Background: The membrane proteases are enzymatic proteins that have transmembrane domains reminiscent of ion channels.
Results: Two proteases with known structure have ion channel activity, and the activity is altered in catalytic dead mutants.
Conclusion: Proteases can have dual functionality as enzymes and ion channels.
Significance: Other higher-order proteases, including presenilin may have dual functionality as both ion channels and proteases.
Keywords: Alzheimer disease, calcium channel, γ-secretase, ion channel, secretase, chanzymes, proteases
Abstract
The GxGD proteases function to cleave protein substrates within the membrane. As these proteases contain multiple transmembrane domains typical of ion channels, we examined if GxGD proteases also function as ion channels. We tested the putative dual function by examining two archeobacterial GxGD proteases (PSH and FlaK), with known three-dimensional structures. Both are in the same GxGD family as presenilin, a protein mutated in Alzheimer Disease. Here, we demonstrate that PSH and FlaK form cation channels in lipid bilayers. A mutation that affected the enzymatic activity of FlaK rendered the channel catalytically inactive and altered the ion selectivity, indicating that the ion channel and the catalytic activities are linked. We report that the GxGD proteases, PSH and FlaK, are true “chanzymes” with interdependent ion channel and protease activity conferred by a single structural domain embedded in the membrane, supporting the proposal that higher-order proteases, including presenilin, have channel function.
Introduction
The conserved GxGD membrane protease family has received considerable interest, as mutations in the mammalian GxGD membrane protease, presenilin, are the leading cause of early onset familial Alzheimer disease (FAD).4 This family of proteases cuts transmembrane protein substrates using a pair of aspartate residues (1, 2), where one aspartate residue constitutes the defining catalytic motif, GxGD. Due to the association of presenilin with FAD, efforts have been made to determine the structure of this protein. However, the full structure of mammalian presenilin or the γ-secretase complex, within which presenilin contributes the catalytic residues (3–5), has proved elusive. Although a cryo-EM structure for the human γ-secretase complex was recently reported, the resolution was not sufficient to probe the exact transmembrane arrangement of presenilin (6). Recently, two crystal structures in the GxGD protease family, both from archeobacterial sources, have been reported. The first, FlaK, is a preflagellin peptidase with its structure solved at 3.6 Å resolution (7). Unlike presenilin which has 9 transmembrane domains, FlaK has 6. The second crystal structure is a presenilin homolog (PSH) from M. marisnigri, whose function in the archeobacteria is currently unknown (8, 9). Like presenilin, it has 9 transmembrane domains, and has 19% identity and over 50% similarity to presenilin-1 (PS1) (9). The structural fold of the catalytic regions of FlaK and PSH is consistent with the predicted structure of presenilin (7, 9).
Interestingly, it has been suggested that presenilin can act as a Ca2+ ion channel, and the dysregulation of Ca2+ activity contributes to the progression of FAD (10), although the mechanism is controversial (11–18). Traditionally proteins are thought to have one function. As it is not generally thought that proteases can have dual functions as ion channels, we investigated if ion channel activity is present in the GxGD proteases, namely PSH and FlaK, i.e.: if they are chanzymes.
We took advantage of the published crystal structures of both of these proteins, and used them as surrogates to investigate if proteases, and by extension, presenilin can also be ion channels. Here, we demonstrate that both PSH and FlaK form cation channels in lipid bilayers. We use a series of mutations in both PSH and FlaK to interrogate the channel activity, and to determine the relationship to the protein secretase function. We also test the ability of FlaK to modify calcium signaling in mammalian cells. A mutation that affected the enzymatic activity of FlaK, rendered the channel catalytically dead and altered the ion selectivity, indicating that the ion channel and the catalytic activities are linked. We report that the GxGD proteases, PSH and FlaK, are the first true “chanzymes” with interdependent ion channel and protease activity conferred by a single structural domain embedded in the membrane.
EXPERIMENTAL PROCEDURES
Constructs
A pET21 bacterial expression was used to express PSH (incorporating the 5 mutations previously identified to increase protein stability: D40N, E42S, A147E, V148P, A229V) and wild type FlaK (WT FlaK) for protein production (7, 9). QuiKChange mutagenesis (Agilent, Santa Clara, CA) was used to generate the mutations D79N FlaK, 2Cys-FlaK, and PSH D162→A; D220→A.
Protein Construction
Plasmids were transformed into BL21 DE3 cells. Cells were grown to an A600 of ∼0.6, and then protein production induced by the addition of 1 mm IPTG. Spheroplasts were made and the protein was purified as previously described (9). Protein was run over Cobalt gel-filtration columns to separate out the protein from contaminants. The purity of the proteins and all mutants were examined by SEC-MALS, and also on Coomassie Blue gels. Cobalt column purified PSH was applied to partial proteolysis by endoproteinase Glu-C (V8 protease from Sigma) at room temperature for 30 min at 0.03 mg/ml. The processed protein was further purified by size exclusion chromatography. The peak fractions were pooled for functional studies.
Consistent with purifications previously published results, no observable contaminants which could impair experiments was detected (7). The proteolytic activity of the proteins was also assessed; either by testing as previously reported, or with mammalian cell lysate (for an example, see Fig. 4).
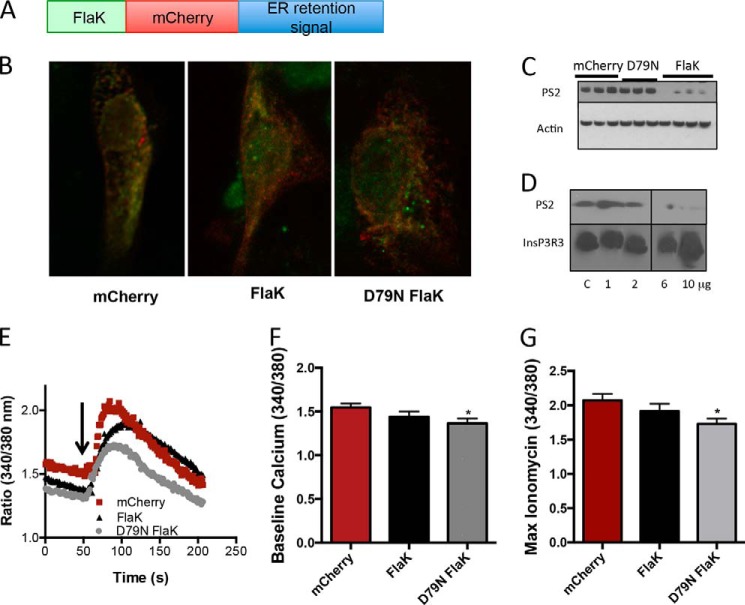
FIGURE 4.
FlaK expression in mammalian cells. A, schematic diagram of the FlaK-mCherry construct. B, distribution of the FlaK construct in LLC-PK1 cells was assessed by staining for the His tag (red), which was downstream of the mCherry tag. The endoplasmic localization was confirmed by overlap with endoplasmic reticulum resident protein calnexin (green). C, expression of FlaK, but not D79N FlaK or the mCherry control resulted in decreased expression of presenilin 2. D, incubation LLC-PK1 cell lysates with purified FlaK protein causes degradation of presenilin 2 but not InsP3R1. C stands for the buffer control. E, Ca2+ response (assessed with Fura2-AM) after the addition of 5 μm ionomycin in MEF cells transfected with mCherry control vector, FlaK, or mFlaK, Arrow. F, quantification of the resting Ca2+ in MEF cells. G, maximum Ca2+ response after the addition of 5 μm ionomycin. n = 19–32 cells from at least three independent experiments.
Lipid Bilayer Studies
Purified PSH or FlaK protein (7) (2–4 ng/ml) was added to lipid bilayers containing phosphatidylethanolamine and phosphatidylcholine (3:1 w/w) dissolved in decane (40 mg lipid ml−1) (Avanti Polar Lipids, Alabaster, AL). All experiments were performed with 250 mm NaCl, KCl, or 50 mm CaCl2 on the cis side and 50 mm NaCl, KCl, or 10 mm CaCl2 on the trans side. All solutions had a pH of 7.35 and contained 10 mm Tris. Experiments were performed under voltage clamp conditions on a Bilayer Clamp BC-525C (Warner Instruments, Hartford, CT).
Recordings were filtered at 20 kHz and digitized at 1 kHz for single channel analysis using pClamp 9.0 software (Molecular Devices, Sunnyvale, CA). Open probability was determined by using current recordings from 30 s of continuously recorded data. Current amplitudes were determined by running single channel searches over at least 30 s continually recorded data, and included more than 100 channel openings per recording. All representative current traces presented were further filtered at 500 Hz and are 5 s long. For esthetic purposes, regularly timed equipment resets have been digitally removed.
Calcium Imaging
Murine embryonic fibroblasts (MEFs) or LLC-PK1 cells were transfected wild type FlaK (WT FlaK) with a C-terminal His tag cloned into pCMV-ER (Invitrogen), upstream of mCherry. QuiKChange mutagenesis was used to generate D79N FlaK and 2Cys FlaK (Agilent). Cells were preincubated in Fura-2AM (5 μm with 0.1% pluronic acid and 2.5 μm probenecid, Invitrogen) and incubated in imaging buffer (in mm: 130 NaCl, 4.7 KCl, 1.2 KH2PO4, 1 MgSO4, 1.25 CaCl2 5 dextrose, and 20 HEPES, pH 7.4) at 37°. Ionomycin (5 μm) was used to empty stores in the presence of imaging buffer with the Ca2+ replaced with 1 mm EGTA and 1.25 mm MgCl2. Fura-2AM was excited with a Lamba DG4 fitted with 340/380 nm excitation filters (Sutter), and images collected with an OrcaER CCD camera (Hanamatsu) mounted on an Olympus microscope and using MetaMorph software (Molecular Devices). One-tailed unpaired Student's t test was used to determine significance with p < 0.05.
RESULTS
PSH Forms a Monovalent Cation Channel
As PSH and FlaK are proteases containing the same GxGD catalytic motif found in presinilin (Fig. 1A), we tested the idea that PSH and FlaK are ion channels. We started by reconstituting purified PSH protein (including the 5 stabilizing mutations that were made in the crystallized structure (9), but do not alter enzymatic activity, to enable direct comparisons with the solved structure) into a lipid bilayer. Downward deflecting currents of ∼1.5 pA at 0 mV were detected with a 250:50 mm NaCl gradient, indicating that PSH conducted Na+ (Fig. 1B). Currents were recorded over a range of voltages, with a conductance of ∼35 pS calculated for Na+ (Fig. 1C). There was no indication that PSH could conduct anions. As prokaryotic cells are very small, and do not contain an endoplasmic reticulum (ER), they do not buffer Ca2+ as efficiently as mammalian cells. Thus, they are not expected to have Ca2+ selective channels. Consistent with this, no currents were observed when the bath solution was 50:10 mm CaCl2 cis:trans, respectively, over a range of voltages.
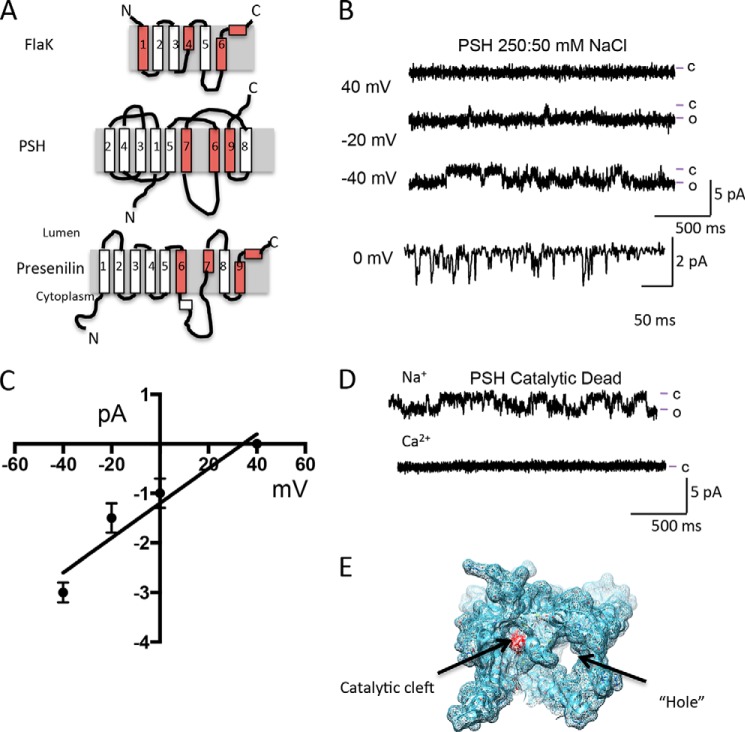
FIGURE 1.
The GxGD protease PSH forms a channel. A, cartoon depiction of PSH, FlaK, and presenilin. Helices containing residues critical for protease activity and formation of the catalytic cleft are colored red. B, reconstitution of PSH into the lipid bilayer results in currents. Lines to the side of the traces represent the open (o) and closed (c) current levels. Current traces shown were filtered at 250 Hz. C, current-voltage relationship of PSH with 250 mm NaCl on the cis side and 50 mm NaCl on the trans side suggests that PSH is a monovalent cation selective ion channel. Current amplitude values are calculated from at least 200 openings at each voltage from at least three experiments using at least two different protein preparations. D, Na+ currents are still present in a catalytic dead mutant version of PSH at 0 mV. Note that there are no current openings observed with Ca2+ as the current carrier. E, depiction of the hole and the catalytic cleft in PSH (PDB: 4HYG and 4HYD).
Catalytic Dead PSH Still Forms a Monovalent Cation Channel
To determine if the catalytic site in PSH influences channel activity, we engineered a double AA mutation at both conserved catalytic aspartates, which has previously been demonstrated to abolish all catalytic activity (8, 9). In presenilin, the catalytically important aspartic residues are also associated with endoproteolysis, and disruption of these residues is associated with FAD (1). In the bilayer, the catalytic dead mutant conducted Na+ and K+ but not Ca2+ (Fig. 1D).
In the crystal structure of PSH, a “hole” comprising of helices 2 and 3 was observed (9). The “hole” spanned the distance of the bilayer, and had a diameter that would be suitable for hydrated ions (ie: greater than 2 Å) to pass through (9) (Fig. 1E). It was previously speculated that this “hole” could constitute a channel pore (9, 17, 20). It was also interesting to note that the “hole” was lined with positively charged ions, which would hinder the passage of positively charged ions such as Na+. We noted that the helices that are responsible for the “hole” are missing in another crystalized GxGD protease, FlaK. We therefore hypothesized that removal of these helices, as observed in FlaK, would be ideal to test whether a six-transmembrane GxGD protease lacking the “hole” could form a functional ion channel.
FlaK Forms a Monovalent Cation Channel
To test the hypothesis that FlaK also formed an ion channel, we reconstituted purified wild type (WT) FlaK protein into a lipid bilayer. Downward deflecting currents of ∼2 pA at 0 mV were detected with a 250:50 mm NaCl gradient, indicating that WT FlaK conducted Na+ (Fig. 2A). Currents were recorded over a range of voltages to determine the ion and voltage sensitivity of the channel (Fig. 2, A and F). The channel formed by FlaK was nearly ideally cation selective, but not voltage sensitive, as found for PSH. The slope conductance for Na+ was calculated to be 85 pS, larger than PSH. The open probability of PSH at 0 mV was around 5%, and the open dwell time was around 3 ms, although examples of longer opening were also detected (Fig. 2, A–C). A similar current amplitude of 2 pA at 0 mV and a slightly higher slope conductance of 97 pS was obtained when K+ was used as a charge carrier (Fig. 2, D and F). Again, consistent with FlaK being a prokaryotic ion channel protein, no currents were observed when the bath solution was changed to 50:10 mm CaCl2 cis:trans, respectively, over the voltage range of −80 to +80 mV (Fig. 2E).
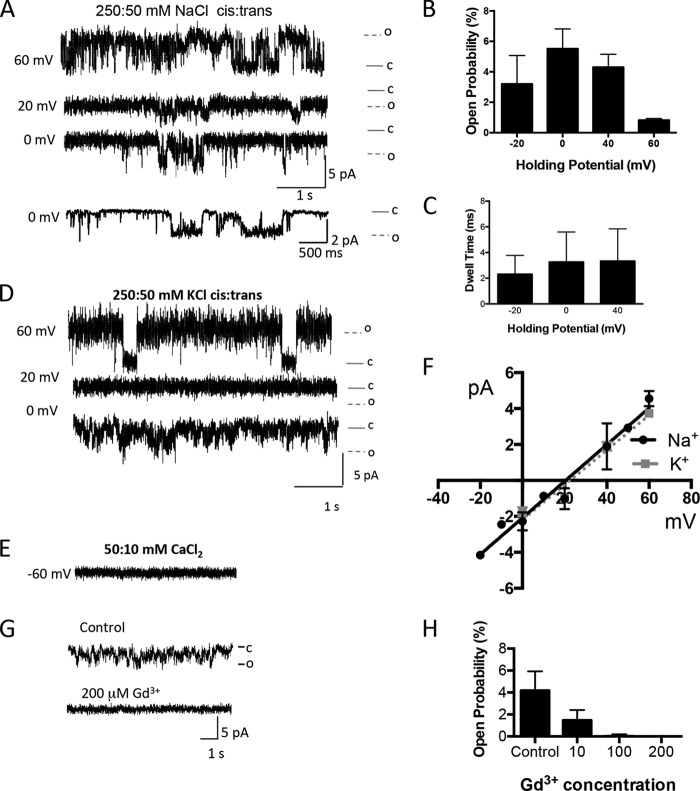
FIGURE 2.
FlaK reconstituted in a lipid bilayer conducts monovalent cations. A, reconstitution of WT FlaK into the lipid bilayer results in Na+ currents. Traces are shown with 250:50 mm NaCl cis:trans over a range of holding potentials. B, open probability of FlaK in 250:50 mm NaCl cis:trans over a range of holding potentials. Data were analyzed from 2–7 experiments at the various voltages from 30–90s records of traces. C, dwell time of FlaK in 250:50 mm NaCl cis:trans over a range of holding potentials. Data were analyzed from 30–90s records of traces, and are averaged over five different experiments. Data presented as mean ± S.D. D, reconstitution of WT FlaK into the lipid bilayer results in K+ currents over a range of holding potentials. Traces are shown with 250:50 mm KCl cis:trans. E, WT FlaK is unable to conduct Ca2+. Example trace is shown at −60 mV with 50:10 mm CaCl2 cis:trans. F, current-voltage relationship of cations with WT FlaK. The current amplitudes were obtained at holding potentials ranging from 0 to 60 mV. Each point represents the average of >100 channel openings, from at least three different experiments from two different protein preparations. G, Na+ current traces are shown in the presence of 0 (Control), 10, and 200 μm Gd3+. Experiments were performed with 250 mm NaCl on the cis side and 50 mm NaCl on the trans side. All representative current traces shown were obtained at 0 mV, filtered at 250 Hz. Downward deflections are channel openings. H, concentration-dependent inhibition of the open probability of FlaK by Gd3+. Experiments were conducted with Gd3+ (n = 3 experiments) added to the cis side. Error bars represent the S.E.
Although there are no known specific inhibitors of FlaK, we used gadolinium, an ion channel blocker of transient receptor potential channels (21). The open probability of WT FlaK at 0 mV was decreased with 10 μm Gd3+ added to the cis side of the membrane (Fig. 2G). Increasing the Gd3+ concentration to 200 μm fully inhibited all channel activity (Fig. 2H). Taken together, this suggests that WT FlaK, like PSH acts as a non-voltage, non-ligand dependent monovalent cation channel in addition to its known function as a GxGD protease. Thus, FlaK, like PSH, is a chanzyme. Moreover, the data suggest that a protein without the helices required to form the “hole” identified in crystal structures still enables the protein to form a channel.
Catalytic Dead FlaK Forms a Divalent Cation Channel
To interrogate if the channel activity of FlaK was linked to its catalytic activity, we created two mutations (Fig. 3A). The D79N mutation completely abolished protease activity (7). Addition of D79N FlaK to the bilayer with a Na+ gradient resulted in channel activity with higher current amplitude compared with WT FlaK, and a near doubling of the slope conductance to 195 pS (Fig. 3, B and C). The increased conductivity of Na+ in the D79N mutant is similar to the reports of increased Ca2+ conductance by mutations in holopresenilin, including the removal of the self-cleavage site of presenilin (10). Interestingly, D79N FlaK was now Ca2+ permeable, with a slope conductance of 120 pS and current amplitude of ∼2 pA at 0 mV (Fig. 3, B and C). The permeability of D79N FlaK was Na+>K+∼Ca2+.
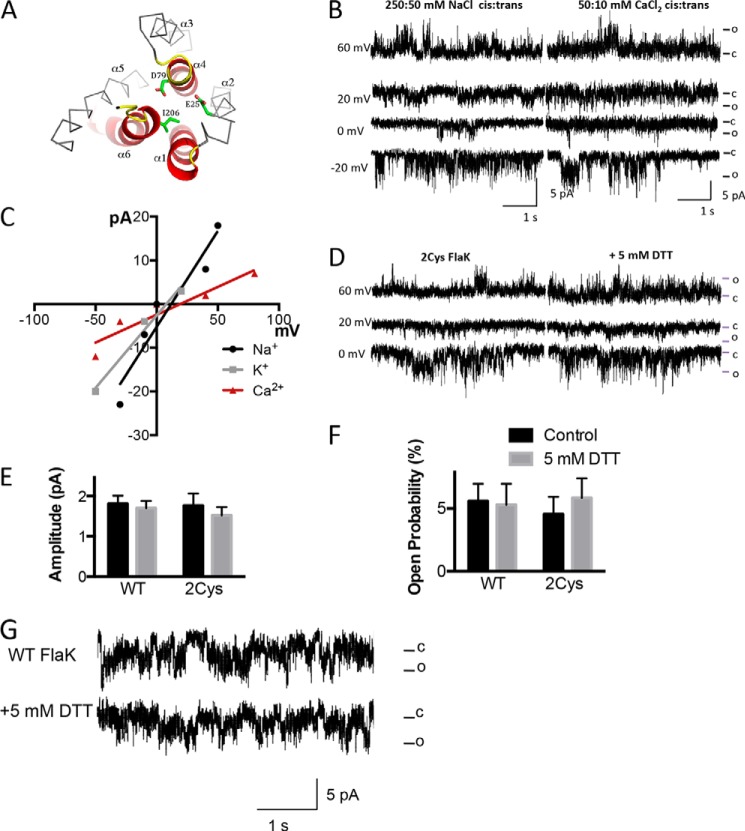
FIGURE 3.
Catalytic dead FlaK variants D79N and 2Cys FlaK have cation channel activity. A, schematic diagram of the mutations made to FlaK to diminish its catalytic activity. In this top view cartoon, without the soluble domain for clarity, the catalytic critical core structure is shown in red. The highly conserved motifs contributing to the catalytic site are in yellow. The D79N, E25C, and I206C mutations made in this work are highlighted in green in a stick model. The helices are labeled α1–6. B, reconstitution of D79N FlaK into the lipid bilayer results in Na+ currents similar to WT FlaK (left traces). D79N FlaK conducts Ca2+ (right traces). Currents were obtained with 50:10 mm Ca2Cl cis:trans. C, current-voltage relationship of D79N FlaK with Na+, K+ or Ca2+ as charge carrier. Each point is the average of >100 channel openings from three different protein preparations. D, insertion of 2Cys FlaK into the lipid bilayer resulted in monovalent cation channel activity (left traces). Breaking the crosslinking disulfide bonds with DTT did not affect channel activity (right traces). Experiments were performed with 250:50 mm NaCl cis:trans. E and F, 5 mm DTT does not have a significant effect on Na+ current amplitude (E) or the Na+ current open probability (F) of the channel formed by WT or 2Cys-FlaK (n = 3–5). Error bars represent the S.E. G, WT FlaK inserted into the bilayer was not affected by the addition of 5 mm DTT. Traces are taken from experiments containing 250:50 mm NaCl cis:trans, at 0 mV holding potential.
To further test that FlaK proteolytic activity was linked to the channel activity, a second mutant, with two cysteine residues that spontaneously cross-link FlaK through α-helices 2 and 6 and has no protease activity was also examined (Fig. 3A) (7). In the bilayer, 2Cys FlaK was able to conduct Na+, but not Ca2+, and the activity was not enhanced by addition of the reducing agent DTT (Fig. 3, D–F), although these agents do restore catalytic activity (7). Addition of reducing agents did not affect WT FlaK current amplitude or single channel activity (Fig. 3G).
The results obtained using the two FlaK mutants D79N and 2Cys suggest that FlaK channel activity can be separated from protease activity, but that the ion selectivity is linked to critical catalytic residues in α-helix 4, which harbors the GxGD motif. The data obtained from the PSH mutant suggest that although the catalytic site can modulate channel activity, disruption of the catalytic site is not sufficient to prevent channel activity. Taken together, the data suggest that the ion channel activity can be modulated by the catalytic properties of the protease, and that these two features are interdependent.
FlaK in Mammalian Cell Alters Calcium Homeostasis
Although planar bilayer experiments provide essential answers on the channel activity of FlaK, we wanted to confirm in a different system that the D79N FlaK mutant altered Ca2+ homeostasis. We therefore expressed FlaK and the D79N FlaK constructs in LLC-PK1 and mouse embryonic fibroblasts (MEFs). Initial experiments showed that expression of FlaK resulted in cellular death, either due to the intrinsic enzymatic activity of FlaK, or due to its monovalent cation conductance. To reduce the effect of a non-selective cation channel, we targeted FlaK to the ER and confirmed its localization and expression levels by including a C-terminal mCherry-His tag and an ER localization sequence (Fig. 4, A and B). To test if FlaK still retained intrinsic catalytic activity in a mammalian environment, we reasoned that FlaK would be capable of cutting endogenous presenilin. Consistent with our hypothesis, we found that expression of presenilin to be decreased in the FlaK transfected cells, but not in the mCherry or the D79N FlaK-transfected cells (Fig. 4C).
To confirm that this was a direct effect of FlaK cutting the protein, and not of some nonspecific effect, we conducted an in vitro assay by incubating increasing amounts of purified FlaK, the same protein used for the lipid bilayer experiments, with cell lysates obtained from LLC-PK1 cells (Fig. 4D). In these experiments, we found that that the presenilin band disappeared, but another ER protein, InsP3R3 remained intact. These results confirmed that the purified FlaK used for the bilayer experiments retained its proteolytic activity, and that FlaK, when expressed in a mammalian cell also retains catalytic activity.
As we had now tested the catalytic activity of FlaK and D79N FlaK, we then went on to test if Ca2+ homeostasis was altered upon expression of the D79N FlaK mutant. In mammalian cells, we found that the resting Ca2+ levels, as measured with the ratiometric Ca2+ indicator Fura 2AM, was significantly lower in D79N FlaK-transfected cells compared with either FlaK or mCherry (control)-transfected cells (Fig. 4, E and F). As our bilayer data suggested that D79N FlaK acts as a leak channel, we reasoned that the ER store concentration of Ca2+ would also be lower in the D79N FlaK-transfected cells. To test this hypothesis, we added 5 μm ionomycin (Fig. 4G), and found that the released Ca2+ from the D79N FlaK-transfected cells was significantly lower than the levels in mCherry or FlaK-transfected cells. These mammalian cell data therefore support the planar lipid bilayer results as they demonstrate that a single mutation that renders FlaK catalytically inactive also modifies Ca2+ homeostasis in mammalian cells.
An Intact Loop for PSH May Be Required for Pore Formation
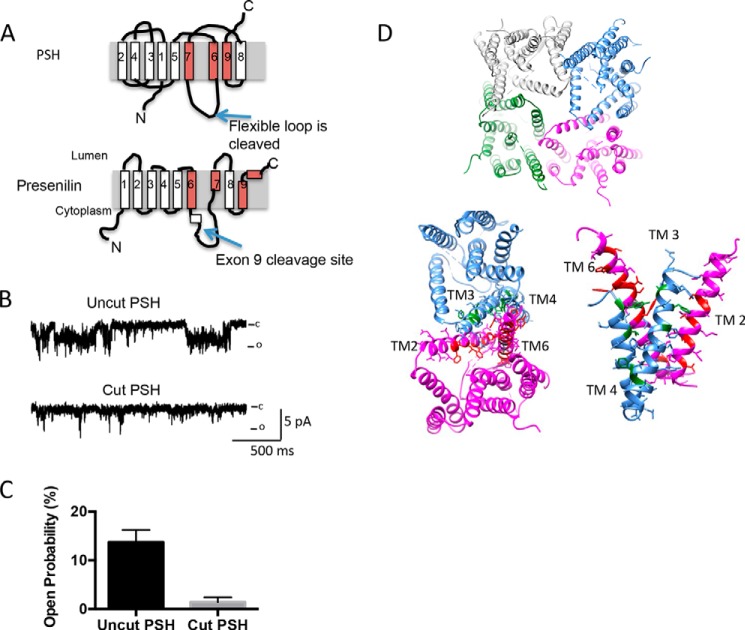
It has been previously reported that presenilin forms a Ca2+ channel, however, a distinguishing feature of the currents was that the amplitude was small, and they were primarily observed as an increase in membrane current noise (10). In contrast, the channels formed by FlaK and PSH had larger amplitudes than the ones previously reported in presenilin (10). One major difference between the proteins used in our study and presenilin, and indeed, the published PSH crystal structure (9), is that 20 amino acids (residues 182–209) on the surface were trimmed by the addition of the V8 protease. The trimmed region is analogous to the extracellular loop that is subjected to presenilinase cleavage in presenilin (exon 9, Fig. 5A). Intriguingly, for presenilin, deletion of exon 9 has been associated with a FAD causing mutation (1). Moreover, disruption of exon 9 alters channel function (10).
FIGURE 5.
The cutting of PSH largely abolishes channel activity. A, schematic diagram of the cut sites. B, insertion of cut PSH (which still is capable of catalytic activity) into the lipid bilayer largely diminishes activity with 250:50 mm NaCl at 0 mV. C, analysis of PSH activity at 0 mV with 250:50 mm NaCl at 0 mV. Analysis is from three different experiments; examining bursts of activity of 30–90 s duration. Error bars represent S.E. D, depiction of how four PSH molecules can arrange to form a channel-like structure (top). Depiction of the interface between two neighboring PSH molecules (bottom left). The corresponding residues in presenilin that are known to be associated with FAD are highlighted in either red or green. These residues were selected from an alignment of PSH and presenilin previously published (9). A side-on view of the interface between two neighboring PSH molecules is shown (bottom right).
We therefore tested to see if disruption of this loop could alter the channel activity. The protein was incubated with V8 protease, and the cleaved product purified. Remarkably, disruption resulted in the near abolition of all channel activity, from around 10% to 1% activity (Fig. 5, B and C). These results suggest that the loop is either critical for the arrangement of helices required to form the pore, or that it contributes to the pore. The published structure suggested that helices 6–9 would be required for the formation of the pore; however, both the cut and uncut versions still have enzymatic activity.
DISCUSSION
Proteins are traditionally thought to have one function. For example, ion channels only conduct ions, and proteases only cut protein substrates. In this study, we have demonstrated that two GxGD proteases, PSH and FlaK, can also form monovalent cation channels. Secondly, we show that disruption of the GxGD catalytic motif (analogous to those that are associated with FAD) alters the ion selectivity of FlaK from monovalent to divalent cations, indicating that the ion channel properties and the catalytic activity are related. We propose that archeobacteria GxGD proteases, are true chanzymes, in that they have both enzymatic and ion channel properties in the same structural domain. Our data provide additional support to the suggestion that presenilin-like proteins have the capability of acting as ion channels.
We demonstrate that the enzymatic activity of FlaK is inextricably linked to the ion channel activity, and both the protease and the ion channel features are essential in maintaining function of this chanzyme. Other than presenilin, only the TRPMs have been identified to act as both enzymes and ion channels (19). However, the kinase activity of TRPM6 and TRPM7 is localized to the C terminus tail of the protein, and is largely independent of the ion channel activity. It remains to be seen whether ion channel features extend to other transmembrane proteins that are not traditionally thought to be ion channels. We speculate that these non-traditional channels could have important functional ramifications. Moreover, similar characteristics of other transmembrane membrane proteins may underlie the unresolved “leak” that is observed across cellular membranes including the plasma and ER membranes (16).
Our results show that WT and mutant PSH and FlaK conduct cations in a bilayer, and disruption of the protease activity can influence ion selectivity. Our results are in agreement with the finding that the holoform of presenilin conducts cations, and supports the presenilin leak hypothesis (10, 22, 23). Our data fit with FlaK and PSH as evolutionarily earlier di-aspartyl membrane proteases that later acquired divalent cation permeability. Indeed, our results demonstrate that a single amino acid change is sufficient to alter the ion selectivity of FlaK, and the mutant FlaK can alter Ca2+ homeostasis in mammalian cells. The change in the store load of Ca2+ is comparable to published reports about leaky Ca2+ channels, for example, mutations to the ryanodine receptor that are associated with heart failure (24). Interestingly, we observed a near abolition of ion channel activity with the cleaved form of PSH, where residues were removed in an analogous position to the internal presenilinase site. We therefore speculate that the cleaved form of presenilin, like PSH, does not readily form an ion channel. This finding is supported by other studies that suggest that the cleaved form of presenilin acts as a dynamic modifier of either the RyR or InsP3R to modulate Ca2+ signaling (25–34).
How can the secretases form a channel? One possibility is oligomerization. Supporting this idea, we superimposed the mutations in presenilin that are disease causing but not on the helices that contain the catalytic residues (Fig. 5D). The superimposition demonstrates that a number of the presenilin mutations are found in the helices that are believed to form the interface between neighboring PSH molecules. We therefore speculate that oligomerization is essential for channel activity, and alteration of this arrangement, either by presenilinase or by removal of specific residues can significantly disrupt channel activity. There is some evidence supporting oligomerization of the secretases: In the crystal structure, FlaK in a detergent mixture of Foscholine-12 and Cymal-6 was packed as a dimer with a quite large interface in one asymmetric unit (7). Similarly, PS1 forms homodimers, where a catalytic site can be made from two inactive presenilin monomers (35–38).
Alternatively, the crystal structures of both FlaK and PSH reveal a water filled vestibule lined by transmembrane segments 1, 2, 6, and 4, which is believed to form the area where protein substrates dock to be cleaved (7). Interestingly, the enzymatic cavity in PSH is larger than what would be necessary to permit monovalent cation permeation (7.5 Å). This large cavity suggests that it would be possible for ions to pass through in the absence of transmembrane rearrangement, which is required for substrate cleavage. Likewise, transmembrane segments 6, 7, 8, and 9 of PS1 are believed to form a similar arrangement (39), where transmembrane 9 of PS1 is a moveable unit, analogous to transmembrane 6 of FlaK (40). Based on the arrangement of the helices in FlaK, it would be possible for small monovalent cations to pass through the cavity. However, our finding that the crosslinked 2Cys FlaK still conducted cations suggests that helices 2 and 6 may not be involved in pore formation, or that movement of these helices may not be required. The rigidity of the 2Cys FlaK mutant also limits the movement of transmembrane 4, which is critical in catalytic activity. It is possible that a single crosslink was not sufficient to block the putative pore, and that additional crosslinks are required.
Our study shows that the archeobacterial proteins PSH and FlaK have dual functions as both ion channels and as proteases, the two activities are interdependent, and the apparent hole in PSH does not contribute to ion activity. We suggest that our data broadens the definition of what constitutes an ion channel, and it remains to be seen whether other transmembrane proteases also have ion channel functionality. Within the context of γ-secretases, these data provide support for all existing Ca2+-dependent hypotheses about presenilin in that GxGD protease family members are chanzymes, where they can act both as an ion channel and as a modulator of Ca2+ homeostasis via enzymatic activity.
Acknowledgments
We thank Michelle Mo and Colleen Feriod for helpful discussions and comments. Yi Xue is thanked for help with protein purification.
This work was supported by National Institutes of Health Grants DK087844 and DK090744 (to B. E. E.).
- FAD
- familial Alzheimer disease
- PSH
- presenilin homolog.
REFERENCES
- 1. Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 2. Brown M. S., Ye J., Rawson R. B., Goldstein J. L. (2000) Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 [DOI] [PubMed] [Google Scholar]
- 3. Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nature Medicine 2, 864–870 [DOI] [PubMed] [Google Scholar]
- 4. Hardy J. (2009) The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J. Neurochem. 110, 1129–1134 [DOI] [PubMed] [Google Scholar]
- 5. Castellani R. J., Smith M. A. (2011) Compounding artefacts with uncertainty, and an amyloid cascade hypothesis that is 'too big to fail'. J. Pathol. 224, 147–152 [DOI] [PubMed] [Google Scholar]
- 6. Lu P., Bai X. C., Ma D., Xie T., Yan C., Sun L., Yang G., Zhao Y., Zhou R., Scheres S. H., Shi Y. (2014) Three-dimensional structure of human γ-secretase. Nature 512, 166–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu J., Xue Y., Lee S., Ha Y. (2011) The crystal structure of GXGD membrane protease FlaK. Nature 475, 528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres-Arancivia C., Ross C. M., Chavez J., Assur Z., Dolios G., Mancia F., Ubarretxena-Belandia I. (2010) Identification of an archaeal presenilin-like intramembrane protease. PloS one 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X., Dang S., Yan C., Gong X., Wang J., Shi Y. (2013) Structure of a presenilin family intramembrane aspartate protease. Nature 493, 56–61 [DOI] [PubMed] [Google Scholar]
- 10. Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S. F., Hao Y. H., Serneels L., De Strooper B., Yu G., Bezprozvanny I. (2006) Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 126, 981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stutzmann G. E. (2005) Calcium dysregulation, IP3 signaling, and Alzheimer's disease. The Neuroscientist 11, 110–115 [DOI] [PubMed] [Google Scholar]
- 12. Mattson M. P. (2010) ER calcium and Alzheimer's disease: in a state of flux. Science Signaling 3, pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Supnet C., Bezprozvanny I. (2011) Presenilins function in ER calcium leak and Alzheimer's disease pathogenesis. Cell Calcium 50, 303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brunello L., Zampese E., Florean C., Pozzan T., Pizzo P., Fasolato C. (2009) Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J. Cell. Mol. Med. 13, 3358–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giacomello M., Barbiero L., Zatti G., Squitti R., Binetti G., Pozzan T., Fasolato C., Ghidoni R., Pizzo P. (2005) Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer's disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol. Dis. 18, 638–648 [DOI] [PubMed] [Google Scholar]
- 16. Bandara S., Malmersjö S., Meyer T. (2013) Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Science Signaling 6, ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bezprozvanny I. (2013) Presenilins and calcium signaling–systems biology to the rescue. Science Signaling 6, pe24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stutzmann G. E., Mattson M. P. (2011) Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol. Rev. 63, 700–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montell C. (2003) Mg2+ homeostasis: the Mg2+nificent TRPM chanzymes. Curr. Biol. CB 13, R799–801 [DOI] [PubMed] [Google Scholar]
- 20. Wolfe M. S. (2013) Structural biology: Membrane enzyme cuts a fine figure. Nature 493, 34–35 [DOI] [PubMed] [Google Scholar]
- 21. Anyatonwu G. I., Ehrlich B. E. (2005) Organic cation permeation through the channel formed by polycystin-2. J. Biol. Chem. 280, 29488–29493 [DOI] [PubMed] [Google Scholar]
- 22. Das H. K., Tchedre K., Mueller B. (2012) Repression of transcription of presenilin-1 inhibits γ-secretase independent ER Ca(2)(+) leak that is impaired by FAD mutations. J. Neurochem. 122, 487–500 [DOI] [PubMed] [Google Scholar]
- 23. Nelson O., Tu H., Lei T., Bentahir M., de Strooper B., Bezprozvanny I. (2007) Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin. Investig. 117, 1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shan J., Xie W., Betzenhauser M., Reiken S., Chen B. X., Wronska A., Marks A. R. (2012) Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circulation Research 111, 708–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheung K. H., Mei L., Mak D. O., Hayashi I., Iwatsubo T., Kang D. E., Foskett J. K. (2010) Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Science Signaling 3, ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheung K. H., Shineman D., Müller M., Cárdenas C., Mei L., Yang J., Tomita T., Iwatsubo T., Lee V. M., Foskett J. K. (2008) Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58, 871–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai C., Lin P., Cheung K. H., Li N., Levchook C., Pan Z., Ferrante C., Boulianne G. L., Foskett J. K., Danielpour D., Ma J. (2006) The presenilin-2 loop peptide perturbs intracellular Ca2+ homeostasis and accelerates apoptosis. J. Biol. Chem. 281, 16649–16655 [DOI] [PubMed] [Google Scholar]
- 28. Leissring M. A., Parker I., LaFerla F. M. (1999) Presenilin-2 mutations modulate amplitude and kinetics of inositol 1,4,5-trisphosphate-mediated calcium signals. J. Biol. Chem. 274, 32535–32538 [DOI] [PubMed] [Google Scholar]
- 29. Leissring M. A., Paul B. A., Parker I., Cotman C. W., LaFerla F. M. (1999) Alzheimer's presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J. Neurochem. 72, 1061–1068 [DOI] [PubMed] [Google Scholar]
- 30. Leissring M. A., Yamasaki T. R., Wasco W., Buxbaum J. D., Parker I., LaFerla F. M. (2000) Calsenilin reverses presenilin-mediated enhancement of calcium signaling. Proc. Natl. Acad. Sci. U. S. A. 97, 8590–8593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shilling D., Mak D. O., Kang D. E., Foskett J. K. (2012) Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J. Biol. Chem. 287, 10933–10944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stutzmann G. E., Smith I., Caccamo A., Oddo S., Laferla F. M., Parker I. (2006) Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 26, 5180–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stutzmann G. E., Smith I., Caccamo A., Oddo S., Parker I., Laferla F. (2007) Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Ann. NY Acad. Sci. 1097, 265–277 [DOI] [PubMed] [Google Scholar]
- 34. Bruno A. M., Huang J. Y., Bennett D. A., Marr R. A., Hastings M. L., Stutzmann G. E. (2012) Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer's disease. Neurobiology Aging 33, 1001 e1001–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cervantes S., Gonzàlez-Duarte R., Marfany G. (2001) Homodimerization of presenilin N-terminal fragments is affected by mutations linked to Alzheimer's disease. FEBS Lett. 505, 81–86 [DOI] [PubMed] [Google Scholar]
- 36. Cervantes S., Saura C. A., Pomares E., Gonzàlez-Duarte R., Marfany G. (2004) Functional implications of the presenilin dimerization: reconstitution of γ-secretase activity by assembly of a catalytic site at the dimer interface of two catalytically inactive presenilins. J. Biol. Chem. 279, 36519–36529 [DOI] [PubMed] [Google Scholar]
- 37. Hébert S. S., Godin C., Tomiyama T., Mori H., Lévesque G. (2003) Dimerization of presenilin-1 in vivo: suggestion of novel regulatory mechanisms leading to higher order complexes. Biochem. Biophys. Res. Commun. 301, 119–126 [DOI] [PubMed] [Google Scholar]
- 38. Herl L., Lleo A., Thomas A. V., Nyborg A. C., Jansen K., Golde T. E., Hyman B. T., Berezovska O. (2006) Detection of presenilin-1 homodimer formation in intact cells using fluorescent lifetime imaging microscopy. Biochem. Biophys. Res. Commun. 340, 668–674 [DOI] [PubMed] [Google Scholar]
- 39. Sato C., Morohashi Y., Tomita T., Iwatsubo T. (2006) Structure of the catalytic pore of γ-secretase probed by the accessibility of substituted cysteines. J. Neurosci. 26, 12081–12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nelson O., Supnet C., Tolia A., Horré K., De Strooper B., Bezprozvanny I. (2011) Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J. Biol. Chem. 286, 22339–22347 [DOI] [PMC free article] [PubMed] [Google Scholar]