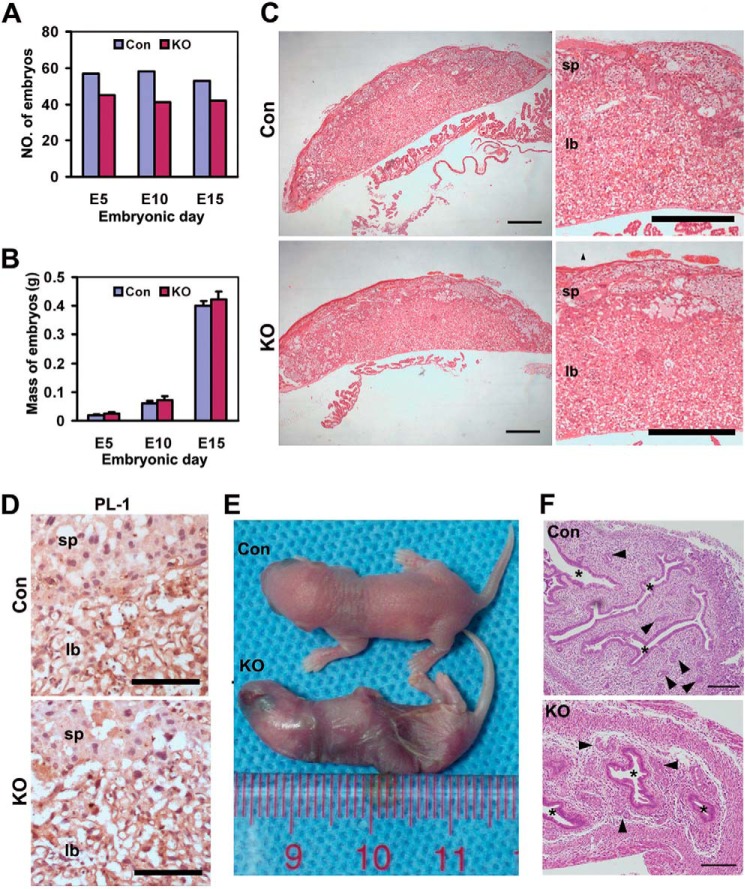
Because recombination mediated by Zp3-Cre specifically occurs in oocytes, it is not only an excellent tool to understand the physiological roles of target genes during folliculogenesis and oogenesis, but also widely used to explore maternal gene effects during embryonic development (34–37). Notably, a recent study on multiallelic disruption of the Rictor gene revealed that mTORC2 is essential for fetal growth and viability (38). To further determine whether maternal Rictor is necessary for embryonic development, sexually mature female cKO (Zp3-Cre+, Rictorloxp/loxp; loss of Rictor in ovulated oocytes) and control (Zp3-Cre−, Rictorloxp/loxp; normal ovulated oocytes) mice were crossed with wild-type C57BL/6J males with proven fertility. In this mating scheme, embryos derived from the oocytes of cKO mice were devoid of maternal Rictor, giving rise to embryos with the genotype −/Rictor. However, we failed to observe visible physical or histological differences between embryos of control and cKO mice (Fig. 5). Although cKO mice had lower numbers of embryos at each scheduled time point (E5, E10, or E15), the total number of embryos within either the control or the cKO group was relatively stable throughout the duration of observation (E5 to E15) (Fig. 6A), indicating that maternal Rictor deficiency is acceptable for normal embryonic development. This assumption was supported by the finding that the average mass of embryos at each time point is comparable between control and cKO mice (Fig. 6B). In addition, histological data revealed no obvious morphological or structural abnormalities in E16 placentas from cKO mice (Fig. 6C), and placental lactogen 1 (PL-1), a marker for trophoblast giant cells, exhibited similar expression and distribution patterns to those of control mice (Fig. 6D). Although cKO mice were susceptible to delivery of dead pups, no physically visible developmental defects were observed in the pups (Fig. 6E). However, specific histological differences were evident between the uteri of control and cKO mice, with smaller cavities and fewer secretory glands in cKO mice (Fig. 6F), which possibly led to abortion or dystocia. These results support the theory that paternal Rictor alone is sufficient for embryonic development.
FIGURE 6.
Maternal Rictor is not required for embryonic development. A, total number of embryos collected at the indicated time points in control (Con) and cKO mice. Six pregnant mice for each group were analyzed. B, comparable placental weights between control and cKO mice. Bars indicate mean ± S.E. C, H&E images revealed no obvious morphological differences between control and cKO placentas. sp and ib, scale bar = 500 μm. D, immunohistochemistry analysis of placental lactogen 1 (PL-1) in control and cKO mice. sp, spongiotrophoblast layer; lb, labyrinth layer. Scale bar = 100 μm. E, overview of the physical appearance of newborn pups from control and cKO groups. F, representative H&E images revealed smaller cavities (*) and fewer glands (arrowhead) in the uteri of cKO mice. Scale bar = 200 μm.
DISCUSSION
In this study, using a POF mouse model induced by the ovotoxicant, 4-VCD, and a mutant mouse model with oocyte-specific deletion of Rictor, we showed that Rictor/mTORC2 functions in oocytes to protect follicles from apoptosis. Upon inhibition of Rictor in oocytes, follicular atresia was accelerated and follicle loss was enhanced. We additionally identified an underlying Rictor/mTORC2/Akt signaling axis controlling follicle survival during folliculogenesis, which inhibits Foxo3a and induces a decrease in the protein levels of pro-apoptotic Bad, Bax, and cleaved PARP. Interestingly, Rictor-cKO mice demonstrated a distinctive ovarian phenotype of POF, featuring a similar decrease in the follicular reservoir, fertility, and serum estrogen level, along with mild elevation of FSH and LH. Our findings have broad physiological and clinical implications and contribute to in-depth understanding of both normal ovarian physiology and development of ovarian diseases.
In humans, POF is an early ovarian dysfunction clinically defined as the cessation of ovarian function with hypoestrogenism and elevated gonadotropin before or at 40 years of age. This condition is characterized by the presence of primary or secondary amenorrhea for at least 4 months, low serum estrogen, and elevated serum gonadotropin concentrations (e.g. FSH>40 IU/liter), and above all, loss of fertility (6, 7). A wide spectrum of pathogenic mechanisms lead to the development of POF, including genetic (e.g. X-chromosome abnormalities), autoimmune, metabolic (galactosaemia), infectious (mumps), and iatrogenic (anticancer treatment) causes (39). However, the majority of cases of POF are idiopathic. Studies on POF have been hindered by inaccessibility of the ovary and occurrence of follicular depletion before the onset of symptoms. Thus, several POF models have been generated with mice via genetic manipulation. For example, deletion of Pten from mouse oocytes causing overactivation of PI3K signaling leads to premature activation of the entire pool of primordial follicles (16). Deletion of Tsc1 (20) or Tsc2 (21) in oocytes induces overactivation of mTORC1 signaling and results in global activation of all primordial follicles around the time of puberty, ending with follicular depletion in early adulthood. In contrast, POF of Rictor-cKO mice was caused by excessive follicular atresia, which occurred at every developmental stage, rather than entire overactivation of the primordial follicle pool in the three mutant mice. Furthermore, the cKO mice exhibited progressive loss of follicles and fertility and became infertile at the age of 8 months. The phenotypic features are similar to the natural and time point pathological changes in humans. Our data suggest that increased follicle apoptosis resulting from genetic variations or mutations also results in POF in mice, advancing our understanding of the pathological processes of POF. Indeed, chemo- or radiotherapy-induced follicular apoptosis contributes to many cases of POF (40).
It is hypothesized that resting primordial follicles are under constant inhibitory local influence to remain dormant (41). PTEN (phosphatase and tensin homolog), TSC1, and TSC2 in oocytes are part of the inhibitory mechanisms that maintain quiescence of primordial follicles. Other similar inhibitory molecules include the cyclin-dependent kinase (Cdk) inhibitor, p27kip1 (p27 or Cdkn1b) (42). In this sense, mTORC1 appears constantly suppressed in oocytes, and selective activation of primordial follicles occurs as a result of selective elevation of mTORC1 activity. In contrast, mTORC2 is believed to be persistently active in oocytes to maintain follicular survival, and apoptosis is enhanced when mTORC2 is inhibited under antagonistic conditions, such as ovotoxicant-induced injury or deficit in survival factors. Taken together, the data indicate that mTORC1 signaling and mTORC2 signaling in oocytes act synergistically to regulate follicular activation, development, and atresia. Further clarification of the mTOR pathway may facilitate the development of improved contraceptives for adjusting follicular activation and survival to an optimal level, thus preserving the follicular reserve pool until fertility is desired.
Rictor/mTORC2 was recently shown to be essential for fetal growth and viability in mice using a multiallelic gene-targeting strategy. Rictor-null mice exhibited placental defects and embryonic lethality (38). However, neither embryonic nor placental defects were observed in embryos from Rictor-cKO mice. This discrepancy led to the assumption that maternal Rictor is not essential for preimplantation embryonic development. To date, it is widely accepted that the first phase of embryonic development is dependent on maternal transcripts and proteins accumulated during oogenesis until broad embryonic genome activation after combination and reprogramming of maternal and paternal genomes (43, 44). The finding that embryos from cKO mice develop normally suggests that maternal Rictor is dispensable for oocyte-to-embryo transition or that transcripts controlled by Rictor/mTORC2 accumulated before the primary follicle are sufficient for preimplantation embryonic development because Zp3-Cre has been shown to express Cre recombinase in oocytes at the primary and/or later follicular stages (34).
In summary, our results indicate that maternal Rictor is not required for preimplantation embryonic development. However, disruption of Rictor in oocytes causes early depletion of functional ovarian follicles, aberrant gonadal hormone secretion, and secondary subfertility in cKO mice, reminiscent of POF phenotypes. Based on the collective findings, we conclude that Rictor/mTORC2 plays a critical role in folliculogenesis, follicle survival, and female fertility and that its inactivation in oocytes causes POF.
Acknowledgment
We thank Professor Mark A. Magnuson (Vanderbilt University) for kindly providing RictorloxP/loxP mice.
This study was supported by The State Key Development Program for Basic Research of China (Grant 2013CB945203), the National Natural Sciences Foundation of China (Grants 81270088, 81301848, U1301222, 31271271, and 31401224), and the Program for Changjiang Scholars and Innovative Research Team in University (Grant IRT1142).
- POF
- premature ovarian failure
- cKO
- conditional knock out
- LH
- luteinizing hormone
- mTOR
- mechanistic target of rapamycin
- mTORC1/2
- mTOR complex 1/2
- PARP
- poly ADP-ribose polymerase
- 4-VCD
- 4-vinylcyclohexene diepoxide
- E
- embryonic day
- p
- phosphorylated.
REFERENCES
- 1. Richards J. S., Pangas S. A. (2010) The ovary: basic biology and clinical implications. J. Clin. Invest. 120, 963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards J. S., Pangas S. A. (2010) New insights into ovarian function. Handb. Exp. Pharmacol. 198, 3–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adhikari D., Liu K. (2009) Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 30, 438–464 [DOI] [PubMed] [Google Scholar]
- 4. Kim J. Y. (2012) Control of ovarian primordial follicle activation. Clin. Exp. Reprod. Med. 39, 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eppig J. J. (2001) Oocyte control of ovarian follicular development and function in mammals. Reproduction 122, 829–838 [DOI] [PubMed] [Google Scholar]
- 6. Shelling A. N. (2010) Premature ovarian failure. Reproduction 140, 633–641 [DOI] [PubMed] [Google Scholar]
- 7. Nelson L. M. (2009) Primary ovarian insufficiency. N. Engl. J. Med. 360, 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laplante M., Sabatini D. M. (2009) mTOR signaling at a glance. J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai X., Jiang Y. (2010) Key factors in mTOR regulation. Cell. Mol. Life Sci. 67, 239–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 12. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 13. Shahbazian D., Roux P. P., Mieulet V., Cohen M. S., Raught B., Taunton J., Hershey J. W., Blenis J., Pende M., Sonenberg N. (2006) The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation andactivity. EMBO J. 25, 2781–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and Raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 15. Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 [DOI] [PubMed] [Google Scholar]
- 16. Reddy P., Liu L., Adhikari D., Jagarlamudi K., Rajareddy S., Shen Y., Du C., Tang W., Hämäläinen T., Peng S. L., Lan Z. J., Cooney A. J., Huhtaniemi I., Liu K. (2008) Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319, 611–613 [DOI] [PubMed] [Google Scholar]
- 17. Zheng W., Nagaraju G., Liu Z., Liu K. (2012) Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell. Endocrinol. 356, 24–30 [DOI] [PubMed] [Google Scholar]
- 18. Jagarlamudi K., Liu L., Adhikari D., Reddy P., Idahl A., Ottander U., Lundin E., Liu K. (2009) Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signalingin oocytes in controlling follicular activation. PLoS One 4, e6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sobinoff A. P., Nixon B., Roman S. D., McLaughlin E. A. (2012) Staying alive: PI3K pathway promotes primordial follicle activation and survival in response to 3MC-induced ovotoxicity. Toxicol. Sci. 128, 258–271 [DOI] [PubMed] [Google Scholar]
- 20. Adhikari D., Zheng W., Shen Y., Gorre N., Hämäläinen T., Cooney A. J., Huhtaniemi I., Lan Z. J., Liu K. (2010) Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 19, 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adhikari D., Flohr G., Gorre N., Shen Y., Yang H., Lundin E., Lan Z., Gambello M. J., Liu K. (2009) Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol. Hum. Reprod. 15, 765–770 [DOI] [PubMed] [Google Scholar]
- 22. Adhikari D., Risal S., Liu K., Shen Y. (2013) Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One 8, e53810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keating A. F., Fernandez S. M., Mark-Kappeler C. J., Sen N., Sipes I. G., Hoyer P. B. (2011) Inhibition of PIK3 signaling pathway members by the ovotoxicant 4-vinylcyclohexene diepoxidein rats. Biol. Reprod. 84, 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoyer P. B., Sipes I. G. (2007) Development of an animal model for ovotoxicity using 4-vinylcyclohexene: a case study. Birth Defects Res. B Dev. Reprod Toxicol. 80, 113–125 [DOI] [PubMed] [Google Scholar]
- 25. Takai Y., Canning J., Perez G. I., Pru J. K., Schlezinger J. J., Sherr D. H., Kolesnick R. N., Yuan J., Flavell R. A., Korsmeyer S. J., Tilly J. L. (2003) Bax, caspase-2, and caspase-3 are required for ovarian follicle loss caused by 4-vinylcyclohexene diepoxide exposure of female mice in vivo. Endocrinology 144, 69–74 [DOI] [PubMed] [Google Scholar]
- 26. Hu X., Christian P. J., Thompson K. E., Sipes I. G., Hoyer P. B. (2001) Apoptosis induced in rats by 4-vinylcyclohexene diepoxide is associated with activation of the caspase cascades. Biol. Reprod. 65, 87–93 [DOI] [PubMed] [Google Scholar]
- 27. Hoyer P. B., Devine P. J., Hu X., Thompson K. E., Sipes I. G. (2001) Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol. Pathol. 29, 91–99 [DOI] [PubMed] [Google Scholar]
- 28. Sun Q. Y., Liu K., Kikuchi K. (2008) Oocyte-specific knockout a novel in vivo approach for studying gene functions during folliculogenesis, oocyte maturation, and embryogenesis. Biol. Reprod. 79, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 29. Chen Z. G., Luo L. L., Xu J. J., Zhuang X. L., Kong X. X., Fu Y. C. (2010) Effect of plant polyphenols on ovarian follicular reserve in aging rats. Biochem. Cell Biol. 88, 737–745 [DOI] [PubMed] [Google Scholar]
- 30. Treins C., Warne P. H., Magnuson M. A., Pende M., Downward J. (2010) Rictor is a novel target of p70 S6 kinase-1. Oncogene 29, 1003–1016 [DOI] [PubMed] [Google Scholar]
- 31. Julien L. A., Carriere A., Moreau J., Roux P. P. (2010) mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 30, 908–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dibble C. C., Asara J. M., Manning B. D. (2009) Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 29, 5657–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stocco C., Telleria C., Gibori G. (2007) The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 28, 117–149 [DOI] [PubMed] [Google Scholar]
- 34. de Vries W. N., Binns L. T., Fancher K. S., Dean J., Moore R., Kemler R., Knowles B. B. (2000) Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis 26, 110–112 [PubMed] [Google Scholar]
- 35. Hirasawa R., Chiba H., Kaneda M., Tajima S., Li E., Jaenisch R., Sasaki H. (2008) Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 22, 1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang F., Kaneda M., O'Carroll D., Hajkova P., Barton S. C., Sun Y. A., Lee C., Tarakhovsky A., Lao K., Surani M. A. (2007) Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 21, 644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Vries W. N., Evsikov A. V., Haac B. E., Fancher K. S., Holbrook A. E., Kemler R., Solter D., Knowles B. B. (2004) Maternal β-catenin and E-cadherin in mouse development. Development 131, 4435–4445 [DOI] [PubMed] [Google Scholar]
- 38. Shiota C., Woo J. T., Lindner J., Shelton K. D., Magnuson M. A. (2006) Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell. 11, 583–589 [DOI] [PubMed] [Google Scholar]
- 39. Goswami D., Conway G. S. (2005) Premature ovarian failure. Hum. Reprod. Update 11, 391–410 [DOI] [PubMed] [Google Scholar]
- 40. Tilly J. L. (2001) Commuting the death sentence: how oocytes strive to survive. Nat. Rev. Mol. Cell. Biol. 2, 838–848 [DOI] [PubMed] [Google Scholar]
- 41. Reddy P., Zheng W., Liu K. (2010) Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol. Metab. 21, 96–103 [DOI] [PubMed] [Google Scholar]
- 42. Rajareddy S., Reddy P., Du C., Liu L., Jagarlamudi K., Tang W., Shen Y., Berthet C., Peng S. L., Kaldis P., Liu K. (2007) p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol. Endocrinol. 21, 2189–2202 [DOI] [PubMed] [Google Scholar]
- 43. Latham K. E., Schultz R. M. (2001) Embryonic genome activation. Front. Biosci. 6, 748–759 [DOI] [PubMed] [Google Scholar]
- 44. Latham K. E. (1999) Mechanisms and control of embryonic genome activation in mammalian embryos. Int. Rev. Cytol. 193, 71–124 [DOI] [PubMed] [Google Scholar]