Background: The Hippo signaling pathway is regulated by mechanical signals from the cytoskeleton.
Results: Perturbation of the spectrin cytoskeleton lead to loss of Hippo signaling phenotypes in Drosophila and mammalian cells.
Conclusion: β-Spectrin regulates Hippo signaling activity in Drosophila and mammalian cells.
Significance: The spectrin cytoskeleton can function as a tension-sensing system and feed into the Hippo pathway.
Keywords: Cell Signaling, Drosophila, Epithelial Cell, Hippo Pathway, Spectrin
Abstract
Emerging evidence suggests functional regulation of the Hippo pathway by the actin cytoskeleton, although the detailed molecular mechanism remains incomplete. In a genetic screen, we identified a requirement for β-Spectrin in the posterior follicle cells for the oocyte repolarization process during Drosophila mid-oogenesis. β-spectrin mutations lead to loss of Hippo signaling activity in the follicle cells. A similar reduction of Hippo signaling activity was observed after β-Spectrin knockdown in mammalian cells. We further demonstrated that β-spectrin mutations disrupt the basal actin network in follicle cells. The abnormal stress fiber-like actin structure on the basal side of follicle cells provides a likely link between the β-spectrin mutations and the loss of the Hippo signaling activity phenotype.
Introduction
The evolutionarily conserved Hippo signaling pathway has been discovered to have an essential function in organ size control (1). It was later found to also play important roles in processes such as differentiation and morphogenesis in various developmental contexts (1–3). The upstream regulatory network of the Hippo pathway is complex in contrast to its well defined core pathway components (2). Importantly, multiple lines of evidence have pointed to the actin cytoskeleton as an essential regulator of Hippo signaling activity (4–10). These observations are consistent with the hypothesis that cells in tissues and organs can sense mechanical signals such as geometry and tension and that these mechanical signals are passed through the actin cytoskeleton to the Hippo pathway for coordination of cell behaviors. However, the mechanistic link between the actin cytoskeleton and the Hippo pathway remains largely unclear.
Here we identify a novel function of β-Spectrin, an actin cross-linking protein (11–13), in the Hippo signaling pathway. Spectrins, originally identified from human erythrocyte ghosts in 1968 (14), are filamentous proteins that are organized into a polygonal meshwork underneath the red blood cell membrane (15, 16). The spectrin-based cytoskeleton confers high deformability and elasticity to the cell membrane for red blood cells to endure the mechanical and osmotic stresses as they circulate through the body. Defects in the spectrin network are associated with many forms of hemolytic anemia (17–20). The spectrin cytoskeletal network exists in most metazoan cell types (21–24), where it has been found to be important for the formation of cell membrane microdomains (25–28), axonal growth (29, 30), and synapse development (31–34). Although spectrins exhibit diverse functions in different tissues and organs, the spectrin network seems to have a general structural and supporting function related to the mechanical properties of cells (24, 35). A recent study has demonstrated that the spectrin cytoskeleton in the Caenorhabditis elegans touch sensory neurons is required for maintaining neuronal prestress status, protecting these neurons from mechanical stress, and enhancing the mechanical sensitivity of these neurons (36).
In this study, we demonstrate that β-spectrin mutations disrupt Hippo signaling activity in the follicular epithelial cells during Drosophila oogenesis. We also show that the requirement of β-Spectrin in the Hippo signaling pathway is conserved in mammalian cells. Although the link between actin stress fibers and YAP/TAZ activity has been established previously in mammalian cell cultures (4–6, 9), here we are able to demonstrate a link between the formation of abnormal stress fibers and loss of Hippo signaling activity in β-spectrin mutant cells in an in vivo developmental context.
EXPERIMENTAL PROCEDURES
Fly Strains
β-spectrin alleles were isolated from a genetic mosaic screen described previously (37). The following fly strains were used: Kinesin-β-gal (38), Staufen-GFP (39), mirror-lacZ (40), kekkon-lacZ (41), expanded-lacZ (42), diap1-lacZ (43), E-cadherin-GFP (44), and Utrophin-GFP (45). Fly lines for mapping, traffic jam-Gal4 (tj-Gal4), nubbin-Gal4 (nub-Gal4), and transgenic UAS-RNAi lines, including EGFP RNAi, α-spectrin RNAi, mer RNAi, and yki RNAi, were obtained from the Bloomington Stock Center. Mosaic clones in the ovarian follicle cells and imaginal disc cells were generated using the FRT/FLP (flippase/flippase recognition target) system.
Mutation Mapping
The FY18 complementation group was mapped through recombination mapping to the region proximal of forked (f) at 15F4-F7. Duplication mapping showed that the FY18 complementation group could be rescued by Dp (1;Y)w73, which further narrowed the mutations to chromosomal region 15F-16F. The mutant alleles were then balanced over an FM7, Kr>GFP chromosome. The genomic DNA from homozygous mutant embryos was sequenced for mutations of candidate genes in the region. The sequence of the starting chromosome y w FRT19A was used as the reference sequence.
Immunofluorescence Staining
Adult females were dissected and ovaries were prepared for immunofluorescence staining according to standard procedures. Third-instar larvae were dissected for imaginal disc staining. The following primary antibodies were used: mouse anti-FasII (catalog no. 1D4, 1:500, DSHB), mouse anti-Cut (catalog no. 2B10, 1:20, DSHB), mouse anti-α-spectrin (catalog no. 3A9, 1:10, DSHB), mouse anti-Dlg (catalog no. 4F3, 1:100, DSHB), mouse anti-Armadillo (catalog no. N2 7A1, 1:100, DSHB), mouse anti-β-gal (catalog no. 40-1a, 1:200, DSHB), mouse anti-Gurken (catalog no. 1D12, 1:10, DSHB), guinea pig anti-Expanded (1:200) (46), guinea pig anti-Merlin (1:200) (47), rabbit anti-atypical PKC (1:1000, Santa Cruz Biotechnology), and rabbit anti-β-spectrin (1:200) (48). Alexa Fluor 647-conjugated secondary antibodies from Invitrogen were used at 1:500. Alexa Fluor 546-phalloidin (1:1000, Invitrogen) and Hoechst (1:10000, Invitrogen) were used to visualize F-actin and DNA, respectively. All images were taken on a Leica TCS SP8 confocal microscope and processed by ImageJ, Adobe Photoshop, and Illustrator.
MCF10A cells were seeded in 6-well plates onto glass coverslips and cultured until the desired density was reached. Cells were then fixed and permeabilized with 4% formaldehyde and 0.1% Triton X-100, respectively. Next, MCF10A cells were blocked using 10% goat serum, followed by incubation with primary and secondary antibodies. The primary antibodies used were mouse YAP antibody (1:100, Santa Cruz Biotechnology) and rabbit polyclonal SPTBN2 antibody (1:100, Proteintech). All images were taken using a Zeiss fluorescence microscope and processed by Adobe software.
Time-lapse Imaging
The egg chambers were dissected and cultured for live imaging following the protocol described before (49). All time-lapse imaging was performed on a Leica TCS SP8 confocal microscope using a ×40 water immersion lens with a 488-nm optically pumped semiconductor lasers. Image z stacks were taken at 1-μm steps from the egg chamber surface to 8 μm below the surface. Images were taken at 30-s intervals from 30 min to 2 h.
S2 Cell Culture, Luciferase Assay, and Quantitative RT-PCR
S2 cells were cultured at 28 °C in Schneider's Drosophila medium (Invitrogen) supplemented with 10% fetal bovine serum and 0.5× penicillin-streptomycin (Invitrogen). S2 cells were seeded in the presence of the indicated dsRNA, followed by transient transfection with the Yki-pMT, Renilla-pMT, and 3xSd2-Luciferase plasmids (gifts from Dr. Georg Halder) by Cellfectin II (Invitrogen). After 24 h, copper sulfate was added to a final concentration of 500 μm, and luciferase activity was measured after 3-day incubation using the Promega Dual-Glo kit. The knockdown efficiency for β-spectrin was assayed by qRT-PCR.2 The primer sequences for the β-spectrin gene were 5′ACTTCGGTGCGTGCATCTCG3′ and 5′TTGATGCGTTGTCGCTCAGC3′.
MCF10A Cell Culture, Stable Cell Line Generation, and Confirmation
MCF10A cells were cultured in DMEM/F12 medium supplemented with horse serum (5%, Sigma), penicillin/streptomycin (1%, Fisher), amphotericin (0.2%, Gemini Bio Products), EGF (20 ng/ml, Novoprotein), hydrocortisone (500 ng/ml, Sigma), insulin (10 μg/ml, Sigma), and cholera toxin (100 ng/ml, Sigma). To generate stable SPTBN2 knockdown cell lines, lentivirus plasmids containing three different SPTBN2-targeting shRNA constructs were purchased from Sigma (catalog nos. TRCN0000117127, TRCN0000117128, and TRCN0000117129), and lentiviruses were packaged in HEK293T cells. Virus-containing medium were collected from HEK293T cells 2 days after transfection and used with 2 μg/ml Polybrene to infect MCF10A cells. 1 μg/ml puromycin was used to select infected MCF10A cells for stable cell lines. To confirm SPTBN2 knockdown efficiency in MCF10A cells, the SPTBN2 mRNA level was tested using qRT-PCR (primer sequences 5′TTTCAACGCCATCGTGCATAA3′ and 5′TGGTCCACATTCACGTCTTCG3′), and SPTBN2 protein was detected by Western blotting using rabbit polyclonal SPTBN2 antibody (1:1000, Proteintech).
RESULTS
β-spectrin Mutations in Posterior Follicle Cells (PFCs) Lead to Oocyte Polarity Defects during Mid-oogenesis
In a Drosophila egg chamber, the oocyte resides in a posterior position, surrounded by somatic epithelial follicle cells (FCs). The oocyte establishes the anterior-posterior axis and the dorsal-ventral axis polarity of the egg and embryo during oogenesis (50). The polarization processes are dependent on the interactions between the oocyte and the FCs. In particular, during mid-oogenesis, the oocyte dorsal-ventral asymmetry is established when an unknown signal from the PFCs triggers the oocyte cytoskeleton to repolarize, and then the oocyte nucleus migrates from the posterior end to the future dorsal-anterior corner of the oocyte (50). Multiple signaling pathway activities in the PFCs, including JAK/STAT (40), EGFR (51, 52), Notch (53, 54), and Hippo (55–57), are required for this dorsal-ventral symmetry-breaking event during mid-oogenesis.
In a genetic screen for mutants affecting oocyte polarity (58), a complementation group of six lethal alleles was isolated as required in the PFCs for oocyte dorsal-ventral asymmetry formation. Through recombination and duplication mapping, we mapped the lethality of these alleles to chromosomal region 15F-16F. Sequencing of these alleles showed that they all contained mutations that lead to premature stop codons in the β-spectrin gene (Fig. 1A). All six alleles exhibited similar phenotypes with comparable severity and penetrance. We therefore used β-spectrinFY18 as a representative allele for the rest of the study. When the PFCs were homozygous mutant for β-spectrin, the oocyte nucleus frequently remained at the posterior end of the oocyte during mid-oogenesis (Fig. 1, B and C, 35.8%, n = 229). To confirm that β-spectrin mutations lead to a general disruption of oocyte polarity instead of specifically affecting the oocyte nucleus migration event, we further examined a panel of oocyte polarity markers. We first examined a microtubule polarity marker, Kinesin-β-gal. Kinesin-β-gal forms a crescent at the posterior end of the oocyte in a wild-type egg chamber after mid-oogenesis (38) (Fig. 1B). Kinesin-β-gal appeared frequently in aggregates or as a diffused cloud in the center of the oocyte in the presence of β-spectrin mutant PFCs (Fig. 1C, 85.4%, n = 64). A second oocyte polarity marker we examined was Staufen. Staufen typically localizes at the posterior end of the oocyte after mid-oogenesis for anchoring the maternal RNAs (59) (Fig. 1D). When the PFCs were mutant for β-spectrin, Staufen also appeared dispersed in the center of the oocyte (Fig. 1E, 91.1%, n = 90). Taken together, these results indicate that β-Spectrin is required in the PFCs for the oocyte polarization process during mid-oogenesis.
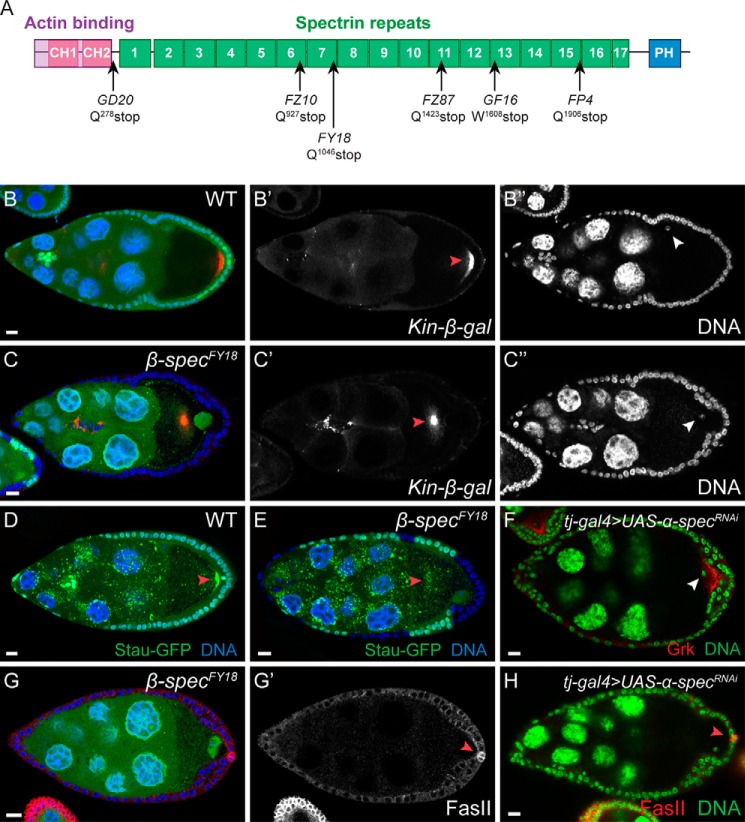
FIGURE 1.
β-spectrin mutations lead to abnormal oocyte polarization. Follicle cell clones are marked by the absence of GFP (green) unless noted otherwise. Scale bars = 10 μm unless noted otherwise. A, schematic of CG5870, the Drosophila β-spectrin protein. All six alleles, including GD20, FZ10, FY18, FZ87, GF16, and FP4, contain premature stop codons at Gln-278, Gln-927, Gln-1046, Gln-1423, Trp-1608, and Gln-1906, respectively. Protein domains are annotated through SMART (75). CH, Calponin homology domain; PH, pleckstrin homology domain. B and C, a wild-type egg chamber (B) and an egg chamber with β-spectrinFY18 mutant PFCs (C) expressing Kinesin-β-gal are stained for β-gal (red) and DNA (blue). In the stage 8 wild-type egg chamber, the oocyte nucleus migrates to the dorsal-anterior corner (B, blue; B”, gray, white arrowhead), and Kinesin-β-gal forms a crescent at the posterior of the oocyte (B, red; B', gray, red arrowhead). In the egg chamber containing β-spectrinFY18 mutant PFCs, the oocyte nucleus stays at the posterior (C, blue; C”, gray, white arrowhead), and Kinesin-β-gal (Kin-β-gal) congregates at the center of the oocyte (C, red; C', gray, red arrowhead). D and E, a wild-type egg chamber (D) and an egg chamber with β-spectrinFY18 mutant PFCs (E) expressing Staufen-GFP (Stau-GFP, green) and stained for DNA (blue). In the wild-type egg chamber, Staufen-GFP is tightly localized at the posterior of the oocyte (red arrowhead). In the egg chamber containing β-spectrinFY18 mutant PFCs, Staufen-GFP appears dispersed in the center of the oocyte (red arrowhead). F, an egg chamber with follicle cells expressing an RNAi construct against α-spectrin stained for Gurken (Grk, red) and DNA (green). Note that the oocyte nucleus (white arrowhead) stays at the posterior end of the oocyte. Grk is mislocalized at the posterior end of the oocyte together with the oocyte nucleus. G, an egg chamber with β-spectrinFY18 mutant PFCs stained for Fas II (G, red; G', gray) and DNA (blue). The posterior polar cells marked by Fas II staining (red arrowhead) remain in direct contact with the oocyte, whereas the oocyte polarity is abnormal, marked by the posterior localization of the oocyte nucleus. H, an egg chamber with follicle cells expressing an RNAi construct against α-spectrin stained for Fas II (red) and DNA (green). Note that the oocyte remains in direct contact with the posterior polar cells marked by Fas II (red arrowhead).
The Drosophila genome contains three spectrin genes, including α-spectrin (60), β-spectrin (61) and β-heavy-spectrin (62). It has been shown previously that α-spectrin mutations in the PFCs lead to disruption of oocyte polarity (63). In the report, the authors attributed the oocyte polarity defects to a loss of direct contact between the polar follicle cells and the oocyte, caused by hyperplasia of the α-spectrin mutant cells. Although in our experiments 55.9% of the β-spectrin mutant PFCs exhibited a hyperplasia phenotype (n = 228), a significant portion of the β-spectrin mutant cells maintained a monolayered or bilayered epithelial structure even though they exhibited an oocyte polarity defect. When we marked the posterior polar cells with Fas II, in 25 of 27 egg chambers where we observed oocyte polarity defects in the presence of β-spectrin mutant PFCs, the posterior polar cells remained in close contact with the oocyte (Fig. 1G). Similarly, when we knocked down α-spectrin in the follicle cells by RNAi, we observed oocyte polarity defects similar to those in the β-spectrin mutants (Fig. 1F). Nevertheless, in these cases, the oocyte was frequently observed as being in direct contact with the posterior polar cells (Fig. 1H). Therefore, we concluded that the oocyte polarity defects in β-spectrin mutants are unlikely to be caused by the separation of the posterior polar cells and the oocyte.
β-spectrin Mutations Lead to Loss of Hippo Signaling Activity in Follicle Cells
The oocyte polarity defects in the presence of β-spectrin mutant PFCs suggested that β-spectrin mutations might affect one (or more) of the four signaling pathways required in the PFCs for the oocyte polarization process, namely the JAK/STAT, EGFR, Notch, and Hippo signaling pathways. We therefore examined well established signaling reporters for each of these pathways.
First, we examined the expression of mirror-lacZ (mirr-lacZ), a JAK/STAT signaling activity reporter normally repressed in PFCs by JAK/STAT signaling activity during mid-oogenesis (40) (Fig. 2A). In the PFCs mutant for β-spectrin, the mirr-lacZ reporter remained repressed (Fig. 2B), indicating that JAK/STAT signaling was not affected in β-spectrin mutant PFCs.
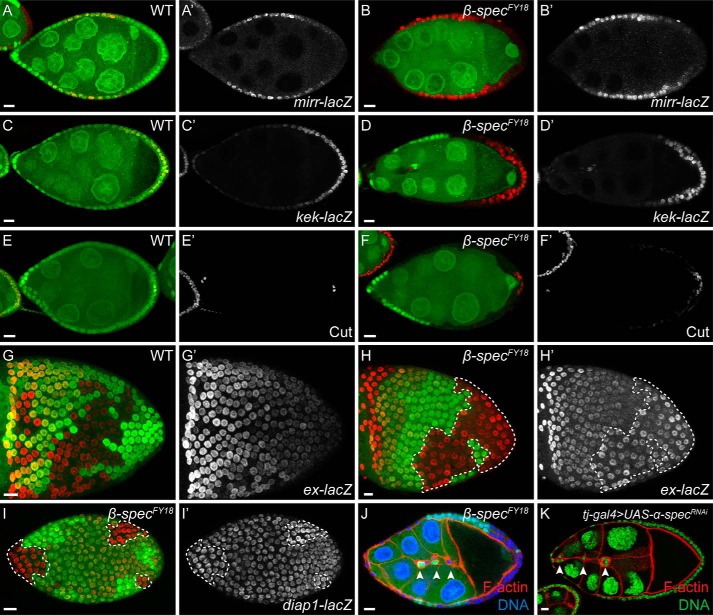
FIGURE 2.
β-spectrin mutations lead to loss of Hippo signaling activity in follicle cells. Follicle cell clones are marked by the absence of GFP (green) unless noted otherwise. Scale bars = 10 μm unless noted otherwise. A and B, the posterior repression of mirror-lacZ, a JAK/STAT signaling reporter, is similar in a wild-type egg chamber (A, red; A', gray) and in an egg chamber containing the β-spectrin mutant PFCs (B, red; B', gray). C and D, the posterior expression pattern of kekkon-lacZ, an EGFR signaling reporter, is similar in a wild-type egg chamber (C, red; C', gray) and in an egg chamber where the PFCs are mutant for β-spectrin (D, red; D', gray). E and F, the expression of Cut, a Notch signaling reporter, is down-regulated in a wild-type stage 7/8 egg chamber (E, red; E', gray). The β-spectrin mutant PFCs retain Cut expression (F, red; F', gray). G and H, the expression level of expanded-lacZ (ex-lacZ), a Hippo signaling reporter, exhibits a gradient pattern from the anterior to the posterior in a wild-type egg chamber (G, red; G', gray). The β-spectrin mutant follicle cells show elevated expression levels of ex-lacZ, regardless of the clone position (H, red; H', gray; clone boundaries are marked by dashed lines). I, the β-spectrin mutant FCs exhibit elevated expression levels of diap1-lacZ, another Hippo signaling reporter (I, red; I', gray; clone boundaries are marked by dashed lines). J, an egg chamber containing β-spectrinFY18 mutant border cells stained with phalloidin (red) and DNA (blue). Border cells fail to migrate as a cluster of cells (white arrowheads). K, an egg chamber in which α-spectrin is depleted in the follicle cells stained with phalloidin (red) and DNA (green). The border cells fail to migrate as a cluster of cells (white arrowheads).
Next, we examined the expression of a kekkon-lacZ (kek-lacZ) reporter, which is normally induced in PFCs in response to EGFR signaling activation during mid-oogenesis (41) (Fig. 2C). The kek-lacZ reporter was activated when the PFCs were mutant for β-spectrin (Fig. 2D), indicating that EGFR signaling was also unaffected by β-spectrin mutations.
Notch signaling activity is required for the follicle cells to undergo a mitotic cycle-to-endocycle switch during mid-oogenesis (53, 54). When Notch signaling activity is compromised in follicle cells, the follicle cells continue to divide and fail to differentiate properly along the oocyte anterior-posterior axis. In the presence of loss of function Notch signaling mutant PFCs, oocyte polarity is also disrupted. We therefore examined the expression of Cut, a Notch signaling activity target. Cut expression is normally repressed by Notch signaling activity after stage 6 of oogenesis (Fig. 2E) (64). The β-spectrin mutant PFCs frequently retained a high level of Cut expression (Fig. 2F, 59.3%, n = 27). It is noteworthy that β-spectrin mutant FCs on the lateral side of the egg chamber did not retain a high Cut expression level (Fig. 2F). In other words, β-spectrin mutations affected Notch signaling specifically in PFCs, unlike the core Notch signaling pathway mutants, which affect all follicle cells regardless of position along the oocyte anterior-posterior axis. It has been shown previously that such a PFC-specific loss of Notch signaling phenotype is typically exhibited in Hippo pathway mutants (55–57). Therefore, we examined the Hippo signaling activity reporters expanded-lacZ (ex-lacZ) (65) and diap1-lacZ (66) in follicle cells. Hippo signaling activity normally represses the expression of expanded-lacZ (Fig. 2G). The β-spectrin mutant clones frequently showed elevated expression levels of ex-lacZ, regardless of the clone position in the egg chamber (Fig. 2H, 67.6%, n = 37). Similarly, the expression level of diap1-lacZ was also up-regulated in β-spectrin mutant cells (Fig. 2I, 69.2%, n = 26). Moreover, Hippo signaling activity has been shown recently to be required for border cell migration in the follicular epithelium (67). When border cells were mutant for β-spectrin (Fig. 2J) or when α-spectrin was knocked down in border cells by RNAi (Fig. 2K), the border cells exhibited migration defects characteristic of those produced by the Hippo pathway mutants. Taken together, our results indicated that β-spectrin mutations lead to oocyte polarity defects through disrupting Hippo signaling activity in follicle cells.
To test whether the function of β-Spectrin in the Hippo signaling pathway is specific to the follicular epithelium in Drosophila, we first examined spectrin function in the imaginal discs from third-instar larvae. We checked the expression of two Hippo signaling reporters, cyclin E-lacZ and diap1-lacZ (66), in the eye and wing imaginal discs. We found that the expression level of these reporters in the β-spectrin mutant cells was comparable with that of wild-type cells (data not shown), indicating that β-spectrin mutations do not significantly affect Hippo signaling activity in the eye and wing imaginal discs. Interestingly, when we knocked down α-spectrin in the wing discs through RNAi, we observed a 1.23-fold increase in adult wing size in comparison with the control (Fig. 3, A–E), indicating an effect on growth control, most likely via an effect on the Hippo signaling pathway. β-Spectrin is specifically localized to the lateral domain of epithelial cells, whereas α-Spectrin is localized both apically and laterally (63, 68). Therefore, depletion of α-Spectrin by RNAi might have a stronger effect on the disruption of the spectrin network and Hippo signaling activity in the imaginal disc cells.
FIGURE 3.
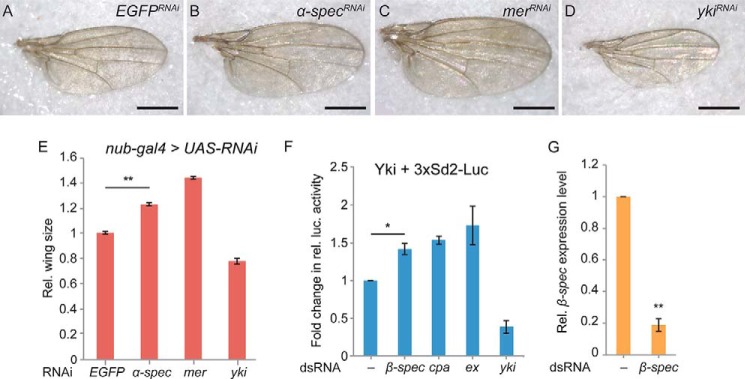
Perturbation of the spectrin network affects adult wing size and Yki activity in S2 cells. A–D, adult wings from flies expressing nub-Gal4 UAS-Dcr2 and UAS-EGFP-RNAi (A), UAS-α-spectrin-RNAi (B), UAS-mer-RNAi (C), and UAS-yki-RNAi (D). Scale bar = 500 μm. E, quantification of the relative (Rel.) wing size for each genotype (n ≥ 8/genotype; **, p < 0.0001; Student's t test). F, a luciferase (luc.) reporter assay to measure Yorkie activity in S2 cells. Drosophila S2 cells incubated with the indicated dsRNA were transiently transfected with Yki-pMT, Ren-pMT, and 3xSd2-Luc plasmids. Luciferase readings are normalized to the Renilla luciferase control. Data represent the average of four independent experiments (n = 4; *, p < 0.01; Student's t test). G, qRT-PCR analysis of the relative β-spectrin transcript levels in the control and β-spectrin RNAi cells normalized by rp49 mRNA level (n = 3; **, p < 0.0001; Student's t test). Error bars indicate mean ± S.E.
In Drosophila S2 cells where Yorkie (Yki) was expressed under the control of a methallothionein promoter, the luciferase activity of a reporter, 3XSd2-Luc, was used successfully to monitor Yki activity (8). When we treated these cells with dsRNA against β-spectrin, we observed a 1.4-fold increase in relative luciferase activity compared with that of the DMSO-treated control group. The increase was comparable with that of the positive control expanded (ex) and capping protein α (cpa) RNAi (Fig. 3, F and G). These results indicated that the requirement of β-Spectrin for Hippo signaling activity is not limited to the follicle cells in Drosophila.
β-Spectrin Is Required for Hippo Signaling Activity in Mammalian Cells
Two recent affinity purification and MS-based proteomic studies have included spectrins in the Hippo pathway protein-protein interaction network (69, 70). Specifically, in cultured human cells, an interaction between the bait protein Salvador homolog 1 (SAV1) and SPTAN1, a human α-Spectrin isoform, has been reported (69). Therefore, we wanted to test whether β-Spectrin has a conserved role in regulating the Hippo pathway in mammals.
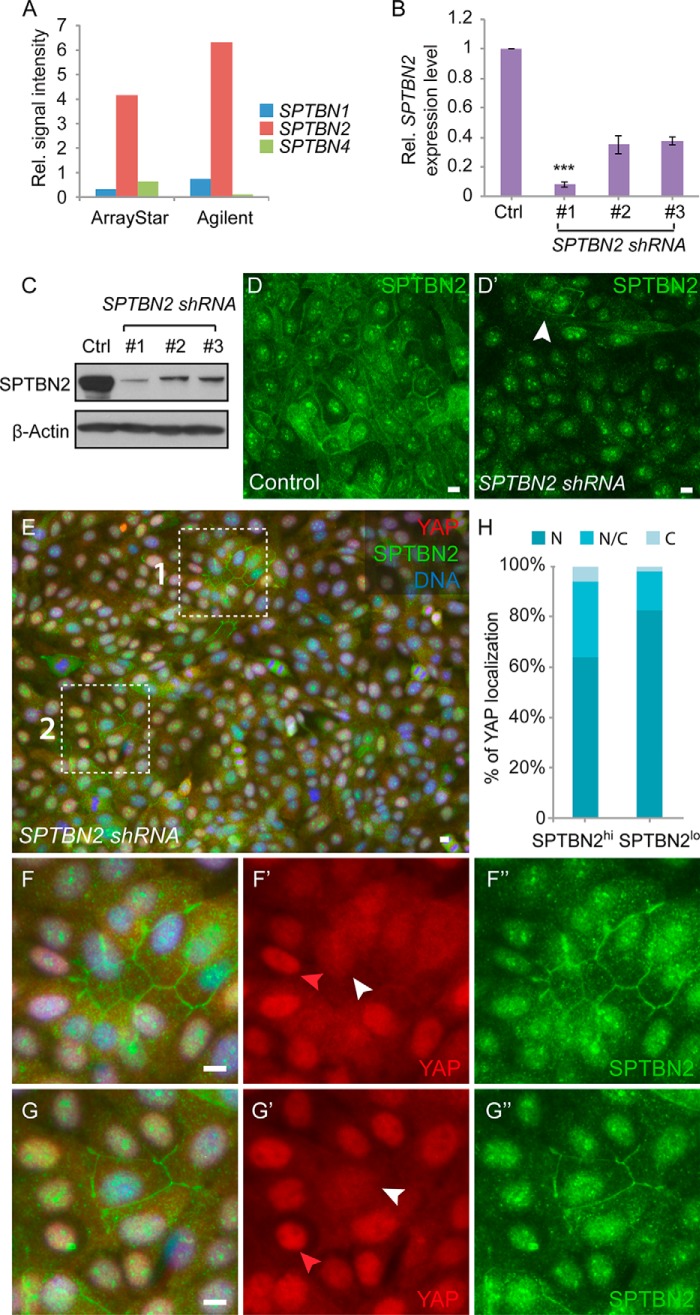
We tested the effect of β-spectrin knockdown on Hippo signaling activity in human mammary epithelial MCF10A cells. When MCF10A cells are grown at a low cell density, the YAP/TAZ proteins (the mammalian homologs of Yorkie) localize in the cell nucleus and promote proliferation. When MCF10A cell culture reaches a high cell density, the YAP/TAZ proteins translocate to the cytoplasm, and cell proliferation is inhibited (9). In human cells, five genes, SPTB, SPTBN1, SPTBN2, SPTBN4, and SPTBN5, encode β-spectrins. On the basis of previous studies (71), we focused on SPTBN1, SPTBN2, and SPTBN4, which are most likely to be functional in MCF10A epithelial cells because SPTB and SPTBN5 are known to be more restrictively expressed in neuronal cells and other specific cell types (71). Microarray gene expression profiling of MCF10A cells further demonstrated that SPTBN2 is most highly expressed among the three β-spectrin genes (Fig. 4A). Therefore, we chose to knock down SPTBN2 to investigate the potential role of β-spectrin in regulating Hippo signaling activity. MCF10A cells were transduced with lentiviruses containing a scrambled control shRNA construct or three different SPTBN2-targeting shRNA constructs, and the knockdown efficiency was confirmed by qRT-PCR (Fig. 4B) and Western blotting (Fig. 4C). In control cells, SPTBN2 is mostly localized on the cytoplasmic membrane, although the antibody against SPTBN2 also shows nonspecific nucleus staining (Fig. 4D). The membrane localization of SPTBN2 was lost in most cells from the SPTBN2 knockdown population (denoted as SPTBN2lo cells) (Fig. 4D'). However, the knockdown was less efficient in a few cells so that they maintained SPTBN2 membrane staining (denoted as SPTBN2hi cells) (Fig. 4D', arrowhead). To normalize the effect of local cell density on YAP localization as mentioned previously, we randomly picked clusters of SPTBN2-expressing SPTBN2hi cells in the knockdown population and compared them with their neighboring SPTBN2lo cells. As shown in Fig. 4, E–H, compared with SPTBN2lo cells, SPTBN2hi cells showed reduced nuclear localization of YAP, indicating that SPTBN2 regulates YAP localization and activity in MCF10A cells.
FIGURE 4.
SPTBN2 is required for Hippo signaling activity in MCF10A cells. Scale bars = 10 μm unless noted otherwise. A, microarray analyses of SPTBN1, SPTBN2, and SPTBN4 mRNA levels in MCF10A cells using microarray chips from ArrayStar and Agilent. Rel., relative. B, qRT-PCR analysis of SPTBN2 mRNA in SPTBN2 knockdown MCF10A cells. Stable MCF10A cell lines with SPTBN2 knockdown were generated using a lentivirus containing three different shRNA constructs. Results were normalized to the GAPDH mRNA level. Data represent the average of three independent experiments (n = 3; ***, p < 0.00001; Student's t test). Error bars represent mean ± S.E. Ctrl, control. C, Western blot analysis of MCF10A SPTBN2 knockdown cells. D, MCF10A control (D) and SPTBN2 knockdown cells (D') stained for SPTBN2 (green). The SPTBN2 membrane staining is largely absent in the SPTBN2 knockdown cells (D'), except in a few cells (white arrowhead, D') where the knockdown efficiency is low. Note that the nucleus signal is nonspecific staining of the primary antibody. E–G, MCF10A cells with SPTBN2 knockdown were stained for YAP (red), SPTBN2 (green), and DNA (blue) (E). Regions 1 and 2 (dashed boxes) are shown in higher magnification in F and G, respectively. Red arrowheads indicate the nuclear localization of YAP in SPTBN2low cells. White arrowheads indicate the nuclear/cytoplasmic localization of YAP in SPTBN2hi cells. H, quantification of cytoplasmic (C), nuclear and cytoplasmic (N/C), or nuclear (N) YAP localization in SPTBN2hi cells (n = 278) and SPTBN2low cells (n = 562).
β-spectrin Mutations Lead to Assembly of Abnormal Actin Stress Fibers in the Follicle Cells
Hippo signaling activity is regulated by a plethora of upstream components, including cell polarity components, cell-cell adhesion molecules, G protein-coupled receptor signaling, and the actin cytoskeleton (2). To understand how β-Spectrin affects Hippo signaling activity, we examined major upstream regulators in β-spectrin mutant cells.
β-Spectrin is specifically localized to the lateral domain of the follicle cells (63) (Fig. 5, A and B). It is possible that β-Spectrin might affect the Hippo signaling pathway through disruption of the cell polarity system. We first examined localization of the cell apical components such as α-Spectrin and aPKC. Both α-Spectrin and aPKC remained apically localized in β-spectrin mutant follicle cells (Fig. 5, C and D). We also examined the apically localized protein Merlin and Expanded, two known upstream regulators of the Hippo signaling pathway. Similarly, the apical localization of Merlin and Expanded remained unchanged in the β-spectrin mutant cells (Fig. 5, E–G). Next, we examined the adhesion junctional components Ecadherin (Ecad) and Armadillo (Arm, the Drosophila β-Catenin homolog). Similarly, the junctional localization of Ecad and Arm was largely unaffected in β-spectrin mutant cells (Fig. 5, H and I). Third, we examined basolateral proteins such as Discs large (Dlg), Na+, and K+-ATPase, and their localization was also unaffected in the β-spectrin mutant cells (Fig. 5, J and K). These results suggest that, although β-Spectrin is part of the cell polarity system, mutations in β-spectrin do not disrupt general apical-basal polarity in follicle cells.
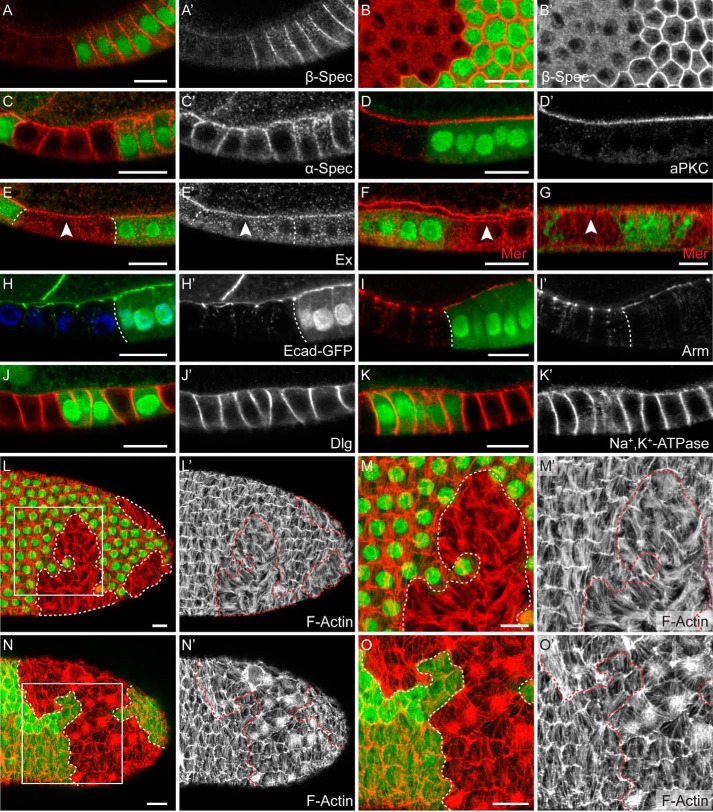
FIGURE 5.
Mutations in β-spectrin lead to defects in the actin cytoskeleton network. Follicle cell and imaginal disc cell clones are marked by the absence of GFP (green) unless noted otherwise. Scale bars = 10 μm unless noted otherwise. A and B, FCs stained for β-Spectrin (β-Spec; A and B, red; A' and B', gray). A, lateral view. B, surface view. β-Spectrin is localized on the lateral side of wild-type cells, and the staining is absent in the β-spectrin mutant clones. C and D, follicle cells stained for α-Spectrin (C, red; C', gray) and aPKC (D, red; D', gray). The apical localization pattern of α-Spectrin and aPKC is unaffected in β-spectrin mutant cells. E–G, follicle cells (E and F) and eye imaginal disc cells (G) stained for Expanded (E, red; E', gray) and Merlin (F and G, red). Merlin and Expanded remain apically localized in β-spectrin mutant cells. H and I, follicle cells expressing E-cadherin-GFP (H, green; H', gray) and stained for DNA (H, blue) and follicle cells stained for Armadillo (Arm; I, red; I', gray). The junctional localization of E-cadherin and Arm is largely unaffected by β-spectrin mutations. J and K, follicle cells stained for Discs Large (Dlg; J, red; J', gray) and Na+ and K+-ATPase (K, red; K', gray). The basolateral localization of Dlg and Na+ and K+-ATPase remains unchanged in β-spectrin mutant cells. L–O, follicle cells stained with phalloidin to view the basal F-actin network (L–O, red; L'–O', gray). Clone boundaries are marked by dashed white lines in L–O and dashed red lines in L'–O'. M and O, enlarged images from the boxed regions in L and N, respectively. Note that, in the β-spectrin mutant cells, the basal F-actin filaments lose the planar polarized parallel bundle structure (L and M) and often form patches with intensified phalloidin staining (N and O).
Spectrins are well known actin cross-linking proteins (11–13). It is possible that β-spectrin mutations might affect the actin cytoskeletal structure in follicle cells. Interestingly, phalloidin staining revealed an abnormal actin cytoskeletal structure on the basal side of β-spectrin mutant follicle cells (Fig. 5, L–O). The actin filaments on the basal side of wild-type follicle cells are oriented perpendicularly to the anterior-posterior axis of the egg chamber after mid-oogenesis (72). These bundled actin filaments, which are part of the planar cell polarity system in the follicular epithelium, resemble stress fibers in cultured cells. In the β-spectrin mutant cells, the actin filaments lose the planar polarized orientation (Fig. 5, L and M) and frequently form actin patches with intensified phalloidin staining on the basal side of the epithelium (Fig. 5, N and O). We further analyzed the actin dynamics in the β-spectrin mutant cells through time-lapse imaging of cultured live stage 8–9 egg chambers. The F-actin filaments were visualized using an Utrophin-GFP marker (45). Although the basal F-actin filaments appear relatively stable in wild-type follicle cells, as reported previously (supplemental Movie 1) (73), in β-spectrin mutant cells, the F-actin filaments failed to assemble into parallel bundles and, instead, initiated radial contractions over time (supplemental Movie 2).
DISCUSSION
The Link between the Basal F-actin Phenotype and the Hippo Signaling Defects in β-spectrin Mutant Cells
Multiple lines of evidence in cultured cells have linked actin stress fibers and YAP/TAZ activity. For example, YAP/TAZ nuclear localization coincided with the presence of abundant stress fibers in cultured cells (5). YAP/TAZ activity decreased in cells treated with the actin depolymerization drugs cytochalasin D and latrunculin A/B, which also reduced stress fiber formation (5, 6). YAP/TAZ activity increased in cultured cells overexpressing Diaphanous, which induced stress fiber formation (4, 8). Several actin binding proteins, including Cofilin 1/2, Capzb, and Gelsolin, were identified as inhibitors of YAP/TAZ activity. Depletion of these proteins caused an increase in both stress fiber formation and YAP/TAZ activity (9).
Follicle cells maintain basal F-actin filaments structurally similar to stress fibers in cultured cells (72). In live β-spectrin mutant cells, the F-actin filaments fail to assemble into a planar polarized network and seem to undergo radial contractions, possibly as a result of repeated actin assembly-disassembly cycles. In the fixed β-spectrin mutant cells, we observed basal actin patches characterized by intense phalloidin staining and lack of planar polarized organization. In light of previous observations in cell cultures linking the formation of actin stress fibers and YAP/TAZ activity, it seems likely that the basal actin phenotype and the Hippo signaling phenotype in the β-spectrin mutant cells are tightly linked.
Spectrins as General Tension-sensing Molecules underneath the Cell Membrane
The Hippo signaling pathway is known to link with the cytoskeletal tension in cultured cells (2). Importantly, it has been shown recently that actomyosin-based cellular tension is critical for Hippo signaling activity in vivo (10).
Spectrins are long and flexible molecules that form a network underneath the cell membrane. The spectrin function is well characterized in red blood cells as providing cells with mechanical properties, including elasticity and deformability, that allow these cells to survive circulation (74). In other cell types, the spectrin function is much less clear. It has been shown that cytoskeletal tension regulates Hippo activity via promoting Ajuba and Wts complex formation at the adherent junctions in a tension-dependent manner (10). We examined Ajuba and Wts complex localization in follicle cells, and they are both junctionally localized in the β-spectrin mutant cells. However, using confocal microscopy, we did not reliably detect a difference of Wts/Ajuba junctional fluorescence intensity between β-spectrin mutant cells and wild-type follicle cells (data not shown). It is possible that the spectrin cytoskeleton is part of a cellular tension-sensing system that feeds into the Hippo regulation network. Although we demonstrated a link between spectrin function and actin network organization, a possible link between spectrin function and myosin-based cellular tension still needs further investigation.
Acknowledgments
We thank Dr. Trudi Schupbach and Dr. Natalie Denef for designing and conducting the original mutant screen and for sharing the β-spectrin mutants. We also thank Dr. Georg Halder, Dr. Richard Fehon, Dr. Christian Klambt, Dr. Lei Zhang, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for reagents; Dr. Mingjie Zhang, Dr. Zilong Wen, and Dr. Karl Herrup for sharing confocal microscopes; and Dr. Trudi Schupbach, Dr. Adam Martin, and Dr. Toyotaka Ishibashi for helpful comments on the manuscript.
This work was supported by Research Grants Council of the Hong Kong Special Administrative Region Grants 16103314, AoE/M-09/12, and IGN12SC01.

This article contains supplemental Movies 1 and 2.
- qRT-PCR
- quantitative RT-PCR
- PFC
- posterior follicle cell
- EGFR
- EGF receptor.
REFERENCES
- 1. Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu F. X., Guan K. L. (2013) The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varelas X. (2014) The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 [DOI] [PubMed] [Google Scholar]
- 4. Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 [DOI] [PubMed] [Google Scholar]
- 5. Wada K., Itoga K., Okano T., Yonemura S., Sasaki H. (2011) Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 [DOI] [PubMed] [Google Scholar]
- 6. Zhao B., Li L., Wang L., Wang C. Y., Yu J., Guan K. L. (2012) Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernández B. G., Gaspar P., Brás-Pereira C., Jezowska B., Rebelo S. R., Janody F. (2011) Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138, 2337–2346 [DOI] [PubMed] [Google Scholar]
- 8. Sansores-Garcia L., Bossuyt W., Wada K., Yonemura S., Tao C., Sasaki H., Halder G. (2011) Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. (2013) A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 [DOI] [PubMed] [Google Scholar]
- 10. Rauskolb C., Sun S., Sun G., Pan Y., Irvine K. D. (2014) Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 158, 143–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pinder J. C., Bray D., Gratzer W. B. (1975) Actin polymerisation induced by spectrin. Nature 258, 765–766 [DOI] [PubMed] [Google Scholar]
- 12. Tilney L. G., Detmers P. (1975) Actin in erythrocyte ghosts and its association with spectrin: evidence for a nonfilamentous form of these two molecules in situ. J. Cell Biol. 66, 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen C. M., Tyler J. M., Branton D. (1980) Spectrin-actin associations studied by electron microscopy of shadowed preparations. Cell 21, 875–883 [DOI] [PubMed] [Google Scholar]
- 14. Marchesi V. T., Steers E., Jr. (1968) Selective solubilization of a protein component of the red cell membrane. Science 159, 203–204 [DOI] [PubMed] [Google Scholar]
- 15. Byers T. J., Branton D. (1985) Visualization of the protein associations in the erythrocyte membrane skeleton. Proc. Natl. Acad. Sci. U.S.A. 82, 6153–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsukita S., Tsukita S., Ishikawa H. (1980) Cytoskeletal network underlying the human erythrocyte membrane: thin-section electron microscopy. J. Cell Biol. 85, 567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palek J., Lux S. E. (1983) Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin. Hematol. 20, 189–224 [PubMed] [Google Scholar]
- 18. Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. (1985) Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. Nature 314, 380–383 [DOI] [PubMed] [Google Scholar]
- 19. Agre P., Orringer E. P., Bennett V. (1982) Deficient red-cell spectrin in severe, recessively inherited spherocytosis. New Engl. J. Med. 306, 1155–1161 [DOI] [PubMed] [Google Scholar]
- 20. Mohandas N., Gallagher P. G. (2008) Red cell membrane: past, present, and future. Blood 112, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett V., Davis J., Fowler W. E. (1982) Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature 299, 126–131 [DOI] [PubMed] [Google Scholar]
- 22. Burridge K., Kelly T., Mangeat P. (1982) Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J. Cell Biol. 95, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glenney J. R., Jr., Glenney P., Osborn M., Weber K. (1982) An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell 28, 843–854 [DOI] [PubMed] [Google Scholar]
- 24. Bennett V., Baines A. J. (2001) Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81, 1353–1392 [DOI] [PubMed] [Google Scholar]
- 25. Nelson W. J., Shore E. M., Wang A. Z., Hammerton R. W. (1990) Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 110, 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee J. K., Coyne R. S., Dubreuil R. R., Goldstein L. S., Branton D. (1993) Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J. Cell Biol. 123, 1797–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKeown C., Praitis V., Austin J. (1998) sma-1 encodes a βH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 125, 2087–2098 [DOI] [PubMed] [Google Scholar]
- 28. Mangeat P. H., Burridge K. (1984) Immunoprecipitation of nonerythrocyte spectrin within live cells following microinjection of specific antibodies: relation to cytoskeletal structures. J. Cell Biol. 98, 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammarlund M., Davis W. S., Jorgensen E. M. (2000) Mutations in β-spectrin disrupt axon outgrowth and sarcomere structure. J. Cell Biol. 149, 931–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moorthy S., Chen L., Bennett V. (2000) Caenorhabditis elegans β-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J. Cell Biol. 149, 915–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao Y., Perkins E. M., Clarkson Y. L., Tobia S., Lyndon A. R., Jackson M., Rothstein J. D. (2011) β-III spectrin is critical for development of Purkinje cell dendritic tree and spine morphogenesis. J. Neurosci. 31, 16581–16590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Featherstone D. E., Davis W. S., Dubreuil R. R., Broadie K. (2001) Drosophila α- and β-spectrin mutations disrupt presynaptic neurotransmitter release. J. Neurosci. 21, 4215–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pielage J., Fetter R. D., Davis G. W. (2005) Presynaptic spectrin is essential for synapse stabilization. Curr. Biol. 15, 918–928 [DOI] [PubMed] [Google Scholar]
- 34. Pielage J., Fetter R. D., Davis G. W. (2006) A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J. Cell Biol. 175, 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu K., Zhong G., Zhuang X. (2013) Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krieg M., Dunn A. R., Goodman M. B. (2014) Mechanical control of the sense of touch by β-spectrin. Nat. Cell Biol. 16, 224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Denef N., Chen Y., Weeks S. D., Barcelo G., Schüpbach T. (2008) Crag regulates epithelial architecture and polarized deposition of basement membrane proteins in Drosophila. Dev. Cell 14, 354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clark I., Giniger E., Ruohola-Baker H., Jan L. Y., Jan Y. N. (1994) Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr. Biol. 4, 289–300 [DOI] [PubMed] [Google Scholar]
- 39. Schuldt A. J., Adams J. H., Davidson C. M., Micklem D. R., Haseloff J., St Johnston D., Brand A. H. (1998) Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev. 12, 1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xi R., McGregor J. R., Harrison D. A. (2003) A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev. Cell 4, 167–177 [DOI] [PubMed] [Google Scholar]
- 41. Pai L. M., Barcelo G., Schüpbach T. (2000) D-cbl, a negative regulator of the Egfr pathway, is required for dorsoventral patterning in Drosophila oogenesis. Cell 103, 51–61 [DOI] [PubMed] [Google Scholar]
- 42. Boedigheimer M., Laughon A. (1993) Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development 118, 1291–1301 [DOI] [PubMed] [Google Scholar]
- 43. Hay B. A., Wassarman D. A., Rubin G. M. (1995) Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83, 1253–1262 [DOI] [PubMed] [Google Scholar]
- 44. Oda H., Tsukita S. (2001) Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J. Cell Sci. 114, 493–501 [DOI] [PubMed] [Google Scholar]
- 45. Rauzi M., Lenne P. F., Lecuit T. (2010) Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 [DOI] [PubMed] [Google Scholar]
- 46. Maitra S., Kulikauskas R. M., Gavilan H., Fehon R. G. (2006) The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 16, 702–709 [DOI] [PubMed] [Google Scholar]
- 47. McCartney B. M., Fehon R. G. (1996) Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J. Cell Biol. 133, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hülsmeier J., Pielage J., Rickert C., Technau G. M., Klämbt C., Stork T. (2007) Distinct functions of α-Spectrin and β-Spectrin during axonal pathfinding. Development 134, 713–722 [DOI] [PubMed] [Google Scholar]
- 49. Prasad M., Jang A. C., Starz-Gaiano M., Melani M., Montell D. J. (2007) A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat. Protoc. 2, 2467–2473 [DOI] [PubMed] [Google Scholar]
- 50. Roth S., Lynch J. A. (2009) Symmetry breaking during Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 1, a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. González-Reyes A., Elliott H., St Johnston D. (1995) Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 375, 654–658 [DOI] [PubMed] [Google Scholar]
- 52. Roth S., Neuman-Silberberg F. S., Barcelo G., Schüpbach T. (1995) cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 81, 967–978 [DOI] [PubMed] [Google Scholar]
- 53. Deng W. M., Althauser C., Ruohola-Baker H. (2001) Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128, 4737–4746 [DOI] [PubMed] [Google Scholar]
- 54. López-Schier H., St Johnston D. (2001) Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 15, 1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meignin C., Alvarez-Garcia I., Davis I., Palacios I. M. (2007) The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17, 1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Polesello C., Tapon N. (2007) Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864–1870 [DOI] [PubMed] [Google Scholar]
- 57. Yu J., Poulton J., Huang Y. C., Deng W. M. (2008) The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE 3, e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yan Y., Denef N., Tang C., Schüpbach T. (2011) Drosophila PI4KIIIα is required in follicle cells for oocyte polarization and Hippo signaling. Development 138, 1697–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. St Johnston D., Beuchle D., Nüsslein-Volhard C. (1991) Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell 66, 51–63 [DOI] [PubMed] [Google Scholar]
- 60. Byers T. J., Dubreuil R., Branton D., Kiehart D. P., Goldstein L. S. (1987) Drosophila spectrin: II: conserved features of the α-subunit are revealed by analysis of cDNA clones and fusion proteins. J. Cell Biol. 105, 2103–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. (1989) Sequence similarity of the amino-terminal domain of Drosophila β spectrin to α actinin and dystrophin. J. Cell Biol. 109, 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dubreuil R. R., Byers T. J., Stewart C. T., Kiehart D. P. (1990) A β-spectrin isoform from Drosophila (β H) is similar in size to vertebrate dystrophin. J. Cell Biol. 111, 1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee J. K., Brandin E., Branton D., Goldstein L. S. (1997) α-Spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development 124, 353–362 [DOI] [PubMed] [Google Scholar]
- 64. Sun J., Deng W. M. (2005) Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development 132, 4299–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hamaratoglu F., Willecke M., Kango-Singh M., Nolo R., Hyun E., Tao C., Jafar-Nejad H., Halder G. (2006) The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8, 27–36 [DOI] [PubMed] [Google Scholar]
- 66. Harvey K. F., Pfleger C. M., Hariharan I. K. (2003) The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457–467 [DOI] [PubMed] [Google Scholar]
- 67. Lucas E. P., Khanal I., Gaspar P., Fletcher G. C., Polesello C., Tapon N., Thompson B. J. (2013) The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 201, 875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pesacreta T. C., Byers T. J., Dubreuil R., Kiehart D. P., Branton D. (1989) Drosophila spectrin: the membrane skeleton during embryogenesis. J. Cell Biol. 108, 1697–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Couzens A. L., Knight J. D., Kean M. J., Teo G., Weiss A., Dunham W. H., Lin Z. Y., Bagshaw R. D., Sicheri F., Pawson T., Wrana J. L., Choi H., Gingras A. C. (2013) Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 6, rs15. [DOI] [PubMed] [Google Scholar]
- 70. Kwon Y., Vinayagam A., Sun X., Dephoure N., Gygi S. P., Hong P., Perrimon N. (2013) The Hippo signaling pathway interactome. Science 342, 737–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Machnicka B., Grochowalska R., Bogusławska D. M., Sikorski A. F., Lecomte M. C. (2012) Spectrin-based skeleton as an actor in cell signaling. Cell. Mol. Life Sci. 69, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gutzeit H. O. (1990) The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur. J. Cell Biol. 53, 349–356 [PubMed] [Google Scholar]
- 73. He L., Wang X., Tang H. L., Montell D. J. (2010) Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 12, 1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Delaunay J. (2007) The molecular basis of hereditary red cell membrane disorders. Blood Rev. 21, 1–20 [DOI] [PubMed] [Google Scholar]
- 75. Schultz J., Milpetz F., Bork P., Ponting C. P. (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U.S.A. 95, 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]